Abstract
Two forms of NAD-dependent d-mandelate dehydrogenase (d-ManDHs) were purified from Enterococcus faecalis IAM 10071. While these two enzymes consistently exhibited high activity toward large 2-ketoacid substrates that were branched at the C3 or C4 position, they gave distinctly different Km and Vmax values for these substrates and had distinct molecular weights by gel electrophoresis and gel filtration.
Lactic acid bacteria possess NAD-dependent l- or d-lactate dehydrogenase (l- or d-LDH; EC 1.1.1.27 or 1.1.1.28, respectively), or both, fermenting the corresponding stereoisomer of lactic acid (13). The two LDHs are distinct from each other in both substrate stereospecificity and protein structure (6, 8, 19, 25, 27, 28), and d-LDH shares a common protein structure with various d-2-hydroxyacid dehydrogenases (11, 14, 15, 24), and even with other dehydrogenases, such as formate (21, 23, 29) and l-alanine (4) dehydrogenases.
Instead of or sometimes together with (7) d-LDH, some lactic acid bacteria have d-LDH-related enzymes that have alternative functions. It is known that a d-LDH-related enzyme called VanH is involved in the vancomycin resistance of vancomycin-resistant Enterococcus (1, 10). VanH has a broader substrate specificity than the usual d-LDHs and is able to utilize 2-ketobutyrate as well as pyruvate. On the other hand, certain Lactobacilli are known to have d-2-hydroxyisocaproate dehydrogenases (d-HicDHs), which utilize still larger aliphatic or aromatic substrates than d-LDHs (7, 16, 18, 22), although their actual physiological role is uncertain. Furthermore, the enzymes purified from Lactobacillus curvatus (17), Enterococcus faecalis (30), and the yeast Rhodotorula graminis (2, 3, 5, 12) have been reported to efficiently catalyze the conversion between benzoylformic acid (C6H5-CO-COO−) and d-mandelic acid (C6H5-CHOH-COO−) and are called d-mandelate dehydrogenases (d-ManDHs) (17), although their crucial relationship to d-LDH remains uncertain (5, 12). d-LDH-related enzymes such as d-HicDHs and d-ManDHs are promising for industrial application, because optically active 2-hydroxyacids are valuable for the synthesis of useful compounds (16-18, 30). In the process of screening lactic acid bacteria for d-ManDH activity, we found that E. faecalis IAM 10071 contains two distinct forms of an enzyme that exhibit marked d-ManDH activity.
For the screening of 28 lactic acid bacteria (Table 1), the enzyme assay was performed at 30°C in 0.1 M sodium phosphate buffer (pH 7.5) containing 0.12 mM sodium NADH and 14.7 mM sodium benzoylformate. One unit was defined as the catalytic rate of 1 μmol of substrate per min. Protein concentrations were determined with Bio-Rad protein assay protein reagent according to the method of Bradford (9), with bovine serum albumin as a standard.
TABLE 1.
Strains of lactic acid bacteria used in this study
| Species | Strain(s)a |
|---|---|
| Enterococcus | |
| E. faecalis | IAM10065, IAM10071, IFO12964, SBT1106, SBT1120, SBT1123, SBT1124 |
| E. faecium | IAM1262 |
| E. hirae | IFO3181 |
| Lactobacillus | |
| L. acidophilus | SBT2062, SBT2159 |
| L. brevis | IAM1082, IAM1381 |
| L. delbrueckii | SBT2142 |
| L. delbrueckii subsp. bulgaricus | SBT0565 |
| L. delbrueckii subsp. delbrueckii | IAM1085, IAM1149, IAM1174, IFO3202 |
| L. fermentum | SBT2558 |
| L. gasseri | SBT1752 |
| L. lactis subsp. lactis | IAM1173, IAM1371, IAM12476, SBT1207 |
| L. salivaris subsp. salivaris | SBT2670 |
| Lactococcus | |
| L. lactis subsp. cremoris | SBT1307 |
| L. lactis subsp. lactis | SBT1207 |
IAM, Institute of Applied Microbiology Culture Collection, Center for Cellular and Molecular Research, Institute of Molecular and Cellular Biosciences. The University of Tokyo; SBT, Snow Brand Milk Products Co., Ltd., Tokyo, Japan; IFO, Institute for Fermentation, Osaka, Japan.
Test tubes containing 8 ml of liquid Lactobacillus MRS medium (Difco, Detroit, Mich.) were inoculated with three loopfulls of cells from fresh slant cultures on MRS medium and incubated at 37°C for 3 days with agitation. The cells were harvested by centrifugation at 11,000 × g for 10 min and then washed with 0.1 M sodium phosphate buffer (pH 7.0). After being lyophilized, the cells were stored at −20°C until use. The dried cells were suspended in the same buffer containing 2 mM 2-mercaptoethanol and then were disrupted by sonication on ice. Clear supernatants of the cell-free extracts of the bacteria, which contained 12 to 33 μg of protein per ml, were obtained by centrifugation at 11,000 × g for 10 min and examined for NADH-dependent benzoylformate-reducing activity. Among cell extracts of 28 lactic acid bacteria examined (Table 1) the extract of E. faecalis IAM 10071 exhibited the highest specific activity for NADH-dependent benzoylformate reduction (5.6 U/mg). Except for Lactobacillus fermentum SBT 2558 (1.2 U/mg), the other strains showed no or much weaker activity than E. faecalis IAM 10071.
During purification of E. faecalis IAM 10071 enzymes, the assay was carried out at 30°C in 50 mM Tris-HCl buffer (pH 7.5) containing 0.1 mM sodium NADH and 10 mM sodium benzoylformate. For the determination of kinetic parameters, the enzyme reactions were performed in the presence of various concentrations of 2-ketoacids or d-mandelate and a saturating amount of NADH (0.1 mM) or NAD+ (5 mM), respectively. Kinetic parameters were calculated from plots of [S]/v versus [S]. The purity of the enzyme preparations was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli (20).
E. faecalis IAM 10071 cells were grown at 37°C in MRS broth (1 liter), harvested in the early stationary phase, and then washed with 50 mM Tris-HCl buffer (pH 7.5), which was used for all of the enzyme purification steps. After the addition of ammonium sulfate to 25% saturation and centrifugation, the clear supernatant from approximately 10 g (wet weight) of the packed cells was applied to a column of Butyl Toyopearl 650M gel (Tosoh, Tokyo, Japan), which had been equilibrated with buffer containing ammonium sulfate (25% saturation), and eluted with a linear gradient of ammonium sulfate from 25 to 0% saturation. This and all of the following column chromatographies were performed at room temperature with a BioLogic system (Bio-Rad).
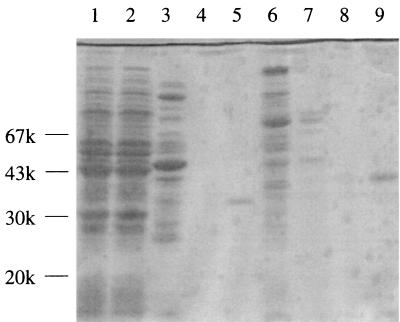
Two obvious peaks of benzoylformate-reducing activity were reproducibly obtained by Butyl Toyopearl 650M column chromatography, even after repeated single-colony isolation of E. faecalis IAM 10071 cells. Because the strain contains at least two distinct forms of d-ManDH, the two apparent d-ManDHs were tentatively named d-ManDH1 and d-ManDH2 according to the order of their elution and further purified separately. After dialysis, the pool for d-ManDH1 was loaded onto a column of Blue Sepharose CL-6B gel (Amersham-Pharmacia Biotech). The gel was washed with buffer containing 100 mM NaCl, and then the active enzyme fraction was eluted in the presence of 1 mM NADH. Unlike d-ManDH1, d-ManDH2 could not be absorbed onto Blue Sepharose CL-6B gel under the same conditions. The d-ManDH2 pool was therefore loaded onto a DEAE-Sepharose CL-6B gel column and then eluted with a linear NaCl concentration gradient from 0 to 0.5 M. Both the d-ManDH1 and d-ManDH2 samples were highly diluted in these steps, because they were broadly eluted from the Blue Sepharose and DEAE-Sepharose, respectively. Therefore, both of the enzyme samples were concentrated and concomitantly further purified by repeated Resource Q (Amersham-Pharmacia Biotech) column chromatography with a linear gradient of 0 to 1 M NaCl. The finally purified d-ManDH1 and d-ManDH2 samples each gave a single major protein band (at least 90% of the total proteins) with distinct mobilities on SDS-PAGE (Fig. 1). The data for the purification of the two d-ManDHs are summarized in Table 2.
FIG. 1.
SDS-PAGE showing the purity of the d-ManDHs at each purification step. Lanes: 1, crude extract of E. faecalis IAM 10071 cells; 2, supernatant of the 25% ammonium sulfate pool; 3 and 6, Butyl Toyopearl M650 pools for ManDH1 and ManDH2, respectively; 4, Blue Sepharose pool for ManDH1; 5, Resource Q pool for ManDH1; 7, DEAE-Sepharose pool for ManDH2; 8 and 9, first and second Resource Q pools for ManDH2, respectively. The gel was calibrated with a low-molecular-weight electrophoresis calibration kit (Amersham-Pharmacia Biotech).
TABLE 2.
Purification of d-ManDH1 and d-ManDH2 from E. faecalis IAM 10071
| Step | Total activity (U) | Total protein (mg) | Sp act (U/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude extract | 1,372 | 293 | 4.7 | 100 | 1 |
| Ammonium sulfate | 1,173 | 275 | 4.2 | 86 | 0.9 |
| d-ManDH1 | |||||
| Butyl Toyopearl | 145 | 8.8 | 16.4 | 10.5 | 3.5 |
| Blue Sepharose | 104 | 0.32 | 328 | 7.6 | 69.9 |
| Resource Q | 51 | 0.066 | 774 | 3.7 | 164.0 |
| d-ManDH2 | |||||
| Butyl Toyopearl | 529 | 15.9 | 33.3 | 38.6 | 7.1 |
| DEAE-Sepharose | 77 | 0.73 | 105 | 5.6 | 22.3 |
| Resource Q | 36 | 0.094 | 378 | 2.6 | 80.7 |
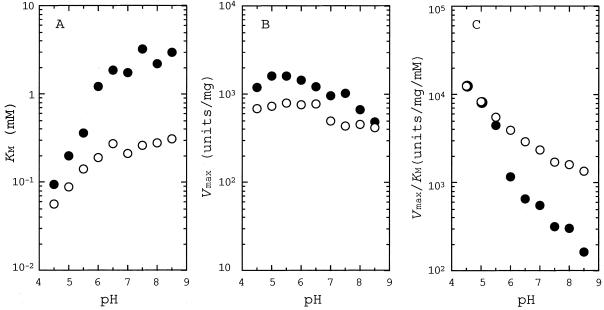
Figure 2 shows the pH profiles of kinetic parameters (Km, Vmax, and Vmax/Km values) for benzoylformate reduction by d-ManDH1 and d-ManDH2 in the pH range of 4.5 to 8.5. For both enzymes, the Km values were apparently constant above pH 6.5, but were greatly reduced below pH 6.0 (Fig. 2A), while the Vmax values were less affected by pH in both d-ManDH1 and d-ManDH2 (Fig. 2B). Thus, d-ManDH1 and d-ManDH2 consistently showed the maximal catalytic efficiencies (Vmax/Km) at pH 4.5, or possibly below pH 4.5 (Fig. 2C), although the former showed slightly larger Km and Vmax values than the latter in the whole pH range (Fig. 2B). On the other hand, d-ManDH2 exhibited maximal reaction velocity at a slightly higher temperature (35 to 45°C) than d-ManDH1 (30 to 40°C) at pH 7.5 (data not shown).
FIG. 2.
pH dependence of Km (A [Vmax versus pH]), Vmax (B [Km versus pH]), and Vmax/Km (C [Vmax/Km versus pH]) on benzoylformate reduction by d-ManDH1 (solid circles) and d-ManDH2 (open circles). The buffers used for these assays were 50 mM sodium acetate (pH 4.5 to 5.5), sodium phosphate (pH 6.0 to 7.0), and Tris-HCl (pH 7.5 to 8.5).
Table 3 shows the kinetic parameters of d-ManDH1 and d-ManDH2 for various substrates, together with the reported Km values for known bacterial d-ManDHs (17, 30). Both enzymes consistently exhibited no or very little activity toward small 2-ketoacid substrates, such as pyruvate, hydroxypyruvate, and 2-ketobutyrate, and much higher activity toward substrates with larger aliphatic or aromatic side chains. The two enzymes also consistently exhibited higher activity (smaller Km and larger Vmax) for 2-ketoacid substrates branched at the C3 or C4 position than unbranched substrates; i.e., 2-ketoisovalerate and 2-ketoisocaproate were more favorable than 2-ketovalerate and 2-ketocaproate, respectively. Among aromatic substrates, the two enzymes preferred benzoylformate to phenylpyruvate by 9- and 17-fold, respectively. The coenzyme NADH could not be replaced by NADPH in the benzoylformate reduction by either of the two enzymes. Both of the enzymes effectively catalyzed d-mandelate oxidation, but there was no detectable l-mandelate oxidation. d-ManDH2 showed generally smaller Km values and lower Vmax values than d-ManDH1 for most of the substrates examined.
TABLE 3.
Kinetic parameters for E. faecalis IAM 10071 d-ManDHs
| Substrate |
E. faecalis IAM 10071a
|
Km (mM) for:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
d-ManDH1
|
d-ManDH2
|
L. curvatusd-ManDH (17) | E. faecalisd-ManDH (30) | ||||||
| Km (mM) | Vmax (U/mg) | Vmax/Km (U/mg/mM) | Km (mM) | Vmax (U/mg) | Vmax/Km (U/mg/mM) | ||||
| Pyruvate | − | − | − | + | + | 0.062 | |||
| Hydroxypyruvate | + | + | 0.46 | + | + | 0.12 | |||
| 2-Ketobutyrate | 27 | 3 | 0.11 | 4.0 | 40 | 10 | 0.55 | 7.7 | |
| 2-Ketovalerate | 2.8 | 345 | 122 | 0.4 | 120 | 300 | 0.17 | 3.3 | |
| 2-Ketoisovalerate | 0.5 | 840 | 1,800 | 0.15 | 300 | 2,000 | 0.18 | 0.4 | |
| 2-Ketocaproate | 4.2 | 360 | 86 | 0.60 | 120 | 200 | 0.10 | 2.3 | |
| 2-Ketoisocaproate | 0.6 | 1,300 | 2,100 | 0.11 | 430 | 3,900 | 0.09 | 0.7 | |
| Benzoylformate | 2.4 | 830 | 350 | 0.26 | 440 | 1,700 | 0.22 | 3.2 | |
| Phenylpyruvate | 11 | 440 | 40 | 3.4 | 350 | 105 | 0.15 | 7.0 | |
| d-Mandelate | 4.8 | 37 | 7.7 | 0.76 | 23 | 30 | 0.5 | ||
−, enzyme activity was undetectable; +, enzyme activity was detectable, but too weak to determine exact Km and Vmax values individually.
d-ManDH1 and d-ManDH2 appear to be distinguishable from the reported d-HicDHs of Lactobacillus casei (16, 18) and Lactobacillus delbrueckii subsp. bulgaricus (7) in their substrate specificity, although these enzymes consistently prefer 2-ketoisocaproate among 2-ketoacid substrates (Table 3). First, it has been reported (18) that L. casei d-HicDH can not catalyze d-mandelate oxidation, unlike these d-ManDHs, although its activity toward benzoylformate is uncertain. Second, the two Lactobacillus d-HicDHs can hardly utilize C3-branched 2-ketoacid substrates, such as 2-ketoisovalerate, compared with unbranched substrates, such as 2-ketovalerate; i.e., they show about a 400-fold larger Km for the former substrates than for the latter (7, 16). Therefore, the two newly isolated enzymes should be called d-ManDHs rather than d-HicDHs, as in the case of the three reported enzymes that effectively catalyze the conversion of benzoylformate to d-mandelate (5, 17, 30).
Among the reported d-ManDHs, the E. faecalis IFO 12964 (30) and L. curvatus DSM 20019 (17) enzymes are known to be highly active toward both aromatic and aliphatic substrates, like d-ManDH1 and d-ManDH2, while the other yeast Rhodotorula enzyme is unique because of its strict specificity for aromatic substrates such as benzoylformate and phenylpyruvate (2). In particular, the E. faecalis IFO 12964 enzyme resembles d-ManDH1 and d-ManDH2 in its preference for C3-branched substrates (2-ketoisovalerate) to unbranched substrates (2-ketovalerate) and in its maximal activity for benzoylformate reduction at pH 4.5 (30). Among the present three Enterococcus enzymes, d-ManDH2 exhibits smaller Km values for most substrates under essentially the same assay conditions (pH 7.5, 30°C), while the other two enzymes are also similar to each other in their Km values (Table 2).
It is known that Lactobacillus d-HicDHs (11) and d-LDHs (19, 25, 27) as well as possibly Enterococcus VanH (26), have dimeric structures that comprise two identical subunits of around 330 amino acids. The molecular masses of E. faecalis IFO 12964 (30), L. curvatus DSM 20019 (17), and R. graminis KGX 39 (2) d-ManDHs were found to be 34, 30, and 38 kDa by SDS-PAGE, and 72, 60 and 77 kDa by gel filtration, respectively, suggesting that all of them are also dimeric enzymes with identical subunits. On the other hand, the apparent molecular masses of d-ManDH1 and d-ManDH2 were estimated to be 32 and 40 kDa, respectively, by SDS-PAGE with a low-molecular-weight electrophoresis calibration kit (Amersham-Pharmacia Biotech) and 110 and 130 kDa, respectively by gel filtration with HiLoad 16/60 Superdex 200pg (Amersham-Pharmacia Biotech), and low- and high-molecular-weight gel filtration calibration kits (Amersham-Pharmacia Biotech). These results suggest that these newly isolated d-ManDHs may have distinct quarternary structures from those of the known d-ManDHs or d-LDHs and d-HicDHs.
This is the first report that one bacterial strain contains at least two distinct forms of d-ManDH. At this stage, detailed structural analysis of d-ManDH1 and d-ManDH2 is highly desirable to clarify their relationship to each other and to other known d-LDH-related enzymes.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research to H.T. from the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Arthur, M., C. Molinas, S. Dutka-Malen, and P. Courvalin. 1991. Structural relationship between the vancomycin resistant protein VanH and 2-hydroxycarboxylic acid dehydrogenase. Gene 103:133-134. [DOI] [PubMed] [Google Scholar]
- 2.Baker, D. P., and C. A. Fewson. 1989. Purification and characterization of d(−)-mandelate dehydrogenase from Rhodotorula graminis. J. Gen. Microbiol. 135:2035-2044. [Google Scholar]
- 3.Baker, D. P., C. Kleanthous, J. N. Keen, E. Weinhold, and C. A. Fewson. 1992. Mechanistic and active-site studies on d(−)-mandelate dehydrogenase from Rhodotorula graminis. Biochem. J. 281:211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker, P. J., Y. Sawa, H. Shibata, S. E. Sedelnikova, and D. W. Rice. 1998. Analysis of the structure and substrate binding of Phormidium lapideum alanine dehydrogenase. Nat. Struct. Biol. 5:561-567. [DOI] [PubMed] [Google Scholar]
- 5.Basak, A. K., D. P. Baker, C. A. Fewson, and D. I. Stuart. 1993. Preliminary crystallographic study of d(−)-mandelate dehydrogenase from Rhodotorula graminis. J. Mol. Biol. 233:781-783. [DOI] [PubMed] [Google Scholar]
- 6.Bernard, N., T. Ferain, D. Garmyn, P. Hols, and J. Delcour. 1991. Cloning of the d-lactate dehydrogenase gene from Lactobacillus delbrueckii subsp.bulgaricus by complementation in Escherichia coli. FEBS Lett. 290:61-64. [DOI] [PubMed] [Google Scholar]
- 7.Bernard, N., K. Johonsen, T. Ferain, D. Garmyn, P. Hols, J. J. Holbrook, and J. Delcour. 1994. NAD+-dependent d-2-hydroxyisocaproate dehydrogenase of Lactobacillus delbrueckii subsp. bulgaricus. Gene cloning and enzyme characterization. Eur. J. Biochem. 224:439-446. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmik, T., and J. L. Steele. 1994. Cloning, characterization and insertional inactivation of the Lactobacillus helveticus d(−)-lactate dehydrogenase gene. Appl. Microbiol. Biotechnol. 41:432-439. [DOI] [PubMed] [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 10.Bugg, T. D., G. D. Wright, S. Dutka-Malen, M. Arthur, P. Courvalin, and C. T. Walsh. 1991. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 30:10408-10415. [DOI] [PubMed] [Google Scholar]
- 11.Dengler, U., K. Niefind, M. Kieß, and D. Schomburg. 1997. Crystal structure of a ternary complex of d-2-hydroxyisocaproate dehydrogenase from Lactobacillus casei, NAD+ and 2-oxoisocaproate at 1.9 Å resolution. J. Mol. Biol. 267:640-660. [DOI] [PubMed] [Google Scholar]
- 12.Fewson, C. A., D. P. Baker, R. M. Chalmers, J. N. Keen, J. D. Hamilton, A. J. Scott, and E. M. Yasin. 1993. Relationships amongst some bacterial and yeast lactate and mandelate dehydrogenases. J. Gen. Microbiol. 139:1345-1352. [DOI] [PubMed] [Google Scholar]
- 13.Garvie, E. I. 1980. Bacterial lactate dehydrogenases. Microbiol. Rev. 44:106-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg, J. D., T. Yoshida, and P. Brick. 1994. Crystal structure of an NAD-dependent d-glycerate dehydrogenase at 2.4 ÅA resolution. J. Mol. Biol. 236:1123-1140. [DOI] [PubMed] [Google Scholar]
- 15.Grant, G. A. 1989. A new family of 2-hydroxyacid dehydrogenases. Biochem. Biophys. Res. Commun. 165:1371-1374. [DOI] [PubMed] [Google Scholar]
- 16.Hummel, W., H. Schütte, and M.-R. Kula. 1985. d-2-Hydroxyisocaproate dehydrogenase from Lactobacillus casei. A new enzyme suitable for stereospecific reduction of 2-ketocarboxylic acids. Appl. Microbiol. Biotechnol. 21:7-15. [Google Scholar]
- 17.Hummel, W., H. Schütte, and M.-R. Kula. 1988. d-(−)-Mandelic acid dehydrogenase from Lactobacillus curvatus. Appl. Microbiol. Biotechnol. 28:433-439. [Google Scholar]
- 18.Kallwass, H. K. W. 1992. Potential of R-2-hydroxyisocaproate dehydrogenase from Lactobacillus casei for stereospecific reductions. Enzyme Microb. Technol. 14:28-35. [Google Scholar]
- 19.Kochhar, S., P. E. Hunziker, P. Leong-Morgenthaler, and H. Hottinger. 1992. Primary structure, physicochemical properties, and chemical modification of NAD+-dependent d-lactate dehydrogenase. Evidence for the presence of Arg-235, His-303, Tyr-101, and Trp-19 at or near the active site. J. Biol. Chem. 267:8499-8513. [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Lamzin, V. S., Z. Dauter, V. O. Popov, E. H. Harutyunyan, and K. S. Wilson. 1994. High resolution structure of holo and apo formate dehydrogenases. J. Mol. Biol. 236:759-785. [DOI] [PubMed] [Google Scholar]
- 22.Lerch, H. P., H. Blocker, H. K. W. Kallwass, J. Hoppe, H. Tsai, and J. Collins. 1989. Cloning, sequencing and expression in Escherichia coli of the d-2-hydroxyisocaproate dehydrogenase gene of Lactobacillus casei. Gene 78:47-57. [DOI] [PubMed] [Google Scholar]
- 23.Popov, V. O., and V. S. Lamzin. 1994. NAD+-dependent formate dehydrogenase. Biochem. J. 301:625-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuller, D. J., G. A. Grant, and L. J. Banaszak. 1995. Allosteric ligand site in the Vmax-type cooperative enzyme phosphoglycerate dehydrogenase. Nat. Struct. Biol. 2:68-76. [DOI] [PubMed] [Google Scholar]
- 25.Stoll, V. S., M. S. Kimber, and E. F. Pai. 1996. Insights into substrate binding by d-2-ketoacid dehydrogenases from the structure of Lactobacillus pentosus d-lactate dehydrogenase. Struct. Curr. Biol. 4:437-447. [DOI] [PubMed] [Google Scholar]
- 26.Stoll, V. S., A. V. Manohar, W. Gillon, E. L. MacFarlane, R. C. Hynes, and E. F. Pai. 1998. A thioredoxin fusion protein of VanH, a d-lactate dehydrogenase from Enterococcus faecium: cloning, expression, purification, kinetic analysis, and crystallization. Protein Sci. 7:1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taguchi, H., and T. Ohta. 1991. d-Lactate dehydrogenase is a member of the d-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, sequencing, and expression in Escherichia coli of the d-lactate dehydrogenase gene of Lactobacillus plantarum. J. Biol. Chem. 266:12588-12594. [PubMed] [Google Scholar]
- 28.Tobey, K. L., and G. A. Grant. 1986. The nucleotide sequence of the serA gene of Escherichia coli and the amino acid sequence of the encoded protein, d-3-phosphoglycerate dehydrogenase. J. Biol. Chem. 261:12179-12183. [PubMed] [Google Scholar]
- 29.Vinals, K., E. Depiereux, and E. Faytmans. 1993. Prediction of structurally conserved regions of d-specific hydroxy acid dehydrogenases by multiple alignment with formate dehydrogenase. Biochem. Biophys. Res. Commun. 192:182-188. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki, Y., and H. Maeda. 1986. Enzymatic synthesis of optically pure (r)-(−)-mandelic acid and other 2-hydroxycarbonic acids: screening for the enzyme, and its purification, characterization and use. Agric. Biol. Chem. 50:2621-2631. [Google Scholar]