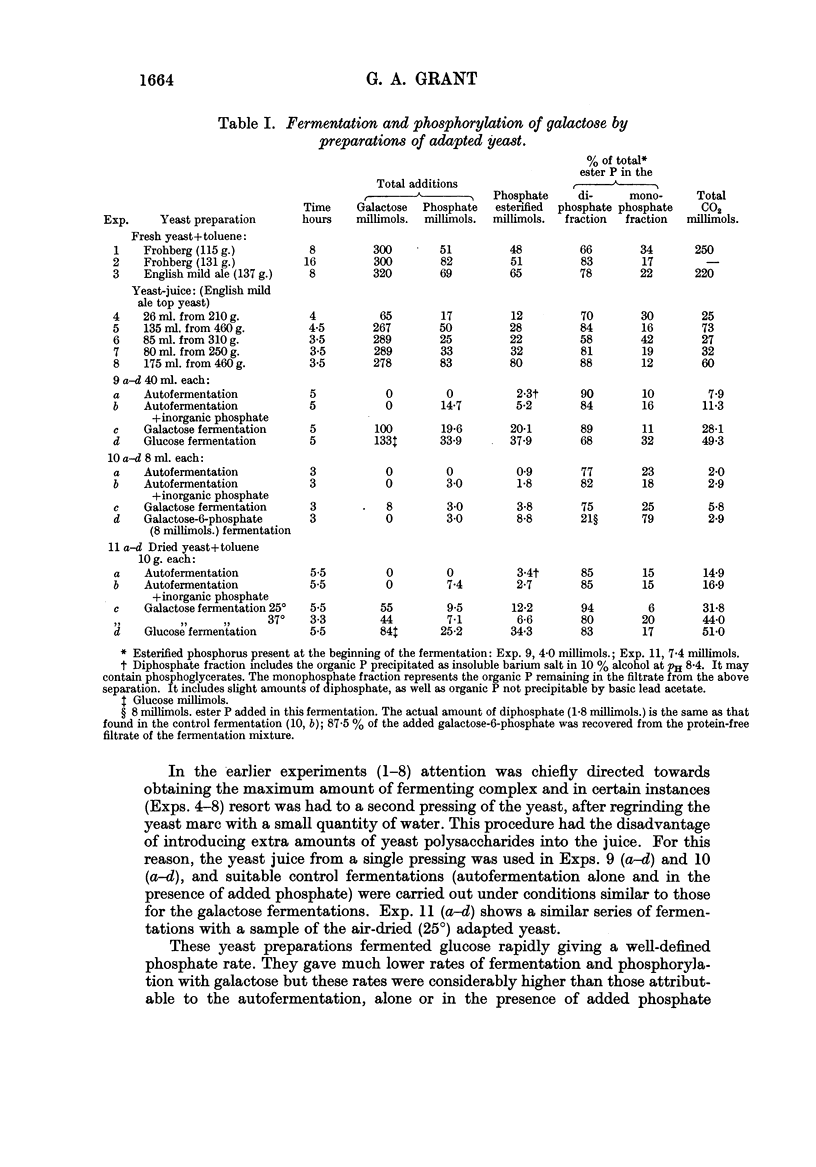
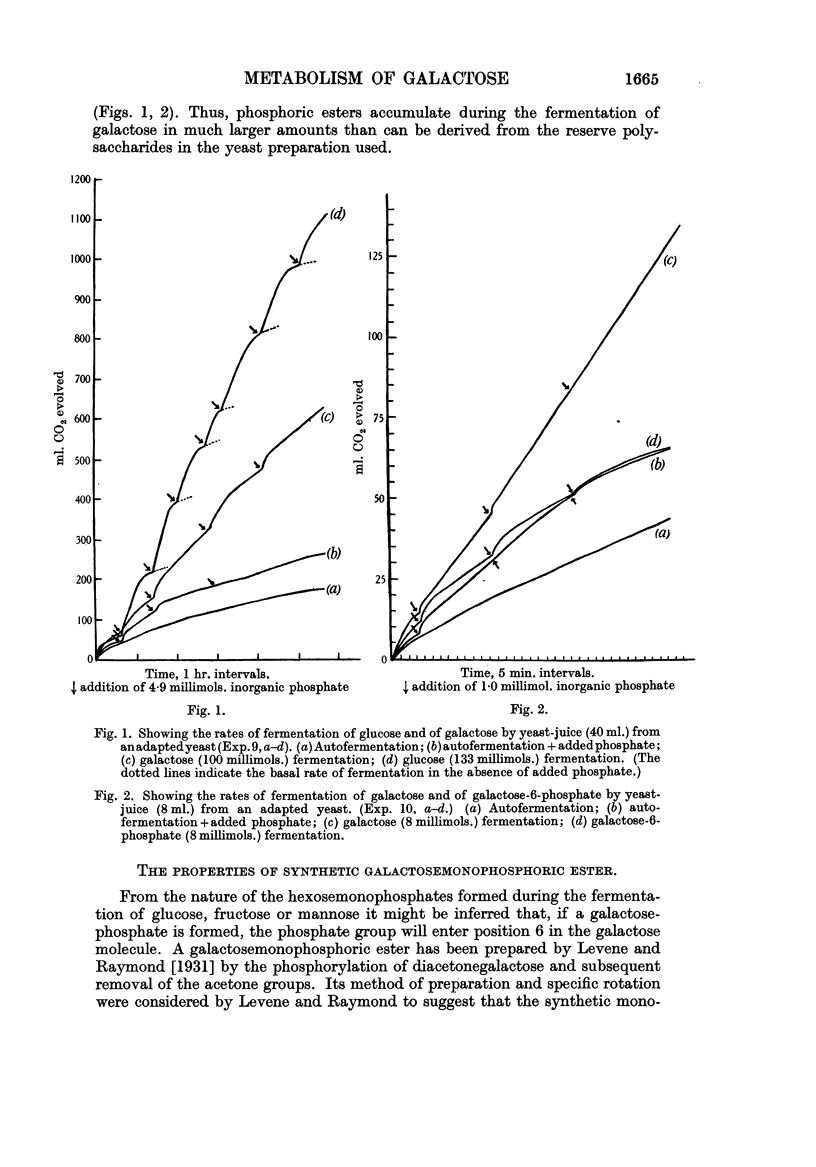
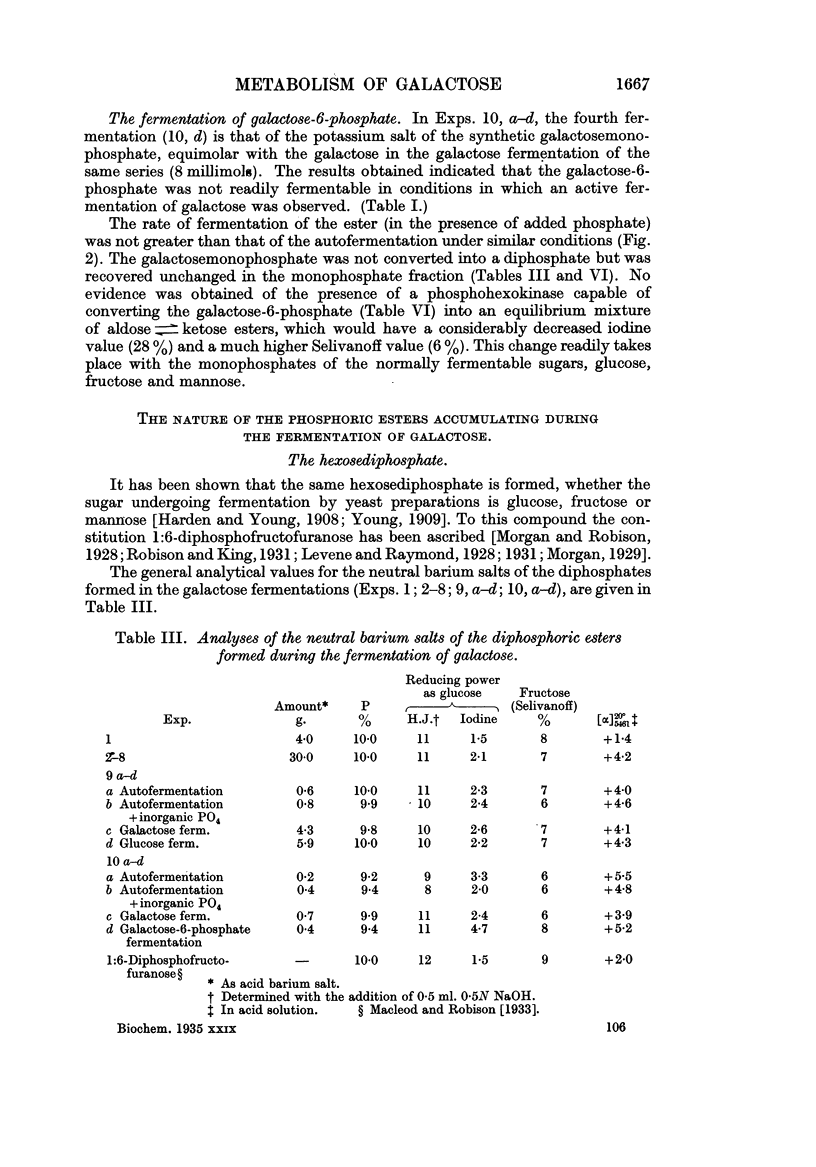
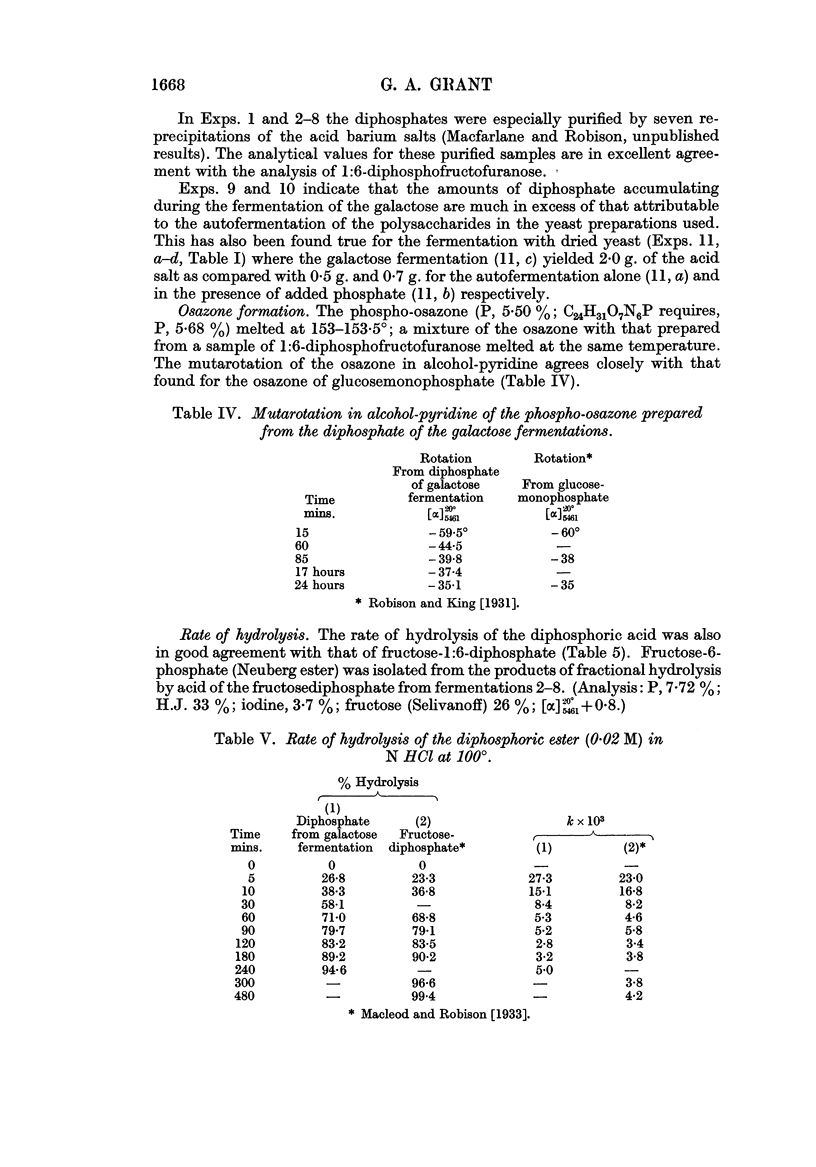
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford C. A. The glycolytic mechanisms of brain. Biochem J. 1933;27(3):903–910. doi: 10.1042/bj0270903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding V. J., Grant G. A., Glaister D. The metabolism of galactose: The behaviour of the rat towards moderate amounts of galactose. Biochem J. 1934;28(1):257–263. doi: 10.1042/bj0280257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott C. M., Robison R. Mannosemonophosphate: The fermentation of mannose by dried yeast. Biochem J. 1934;28(5):1844–1853. doi: 10.1042/bj0281844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod M., Robison R. The hydrolysis of hexosediphosphoric ester by bone phosphatase: A new fructosemonophosphate. Biochem J. 1933;27(1):286–297. [PMC free article] [PubMed] [Google Scholar]
- Martland M., Robison R. The preparation and use of the bone phosphatase. Biochem J. 1929;23(2):237–242. doi: 10.1042/bj0230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T., Robison R. Constitution of hexosediphosphoric acid. Part II: The dephosphorylated alpha- and beta-methylhexosides. Biochem J. 1928;22(5):1270–1276. doi: 10.1042/bj0221270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison R. A New Phosphoric Ester Produced by the Action of Yeast Juice on Hexoses. Biochem J. 1922;16(6):809–824. doi: 10.1042/bj0160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison R., King E. J. Hexosemonophosphoric esters. Biochem J. 1931;25(1):323–338. doi: 10.1042/bj0250323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison R., Morgan W. T. The phosphoric esters of alcoholic fermentation. Biochem J. 1930;24(1):119–132. doi: 10.1042/bj0240119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankó B., Robison R. The hydrolysis of hexosediphosphoric ester by bone phosphatase: (a) The participation of phosphohexokinase; (b) the isolation of pure fructose-1-phosphate. Biochem J. 1935 Apr;29(4):961–972. doi: 10.1042/bj0290961. [DOI] [PMC free article] [PubMed] [Google Scholar]


