Abstract
Listeria monocytogenes is a psychrotrophic food-borne pathogen that is problematic for the food industry because of its ubiquitous distribution in nature and its ability to grow at low temperatures and in the presence of high salt concentrations. Here we demonstrate that the process of adaptation to low temperature after cold shock includes elevated levels of cold shock proteins (CSPs) and that the levels of CSPs are also elevated after treatment with high hydrostatic pressure (HHP). Two-dimensional gel electrophoresis combined with Western blotting performed with anti-CspB of Bacillus subtilis was used to identify four 7-kDa proteins, designated Csp1, Csp2, Csp3, and Csp4. In addition, Southern blotting revealed four chromosomal DNA fragments that reacted with a csp probe, which also indicated that a CSP family is present in L. monocytogenes LO28. After a cold shock in which the temperature was decreased from 37°C to 10°C the levels of Csp1 and Csp3 increased 10- and 3.5-fold, respectively, but the levels of Csp2 and Csp4 were not elevated. Pressurization of L. monocytogenes LO28 cells resulted in 3.5- and 2-fold increases in the levels of Csp1 and Csp2, respectively. Strikingly, the level of survival after pressurization of cold-shocked cells was 100-fold higher than that of cells growing exponentially at 37°C. These findings imply that cold-shocked cells are protected from HHP treatment, which may affect the efficiency of combined preservation techniques.
As the demand for fresh food products with extended shelf lives is increasing, the food processing industry is using novel preservation methods to satisfy this demand. Cold storage is widely used, and new processing techniques, such as treatment with high hydrostatic pressure (HHP), are emerging. The use of combinations of mild preservation methods (hurdle technology) is becoming more prevalent. Such combinations of treatments subject bacteria to sublethal stresses which the bacteria must overcome to survive or grow in food products. However, the physiological mechanisms by which microorganisms adapt to sublethal stresses are not well understood. The food-borne human pathogen Listeria monocytogenes is known for its ability to survive when it is subjected to a variety of environmental stresses, such as high osmotic pressures (NaCl concentrations up to 10%) and temperatures as low as −0.1°C (30). This organism can cause listeriosis in immunocompromised individuals and accounts for almost 35% of all deaths in the United States due to known food-borne bacterial pathogens (19). Many outbreaks have been associated with ready-to-eat foods which may have undergone some form of minimal processing.
Mechanisms that allow low-temperature growth of microorganisms involve maintenance of cellular membrane fluidity, uptake or synthesis of compatible solutes, maintenance of the structures of macromolecules, such as ribosomes, and maintenance of protein synthesis. In L. monocytogenes, adaptation of membranes to low temperatures is accomplished by altering the branching in the methyl end of the fatty acid from iso branching to anteiso branching and by decreasing the fatty acid chain length, which results mainly in an increase in the amount of anteiso-C15:0 fatty acids (1). The osmolytes betaine and carnitine accumulate and stimulate growth at low temperatures (5, 13, 17). In L. monocytogenes the 70S ribosomal particle structure becomes unstable upon cold shock (4), and this instability must be overcome for normal protein synthesis to occur. In Escherichia coli, protein synthesis after cold shock is related to the synthesis of so-called cold shock proteins (CSPs) (23). However, production of such proteins has never been monitored in L. monocytogenes, although csp genes have been described for two strains. Two csp genes were identified in the L. monocytogenes ATCC 23074 genome by using universal csp gene primers (11), and two csp genes of L. monocytogenes EGD have been sequenced (accession numbers LMO012349 and LMO012350) (E. M. Busch and T. Chakraborty, unpublished data).
CSPs are 7-kDa proteins, and families of CSPs have been found in nearly all bacteria (10, 14, 23, 34). Several functions have been postulated for CSPs. These molecules can act as RNA chaperones and minimize secondary folding (14, 15). They may also act as transcription activators or antiterminators, thereby stimulating production of non-7-kDa cold-induced proteins (2, 8, 18). Furthermore, it has been suggested that CSPs play a role in protection against freezing (33). CSPs are not induced only at low temperature; they are also induced by other environmental stresses. CspA, the major CSP in E. coli, was found to be expressed when the organism left the stationary phase (9). Furthermore, of the nine CSPs found in E. coli, five were not induced at low temperatures. CspD, for example, is induced when there is nutritional stress and during the stationary growth phase (35). Bacillus subtilis possesses three CSPs, CspB, CspC, and CspD, and upon cold shock all three of these CSPs are induced. However, CspB and CspC are also induced under stationary-phase conditions (16).
Currently, the use of high-pressure technology for mild preservation of food products is being explored. The main advantage of this technology, which has been proposed as a valuable alternative to conventional heat treatments, is that it preserves the characteristics (vitamins and flavor) of the initial product. Inactivation of L. monocytogenes by high pressure has been described in a number of papers (22, 28). However, the physiological responses of microorganisms to sublethal pressures are not well understood. It has been proposed that HHP treatments target cellular membranes and ribosomes and that the latter adopt a less stable conformation upon pressurization (20, 25). In E. coli production of CspA is induced after HHP treatment, as is production of certain cold-induced proteins and heat shock proteins (31). Whenever mild preservation methods are combined, it is essential to determine if bacterial adaptive responses to stress conditions (e.g., low temperature) can affect the susceptibility of the bacteria to other stress conditions. Here we show that the process of adaptation of L. monocytogenes LO28 to low temperature includes induction of CSPs and that induction of CSPs also occurs upon HHP treatment. We found that the level of survival after HHP treatment of cold-shocked cells was higher than the level of survival of HHP-treated cells growing exponentially at 37°C, which may have implications for the efficiency of combined preservation techniques.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. monocytogenes LO28 (serotype 1/2c) was grown in brain heart infusion broth at 37°C. Cell growth was monitored spectrophotometrically by measuring the optical density at 620 nm (OD620). At an OD620 of 0.4 the culture was cold shocked at 10°C.
Cold adaptation and freeze survival.
To study the effect of cold shock on freeze survival of L. monocytogenes, a freeze-thaw challenge analysis was performed with aliquots removed before and 1, 2, 3, 4, and 20 h after the cold shock. Samples (10 ml in 50-ml tubes) were withdrawn, concentrated with a centrifuge (15 min, 3,000 × g), and suspended in fresh medium, and the numbers of CFU were determined; after this the samples were frozen at exactly −20°C for 20 h. After this freezing period the samples were thawed for 4 min at 37°C in a water bath, and the numbers of CFU were determined. In addition, after thawing growth at 37°C was monitored by measuring the OD620.
Protein extraction.
Samples of 10-ml bacterial suspensions were removed, centrifuged, and suspended in water to an OD620 of 10. Total cellular proteins were extracted from the cells with an MSK cell homogenizer (B. Braun Biotech International, Melsungen, Germany) and zirconium beads (diameter, 0.1 mm; Biospec Products) by using eight 1-min treatments; the preparations were cooled on ice between treatments. Then the zirconium beads were allowed to sediment by gravity, and the supernatant, containing the cellular proteins, was analyzed by two-dimensional gel electrophoresis (2D-E).
Protein analysis by 2D-E and one-dimensional SDS-PAGE.
2D-E was performed essentially as described previously (21, 33) by using a Pharmacia 2D-E system (Pharmacia Biotech, Uppsala, Sweden). Before the samples were loaded onto the isoelectric focusing (IEF) gel, 12.5 μl of a protein solution (40 μg of protein) was treated with 12.5 μl of lysis solution (9 M urea, 2% 2-mercaptoethanol, 2% IPG buffer 3-10L [Pharmacia Biotech], 2% Triton X-100, 6 mM Pefabloc SC [Merck, Darmstadt, Germany]). This preparation was put on ice, and 25 μl of sample solution (8 M urea, 2% 2-mercaptoethanol, 2% IPG buffer 3-10L, 0.5% Triton X-100, a few grains of bromophenol blue) was added. The mixture (total volume, 50 μl) was loaded onto the acidic end of a first-dimension IEF gel with isoelectric points ranging from 3 to 10 (Immobiline Dry strips; Pharmacia Biotech). IEF gels with pI values ranging from 4.0 to 5.0 were loaded with 100 μg of protein, and the lysis buffer and the sample solution were prepared with IPG buffer 3.5-5.0L. Both IEF gels were linear, as stated by the manufacturer (Pharmacia Biotech). For the second dimension, homogeneous sodium dodecyl sulfate (SDS)-15% polyacrylamide gel electrophoresis (PAGE) gels or SDS-12 to 14% PAGE gels (ExcelGel; Pharmacia Biotech) were used. A high-molecular-mass marker (Pharmacia Biotech) that produced bands at 97, 66, 45, 30, 20.1, and 14.4 kDa and a low-molecular-mass marker (Pharmacia Biotech) that produced bands at 16.9, 14.4, 10.7, 8.2, 6.2, and 2.5 kDa were used. The gels were silver stained as described by Blum et al. (7) and were analyzed by using PDQuest software (Bio-Rad, Richmond, Calif.). Representative gels obtained in duplicate experiments are shown below. The gels were standardized by calculating the intensity of each spot as a percentage of the total intensity of the spots visualized on a gel, and then induction factors were calculated. One-dimensional Tricine-SDS-PAGE for separation of low-molecular-weight proteins was performed as described by Schägger and Von Jagow (27).
Immunoblotting with anti-CspB.
A 500-μg portion of total protein was loaded onto a 2D-E gel (and 30 μg of total protein was loaded onto a one-dimensional gel) for immunoblotting with rabbit anti-CspB antibody of B. subtilis, which was kindly provided by P. Graumann (Philipps-Universität Marburg, Marburg, Germany). The proteins were blotted on a nitrocellulose membrane (Bio-Rad) by using a transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol; pH 8.3) and incubated overnight with the anti-CspB antibody. The membrane was treated with a goat anti-rabbit solution (Bio-Rad) and stained with 3,3-diaminobenzidine-tetrahydrochloride-H2O2.
Southern blot hybridization.
Chromosomal DNA of L. monocytogenes was isolated as described previously (29). Restriction enzyme PstI was purchased from GIBCO/BRL Life Technologies, New England Biolabs. Radiolabeling of PCR products, agarose gel electrophoresis, and Southern blot hybridization were performed by using established procedures (26). PCR reagents (Taq polymerase and deoxynucleoside triphosphates) were purchased from Boehringer GmbH (Mannheim, Germany) and were used according to the manufacturer's instructions. By using universal csp gene primers (12) and PCR a product carrying a partial csp gene was obtained. This product was sequenced with an ALF automatic sequencer (Pharmacia Biotech) used in combination with an AutoRead sequencing kit (Pharmacia Biotech) with fluorescein-labeled primers. The radiolabeled PCR product was used as a probe for Southern hybridization. The following stringency conditions were used: incubation for 4 min at 65°C with 5× SSC-0.1% SDS, followed by washing with 2× SSC-0.1% SDS for 4 min at 65°C and with 0.5× SSC-0.1% SDS for 3 min at 65°C (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
HHP treatment.
Suspensions (30 ml) of exponentially growing L. monocytogenes (OD620, 0.4) were placed in plastic bags. These samples were pressure treated for 10 min at 50, 100, or 200 MPa at 30°C in an HHP apparatus (Resato, Roden, The Netherlands). The suspensions were depressurized within seconds, and total cellular proteins were extracted and analyzed by 2D-E. Growth of the cells after the pressure treatment was examined by measuring OD620 at 37°C. The levels of survival after treatment for 20 min at 200, 250, 300, and 350 MPa were determined for exponentially growing cells and cells that were cold shocked at 10°C.
RESULTS
Growth after cold shock and freeze survival.
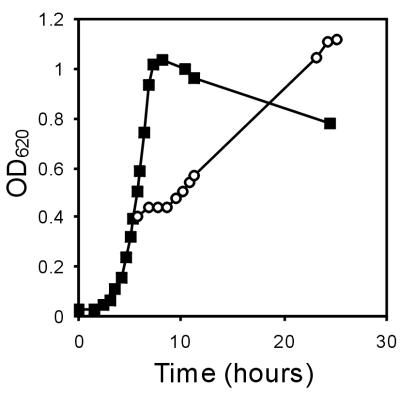
The growth of L. monocytogenes LO28 was examined after the organism was cold shocked by transfer from 37°C to 10°C. L. monocytogenes LO28 grew in brain heart infusion broth at 37°C at a rate of 0.6 h−1. When exponentially growing cells were cold shocked at 10°C, an acclimation phase lasting about 3 h occurred before growth resumed at a rate of about 0.2 h−1 (Fig. 1). Since low-temperature processing of foods is frequently followed by freezing, cells that were cold shocked at 10°C for 4 h were subjected to freezing. L. monocytogenes LO28 cells that were grown exponentially at 37°C exhibited about 50% survival after freezing. However, when cells were cold shocked for 4 h at 10°C before freezing, the level of survival after freezing was 90%. After L. monocytogenes cells that were grown at 37°C were thawed, growth resumed after a lag period of about 2 h. However, after cold-shocked cells were thawed, growth at 37°C resumed without a lag period (data not shown). This indicates that cell history is an important parameter for survival after freezing and subsequent growth.
FIG. 1.
Growth of L. monocytogenes LO28 at 37°C (▪) and after cold shock at 10°C (○).
Identification and production of CSPs.
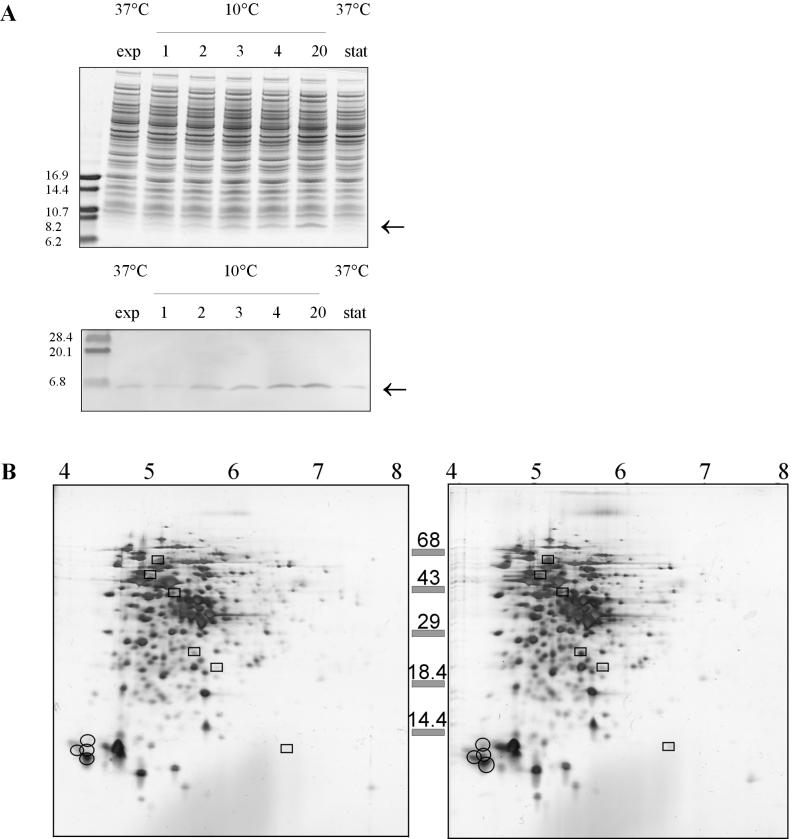
To study the production of CSPs in L. monocytogenes, one-dimensional gel electrophoresis was performed after a cold shock. Two hours after the cold shock, elevated levels of 7-kDa proteins were found in L. monocytogenes (Fig. 2A), and the levels increased even more upon growth at 10°C. A Western immunoblot analysis performed with anti-CspB of B. subtilis indicated that the level of CSPs increased after the cold shock. The level increased 1.5-fold 2 h after the cold shock and almost 4-fold after 20 h of incubation at 10°C. The CSP levels were not elevated in stationary-phase cultures incubated at 37°C.
FIG. 2.
Analysis of L. monocytogenes LO28 proteins. (A) Upper panel, one-dimensional gel electrophoresis of mid-exponential-phase cells (exp) (37°C), cells cold shocked at 10°C for 1, 2, 3, 4, and 20 h, and cells in the stationary phase (stat) at 37°C; lower panel, Western blot of an identical one-dimensional gel with anti-CspB from B. subtilis. (B) 2D-E of cell extracts of L. monocytogenes LO28 with pI values ranging from 3 to 10. Proteins induced at least threefold are enclosed in boxes, and the CSPs are circled. Left panel, exponential-phase cells at 37°C; right panel, cells 4 h after cold shock at 10°C.
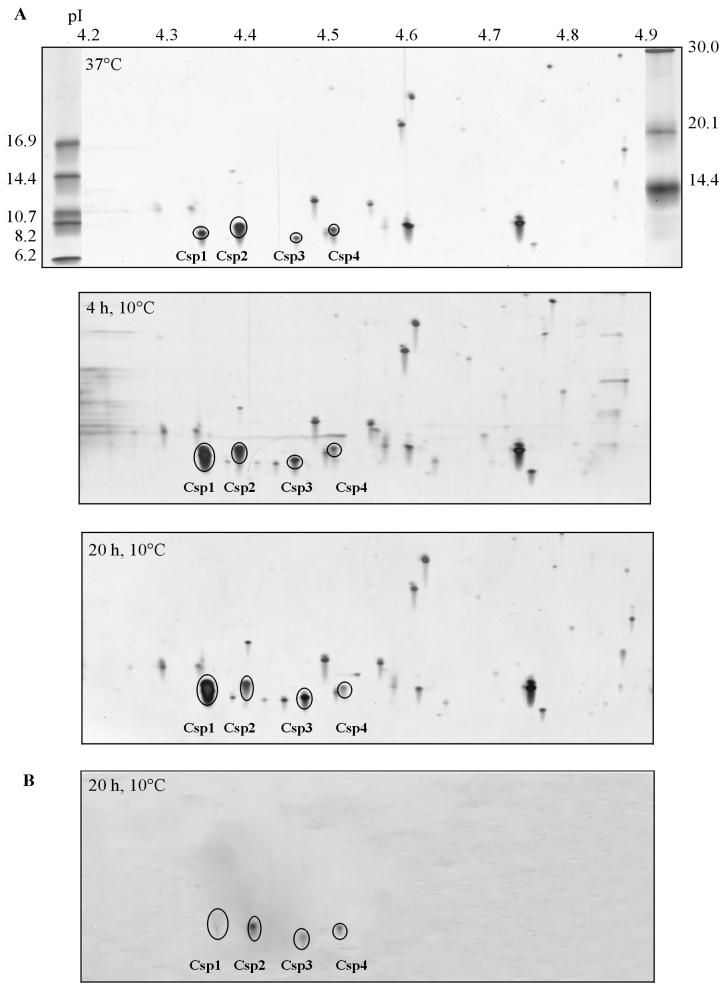
To study the presence of CSPs in more detail, we performed 2D-E with proteins extracted from cells in the exponential phase and from cells that were cold shocked for 4 h (cells in the early exponential phase). For the first dimension, a pI range of 3 to 10 was used. Increased levels of production of 7-kDa proteins were observed in the acidic region of the gel (Fig. 2B), and a Western immunoblot analysis indicated that four putative CSPs were present in this region (data not shown). The levels of six other proteins were found to have increased at least threefold upon cold shock (Fig. 2B). To further characterize CSPs from L. monocytogenes LO28, 2D-E with a pI range of 4 to 5 was performed. This was followed by Western immunoblotting performed with anti-CspB of B. subtilis, which is known to react with all CSPs in B. subtilis (15). In this way four spots were visualized, and these four spots were designated Csp1, Csp2, Csp3, and Csp4 (Fig. 3). Based on migration in 2D-E gels, the pI values for Csp1, Csp2, Csp3, and Csp4 are 4.32, 4.35, 4.43, and 4.51, respectively, and the molecular masses are 7.4, 8.0, 7.0, and 7.6 kDa, respectively. The proteins encoded by the cspL and cspLB genes of L. monocytogenes EGD (accession numbers LMO012349 and LMO012350, respectively) (Busch and Chakraborty, unpublished) had calculated isoelectric points of 4.32 and 4.35, respectively, and molecular masses of 7.3 and 7.5 kDa, respectively. These calculated pI values and molecular masses match the values obtained for Csp1 and Csp2 of LO28.
FIG. 3.
Identification of CSPs in L. monocytogenes LO28. (A) Separation of cell extracts from cells in the exponential growth phase at 37°C (upper panel), from cells 4 h after cold shock at 10°C (middle panel), and from cells 20 h after cold shock at 10°C (lower panel). (B) Western blot, obtained with anti-CspB from B. subtilis, of cell extract from L. monocytogenes LO28 cells 20 h after cold shock at 10°C.
By using universal csp gene primers and L. monocytogenes LO28 total DNA as the template, a PCR product carrying a partial csp gene was obtained. The nucleic acid sequence was 100% identical to a partial cspL gene sequence of L. monocytogenes ATCC 23074 (11) and a cspL sequence of L. monocytogenes EGD (Busch and Chakraborty, unpublished). Southern blotting of L. monocytogenes LO28 genomic DNA PstI fragments by using the PCR product as the probe revealed four hybridizing bands (Fig. 4), one major band and three less intense hybridizing fragments. This indicated that there are four csp genes on the L. monocytogenes LO28 chromosome, corresponding to the four CSPs found on 2D-E gels.
FIG. 4.
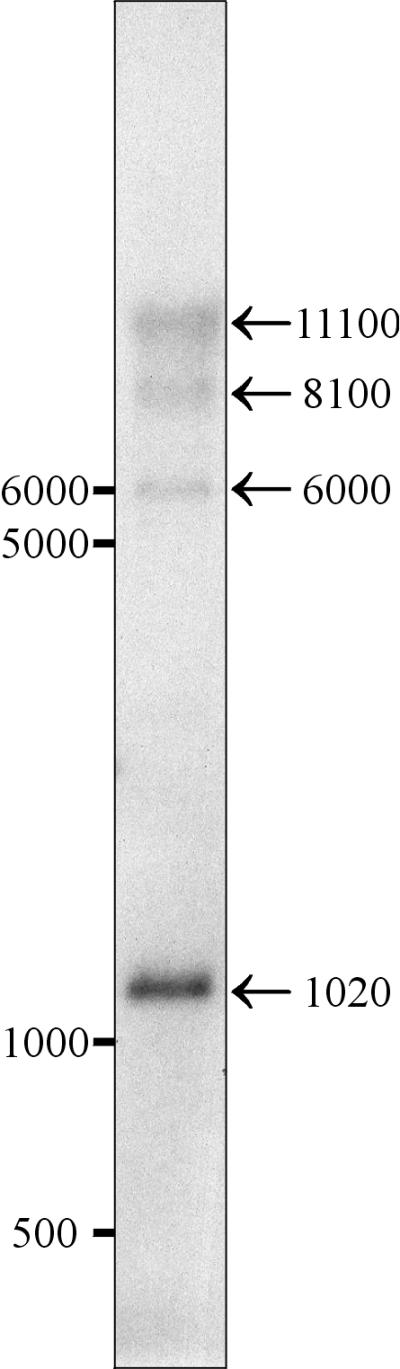
csp genes in L. monocytogenes LO28: Southern blot of L. monocytogenes LO28 PstI fragments in which the csp PCR product was used as the probe. The sizes of markers (in base pairs) are indicated on the left, and the sizes of the hybridizing fragments (in base pairs) are indicated on the right.
CSP production has never been monitored in L. monocytogenes. In this study we found that production of Csp1 and Csp3 increased after a cold shock. Four hours after the cold shock the levels of Csp1 and Csp3 were 6.5- and 2-fold higher, respectively (Table 1), and 20 h after the cold shock the levels of Csp1 and Csp3 were 10- and 3.5-fold higher, respectively. The levels of Csp2 and Csp4 were not elevated upon cold shock. We observed no increases in the levels of CSPs for L. monocytogenes LO28 cells in the stationary phase at 37°C (data not shown).
TABLE 1.
CSPs in L. monocytogenes LO28 and induction of CSPs after cold shock
| Protein | Mol wt (103)a | pIa | Level in:
|
||
|---|---|---|---|---|---|
| Exponential-phase cells at 37°Cb | Cells 4 h after cold shock at 10°C | Cells 20 h after cold shock at 10°C | |||
| Csp1 | 7.4 | 4.32 | 1 | 6.6 | 9.8 |
| Csp2 | 8.0 | 4.35 | 1 | 0.5 | 0.3 |
| Csp3 | 7.0 | 4.43 | 1 | 2.1 | 3.4 |
| Csp4 | 7.6 | 4.51 | 1 | 0.7 | 0.3 |
Based on migration in a 2D-E gel.
The level of each CSP at 37°C was defined as 1.
Response of L. monocytogenes to HHP treatment.
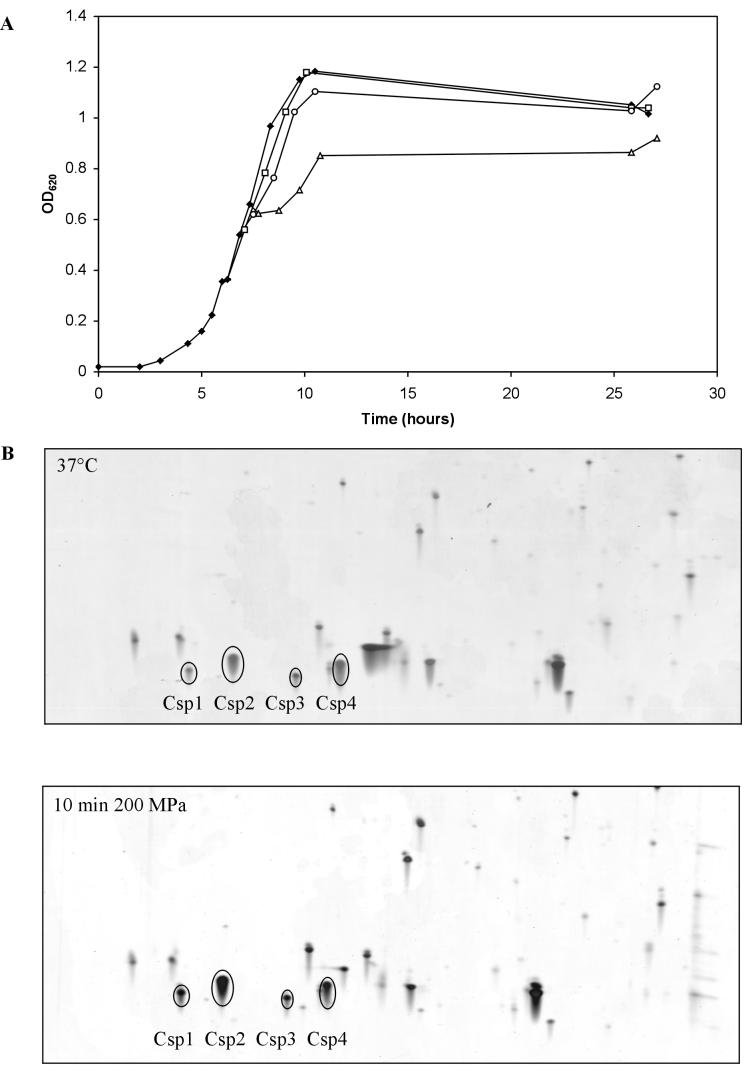
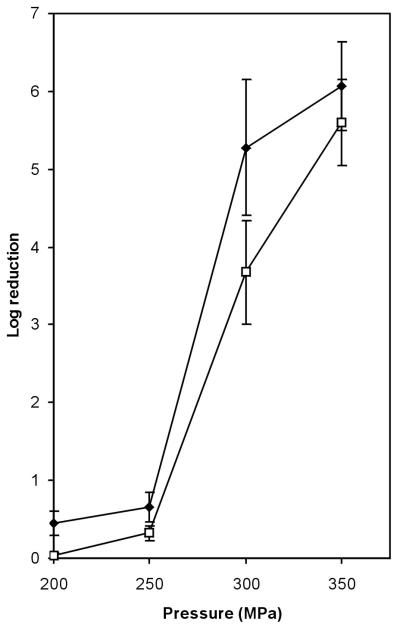
CspA was induced in pressure-treated E. coli cells (31). This indicates that CSPs may have a role in adaptation to pressure treatment. Here we analyzed the response of L. monocytogenes LO28 to hydrostatic pressure treatment by monitoring the growth of pressure-treated cells and performing 2D-E with cell extracts from these cells. After 10 min of treatment at 50 or 100 MPa, growth was hardly affected. However, the OD620 of cells treated at 200 MPa did not increase for about 1 h (Fig. 5A). 2D-E analysis of cell extracts obtained from cells after 10 min of treatment at 200 MPa revealed that the levels of Csp1 and Csp2 were higher than the levels in control cells (Fig. 5B). The Csp1 level was 3.5-fold higher, and the level of Csp2 was 2-fold higher. Since cold shock and high-pressure treatment both increased CSP levels, we examined the possibility of cross-protection. After pressure treatment of cold-shocked cells, the level of survival was indeed higher than the level of survival of exponentially growing cells that were pressure treated. For cells treated for 20 min at 300 MPa the level of survival was 100-fold higher for cold-shocked cells than for cells growing exponentially at 37°C (Fig. 6).
FIG. 5.
Response of L. monocytogenes LO28 to HHP treatment. (A) Growth (OD620) at 37°C (⧫) and growth after 10 min of exposure to 50 MPa (□), 100 MPa (○), and 200 MPa (▵). (B) 2D-E with a pI range of 4 to 5 for cell extracts from untreated cells (upper panel) and cell extracts from cells treated for 10 min at 200 MPa (lower panel).
FIG. 6.
Reduction in the number of viable L. monocytogenes cells after exposure for 20 min to 200, 250, 300, and 350 MPa for cells growing exponentially at 37°C (⧫) and cells exposed to 10°C for 4 h (□).
DISCUSSION
The process of adaptation of L. monocytogenes to low temperature includes maintenance of membrane fluidity (1), uptake of compatible solutes (5, 13, 17), maintenance of ribosomal structure (4), and induction of several proteins (3, 24). In this study we focused on identification of 7-kDa CSPs in L. monocytogenes LO28. CSPs have not been identified previously in 2D-E studies focusing on adaptation of L. monocytogenes to low temperature (3, 24). In the previous studies the low-molecular-weight proteins were not visualized due to the experimental setup used. We describe here identification of four CSPs in L. monocytogenes LO28, designated Csp1, Csp2, Csp3, and Csp4. The Csp1 and Csp3 levels were elevated upon cold shock (6.5- and 2-fold, respectively, after 4 h at 10°C), whereas the levels of Csp2 and Csp4 were not elevated. The levels of Csp1 and Csp3 increased even more after cell growth at a low temperature resumed. The production was greatest during the stationary phase after the cold shock. Nontransient induction after cold shock was also observed for CapA (a CSP homologue) of Arthrobacter globiformis, a psychrotrophic bacterium (6). The nontransient increased production of Csp1 and Csp3 indicates that CSPs are required not only for acclimation but also for growth at low temperatures. Next, we observed that substantial levels of all four CSPs were present in L. monocytogenes LO28 cultures growing at 37°C, which implies that these proteins have a function during normal growth. It has been shown that at least one CSP is required for B. subtilis growth at low and normal temperatures (15).
The survival of L. monocytogenes cells after freezing may pose a danger for public health. Therefore, it is important to monitor the survival of L. monocytogenes cells after freezing at different growth stages. The level of survival after exponentially growing L. monocytogenes LO28 was frozen was approximately 50%. However, when cells were cold shocked before freezing, the level of survival was 90%. This phenomenon of adaptation to freezing by preexposure to a low temperature has been described for a number of bacteria, such as B. subtilis (32) and Lactococcus lactis (33). In these organisms CSPs play a role in freeze protection, as shown by CSP-overproducing variants, which exhibited higher levels of survival after freezing (33), and by csp deletion mutants, which exhibited decreased levels of survival after freezing(32). Notably, after a suspension of mid-exponential-phase L. monocytogenes LO28 cells was frozen for 20 h, growth started only after a 2-h lag period, whereas growth of cold-shocked cells resumed immediately. These data indicate that in order to design safe food processing procedures, it is very important to take into account the effects that cell history might have on the bacterial survival potential, as shown here for the freeze survival of L. monocytogenes after low-temperature exposure.
HHP treatment may be a powerful new preservation strategy to reduce the risk of food-borne infections. Here we show that treatment for 10 min at 200 MPa has a severe effect on cellular growth of L. monocytogenes. To analyze the response of L. monocytogenes cells to this treatment, we performed 2D-E with a focus on the production of 7-kDa CSPs. It has been reported that in E. coli the CspA levels increase when the cells are pressure treated (31). We observed increases in the Csp1 and Csp2 levels in L. monocytogenes LO28 after treatment at 200 MPa. The Csp1 levels were up to 10-fold higher after a cold shock and 3.5-fold higher after high-pressure treatment. Strikingly, the level of Csp2 did not increase upon cold shock, while the level of this protein was twofold higher after pressure treatment. Graumann and Marahiel (16) proposed that partial inactivation of ribosomes is the main trigger for induction of CSPs at low temperatures. The effect on ribosomal structure observed after cold shock was similar to the effect observed after high-pressure treatment (20), suggesting that there is overlap in the responses to these two types of stress. Indeed, we observed induction of CSPs both at low temperature and after pressure treatment of L. monocytogenes LO28. This indicates that CSPs might have a role in adaptation to both types of stress. Combining cold shock with pressure treatment resulted in increased survival after the pressure treatment. The induced levels of CSPs after cold shock might protect a cell exposed to high pressure. It is conceivable that cold-adapted ribosomes are less sensitive to the ribosome-deteriorating effects of pressure, resulting in higher levels of survival after pressure treatment. This protection against pressure treatment resulting from low-temperature treatment can be important for processing technology (e.g., if cold storage of food products is combined with pressure treatment).
REFERENCES
- 1.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, W., B. Xia, M. Inouye, and K. Severinov. 2000. Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc. Natl. Acad. Sci. USA 97:7784-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayles, D. O., B. A. Annous, and B. J. Wilkinson. 1996. Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl. Environ. Microbiol. 62:1116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayles, D. O., M. H. Tunick, T. A. Foglia, and A. J. Miller. 2000. Cold shock and its effect on ribosomes and thermal tolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4351-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 6.Berger, F., P. Normand, and P. Potier. 1997. capA, a cspA-like gene that encodes a cold acclimation protein in the psychrotrophic bacterium Arthrobacter globiformis SI55. J. Bacteriol. 179:5670-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 8.Brandi, A., C. L. Pon, and C. O. Gualerzi. 1994. Interaction of the main cold shock protein CS7.4 (CspA) of Escherichia coli with the promoter region of hns. Biochimie 76:1090-1098. [DOI] [PubMed] [Google Scholar]
- 9.Brandi, A., R. Spurio, C. O. Gualerzi, and C. L. Pon. 1999. Massive presence of the Escherichia coli ‘major cold-shock protein' CspA under non-stress conditions. EMBO J. 18:1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etchegaray, J. P., and M. Inouye. 1999. CspA, CspB, and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature under conditions that completely block protein synthesis. J. Bacteriol. 181:1827-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis, K. P., C. E. D. Rees, and G. S. A. B. Stewart. 1995. Identification of a major cold shock protein homologue in Listeria monocytogenes, p. 403-409. In Proceedings of the XIIth International IsoPol Symposium. Promaco Conventions Pty. Ltd., Canning Bridge, Australia.
- 12.Francis, K. P., and G. S. A. B. Stewart. 1997. Detection and speciation of bacteria through PCR using universal major cold-shock protein primer oligomers. J. Ind. Microbiol. Biotechnol. 19:286-293. [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt, P. N. M., L. Tombras Smith, and G. M. Smith. 2000. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J. Bacteriol. 182:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graumann, P., K. Schröder, R. Schmid, and M. A. Marahiel. 1996. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178:4611-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graumann, P., T. M. Wendrich, M. H. W. Weber, K. Schroder, and M. A. Marahiel. 1997. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol. Microbiol. 25:741-756. [DOI] [PubMed] [Google Scholar]
- 16.Graumann, P. L., and M. A. Marahiel. 1999. Cold shock proteins CspB and CspC are major stationary-phase-induced proteins in Bacillus subtilis. Arch. Microbiol. 171:135-138. [DOI] [PubMed] [Google Scholar]
- 17.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaTeana, A., A. Brandi, M. Falconi, R. Spurio, C. L. Pon, and C. O. Gualerzi. 1991. Identification of a cold shock transcriptional enhancer of the Escherichia coli major cold shock gene encoding nucleoid protein H-NS. Proc. Natl. Acad. Sci. USA 88:10907-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niven, G. W., C. A. Miles, and B. M. Mackay. 1999. The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: an in vivo study using differential scanning calorimetry. Microbiology 145:419-425. [DOI] [PubMed] [Google Scholar]
- 21.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson, M. F., M. Quinn, R. Simpson, and A. Gilmour. 1995. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J. Food Prot. 58:524-529. [DOI] [PubMed] [Google Scholar]
- 23.Phadtare, S., J. Alsina, and M. Inouye. 1999. Cold-shock response and cold-shock proteins. Curr. Opin. Microbiol. 2:175-180. [DOI] [PubMed] [Google Scholar]
- 24.Phan-Thanh, L., and T. Gormon. 1995. Analysis of heat and cold shock proteins in Listeria by two-dimensional electrophoresis. Electrophoresis 16:444-450. [DOI] [PubMed] [Google Scholar]
- 25.Russell, N. J., R. I. Evans, P. F. ter Steeg, J. Hellemons, A. Verheul, and T. Abee. 1995. Membranes as a target for stress adaptation. Int. J. Food Microbiol. 28:255-261. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Schägger, H., and G. Von Jagow. 1987. Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 28.Styles, M. F., D. G. Hoover, and D. F. Farkas. 1991. Response of Listeria monocytogenes and Vibrio parahaemolyticus to high hydrostatic pressure. J. Food Sci. 56:1404-1407. [Google Scholar]
- 29.Vos, P., M. Van Asseldonk, F. Van Jeveren, R. J. Siezen, G. Simons, and W. M. De Vos. 1989. A maturation protein is essential for the production of active forms of Lactococcus lactis SK11 serine protease located in or secreted from the cell envelope. J. Bacteriol. 171:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker, S. J., P. Archer, and J. G. Banks. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157-162. [DOI] [PubMed] [Google Scholar]
- 31.Welch, T. J., A. Farewell, F. C. Neidhardt, and D. H. Bartlett. 1993. Stress response of Escherichia coli to elevated hydrostatic pressure. J. Bacteriol. 175:7170-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willimsky, G., H. Bang, G. Fischer, and M. A. Marahiel. 1992. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J. Bacteriol. 174:6326-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wouters, J. A., B. Jeynov, F. M. Rombouts, W. M. de Vos, O. P. Kuipers, and T. Abee. 1999. Analysis of the role of 7 kDa cold-shock proteins of Lactococcus lactis MG1363 in cryoprotection. Microbiology 145:3185-3194. [DOI] [PubMed] [Google Scholar]
- 34.Wouters, J. A., J. W. Sanders, J. Kok, W. M. de Vos, O. P. Kuipers, and T. Abee. 1998. Clustered organization and transcriptional analysis of a family of five csp genes of Lactococcus lactis MG1363. Microbiology 144:2885-2893. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka, K., L. Fang, and M. Inouye. 1998. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol. Microbiol. 27:247-255. [DOI] [PubMed] [Google Scholar]