Abstract
Raw cows' milk naturally infected with Mycobacterium paratuberculosis was pasteurized with an APV HXP commercial-scale pasteurizer (capacity 2,000 liters/h) on 12 separate occasions. On each processing occasion, milk was subjected to four different pasteurization treatments, viz., 73°C for 15 s or 25 s with and without prior homogenization (2,500 lb/in2 in two stages), in an APV Manton Gaulin KF6 homogenizer. Raw and pasteurized milk samples were tested for M. paratuberculosis by immunomagnetic separation (IMS)-PCR (to detect the presence of bacteria) and culture after decontamination with 0.75% (wt/vol) cetylpyridinium chloride for 5 h (to confirm bacterial viability). On 10 of the 12 processing occasions, M. paratuberculosis was detectable by IMS-PCR, culture, or both in either raw or pasteurized milk. Overall, viable M. paratuberculosis was cultured from 4 (6.7%) of 60 raw and 10 (6.9%) of 144 pasteurized milk samples. On one processing day, in particular, M. paratuberculosis appeared to have been present in greater abundance in the source raw milk (evidenced by more culture positives and stronger PCR signals), and on this occasion, surviving M. paratuberculosis bacteria were isolated from milk processed by all four heat treatments, i.e., 73°C for 15 and 25 s with and without prior homogenization. On one other occasion, surviving M. paratuberculosis bacteria were isolated from an unhomogenized milk sample that had been heat treated at 73°C for 25 s. Results suggested that homogenization increases the lethality of subsequent heat treatment to some extent with respect to M. paratuberculosis, but the extended 25-s holding time at 73°C was found to be no more effective at killing M. paratuberculosis than the standard 15-s holding time. This study provides clear evidence that M. paratuberculosis bacteria in naturally infected milk are capable of surviving commercial high-temperature, short-time pasteurization if they are present in raw milk in sufficient numbers.
Mycobacterium paratuberculosis has been causing considerable concern to the dairy industry worldwide in recent years due to the unresolved issue of its potential role in Crohn's disease in humans (9, 10) and the possibility that pasteurization does not effectively control this organism if it is present in raw milk. M. paratuberculosis causes Johne's disease, a chronic, incurable inflammatory bowel condition, in cattle and other domestic ruminants in many countries worldwide (5), with a higher prevalence in dairy herds than in beef herds. The disease has a long incubation period, and clinical signs may not be seen until an animal is 3 to 5 years of age. Asymptomatic animals, which are thought to predominate in an infected herd, can shed M. paratuberculosis in feces and milk for up to 18 months prior to showing any clinical signs of infection, so a farmer may not be aware that a Johne's disease problem exists in his herd. Clinically infected animals can shed as many as 5 × 1012 M. paratuberculosis cells per day in feces (6, 7), and these cells can remain viable for several months in the environment (18). Fecal contamination of milk can, and does, occur during the milking process, the extent of which depends very much on the hygiene practices of the farmer during teat preparation before attachment of the milking cluster. Unfortunately, due to deficiencies in current methodology for the isolation of M. paratuberculosis from milk, it is not possible to accurately determine how many M. paratuberculosis cells are present in naturally infected milk emanating from a dairy herd with Johne's disease (8, 11).
Essentially, two approaches have been taken to date to determine the effect of high-temperature, short-time (HTST) pasteurization on the viability of M. paratuberculosis in milk. Firstly, laboratory pasteurization studies have been carried out using laboratory scale equipment to simulate commercial HTST pasteurization (i.e., 72°C for 15 s) of raw cows' milk spiked with high numbers of laboratory-grown M. paratuberculosis bacteria. Secondly, large-scale testing of commercially pasteurized cows' milk has been undertaken in the United Kingdom to determine whether viable M. paratuberculosis exists in retail pasteurized milk. Neither approach is without its problems, given the slow growth of M. paratuberculosis (incubation times of up to 18 weeks at 37°C are necessary) and the absence of an appropriate selective culture medium for this organism. The findings of several laboratory pasteurization studies have been reported over the past decade (4, 12, 14, 17, 19, 20, 23, 24), and these suggest that M. paratuberculosis is not completely inactivated by pasteurization of milk at 72°C for 15 s, the minimum heat treatment required for milk pasteurization by European Commission legislation. These findings led the United Kingdom dairy industry in 1998 to voluntarily adopt an increased holding time for commercial milk pasteurization, 25 s rather than 15 s at 72°C, in an effort to increase the lethality of the pasteurization process (2). The findings of only two pasteurized milk surveys have been reported to date. Millar et al. (21) carried out a survey of 312 samples of retail pasteurized cows' milk purchased from supermarkets in southern England and Wales from September 1991 to March 1993. With an M. paratuberculosis-specific PCR assay that was unable to distinguish between viable and dead M. paratuberculosis cells, the presence of M. paratuberculosis DNA was detected in 7% of the cartons and bottles of retail pasteurized cows' milk studied overall, although at peak times, up to 25% of the milk samples showed signs of M. paratuberculosis contamination. Despite considerable effort, these authors were unable to provide conclusive evidence that any of the M. paratuberculosis bacteria detected were present in a viable form. Recently, a national survey of bulk raw and commercially pasteurized cows' milk in the United Kingdom was carried out. Preliminary results of M. paratuberculosis testing were reported to the United Kingdom Advisory Committee on the Microbiological Safety of Food in September 2000 (1). A total of 827 raw and commercially pasteurized milk samples from 241 approved dairy processing establishments throughout England, Wales, Scotland, and Northern Ireland were tested over a 17-month period (March 1999 to July 2000). Overall, around 2% of both the raw and pasteurized milk samples tested culture positive for M. paratuberculosis. Information recorded at the time when the milk samples were collected indicated that the majority (70%) of the culture-positive pasteurized milk samples had received a heat treatment of 72 to 74°C for 15 s; the remainder had been heat-treated at 72 to 75°C for 25 to 26 s. There was no indication at any of the dairy processing establishments that pasteurization had not been carried out effectively (i.e., phosphatase test results were always negative), and postprocess contamination of the samples was considered unlikely to have occurred. These findings appear to confirm that M. paratuberculosis has the potential to survive commercial HTST pasteurization processes, even, on occasion, treatments involving the extended 25-s holding time.
It has been suggested that reliable results on the efficacy of commercial pasteurization in relation to M. paratuberculosis will only be obtained by passing naturally infected milk through an industrial-scale HTST pasteurizer that achieves turbulent flow during heating (3). However, this type of experiment presents logistical problems in terms of gaining access to a commercial pasteurizing plant for research purposes and identifying a consistent source of naturally infected raw milk to use for such a study. The study reported here was instigated by the Northern Ireland Dairy Industry in an attempt to provide a definitive answer to the following question: does M. paratuberculosis survive commercial HTST pasteurization? Having identified a small number of local dairy farms producing milk infected with M. paratuberculosis, we used the milk from two of these farms as a source of naturally infected milk for the duration of the pasteurization trials reported here. Commercial-scale HTST pasteurization and homogenization equipment was made available at Loughry College—The Food Centre, Cookstown, Northern Ireland, for use during the study, and a longer holding tube was acquired for the pasteurizer to enable the inclusion of heat treatments involving the extended holding time of 25 s. Loughry College is situated around 50 miles from the laboratory at Queen's University, Belfast (QUB), where the milk samples were to be subsequently tested for the presence of viable M. paratuberculosis. The pasteurization trials took place from December to March. These months correspond to the possible peak period of M. paratuberculosis shedding into milk noted by Millar et al. (21) and were deliberately chosen to ensure that M. paratuberculosis would be present in the raw milk supplying the pasteurizer throughout the period of the study.
MATERIALS AND METHODS
Experimental design.
From December 1999 to March 2000, raw milk potentially naturally infected with M. paratuberculosis obtained from two local dairy farms was subjected to four different pasteurization treatments on 12 separate occasions using commercial-scale HTST pasteurization and homogenization equipment located in a self-contained processing room in Loughry College's Food Technology Centre. In order to remove any potential bias, the four pasteurization treatments were applied in a predetermined random order each week. Raw and pasteurized milk samples were tested for the presence of M. paratuberculosis by immunomagnetic separation (IMS)-PCR (to detect DNA from M. paratuberculosis cells whether they were dead or alive) and decontamination and culture (to detect viable M. paratuberculosis cells if they were present) at QUB.
Raw milk collection.
On the afternoon prior to milk processing, bulk raw cows' milk was collected by road tanker from two dairy farms whose milk had recently tested culture positive for M. paratuberculosis during a survey of bulk raw cows' milk from individual dairy farms throughout Northern Ireland. The milk was maintained at chill temperature (4°C) in the tanker overnight with periodic agitation and delivered to Loughry College on the next morning.
Processing equipment.
The pasteurizer used in these heat trials was an APV HXP pasteurizer (APV UK Limited, Crawley, West Sussex, United Kingdom) with a processing capability of 2,000 liters/h. The holding section of the pasteurizer was a combination of plates and a holding tube (internal diameter, 45 mm), and in order to facilitate the 25-s holding time, an extended holding tube was substituted when required. The 15-s and 25-s holding tube arrangements were tested and certified by personnel from APV UK Ltd. before use in these studies. The pasteurizer's temperature recorder and hot water controller were also calibrated and certified by a competent local company prior to use.
The homogenizer used was an APV Manton Gaulin KF6 that homogenized at a total pressure of 2,500 lb/in2 in two stages (second stage, 600 lb/in2 made up to 2,500 lb/in2 by the first-stage pressure of 1,900 lb/in2). Homogenization took place after milk had passed through the regeneration section of the pasteurizer so the milk temperature was ca. 52 to 53°C during homogenization.
Cleaning of processing plant before, during, and after use.
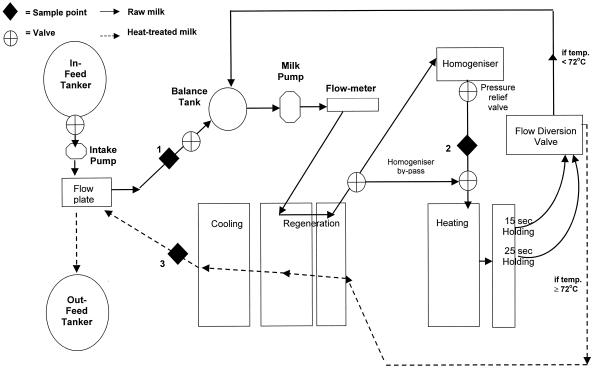
Prior to its first use on any processing day, the processing plant was sterilized in its assembled form by circulation of hot water at 85°C through the entire system (Fig. 1), i.e., the pasteurizer, homogenizer (no pressure on second stage and 1,000 lb/in2 on first stage), and associated pipework, for a minimum of 30 min. On at least five occasions during this time, the pasteurizing plant was run on divert for no more than 50 s on any occasion. The plant was then stabilized by reducing the temperature to 74 to 75°C. The plant was flushed with raw milk before its first use.
FIG. 1.
Schematic diagram showing the layout of the pasteurization and homogenization plant and the locations of raw and pasteurized milk sampling ports.
Between treatments, all of the equipment and pipework used in the previous treatment and any additional equipment and pipework to be used in the next treatment received a full cleaning cycle as follows. (i) The plant and pipework were rinsed for a minimum of 15 min with cold water. Vent plugs on the pasteurizer were loosened to allow drainage of milk deposits. (ii) A 1.5 to 2% (wt/vol) solution of ODC caustic detergent (Kilco Chemicals Ltd., Mallusk, Northern Ireland) was run through the plant at 85°C for 30 min. On at least five occasions during this 30-min period, and for no more than 50 s on any occasion, the pasteurizing plant was run on divert. (iii) The caustic detergent in the plant and pipework was cooled and drained to waste for 15 to 20 min before the entire plant was rinsed with cold water for a minimum of 15 min.
When milk treatments were completed for the day, the cleaning protocol used between treatments (as described above) was repeated. When the plant was completely drained, all valves were dismantled and hand washed in a 0.5% (vol/vol) solution of Delladet detergent-sanitizer (DiverseyLever, Northampton, United Kingdom) at 50 to 55°C and finally rinsed with cold water. The entire processing area (floors, doors, drains, and sinks) was cleaned with Shure Foam 2000 (DiverseyLever) and left for 20 min before thorough rinsing with cold water. The area was then disinfected with Divosan FG (DiverseyLever) and finally rinsed thoroughly with clean, cold water after a contact time of 30 min.
Heat treatment of milk.
Raw milk was pumped from the road tanker to the balance tank of the pasteurizer and then processed by each of the following four treatments: A, 72°C for 15 s with no homogenization; B, 72°C for 15 s with prior homogenization at 2,500 lb/in2 in two stages; C, 72°C for 25 s with no homogenization; D, 72°C for 25 s with prior homogenization at 2,500 lb/in2 in two stages.
The pasteurizer was allowed to stabilize at each temperature and holding time setting for 15 min before samples of pasteurized milk were collected. Once the required number of pasteurized milk samples had been collected, milk pumping was halted and the complete system underwent a full between-treatments cleaning cycle before the next heat treatment was carried out.
Collection of milk and water samples for testing.
Raw and pasteurized milk samples were aseptically collected into sterile plastic sample jars via Micro-Port sample points located at three places on the processing plant, as indicated in Fig. 1, by using sterile sample needles. Unhomogenized raw milk samples (150 ml) were collected via sample point 1, located on pipework prior to entering the plate pack of the pasteurizer. Homogenized raw milk samples (150 ml) were collected via sample point 2, located on the pipeline between the homogenizer and the heating section of the pasteurizer after the homogenizer had been running for 5 min. Pasteurized milk samples (150 ml) were collected via sample point 3, located on pipework leaving the cooling section of the pasteurizer. Each week, three mixed raw milk samples (one unhomogenized and two homogenized), three pasteurized milk samples for each of the four treatments (A to D), and individual bulk tank raw milk samples from the two source dairy farms were collected for testing. In addition, on three occasions during the 12-week trials (weeks 1, 5, and 12), water samples (500 ml) from the rising mains and the cold water line in the processing room at Loughry College were also aseptically collected and submitted for M. paratuberculosis testing. All samples were immediately placed in a lockable insulated box containing frozen ice packs capable of maintaining the temperature of the milk samples at or below 5°C during transit. Samples were transported by road to the testing laboratory at QUB by an assigned Department of Agriculture and Rural Development for Northern Ireland Sampling Officer. Samples arrived at QUB in the late afternoon and were stored overnight at 4°C until milk testing commenced on the next morning.
Detection of M. paratuberculosis in raw and heat-treated milk.
Each milk (or water) sample was opened in turn, and two 50-ml aliquots were aseptically decanted into sterile, disposable centrifuge tubes prior to further processing. Each milk or water sample was subjected to IMS-PCR and decontamination and culture as follows.
IMS-PCR.
One 50-ml portion of each milk (or water) sample was centrifuged (2,500 × g for 15 min), and the pellet was resuspended in 1 ml of sterile phosphate-buffered saline containing 0.05% (wt/vol) Tween 20 (PBS-T) at pH 7.4 prior to IMS (12). Ten microliters of sheep anti-rabbit immunoglobulin G Dynabeads (approximately 106; Dynal UK Ltd., Wirral, Merseyside, United Kingdom) coated with rabbit anti-M. paratuberculosis immunoglobulin G (immunobeads [IMB]) was added to each sample. Samples were incubated at room temperature (21°C) for 30 min with gentle agitation on a Dynal sample mixer. After 30 min, the IMB were removed from the cell suspension by magnetic separation for 10 min. The supernatant was carefully aspirated off, and the IMB were washed three times with 1 ml of PBS-T, with magnetic separation for 2 min between washes. Once IMS was complete, the IMB and any attached M. paratuberculosis cells were resuspended in 800 μl of TEN lysis buffer (2 mM EDTA, 400 mM NaCl, 10 mM Tris/HCl [pH 8.0], 0.6% [wt/vol] sodium dodecyl sulfate) containing 20 μg of proteinase K and incubated overnight at 37°C to weaken the cell wall. On the next morning, each sample was transferred to a blue-capped Fast RNA tube (Anachem Ltd., Luton, Bedfordshire, United Kingdom) containing fine ceramic and silica particles. The tubes were processed in a RiboLyser Cell Disruptor (Hybaid Ltd., Middlesex, United Kingdom) at 6.5 m/s for 45 s to lyse the mycobacterial cells and release DNA. Samples were placed on ice for 10 min before DNA was extracted, purified, and precipitated from each sample with phenol, chloroform-isoamyl alcohol (24:1), and isopropanol, respectively. Precipitated DNA was washed once in 70% (vol/vol) ethanol and resuspended in 50 μl of Tris-EDTA buffer (10 mM Tris, 1 mM EDTA [pH 8.0]). For PCR, 5 μl of this extracted template DNA was added to 45 μl of the PCR mixture. Each reaction mixture consisted of 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 1.75 mM MgCl2, 150 μM each deoxynucleoside triphosphate, 60 pmol each of primers P90 and P91 (22), and 1.25 U of Platinum Taq DNA polymerase (Life Technologies Ltd.) overlaid with 2 drops of light mineral oil. The PCR mixtures were subjected to 37 cycles of 94°C for 30 s, 62°C for 15 s, and 72°C for 1 min with an initial denaturation step of 94°C for 2 min and a final annealing step of 72°C for 5 min. PCR products were visualized after 2% (wt/vol) agarose gel electrophoresis by staining with ethidium bromide (0.5 μg/ml) and viewed on a UV Transilluminator. The size of the amplified product was checked by utilizing ΦX174 replicative-form DNA digested with HaeIII as molecular weight markers (Sigma).
Decontamination and culture.
The second 50-ml portion of each milk sample was centrifuged (2,500 × g for 15 min), and the pellet was resuspended in 10 ml of 0.75% (wt/vol) cetylpyridinium chloride (Sigma). Following incubation at room temperature (approximately 21°C) for 5 h and further centrifugation as described above, the pellet was resuspended in 800 μl of PBS-T. Two slopes of Herrold's egg yolk medium containing mycobactin J (HEYM) at 2 μg/ml were each inoculated with 200 μl of the resuspended pellet. The remaining 400 μl was inoculated into one vial of BACTEC Middlebrook 12B radiometric medium (Becton Dickinson UK Ltd., Cowley, Oxford, United Kingdom) supplemented with 0.5 ml of egg yolk emulsion, 100 μl of PANTA antibiotic supplement (Becton Dickinson), and mycobactin J (Synbiotics Europe SAS, Lyon, France) at 2 μg/ml. Both media were incubated at 37°C for up to 18 weeks. Inoculated vials of BACTEC medium were read on the BACTEC 460 TB machine (Becton Dickinson), and the slopes were examined periodically. When growth was observed in either medium, Ziehl-Neelsen acid-fast staining was carried out to confirm the presence of acid-fast organisms. A series of confirmatory tests were carried out on each suspect acid-fast isolate to determine whether it was M. paratuberculosis. An isolate was only confirmed to be M. paratuberculosis if it exhibited typical colony morphology, was slow-growing, tested IS 900 PCR positive from a colony, and required mycobactin J for growth.
Phosphatase testing.
Throughout the study, pasteurized milk samples were subjected to the phosphatase test to verify that pasteurization had been achieved.
RESULTS
M. paratuberculosis infection status of raw milk.
Viable M. paratuberculosis was cultured from 4 (6.7%) of the 60 raw milk samples examined during this study. M. paratuberculosis DNA was also detected by IMS-PCR in 6.7% of the raw milk samples. Viable M. paratuberculosis was isolated by culture from the raw milk supplying the pasteurizer in weeks 2 and 8 and detected by IMS-PCR in weeks 1, 8, and 9. In weeks 5, 6, 7, 10, 11, and 12, some of the pasteurized milk samples tested IMS-PCR positive for M. paratuberculosis but no M. paratuberculosis infection was detected by either culture or IMS-PCR in the corresponding raw milk in these weeks. Results strongly suggest that M. paratuberculosis was present in greater abundance in the source raw milk in week 8 than in the other weeks of the study. In this week, three of five raw milk samples tested culture positive and strong IMS-PCR signals were obtained with both raw and pasteurized milk samples.
M. paratuberculosis infection status of water at milk processing facility.
Water samples collected from the rising mains and the cold water line in the pasteurizing room at Loughry College on three occasions during the period of the study showed no evidence of contamination with M. paratuberculosis by either culture or IMS-PCR.
Milk processing details.
A summary of the actual pasteurization temperature, holding time, and homogenization pressure data for each heat treatment over the period of the study is provided in Table 1. A Reynold's number of 39,226 was calculated for milk heat treated at 73°C at a flow rate of 2,000 liters/h in a holding tube 45 mm in diameter, confirming that turbulent flow existed in the holding section of the APV HXP pasteurizer.
TABLE 1.
Summary of pasteurization temperature, holding time, and pressure data for pasteurization and homogenization during heat treatments A, B, C, and D over the 12-week period of the study
| Heat treatment | Pasteurization temp (°C)a
|
Avg holding time (s)b | Homogenization pressure (lb/in2)
|
|||
|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | First stage | Second stage | ||
| A | 72.9 | 73.5 | 73.1 ± 0.19 | 15.87 | ||
| B | 73.1 | 73.7 | 73.3 ± 0.23 | 15.87 | 1,900 | 600 |
| C | 73.0 | 74.0 | 73.1 ± 0.27 | 26.56 | ||
| D | 73.0 | 73.5 | 73.3 ± 0.20 | 26.56 | 1,900 | 600 |
Values represent the minimum, maximum, and mean temperatures recorded over the 12-week period of the study for each of the four heat treatments, A to D.
Average holding times were certified by APV UK Ltd. before experiments commenced.
Detection of M. paratuberculosis in heat-treated milk.
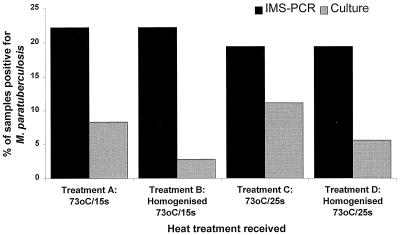
All pasteurized milk samples tested phosphatase negative throughout the study, indicating that proper pasteurization had taken place in all cases. Overall, taking no account of the particular heat treatment applied, viable M. paratuberculosis was cultured from 10 (6.9%) of 144 pasteurized milk samples whereas M. paratuberculosis DNA (from all of the cells present, both viable and heat killed) was detected by IMS-PCR in 30 (20.8%) pasteurized milk samples. HTST pasteurization was therefore shown to have a significant negative impact on M. paratuberculosis viability (P < 0.001). Confirmed isolates of M. paratuberculosis were cultured from one or more of the pasteurized milk samples in 2 of the 12 weeks of the study, from milk heat treated at 73°C for 25 s without prior homogenization (treatment C) in week 4 and from milk heat treated at 73°C for both 15 and 25s, with and without prior homogenization (treatments A, B, C, and D) in week 8. Overall, fewer pasteurized milk samples contained viable M. paratuberculosis if the milk had been homogenized prior to pasteurization (Fig. 2); however, the observed difference between treatments A and C (no homogenization) and treatments B and D (prior homogenization) was not statistically significant (P = 0.19).
FIG. 2.
Comparison of IMS-PCR and culture results of pasteurized, naturally infected milk subjected to four treatments involving heating at 73°C for 15 and 25 s with and without prior homogenization. The observed differences in culture results for treatments A and C (no homogenization) and treatments B and D (prior homogenization), respectively, were not statistically significant (P = 0.19).
DISCUSSION
The study reported here was undertaken to provide an answer to the following question: could M. paratuberculosis present in milk as a result of natural infection survive commercial HTST pasteurization? To our knowledge, this study is the first to produce results for naturally infected milk passed through a commercial-scale pasteurizer under turbulent-flow conditions. All previous pasteurization studies have employed milk artificially spiked with laboratory-grown M. paratuberculosis (4, 12, 14, 17, 19, 20, 23, 24), and a commercial-scale pasteurizer was used in only one of these studies (17). Consequently, the findings of this study are not directly comparable to those of any of the previously published pasteurization studies. The findings of this study clearly indicate that M. paratuberculosis in naturally infected milk is capable of surviving commercial-scale pasteurization at 73°C for 15 and 25 s with and without prior homogenization if the bacterial cells are present in sufficient numbers before heat treatment. Since the water sampled from the processing room at Loughry College showed no signs of M. paratuberculosis contamination when tested on three occasions during the study, it must be assumed that M. paratuberculosis contamination detected in raw or pasteurized milk at any point during this study originated on the source farm(s) and not during processing.
There are recognized deficiencies in the methodologies available for the culture of M. paratuberculosis from milk (11), relating chiefly to potential adverse effects on viability associated with the use of chemical decontamination as a selective step prior to culture (8). The detection and isolation methodologies for M. paratuberculosis adopted during this study, IMS-PCR and decontamination with 0.75% (wt/vol) cetylpyridinium chloride for 5 h prior to culture, respectively, were fully evaluated before use and shown to be capable of detecting low levels of M. paratuberculosis in milk (8, 16). Exactly the same methodologies were adopted during the recent United Kingdom survey of commercially pasteurized cows' milk (1). The use of chemical decontamination prior to culture was deemed necessary during the present study because, due to the scale of milk processing, bulk raw milk had to be used rather than aseptically obtained raw milk from an individual cow, as used in our earlier laboratory pasteurization studies (12, 14). Bulk raw milk has a higher level of background microflora, and therefore, if decontamination was not applied prior to culture, problems of overgrowth of slopes would occur and results would be lost. However, during this study, chemical decontamination was applied to all milk samples on the day after heat processing, not immediately after heat treatment. It is likely that immediately after heat treatment, sublethally heat-injured M. paratuberculosis cells exist in pasteurized milk, but given sufficient time, these sublethally injured cells could recover to fully growth-competent status. If chemical decontamination were to be applied directly after heating, then the heat-injured M. paratuberculosis would have the extra hurdle of chemical treatment to overcome before viability could be demonstrated, and nonisolation would not necessarily indicate that no viable M. paratuberculosis had survived pasteurization. For this reason, it was probably an advantage that milk testing could not take place at Loughry College immediately after heating but only after the milk samples had been transported at 4°C to the laboratory at QUB (elapsed time, 18 to 24 h). The very fact that isolation of viable M. paratuberculosis from pasteurized milk was achieved during this study even though chemical decontamination had been applied is significant.
In our experience of using the IMS-PCR and culture methods in tandem to test milk for the presence of M. paratuberculosis, there was never 100% agreement between the IMS-PCR and culture results and there were some inconsistencies between the IMS-PCR and culture results for milk samples in certain weeks of this study also. For example, sometimes the raw milk tested culture positive for M. paratuberculosis but IMS-PCR negative or vice versa (e.g., in weeks 1, 2, 8, and 9) or a pasteurized sample tested culture positive in week 4 but there was no evidence of M. paratuberculosis infection in the corresponding raw milk, which was both culture and IMS-PCR negative. Unfortunately, there is no simple explanation for this situation. It could possibly be due to the fact that M. paratuberculosis is unlikely to be evenly distributed throughout any milk sample given that it can occur as clumps of cells and single cells. Since two separate 50-ml aliquots of each raw or pasteurized milk sample were independently subjected to IMS-PCR and culture, it is possible, given the low numbers of M. paratuberculosis cells liable to be present in naturally infected milk, that one aliquot contained a clump of M. paratuberculosis cells and the other did not and hence increasing the chances of a positive result in one test. This may explain the inconsistent results for raw milk samples tested in weeks 1, 2, 8, and 9 of this study. In the case of the week 4 results, it is entirely possible that a pasteurized sample, but not the corresponding raw milk, could test culture positive because there is a greater chance of raw milk cultures becoming overgrown by non-acid-fast milk microorganisms during incubation and the growth of M. paratuberculosis being masked or inhibited if a high level of background flora exists in the raw milk, even where decontamination has been applied.
During the present study, similar percentages of raw and pasteurized milk samples tested culture positive for the presence of M. paratuberculosis overall (6.7 and 6.9%, respectively). Although it is recognized that the results of this study and the United Kingdom milk survey are not directly comparable because milk from farms known to be infected was used in the former and milk from the general United Kingdom supply was involved in the latter, a similar trend was noted during the United Kingdom milk survey, where 1.9 and 2.1% of the raw and pasteurized milk samples, respectively, were confirmed culture positive for M. paratuberculosis overall (1). Rather than indicating that commercial HTST pasteurization has no effect on the viability of M. paratuberculosis, we suggest that these results have arisen because sedimentation of M. paratuberculosis cells from pasteurized milk by centrifugation is more efficacious, for whatever reason, than that from raw milk. In the present study, a much higher percentage of pasteurized milk samples tested IMS-PCR positive (20.8%) than tested culture positive (6.9%). Given this differential in the percentages for all M. paratuberculosis cells (living and dead) detected by IMS-PCR and viable M. paratuberculosis cells detected by culture in pasteurized milk samples, these results indicate that HTST pasteurization has a significant negative impact on the viability of M. paratuberculosis cells present in naturally infected milk, as would be expected. However, the fact that survivors were isolated from pasteurized milk in two weeks of the study appears to confirm that commercial HTST pasteurization may not achieve 100% inactivation of M. paratuberculosis 100% of the time. Since the majority of culture-positive pasteurized milk samples were obtained in one particular week of the study, in which the source raw milk tested culture and IMS-PCR positive more frequently than in other weeks, this suggests that the number of cells of M. paratuberculosis present before pasteurization is a key consideration governing its potential survival after pasteurization. Unfortunately, as current isolation methodology does not permit accurate enumeration of M. paratuberculosis cells in milk, we are unable to estimate how many M. paratuberculosis cells might need to be present in milk for survival to occur.
Results of this study indicate that there may be some advantage to milk homogenization before pasteurization in terms of the subsequent heat killing of M. paratuberculosis achieved. We have previously postulated that the existence of M. paratuberculosis in large clumps may assist survival during heating (15). The shear forces existing during homogenization may have the potential to break up clumps, giving rise to dispersed cells, which would be more easily killed by subsequent heating. This may explain the greater killing observed after pasteurization of homogenized milk samples in the present study (Fig. 2). With regard to the effect of a longer holding time at 73°C, contrary to the findings of a previous study in which a longer holding time of 25 s at 72°C was found to be more effective at killing M. paratuberculosis than the legally required holding time of 15 s (13), this study did not demonstrate a significant difference between the effects of a 15-s holding time (treatments A and B) and a 25-s holding time (treatments C and D) at 73°C when applied to naturally infected milk. However, key differences between the earlier study and the present study may account for our previous findings: (i) raw milk spiked with laboratory-grown M. paratuberculosis was used previously rather than naturally infected milk, (ii) turbulent flow did not exist in the heating apparatus, and (iii) the milk was cultured straight after heating, allowing no time for possible repair of sublethal heat injury to take place.
This study may not be entirely representative of large-scale dairy processing operations, in which greater dilution of naturally infected milk from one farm with noninfected milk from a number of other farms is likely to occur during bulk storage prior to pasteurization. However, it would be representative of small-scale producer-pasteurizer operations that still exist in the United Kingdom, in which the milk from a single farm is processed. If that farm happened to be an M. paratuberculosis-infected farm, then the pasteurized milk could contain viable M. paratuberculosis. The findings of this study demonstrate that M. paratuberculosis bacteria in naturally infected milk are capable of surviving commercial HTST pasteurization of milk at 73°C for 15 and 25 s with and without prior homogenization if the bacteria are present in sufficient numbers before heat treatment. These findings reinforce those of the United Kingdom survey of commercially pasteurized milk, which showed that a small proportion (2%) of commercially pasteurized cows' milk contained viable M. paratuberculosis, including milk samples heat treated for the extended holding time of 25 s at 74°C (1). Results of the present study suggest that homogenization prior to pasteurization reduced the incidence of viable M. paratuberculosis bacteria in pasteurized milk but survival was still possible if high enough numbers of these organisms were present in milk. Further optimization of pasteurization time and temperature conditions, homogenization pressures, and investigation of the potential impact of milk processing technologies, such as microfiltration and bactofugation prior to pasteurization, is warranted in light of these findings in order to ensure the elimination of viable M. paratuberculosis from milk.
Acknowledgments
We are grateful to the Northern Ireland Dairy Industry for instigating and funding this research.
Thanks are also due to John Speers, Principal of Loughry College; to John Dooey for technical assistance during milk processing; and to Stewart Todd for transporting the milk and water samples between Loughry College and QUB each week.
REFERENCES
- 1.Advisory Committee on the Microbiological Safety of Food. 2000. Preliminary results from the National Study on the Microbiological Quality and Heat Processing of Cows' Milk: Mycobacterium avium subsp. paratuberculosis. Paper ACM/485, presented to the Advisory Committee on the Microbiological Safety of Food on 19 September 2000. [Online.] http://www.foodstandards.gov.uk/pdf_files/papers/acm485.pdf.
- 2.Amor, D. 1998. Why milk needs reassessing. Food Ind. News August:10.
- 3.Cerf, O., and M. W. Griffiths. 2000. Mycobacterium paratuberculosis heat resistance. Lett. Appl. Microbiol. 30:341-344. [DOI] [PubMed] [Google Scholar]
- 4.Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurisation. J. Vet. Diagn. Investig. 5:629-631. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini, R. J., H. J. Van Kruiningen, and R. S. Merkal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74:218-262. [PubMed] [Google Scholar]
- 6.Clarke, C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and in other species. J. Comp. Pathol. 116:217-261. [DOI] [PubMed] [Google Scholar]
- 7.Cocito, C., P. Gilot, M. Coene, M. De Kesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dundee, L., I. R. Grant, H. J. Ball, and M. T. Rowe. 2001. Comparative evaluation of four decontamination protocols for the isolation of Mycobacterium avium subsp. paratuberculosis from milk. Lett. Appl. Microbiol. 33:173-177. [DOI] [PubMed] [Google Scholar]
- 9.European Commission. 2000. Possible links between Crohn's disease and paratuberculosis—report of the Scientific Committee on Animal Health and Animal Welfare. [Online.] http://www.europa.eu.int/comm/food/fs/sc/scah/out38_en.pdf.
- 10.Food Safety Authority of Ireland. 2000. Mycobacterium paratuberculosis—does it contribute to Crohn's disease? [Online.] http://www.fsai.ie/research/myco.pdf.
- 11.Grant, I. R., and M. T. Rowe. 2001. Methods for the detection and enumeration of viable Mycobacterium paratuberculosis from milk and milk products. Bull. Int. Dairy Fed. 362:41-52. [Google Scholar]
- 12.Grant, I. R., H. J. Ball, and M. T. Rowe. 1998. Effect of high temperature, short time (HTST) pasteurisation on milk containing low numbers of Mycobacterium paratuberculosis. Lett. Appl. Microbiol. 26:166-170. [DOI] [PubMed] [Google Scholar]
- 13.Grant, I. R., H. J. Ball, and M. T. Rowe. 1999. Effect of higher pasteurisation temperatures, and longer holding times at 72oC, on the inactivation of Mycobacterium paratuberculosis in milk. Lett. Appl. Microbiol. 28:461-465. [DOI] [PubMed] [Google Scholar]
- 14.Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Inactivation of Mycobacterium paratuberculosis in cow's milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Investigation of the thermal death of Mycobacterium paratuberculosis during pasteurisation, p. 452-457. In International Dairy Federation General Secretariat (ed.), Heat treatments and alternative methods. International Dairy Federation, Brussels, Belgium.
- 16.Grant, I. R., C. M. Pope, L. M. O'Riordan, H. J. Ball, and M. T. Rowe. 2000. Improved detection of Mycobacterium avium subsp. paratuberculosis in milk by immunomagnetic PCR. Vet. Microbiol. 77(3-4):369-378. [DOI] [PubMed] [Google Scholar]
- 17.Hope, A. F., P. A. Tulk, and R. J. Condron. 1996. Pasteurization of Mycobacterium paratuberculosis in whole milk, p. 377-382. In R. J. Chiodini, M. E. Hines, and M. T. Collins (ed.), Proceedings of the Fifth International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Rehoboth, Mass.
- 18.Jorgensen, J. B. 1977. Survival of Mycobacterium paratuberculosis in slurry. Nord. Veterinaermed. 29(6):267-270. [PubMed] [Google Scholar]
- 19.Keswani, J., and J. F. Frank. 1998. Thermal inactivation of Mycobacterium paratuberculosis in milk. J. Food Prot. 61:974-978. [DOI] [PubMed] [Google Scholar]
- 20.Meylan, M., D. M. Rings, W. P. Shulaw, J. J. Kowalski, S. Bech-Nielsen, and G. F. Hoffsis. 1996. Survival of Mycobacterium paratuberculosis and preservation of immunoglobulin G in bovine colostrum under experimental conditions simulating pasteurization. Am. J. Vet. Res. 57:1580-1585. [PubMed] [Google Scholar]
- 21.Millar, D., J. Ford, J. Sanderson, S. Withey, M. Tizard, T. Doran, and J. Hermon-Taylor. 1996. IS 900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows' milk in England and Wales. Appl. Environ. Microbiol. 62:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss, M. T., J. Sanderson, M. Tizard, J. Hermon-Taylor, F. El-Zaatari, D. Markesich, and D. Graham. 1992. PCR detection of Mycobacterium paratuberculosis in long-term cultures from Crohn's disease tissues. Gut 33:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl. Environ. Microbiol. 63:4975-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]