Abstract
The role of three key nitrogen regulatory genes, glnB (encoding the PII protein), glnZ (encoding the Pz protein), and glnD (encoding the GlnD protein), in regulation of poly-3-hydroxybutyrate (PHB) biosynthesis by ammonia in Azospirillum brasilense Sp7 was investigated. It was observed that glnB glnZ and glnD mutants produce substantially higher amounts of PHB than the wild type produces during the active growth phase. glnB and glnZ mutants have PHB production phenotypes similar to that of the wild type. Our results indicate that the PII-Pz system is apparently involved in nitrogen-dependent regulation of PHB biosynthesis in A. brasilense Sp7.
A wide variety of bacteria can produce a thermoplastic poly-3-hydroxybutyrate (PHB) as an energy and carbon storage compound under unbalanced nutrient conditions (1, 11). Because of the biodegradability and biocompatibility of PHB and because PHB has a wide range of applications, the biochemistry of PHB (16, 17, 18, 19, 21), its uses in genetic and metabolic engineering (9, 15, 20, 22, 26, 35), and its application in tissue engineering (23, 24, 25, 36) and material engineering (13) have been intensively studied.
In most PHB-producing bacteria, production of only a little PHB is observed during the active growth phase of cells. Therefore, a long time is needed for bacteria to reach a high-density non-PHB cell biomass before accumulation of large amounts of PHB can occur (1, 11). Nutrient limitation is necessary to trigger PHB accumulation, and generally ammonia is used as the critical control factor for uncoupling the growth of cells and PHB production. However, some bacteria, such as Azotobacter vinelandii UWD (14), Alcaligenes latus (4, 34), and Pseudomonas putida KT2442 (7), are able to accumulate a large amount of PHB or polyhydroxyalkanoates during exponential growth, but the genetic reasons for this are not well defined.
The PHB-producing abilities of Azospirillum, a genus of free-living nitrogen-fixing bacteria, have been studied previously. Some species, such as Azospirillum brasilense and Azospirillum lipoferum, can accumulate high levels of PHB (up to 88% of the cell dry biomass) under unbalanced nutrient conditions, such as oxygen limitation or a high C/N ratio (8, 29). However, it was observed in a previous study that ntrB and ntrC mutants of A. brasilense Sp7 can grow and produce PHB simultaneously even when a large amount of ammonia is present in the medium, indicating that ntrB and ntrC are involved in regulation of PHB biosynthesis by ammonia in A. brasilense Sp7 (28). Because inactivation of inhibition of PHB production by ammonia has industrial potential for improving process control and productivity (10), further studies were performed to investigate the involvement of other nitrogen regulatory genes in controlling PHB production in A. brasilense Sp7.
The PII and Pz proteins, encoded by the glnB and glnZ genes, respectively, have similar structures but perform different functions (3). These two proteins are involved in sensing the intracellular nitrogen status (2). They occur in two forms, the native form when there is excess nitrogen and the uridylylated form under nitrogen-limiting conditions (2). Uridylylation and deuridylation of the PII and Pz proteins are catalyzed by another nitrogen-sensing protein, GlnD, which functions as uridylyltransferase and uridylyl-removing enzyme and is encoded by the glnD gene (31, 32).
In this study, glnB, glnZ, glnB glnZ, and glnD mutants were examined to determine PHB production under low-C/N-ratio conditions. The bacterial stains used in this study are listed in Table 1. All of the strains were routinely grown in MMAB medium (33) at 30°C. Kanamycin (25 μg/ml), tetracycline (10 μg/ml), spectinomycin (50 μg/ml), and streptomycin (100 μg/ml) were added to the medium when required. Batch fermentation was performed in a 2-liter pH-stat and O2-stat fermentor as described previously (12). The concentration of dissolved oxygen (DO2) was kept constant by varying the airflow into the fermentor based on the measured DO2 level, so the airflow rate could be used as an indicator of the oxygen uptake rate (12).
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristic(s) | Reference |
|---|---|---|
| Sp7 (= ATCC 29145) | Wild type | 30 |
| 7628 | glnB::kan Kmr, A. brasilense Sp7 glnB mutant | 3 |
| 7611 | glnZ::Ω Spr Smr, A. brasilense Sp7 glnZ mutant | 2 |
| 2812 | glnB::kan/glnZ::Ω Kmr Spr Smr, A. brasilense Sp7 glnB glnZ double mutant | 2 |
| FAJ311 | glnD::Tn5-B30 Tcr, A. brasilense Sp7 glnD mutant | 32 |
All of the analytical procedures used to determine cell growth, biomass, and PHB concentration were performed as described previously (28). All the data below are averages based on at least two replicates.
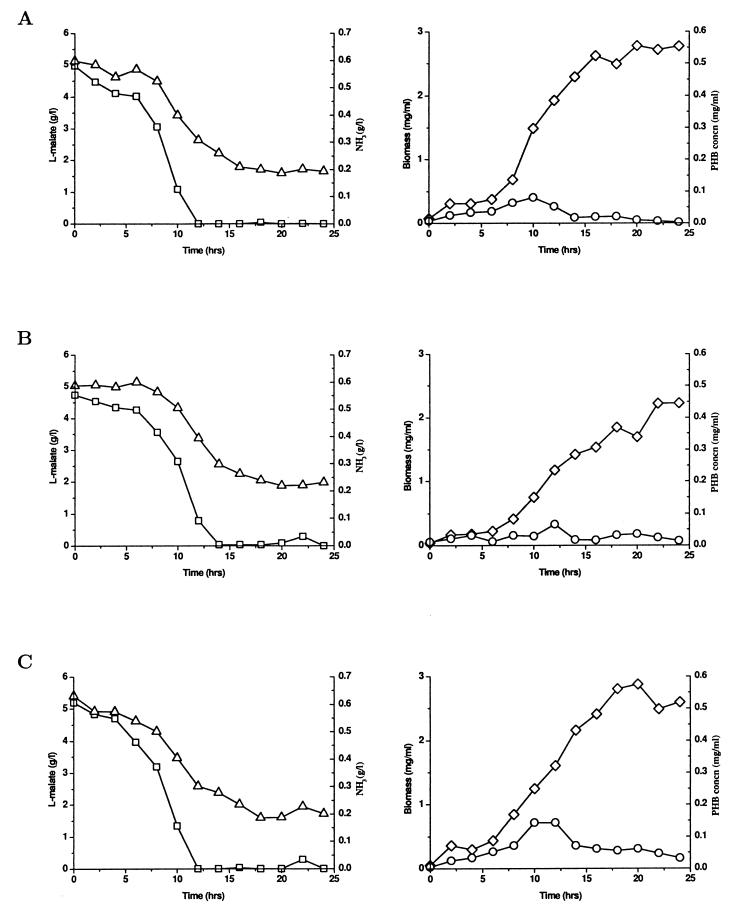
To determine the involvement of glnB, glnZ, and glnD in regulation of PHB production by ammonia in A. brasilense Sp7, the wild-type and mutant strains were grown in a fermentor in order to precisely monitor and control the culture conditions. MMAB medium was supplemented with 10 g of malate per liter and 2 g of NH4Cl per liter (initial C/N ratio, 6.8), and the DO2 concentration was kept at 30%, which has been reported to be the optimal value for PHB production in A. brasilense (29). Under these culture conditions, no nitrogen fixation can occur because the nitrogen fixation process is repressed by the presence of a high concentration of combined nitrogen and a high DO2 concentration. Therefore, the possibility that diazotrophic growth has any effect can be excluded (5, 6). The results obtained are shown in Fig. 1.
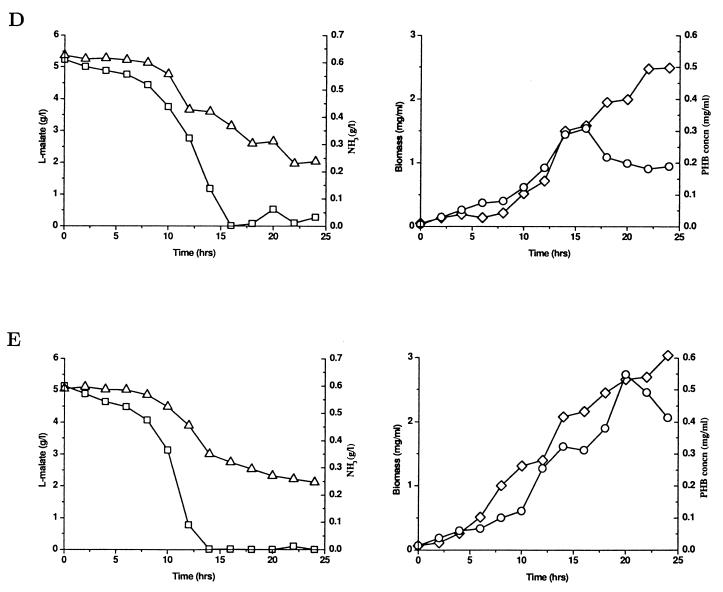
FIG. 1.
Time course of fermentation in A. brasilense Sp7 (A) and its glnB (B), glnZ (C), glnB glnZ (D), and glnD (E) mutants. Symbols: □, l-malate concentration; ▵, ammonia concentration; ◊, biomass concentration; ○, PHB concentration. The data in each panel are the data from one of the reproducible independent experiments performed. All the data are averages based on at least two replicates.
We observed that A. brasilense wild-type strain Sp7 can produce only small amounts of PHB during the active growth phase, which is consistent with a previous report (28). Cell growth enters the stationary phase because of depletion of the carbon source (malate). The PHB concentration decreases during the stationary phase because PHB is probably used as the alternative carbon source for growth maintenance after the supply of malate is exhausted (Fig. 1A). The glnB mutant has a PHB production and cell growth phenotype similar to that of the wild type (Fig. 1B). The glnZ mutant can produce slightly more PHB during the growth phase than the wild type and glnB mutant can produce, but the amount of PHB is still small (Fig. 1C). However, the glnB glnZ double mutant produces a significantly larger amount of PHB during the active growth phase than the wild type produces (Fig. 1D). The glnD mutant can accumulate even more PHB (up to 40% of the cell dry weight) during the growth phase than the glnB glnZ double mutant can accumulate (Fig. 1E). PHB accumulation is obviously associated with cell growth in the glnB glnZ and glnD mutants under nitrogen-excess conditions. Additionally, all four mutants have cell growth and respiration phenotypes (data not shown) similar to those of the wild type under the conditions used in this study, if the effects of other growth-limiting factors caused by mutations on PHB production are excluded. Furthermore, a mathematical modelling analysis has been done to confirm the significant differences in PHB production regulated by ammonia for the wild type and the four mutants (data not shown) (27).
It has been reported that even though the PII and Pz proteins have similar structures and are similarly modified (uridylylated) in response to nitrogen limitation in cells, they are involved differently in nitrogen-dependent regulation of various physiological functions (2). However, the results of an analysis of PHB production in glnB, glnZ, and glnB glnZ mutants suggest that the PII and Pz proteins can complement each other for control of PHB accumulation in A. brasilense Sp7. Surprisingly, the glnD mutant can also produce a large amount of PHB associated with cell growth and is not sensitive to the inhibitory effect of ammonia on PHB accumulation. Under nitrogen-excess conditions, such as those used in this study, the PII and Pz proteins are retained in their native, nonuridylylated forms (2), which are also the only forms present in a glnD mutant regardless of the intracellular nitrogen status (31, 32). The high level of PHB production in the glnD mutant might imply that in A. brasilense Sp7 the glnD gene controls regulation of PHB biosynthesis by ammonia not via the PII and Pz nitrogen-sensing system but via another unknown target gene(s).
Acknowledgments
This study was supported by a predoctoral fellowship from the Research Council, K.U. Leuven.
We acknowledge M. de Zamaroczy for providing the glnB, glnZ, and glnB glnZ mutants of A. brasilense Sp7.
REFERENCES
- 1.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial use of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Zamaroczy, M. 1998. Structural homologues PII and Pz of Azospirillum brasilense provide intracellular signalling for selective regulation of various nitrogen-dependent functions. Mol. Microbiol. 29:449-463. [DOI] [PubMed] [Google Scholar]
- 3.de Zamaroczy, M., A. Paquelin, G. Peltre, K. Forchhammer, and C. Elmerich. 1996. Coexistence of two structurally similar but functionally different PII proteins in Azospirillum brasilense. J. Bacteriol. 178:4143-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hänggi, U. J. 1990. Pilot scale production of PHB with Alcaligenes latus, p. 65-70. In E. A. Dawes (ed.), Novel biodegradable microbial polymers. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 5.Hartmann, A., and R. H. Burris. 1987. Regulation of nitrogenase activity by oxygen in Azospirillum brasilense Sp7 and Azospirillum lipoferum. J. Bacteriol. 169:944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann, A., H. Fu, and R. H. Burris. 1986. Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J. Bacteriol. 165:864-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huisman, G. W., E. Wonink, G. de Koning, H. Preusting, and B. Witholt. 1992. Synthesis of poly(3-hydroxyalkanoates) by mutant and recombinant Pseudomonas strains. Appl. Microbiol. Biotechnol. 38:1-5. [Google Scholar]
- 8.Itzigsohn, R., O. Yarden, and Y. Okon. 1995. Polyhydroxyalkanoate analysis in Azospirillum brasilense. Can. J. Microbiol. 41:73-76. [Google Scholar]
- 9.Leaf, T. A., M. S. Peterson, S. K. Stoup, D. Somers, and F. Srienc. 1996. Saccharomyces cerevisiae expressing bacterial polyhydroxybutyrate synthase produces poly-3-hydroxybutyrate. Microbiology 142:1169-1180. [DOI] [PubMed] [Google Scholar]
- 10.Lee, S.-Y., and H.-N. Chang. 1995. Production of poly(3-hydroxybutyric acid) by recombinant Escherichia coli strains: genetic and fermentation studies. Can. J. Microbiol. 41(Suppl. 1):207-215. [DOI] [PubMed] [Google Scholar]
- 11.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchal, K., J. Sun, V. Keijers, H. Haaker, and J. Vanderleyden. 1998. A cytochrome cbb3 (cytochrome c) terminal oxidase in Azospirillum brasilense Sp7 supports microaerobic growth. J. Bacteriol. 180:5689-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchessault, R. H., T. L. Bluhm, Y. Deslandes, G. K. Hamer, W. J. Orts, P. J. Sundarajan, M. G. Taylor, S. Bloembergen, and D. A. Holden. 1988. Poly(β-hydroxyalkanoates): biorefinery polymers in search of applications. Makromol. Chem. Macromol. Symp. 19:235-254. [Google Scholar]
- 14.Page, W. J., and O. Knosp. 1989. Hyperproduction of poly-β-hydroxybutyrate during exponential growth of Azotobacter vinelandii uwd. Appl. Environ. Microbiol. 55:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park, J.-S., H.-C. Park, T.-L. Huh, and Y.-H. Lee. 1995. Production of poly-β-hydroxybutyrate by Alcaligenes eutrophus transformants harbouring cloned phbCAB genes. Biotechnol. Lett. 17:197-205. [Google Scholar]
- 16.Peoples, O. P., S. Masamune, C. T. Walsh, and A. J. Sinskey. 1987. Biosynthetic thiolase from Zooglea ramigera. III. Isolation and characterization of the structural gene. J. Biol. Chem. 262:97-102. [PubMed] [Google Scholar]
- 17.Peoples, O. P., and A. J. Sinskey. 1989. Fine structural analysis of the Zooglea ramigera phbA-phbB locus encoding β-ketothiolase and acetoacetyl-CoA reductase: nucleotide sequence of phbB. Mol. Microbiol. 3:349-357. [DOI] [PubMed] [Google Scholar]
- 18.Peoples, O. P., and A. J. Sinskey. 1989. Poly-β-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Characterization of the gene encoding β-ketothiolase and acetoacetyl-CoA reductase. J. Biol. Chem. 264:15293-15297. [PubMed] [Google Scholar]
- 19.Peoples, O. P., and A. J. Sinskey. 1989. Poly-β-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J. Biol. Chem. 264:15298-15303. [PubMed] [Google Scholar]
- 20.Poirier, Y., D. E. Dennis, K. Klomparens, and C. Somerville. 1992. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science 256:520-523. [DOI] [PubMed] [Google Scholar]
- 21.Reusch, R. N., and H. L. Sadoff. 1983. d-(−)-Poly-β-hydroxybutyrate in membranes of genetically competent bacteria. J. Bacteriol. 156:778-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert, P., A. Steinbüchel, and H. G. Schlegel. 1988. Cloning of the Alcaligenes eutrophus genes for the synthesis of poly-β-hydroxybutyrate (PHB) and synthesis of PHB in Escherichia coli. J. Bacteriol. 170:5837-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sodian, R., S. P. Hoerstrup, J. S. Sperling, S. H. Daebritz, D. P. Martin, F. J. Schoen, J. P. Vacanti, and J. E. Mayer, Jr. 2000. Tissue engineering of heart valves: in vitro experiences. Ann. Thorac. Surg. 70:140-144. [DOI] [PubMed] [Google Scholar]
- 24.Sodian, R., S. P. Hoerstrup, J. S. Sperling, D. P. Martin, S. Daebritz, J. E. Mayer, Jr., and J. P. Vacanti. 2000. Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. Am. Soc. Artif. Organs J. 46:107-110. [DOI] [PubMed] [Google Scholar]
- 25.Sodian, R., J. S. Sperling, D. P. Martin, A. Egozy, U. Stock, J. E. Mayer, Jr., and J. P. Vacanti. 2000. Fabrication of a trileaflet heart valve scaffold from a polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng. 6:183-188. [DOI] [PubMed] [Google Scholar]
- 26.Steinbüchel, A., E. Hustede, M. Liebergesell, U. Pieper, A. Timm, and H. E. Valentin. 1992. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol. Rev. 102:217-230. [DOI] [PubMed] [Google Scholar]
- 27.Sun, J. 2001. Genetic and quantitative aspects of poly-3-hydroxybutyrate biosynthesis regulated by ammonia in Azospirillum. Ph.D. thesis. Katholieke Universiteit Leuven, Leuven, Belgium.
- 28.Sun, J., X. Peng, J. Van Impe, and J. Vanderleyden. 2000. The ntrB and ntrC genes are involved in the regulation of poly-3-hydroxybutyrate biosynthesis by ammonia in Azospirillum brasilense Sp7. Appl. Environ. Microbiol. 66:113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tal, S., and Y. Okon. 1985. Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can. J. Microbiol. 31:608-613. [Google Scholar]
- 30.Tarrand, J. J., N. R. Krieg, and J. Döbereiner. 1978. A taxonomic study of the Spirillum lipoferum group, with description of a new genus, Azospirillum gen. nov., and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can. J. Microbiol. 24:967-980. [DOI] [PubMed] [Google Scholar]
- 31.Van Dommelen, A. 1998. Ammonium transport in Azospirillum brasilense. Ph.D. thesis. K.U. Leuven, Leuven, Belgium.
- 32.Van Dommelen, A., V. Keijers, E. Somers, and J. Vanderleyden. The Azospirillum brasilense glnD gene encodes a key sensor in the nitrogen regulatory cascade. Mol. Genet. Genom., in press. [DOI] [PubMed]
- 33.Vanstockem, M., K. Michiels, J. Vanderleyden, and A. Van Gool. 1987. Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-mob insertion mutants. Appl. Environ. Microbiol. 53:410-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, F., and S.-Y. Lee. 1997. Poly(3-hydroxybutyrate) production with high polymer content by fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl. Environ. Microbiol. 63:3703-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams, M. D., A. M. Fieno, R. A. Grant, and D. H. Sherman. 1996. Expression and analysis of a bacterial poly(hydroxyalkanoate) synthase in insect cells using baculovirus system. Protein Expr. Purif. 7:203-211. [DOI] [PubMed] [Google Scholar]
- 36.Williams, S. F., D. P. Martin, D. H. Horowitz, and O. P. Peoples. 1999. PHA applications: addressing the price performance issue. I. Tissue engineering. Int. J. Biol. Macromol. 25:111-121. [DOI] [PubMed] [Google Scholar]