Abstract
The genomic diversity of 33 previously assigned strains from six species within the genus Pediococcus was assessed by randomly amplified polymorphic DNA (RAPD) PCR and pulsed-field-gel electrophoresis (PFGE). The RAPD PCR patterns produced by two separate random primers, termed P1 (ACGCGCCCT) and P2 (ATGTAACGCC), were compared by the Pearson correlation coefficient and the unweighted pair group method with arithmetic averages clustering algorithm. Pattern variations between repeat samples set a strain discrimination threshold of less than 70% similarity. P1 and P2 primers alone and in combination produced 14, 21, and 28 distinct patterns, respectively. When each strain was assigned with a type strain with which it shared the highest level of similarity, both primers grouped 17 of the 27 strains to their proposed species. PFGE following genomic digestion with the restriction enzymes ApaI, NotI, and AscI produced 30, 32, and 28 distinct macrorestriction patterns, respectively. Specific DNA fragments within the NotI and AscI macrorestriction patterns for each strain were observed that allowed 27 of the 33 strains to be assigned to their proposed species. For example, following digestion with AscI, all Pediococcus parvulus strains were characterized by two DNA fragments, one of approximately 220 kb and another between 700 and 800 kb. The exceptions correlated with those observed with both RAPD PCR primers and included three P. damnosus and two P. pentosaceus strains that grew at temperatures regarded as nonpermissive for their proposed species but not for those with which they grouped.
Pediococci are gram-positive, facultatively anaerobic cocci belonging to the group of lactic acid bacteria. The genus consists of eight species, Pediococcus acidilactici, P. pentosaceus, P. parvulus, P. dextrinicus, P. damnosus, P. inopinatus, P. halophilus, and P. urinaeequi, although the taxonomic status of the latter two species remains uncertain (10, 13, 16). Lactic acid bacteria have a GRAS (generally regarded as safe) status, and two Pediococcus species, P. acidilactici and P. pentosaceus, have been widely used in the fermentation of vegetables (20), meats (24, 25), dough (32), fruit juices (20), dairy products (5, 22,), and silage (7, 14). Both species have been recovered from the gastrointestinal tracts of poultry (19), ducks (21), and other animals (17), and several commercial probiotic feeds containing either species are currently available (39, 42). Pediocins, inhibitory to a range of food pathogens, have been isolated from both species and P. damnosus (8, 24, 25, 31). Within the brewing industry, pediococci, principally P. damnosus, occur as primary contaminants in pitching yeast and are among the most prevalent spoilage microbes (35).
Various molecular genetic methods have been used to discriminate between strains in the genus Pediococcus. These include the use of specific DNA target probes (23, 27, 28, 29, 37), ribotyping (2, 18, 38), total DNA-DNA hybridization (1, 13), 16S rRNA gene sequencing (2, 10, 21, 33), randomly amplified polymorphic DNA (RAPD) PCR (21, 29, 30, 32) and pulsed-field gel electrophoresis (PFGE) (3, 12, 24).
RAPD PCR uses low-stringency hybridization conditions with a single random oligonucleotide primer and PCR to amplify DNA sequences from the bacterial genome (43, 45). To date, RAPD PCR studies of pediococci have been based on single random primers. An 18-mer primer originally designed to target a pediocin-encoding gene on a plasmid from P. acidilactici was only effective at discriminating strains of P. acidilactici, with 19 strains producing seven distinct RAPD patterns (29, 30). In another study, a 17-mer primer discriminated nine pediococci isolated from the gastrointestinal tracts of ducks into four groups and partial 16S ribosomal sequencing indicated that each group represented a single species (21). A 9-mer primer was used to assign proposed Pediococcus isolates from fermented cereals to one of six species of the genus (32). Although clear clusters involving all but the P. dextrinicus and P. damnosus type strains were found, species classification was not confirmed by any other means and more than 40% of the isolates failed to cluster. It remains unclear if RAPD PCR can be used effectively to discriminate strains and species from across the genus.
PFGE, unlike conventional electrophoresis, can resolve DNA fragments of greater than 30 kb and separate a bacterial genome digested with a rarely cutting restriction enzyme into a discrete number of DNA fragments termed a macrorestriction pattern (26, 40). The use of PFGE to discriminate among pediococci appears to have been limited to P. acidilactici and P. pentosaceus strains (3, 12, 24), and in only one case was a large number of isolates examined (3). For these two species, PFGE discriminated between both strains and species, with the latter discrimination being based on a difference in cutting frequency with the restriction enzyme SmaI (3). However, it is not known how effectively PFGE can discriminate strains and species from across the genus.
The objective of this study was to examine the genomic diversity of a range of strains previously assigned to species within the genus Pediococcus by using RAPD PCR with two separate random primers and PFGE with a number of restriction enzymes. From this analysis, we were able to determine the degree of strain diversity evident with both techniques and identify genomic fingerprints specific for each species.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The strains used in this study are listed in Table 1. Cultures were grown under anaerobic conditions (anaerobic jars with Anaerocult A gas packs [Merck, Darmstadt, Germany]) in modified MRS (mMRS), comprising Lactobacilli MRS medium (Difco, Detroit, Mich.) supplemented with 0.05% (wt/vol) cysteine hydrochloride at temperatures recommended by each strain supplier (Table 1).
TABLE 1.
Pediococcus strains used in this study
| Species and straina | Source | Growth temp (°C)b |
|---|---|---|
| P. acidilactici | ||
| NCIMB 6990 | 37 | |
| NCIMB 700993 | Summer sausage | 30 |
| NCIMB 701851 | Silage | 30 |
| NCIMB 701860 | 30 | |
| NCFB 2767T | Barley | 37 |
| CNCM MA18/5M | 37 | |
| CNCM MA28/6B | 37 | |
| P. pentosaceus | ||
| NCIMB 7837 | Dairy products | 37 |
| NCIMB 12009 | Sake mash | 37 |
| NCIMB 8106 | 30 | |
| NCIMB 8127 | 25 | |
| NCIMB 8952 | 25 | |
| NCIMB 700814 | Pickled cucumber | 30 |
| NCDO 1850 | Silage | 30 |
| NCFB 990T | Dried beer yeast | 37 |
| P. inopinatus | ||
| ATCC 49902T | Brewery yeast | 30 |
| P. damnosus | ||
| NCIMB 8519 | Beer | 25 |
| NCIMB 10563 | Cider | 25 |
| NCIMB 1782 | Marinated herring | 30 |
| NCIMB 701834 | 22 | |
| NCIMB 701835 | 22 | |
| NCIMB 701864 | Japanese beer | 22 |
| NCIMB 701866 | 22 | |
| ATCC 29358T | Lager beer yeast | 26 |
| P. parvulus | ||
| NCIMB 701107 | Cheese | 30 |
| NCIMB 701248 | 30 | |
| NCIMB 701558 | Hay | 30 |
| NCIMB 701559 | Cow shed air | 30 |
| NCIMB 701852 | Silage | 30 |
| ATCC 19371T | Silage | 30 |
| P. dextrinicus | ||
| DSMZ 20293 | Beer | 30 |
| DSMZ 20334 | Silage | 30 |
| DSMZ 20335T | Silage | 30 |
Culture collections: NCIMB, National Collection of Industrial and Marine Bacteria, Aberdeen, United Kingdom; ATCC, American Type Culture Collection, Manassas, Va.; DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany; NCDO, National Collection of Dairy Organisms (now NCIMB); NCFB, National Collection of Food Bacteria (now NCIMB); CNCM, National Collection of Cultures of Microorganisms, Paris, France. A superscript capital T indicates a type strain.
Recommended growth temperature.
Growth at nonpermissive temperatures.
Strains of P. parvulus and P. damnosus were cultured in broth at 25°C, serially diluted in mMRS agar, and incubated at either 25 or 37°C for 3 and 2 days, respectively. Growth was expressed as the number of CFU per milliliter of the broth culture. Strains of P. acidilactici and P. pentosaceus were cultured overnight at 37°C in mMRS broth, inoculated at 0.1% (vol/vol), and incubated at 37 or 50°C for 24 h. Increases in culture turbidity were determined with a Hitachi U-1100 spectrophotometer (Hitachi, Tokyo, Japan) set at a wavelength of 550 nm.
RAPD PCR.
From a stationary-phase culture, determined by spectrophotometer readings, 1.5 ml of cells was centrifuged at 5,000 × g for 5 min and the DNA was extracted by the method detailed in reference 9 as follows. The resulting cell pellet was resuspended in 200 μl of a solution containing 2% (vol/vol) Triton X-100, 1% (wt/vol) sodium dodecyl sulfate, 100 mM NaCl, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 200 μl of phenol-chloroform-isoamyl alcohol. Acid-washed glass beads (0.3 g; Sigma-Aldrich, Dublin, Ireland) were added, mixed with a Sorvall vortex mixer for 2 min, and centrifuged at 10,000 × g for 5 min. The upper layer was removed, and to it 0.1 volume of 3 M sodium acetate and 3 volumes of absolute ethanol at −20°C were added. The mixture was centrifuged at 10,000 × g for 10 min, and the resulting pellet was washed in 70% (vol/vol) ethanol. Centrifugation was repeated, and the pellet was dried at 37°C for 30 min and dissolved in 50 μl of sterile water (9). Two microliters of extracted DNA was used per PCR amplification in a total volume of 50 μl containing 0.25 μM each deoxynucleoside triphosphate (supplied as a 50× deoxynucleoside triphosphate Mastermix; Bioline USA, Inc.), 10 mM (NH4)2SO4, 67 mM Tris-HCl (pH 8.8), 0.1% (vol/vol) Tween 20 (supplied as a 10× NH4 Reaction Buffer; Bioline), and 5 mM MgCl2 (supplied as a 50 mM stock; Bioline). Two random primers, termed P1 (5" ACGCGCCCT 3") and P2 (5" ATGTAACGCC 3") (both synthesized by Sigma-Genosys Ltd.), were previously found to yield approximately 10 distinct DNA fragments of 100 to 1,200 bp for a range of lactic acid bacteria (15). The primers were used in separate RAPD reactions at a concentration of 1 μM. The final mixtures were denatured for 6 min at 94°C in a DNA Thermal Cycler (Hybaid Ltd.), paused for the addition of 1 U of BIOTAQ DNA polymerase (Bioline), and subjected to 35 amplification cycles, each consisting of three 1-min stages of 94, 46, and 72°C. PCR products from a 10-μl sample were separated in a 1.5% agarose gel by using a 100-bp ladder (Amersham-Pharmacia-Biotech, Uppsala, Sweden) as a molecular size standard.
Computer comparisons of RAPD PCR patterns.
From a negative digitized image of each RAPD PCR pattern, the strains were compared by the Pearson product moment correlation coefficient (r) and cluster analysis by the unweighted pair group method with arithmetic averages (21, 32, 34) using the GelCompar version 4 program (Applied Maths, Krotrijk, Belgium). Reproducibility tests were performed by using triplicate DNA preparations for each type strain and a series of independent PCR amplifications. The level of similarity observed between repeats, when included within the dendrogram for all strains, established a discrimination threshold below which RAPD PCR patterns were considered to be different. To determine the ability of RAPD PCR to discriminate Pediococcus species, each strain was assigned to the type strain with which it shared the highest level of similarity.
PFGE.
High-molecular-weight DNA was isolated from 1 ml of a stationary-phase culture, washed once in 1 M NaCl-10 mM Tris-HCl (pH 7.6), and resuspended in 300 μl of the same. The cell suspension was mixed with an equal volume of 2% (wt/vol) low-melting-point agarose (Bio-Rad Laboratories, Richmond, Calif.) in 0.125 M EDTA (pH 7.6), dispensed into molds 10 mm long by 5 mm wide by 1 mm deep, and allowed to solidify at 4°C. The agarose-cell mixture set within each mold was referred to as a plug. Up to three plugs per strain were added to 1 ml of 1 M NaCl-6 mM Tris-HCl-100 mM EDTA-1% (wt/vol) Sarkosyl (Sigma-Aldrich), pH 7.6, containing 10 mg of lysozyme/ml (L-7651; Sigma-Aldrich) and incubated overnight at 37°C. The lysozyme solution was replaced with 1 ml of 0.5 M EDTA-1% (wt/vol) Sarkosyl, pH 8.0, containing 0.5 mg of proteinase K/ml (P-6556; Sigma-Aldrich) and incubated at 37°C overnight. This step was repeated with a fresh proteinase K solution. After two 1-h washes with 1 mM phenylmethylsulfonyl fluoride in 10 mM Tris-Cl-1 mM EDTA (pH 8) at 37°C, the plugs were stored in 10 mM Tris-HCl-100 mM EDTA (pH 8.0) at 4°C. Prior to incubation with selected restriction enzymes, a 1-mm slice (1 mm long by 5 mm wide by 1 mm deep) of the plug was removed and washed three times for 15 min each time in 1 ml of 10 mM Tris-HCl-0.1 mM EDTA (pH 8.0) at room temperature. Each slice was preincubated with 100 μl of the restriction buffer recommended for the enzyme (all supplied by New England Biolabs, Beverly, Mass.) for 30 min at 4°C and then replaced with 100 μl of fresh buffer containing 20 U of the restriction enzyme ApaI, SmaI, AscI, NotI, or SfiI. Restriction digestion was performed overnight at temperatures recommended by the supplier. Plug slices were loaded directly into the wells of a 1% (wt/vol) pulsed-field grade agarose (Bio-Rad Laboratories) gel and sealed with 1% agarose. DNA fragments were resolved with a contour-clamped homogeneous electric field DRIII pulsed-field system (Bio-Rad Laboratories) at 6 V/cm for 18 to 24 h with 0.5× Tris base-borate-EDTA running buffer maintained at 14°C. Linear ramped pulse times were selected depending on the sizes of the DNA fragments to be resolved, 1 to 15 s for fragments of less than 250 kb (resulting from ApaI, SmaI, and selected NotI digestions), 5 to 50 s for fragments of up to 600 kb (resulting from selected AscI digestion), and 10 to 100 s for fragments of up to 1,500 kb (resulting from SfiI and selected AscI and NotI digestions). Gels were stained in distilled water containing 0.5 μg of ethidium bromide/ml for 30 min and destained for 60 min.
Gel photography and analysis.
Both RAPD PCR and PFGE gels were visualized by UV transillumination and photographed with Polaroid film (type 677). Photographs were digitized on a Scanjet 4P scanner with Deskscan II software (Hewlett-Packard). The sizes of PFGE fragments were estimated by using two sets of standards ranging from 2 to 600 kb (low-range PFGE marker no. N0350S) and 225 to 1,900 kb (yeast chromosome marker no. N0345S), both supplied by New England Biolabs.
RESULTS
RAPD PCR strain discrimination.
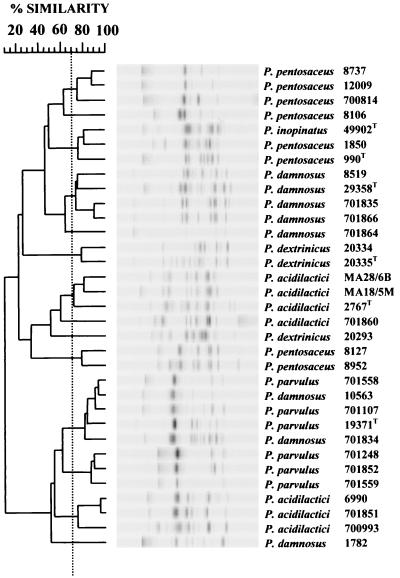
Under the conditions stipulated, RAPD PCR using either the P1 (Fig. 1) or the P2 (Fig. 2) primer produced an average of 9 or 13 DNA fragments per strain, respectively. Fragments ranged from 100 to 2,000 bp, with fragments of greater than 1,000 bp mainly confined to the P2 primer. Repeated RAPD reactions produced patterns with 70 to 95% similarity, and therefore, discrimination was only considered when strains were less than 70% similar. The P1 and P2 primers discriminated 14 and 21 RAPD patterns, respectively. However, five of the P2 patterns were considered borderline, with similarities to other pattern groups ranging from 67 to 69%. Cross-referencing of the RAPD groupings for both primers discriminated the strains further, with a number of strains grouped by one primer being segregated by the other. Overall, 28 distinct RAPD groupings were observed, with only two cases considered borderline.
FIG. 1.
Dendrogram based on computer comparisons of RAPD PCR patterns produced by random primer P1 with Pediococcus strains previously assigned to species within the genus (Table 1). The dendrogram was constructed by using the Pearson product moment correlation coefficient (r) (similarity, 100 × r) and the unweighted pair group algorithm method with arithmetic averages. The strain discrimination threshold (dashed line) was set at a similarity value of 70%. A superscript capital T denotes a type strain.
FIG. 2.
Dendrogram based on computer comparisons of RAPD PCR patterns produced by random primer P2 with pediococcus strains previously assigned to species within the genus (Table 1). The dendrogram was constructed by using the Pearson product moment correlation coefficient (r) (similarity, 100 × r) and the unweighted pair group algorithm method with arithmetic averages. The strain discrimination threshold (dashed line) was set at a similarity value of 70%. A superscript capital T denotes a type strain.
RAPD PCR species assignment.
Both primers assigned 17 of the 27 strains to their proposed species type strain, with 15 of the strains being common to both primers (Fig. 1 and 2). Both primers grouped P. damnosus NCIMB 10563, NCIMB 1782, and NCIMB 701834 with P. parvulus and P. pentosaceus NCIMB 8127 and NCIMB 8952 and P. dextrinicus DSMZ 20293 with P. acidilactici. P. acidilactici NCIMB 6990, NCIMB 70093, and NCIMB 701851 did not group with their proposed type strain but were assigned to different species by each primer.
PFGE strain discrimination.
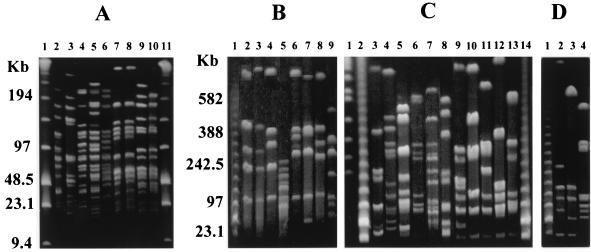
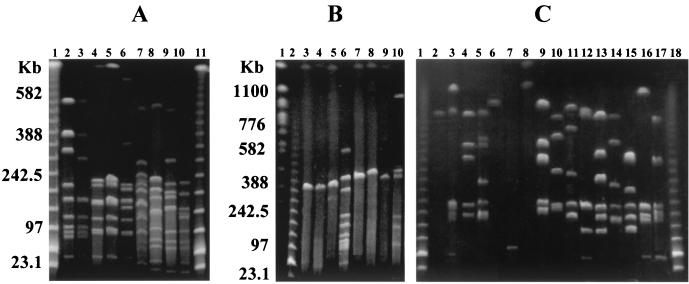
PFGE, following restriction digestion with ApaI, produced 30 distinct macrorestriction patterns. The enzyme produced a high number of DNA fragments of generally less than 150 kb (data not shown). Although not all fragments could be fully resolved, due to the large number of fragments, three pairs of strains, P. acidilactici CNCM MA18/5M and MA28/6B, P. pentosaceus NCIMB 8106 and NCIMB 8952, and P. pentosaceus NCIMB 8127 and NCIMB 8952, appeared to have the same macrorestriction patterns. Restriction digestion with either NotI (Fig. 3) or AscI (Fig. 4) produced 32 and 28 distinct macrorestriction patterns, respectively. Only strains CNCM MA18/5M and MA28/6B were not discriminated by NotI (Fig. 3A, lanes 7 and 8), and additional digestion with SmaI and SfiI also failed to distinguish these two strains (data not shown). It seems highly likely that these two isolates are the same strain.
FIG. 3.
PFGE macrorestriction patterns for the restriction enzyme NotI. Pulse times were 1 to 15 s for the gel shown in panel A and 10 to 100 s for the gels shown in panels B to D. Unless stated otherwise, all strains were NCIMB strains. A superscript capital T denotes a type strain. For species-specific DNA fragments, see Results. Panel A, lanes 2 to 10, P. acidilactici 6990, 700993, 701851, 701860, NCFB 2767T, CNCM MA18/5M, and MA28/6B and P. pentosaceus 8127 and 8952. Panel B, lanes 2 to 9, P. pentosaceus 7837, 8106, 12009, 8127, 700814, NCDO 1850, and NCFB 990T and P. inopinatus ATCC 49902T. Panel C, lanes 3 to 16, P. damnosus 8519, 10563, 1782, 701834, 701835, 701866, 701864, and ATCC 29358T and P. parvulus 701107, 701248, 701558, 701559, 701852, and ATCC 19371T. Panel D, lanes 2 to 4, P. dextrinicus DSMZ 20335T, 20334, and 20293. Panel A, lanes 1 and 11, panel B, lane 1, panel C, lanes 2 and 17, and panel D, lane 1, contained molecular size markers (lambda HindIII fragments with lambda concatemers). Panel C, lane 1, contained Saccharomyces cerevisiae chromosomes.
FIG. 4.
PFGE macrorestriction patterns for AscI. Pulse times were 5 to 50 s for the gel shown in panel A and 10 to 100 s for gels shown in panels B to D. Unless stated otherwise, all strains were NCIMB strains. A superscript capital T denotes a type strain. For species-specific DNA fragments, see Results. Panel A, lanes 2 to 10, P. pentosaceus 8127 and 8952 and P. acidilactici CNCM MA18/5M, MA28/6B, NCFB 2767T, 701860, 701551, 700993, and 6990. Panel B, lanes 2 to 9, P. pentosaceus 7837, 8106, 12009, 8127, 700814, NCDO 1850, and NCFB 990T and P. inopinatus ATCC 49902T. Panel C, lanes 2 to 17, P. damnosus 8519, 10563, 1782, 701834, 701835, 701864, and ATCC 29358T, P. parvulus 701107, 701248, 701558, 701559, 701852, and ATCC 19371T, and P. dextrinicus DSMZ 20293, 20334, and 20335T. Panel A, lanes 1 and 10, panel B, lane 1, and panel C, lanes 1 and 18 contained molecular size markers (lambda HindIII fragments with lambda concatemers). Panel B, lane 1, contained S. cerevisiae chromosomes.
Species discrimination by PFGE.
The NotI and AscI macrorestriction patterns for the majority of strains appeared to characterize each species, and based on PFGE data presented below, 27 of the 33 strains were assigned to their proposed species.
For P. acidilactici, a high frequency of cutting with either NotI (Fig. 3A) or AscI (Fig. 4A) distinguished the species from P. pentosaceus, P. parvulus, P. damnosus, P. dextrinicus, and P. inopinatus. With RAPD PCR for both primers, the P. acidilactici strains fell into two categories. Although PFGE clearly distinguished all of the strains as P. acidilactici, with AscI, the two categories were again apparent, with strains CNCM MA18/5M, MA28/6B, NCFB 2767 (type strain), and NCIMB 701860 (Fig. 4A, lanes 4 to 7) sharing DNA fragments of approximately 80, 130, and 170 kb. Therefore, both approaches suggest that the species can be divided into at least two genotypic groups and this diversity within the species is in agreement with recent studies (29, 30). Interestingly, two P. pentosaceus strains, NCIMB 8127 and NCIMB 8952, which shared the P. acidilactici PFGE patterns for NotI (Fig. 3A, lanes 9 and 10, and B, lane 5) and AscI (Fig. 4A, lanes 2 and 3, and B, lane 6), were also assigned to P. acidilactici by both RAPD PCR primers (Fig. 1 and 2). In addition, broth cultures of all P. acidilactici strains and P. pentosaceus NCIMB 8127 and NCIMB 8952, but not the remaining P. pentosaceus strains, grew at 50°C, a permissive temperature for P. acidilactici only (16).
The general AscI macrorestriction patterns for P. pentosaceus (Fig. 4B, lanes 3 to 9) appeared to be characteristic for the species. Only two or three fragments were evident, with one between 340 and 400 kb and another greater than 1,100 kb. Additional marker fragments were found with NotI (Fig. 3B, lanes 2 to 8) at approximately 110 kb and between 720 and 800 kb.
All P. parvulus strains digested with NotI (Fig. 3C, lanes 11 to 16) produced DNA fragments of approximately 80 and 125 kb and between 280 and 350 kb. Following AscI digestion (Fig. 4C, lanes 9 to 14), they shared fragments of approximately 220 kb and 700 to 800 kb.
The general AscI macrorestriction patterns for P. damnosus (Fig. 4C, lanes 2 and 6 to 8) were characteristic for the species, with very few DNA fragments produced. However, strains NCIMB 1782, NCIMB 701834, and NCIMB 10563 (Fig. 4C, lanes 3 to 5) did not concur with the general P. damnosus pattern. Similarly, digestion with NotI (Fig. 3C, lanes 3, 6, and 10) produced a fragment of approximately 100 kb and two others of 160 to 220 kb for members of the species, with the exception of strains NCIMB 1782, NCIMB 701834, and NCIMB 10563 (Fig. 3C, lanes 4 to 6). With both restriction enzymes, these three strains actually shared fragments associated with P. parvulus and, interestingly, all grouped to P. parvulus when RAPD PCR was used. The two species can be distinguished by growth at 37oC, which is nonpermissive for P. damnosus but not P. parvulus (16). All of the P. parvulus strains tested and strains NCIMB 1782, NCIMB 701834, and NCIMB 10563, but not the remaining P. damnosus strains, grew at 37°C (Table 2).
TABLE 2.
Colony counts of P. parvulus and P. damnosus cultures plated at selected temperatures
| Strain | No. of CFU/ml at:
|
|
|---|---|---|
| 25°C | 37°C | |
| P. parvulus 701248 | 2.4 × 109 | 1.9 × 109 |
| P. parvulus 701107 | 4.2 × 109 | 2.7 × 109 |
| P. parvulus 19371 | 2.4 × 109 | 2.2 × 109 |
| P. damnosus 701864 | 1010 | 104a |
| P. damnosus 29358 | 6.0 × 109 | 104a |
| P. damnosus 701835 | 6.3 × 109 | 104a |
| P. damnosus 1782 | 3.5 × 109 | 3.6 × 109 |
| P. damnosus 701834 | 2.0 × 109 | 1.8 × 109 |
| P. damnosus 10563 | 2.9 × 109 | 3.0 × 109 |
No colonies at the lowest dilution of 104.
Digestion of P. dextrinicus DNA with either NotI (Fig. 3D, lanes 2 to 4) or AscI (Fig. 4C, lanes 15 to 18) failed to identify DNA fragments that were common to only the three P. dextrinicus strains. However, if strain DSMZ 20293 was excluded, the remaining two strains shared fragments of approximately 20, 125, and 160 kb with NotI. When AscI was used, fragments of approximately 45 kb and three of 150 to 250 kb were again common to the two strains but additional strains from the species are required to confirm the species specificity of these fragments.
Only the type strain of P. inopinatus was included in the study. Although it did not conform to any of the other species-specific PFGE data, more strains are needed to clarify if PFGE can be used to identify strains of this species.
Species identification based on either NotI or AscI alone would have resulted in two cases of ambiguous assignment. P. dextrinicus DSMZ 20335 had the characteristic NotI PFGE features (Fig. 3D, lane 1) of P. parvulus and P. dextrinicus. Similarly P. damnosus NCIMB 10563, which appeared to be P. parvulus based on PFGE, RAPD PCR, and growth at selected temperatures, also had the characteristic AscI PFGE features of P. dextrinicus (Fig. 4C, lane 4). However, in both cases, reference to the second restriction enzyme PFGE patterns confirmed their proposed classification.
DISCUSSION
Both RAPD PCR and PFGE revealed a considerable degree of genomic diversity throughout the genus Pediococcus, with most strains being discriminated by either method.
With RAPD PCR, the level of strain discrimination achieved on combination of the data for both primers was higher than that achieved with each primer alone or those previously reported for single primers (21, 29, 30, 32). From the PFGE data, it was apparent that additional genomic differences between strains existed and the inclusion of additional primers might have increased the level of strain discrimination still further. Strain discrimination was only considered when RAPD patterns were less than 70% similar, and this was consistent with an earlier study (21). Such experimental variation is well documented for RAPD PCR (11) and limits both the degree of strain discrimination and the establishment of reference patterns that can be used to verify and track specific strains.
Under the conditions used in the present study, PFGE was found to discriminate more strains than RAPD PCR and this agrees with the findings presented for a number of other genera (4, 6, 41). The level of discrimination achieved across the genus agreed well with that previously reported for 67 P. acidilactici and P. pentosaceus strains isolated from human clinical samples that produced 59 distinct macrorestriction patterns following digestion of genomic DNA with either SmaI or NotI (3). In addition, the high degree of reproducibility observed with PFGE allowed the establishment of reference patterns for the potential tracking of strains. For example, of the 19 P. acidilactici NotI macrorestriction patterns presented for isolates recovered from human clinical samples (3), two isolates, 3144-90 and 562-89, exhibited the same NotI macrorestriction patterns as P. acidilactici NCIMB 700993 and NCIMB 701851, respectively (Fig. 3A, lanes 3 and 4). The AscI macrorestriction pattern for NCIMB 700993 (Fig. 4A, lane 9) also matched four P. acidilactici strains isolated from fermented sausages (24). Since NCIMB 700993 was also isolated from fermented sausage, they may represent the same strain. Indeed, in another study, two of the four isolates were considered to be identical after ribotyping and genomic restriction followed by conventional gel electrophoresis (18). Although pediococci remain uncommon as etiological agents of disease, their natural vancomycin resistance makes them a growing concern as opportunistic agents associated with human infections (3, 36).
In a previous study, proposed pediococci isolated from fermented cereals were assigned at the species level by using RAPD PCR and a primer identical to P1 (32). Grouping was based on a minimum similarity threshold of 33% and resulted in approximately 60% of the isolates being assigned. By using the same similarity threshold, we found that all strains could be assigned to a type strain. This difference may reflect either (i) a novel or non-Pediococcus component within the proposed cereal isolates that were genotypically distinct from the six type strains or (ii) differences in the method of DNA extraction and/or PCR conditions. In addition, from the present study, it was evident that with either primer, approximately 40% of the strains were misidentified. Approximately half of these strains were assigned to the same incorrect species by both primers. This, together with their PFGE fingerprints and growth at selected temperatures, discussed later, implies that they may have been originally misidentified. The remaining strains were assigned to different species by each primer and show that misidentification can arise through assignment by RAPD PCR.
PFGE was recently used to distinguish two Pediococcus species, P. acidilactici and P. pentosaceus, based on a higher frequency of cuts with SmaI (3). We similarly found a higher frequency of cuts for P. acidilactici with NotI and AscI, but reasons for this finding are unclear since the three enzymes do not have a nucleotide sequence in common and the reported G+C contents of the Pediococcus species appear to be very similar (16).
In the present study, we have extended the application of PFGE macrorestriction patterns to identify 21 of the 27 strains assigned to P. acidilactici, P. pentosaceus, P. parvulus, P. damnosus, or P. dextrinicus. The six strains not assigned correctly were grouped to the same incorrect species by RAPD PCR, and it was considered that these strains may have been originally misidentified, but additional classification, by 16S ribosomal sequencing or DNA-DNA hybridization, is required to clarify their taxonomic status. These strains are considered below.
Two P. pentosaceus strains grouped with P. acidilactici and also grew at 50°C, a permissive temperature for P. acidilactici only (16). Problems associated with classification across the two species resulted in the original P. acidilactici type strain being reclassified as P. pentosaceus (1, 13, 16), and in two recent studies, P. acidilactici strains were considered to be P. pentosaceus based on gene-specific PCR and RAPD PCR (30) or whole-cell protein profiles (3).
Three P. damnosus strains grouped with P. parvulus and also grew at 37°C, a nonpermissive temperature for P. damnosus. From a number of recent studies using ribotyping (2, 38) and monoclonal antibody reactions (44), it appears that the assignment of Pediococcus isolates to P. damnosus based on their brewery habitat and carbohydrate fermentation profiles (16, 35) can lead to misidentification. Although none of these involved reclassification as P. parvulus, data from total DNA-DNA hybridization (1) and 16S rRNA sequencing (10) have shown a higher level of genetic relatedness between the two species than with any other species of the genus.
One P. dextrinicus strain, DSMZ 20293, failed to group with any other Pediococcus species by PFGE and was only weakly associated with P. acidilactici by RAPD PCR. Unlike the other P. dextrinicus strains, which were isolated from silage, DSMZ 20293 was recovered from beer, and in a recent ribotyping study, the strain was considered to be a potentially new Pediococcus species (38).
Data from 16S rRNA sequence comparisons and DNA-DNA hybridization reactions between the various type strains of the genus led to the proposal of a three-branched phylogenetic tree comprising P. damnosus with P. parvulus and P. inopinatus, P. acidilactici with P. pentosaceus, and P. dextrinicus alone (10, 13). However, the RAPD PCR and PFGE data for the type strains showed distinct differences between the species. For example, the P. damnosus and P. parvulus type strains produced very different PFGE patterns and were not associated by RAPD PCR but had the highest degree of interspecies relatedness noted in the genus (1, 10).
In summary, the genomic diversity within the genus Pediococcus was determined by using RAPD PCR and PFGE and each technique could prove useful for the rapid classification of pediococci isolated from a variety of sources, including food, feed, silage, beer, and human clinical samples.
Acknowledgments
This work was supported by the European Union Standard Testing and Measures program (SMT4-CT98-2235) and the Irish Government under National Development Plan 2000-2006.
REFERENCES
- 1.Back, W., and E. Stackebrandt. 1978. DNS/DNS-homologies Studien innerhalb der Gattung Pediococcus. Arch. Microbiol. 118:79-85. [Google Scholar]
- 2.Barney, M., A. Volgyi, A. Navarro, and D. Ryder. 2001. Riboprinting and 16S rRNA gene sequencing for identification of brewery Pediococcus isolates. Appl. Environ. Microbiol. 67:553-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros, R. R., M. D. S. Carvalho, J. M. Peralta, R. R. Facklam, and L. M. Teixeira. 2001. Phenotypic and genotypic characterization of Pediococcus strains isolated from human clinical sources. J. Clin. Microbiol. 39:1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bert, F., C. Branger, and N. Lambert-Zechovsky. 1997. Pulsed-field gel electrophoresis is more discriminating than multilocus enzyme electrophoresis and random amplified polymorphic DNA analysis for typing pyogenic streptococci. Curr. Microbiol. 34:226-229. [DOI] [PubMed] [Google Scholar]
- 5.Bhowmik, T., and E. H. Marth. 1990. Role of Micrococcus and Pediococcus species in cheese ripening: a review. J. Dairy Sci. 73:859-866. [Google Scholar]
- 6.Bjorkroth, J., J. Ridell, and H. Korkeala. 1996. Characterisation of Lactobacillus sake strains associated with production of ropy slime by randomly amplified polymorphic DNA (RAPD) and pulsed-field gel electrophoresis (PFGE) patterns. Int. J. Food Microbiol. 31:59-68. [DOI] [PubMed] [Google Scholar]
- 7.Cai, Y., S. Kumai, M. Ogawa, Y. Benno, and T. Nakase. 1999. Characterization and identification of Pediococcus species isolated from forage crops and their application for silage preparation. Appl. Environ. Microbiol. 65:2901-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheun, H., S. Makino, T. Shirahata, and M. Mikami. 2000. The practical application of pediocin produced by Pediococcus acidilactici in food. Biosci. Microflora 19:47-50. [Google Scholar]
- 9.Coakley, M., and R. P. Ross. 1996. Application of the polymerase chain reaction to the rapid analysis of brewery yeast strains. J. Inst. Brew. 102:349-354. [Google Scholar]
- 10.Collins, M. D., A. M. Williams, and S. Wallbanks. 1990. The phylogeny of Aerococcus and Pediococcus as determined by 16S rRNA sequence analysis: description of Tetragenococcus gen. nov. FEMS Microbiol. Lett. 70:255-262. [DOI] [PubMed] [Google Scholar]
- 11.Cusick, S. M., and D. J. O'Sullivan. 2000. Use of a single, triplicate arbitrarily primed PCR procedure for molecular fingerprinting of lactic acid bacteria. Appl. Environ. Microbiol. 66:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel, P. 1995. Sizing of the Lactobacillus plantarum genome and other lactic acid bacteria species by transverse alternating field electrophoresis. Curr. Microbiol. 30:243-246. [Google Scholar]
- 13.Dellaglio, F., L. D. Trovatelli, and P. G. Sarra. 1981. DNA-DNA homology among representatives strains of the genus Pediococcus. Zentbl. Bakteriol. Mikrobiol. Hyg. 1 Abt. Orig. C 2:140-150. [Google Scholar]
- 14.Fitzgerald, L. 2000. Teagasc directory of silage additives. 2000. Todays Farm 10:35-39. [Google Scholar]
- 15.Fitzsimons, N. A., T. M. Cogan, S. Condon, and T. Beresford. 1999. Phenotypic and genotypic characterization of non-starter lactic acid bacteria in mature cheddar cheese. Appl. Environ. Microbiol. 65:3218-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garvie, E. I. 1986. Genus Pediococcus, p. 1075-1079. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt, (ed.), Bergey's manual of systematic bacteriology, vol 2. The Williams & Wilkins Co., Baltimore, Md. [Google Scholar]
- 17.Hudson, J. A., Y. Cai, R. J. Corner, B. Morvan, and K. N. Joblin. 2000. Identification and enumeration of oleic acid and linoleic acid hydrating bacteria in the rumen of sheep and cows. J. Appl. Microbiol. 88:286-292. [DOI] [PubMed] [Google Scholar]
- 18.Jager, K., and S. Harlander. 1992. Characterization of a bacteriocin from Pediococcus acidilactici PC and comparison of bacteriocin-producing strains using molecular typing procedures. Appl. Microbiol. Biotechnol. 37:631-637. [Google Scholar]
- 19.Juven, B. J., R. J. Meinersmann, and N. J. Stern. 1991. Antagonistic effects of lactobacilli and pediococci to control intestinal colonization by human entero-pathogens in live poultry. J. Appl. Bacteriol. 70:95-103. [DOI] [PubMed] [Google Scholar]
- 20.Knorr, D. 1998. Technological aspects related to microorganisms in functional foods. Trends Food Sci. Technol. 9:295-306. [Google Scholar]
- 21.Kurzak, P., M. A. Ehrmann, and R. F. Vogel. 1998. Diversity of lactic acid bacteria associated with ducks. Syst. Appl. Microbiol. 21:588-592. [DOI] [PubMed] [Google Scholar]
- 22.Litopoulou-Tzanetaki, E., D. C. Graham, and Y. Beyatli. 1989. Detection of pediococci and other non-starter organisms in American cheddar cheese. J. Dairy Sci. 72:854-858. [Google Scholar]
- 23.Lonvaud-Funel, A., Y. Guilloux, and A. Joyeux. 1993. Isolation of a DNA probe for identification of glucan-producing Pediococcus damnosus in wines. J. Appl. Bacteriol. 74:41-47. [Google Scholar]
- 24.Luchansky, J. B., K. A. Glass, K. D. Harsono, A. J. Degnan, N. G. Faith, B. Cauvin, G. Baccus-Taylor, K. Arihara, B. Bater, A. J. Maurer, and R. B. Cassens. 1992. Genomic analysis of Pediococcus starter cultures used to control Listeria monocytogenes in turkey summer sausage. Appl. Environ. Microbiol. 58:3053-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattila-Sandholm, T., A. Haikara, and E. Skyttae. 1993. The effect of Pediococcus damnosus and Pediococcus pentosaceus on the growth of pathogens in minced meat. Int. J. Food Microbiol. 13:87-94. [DOI] [PubMed] [Google Scholar]
- 26.Mendez-Alvarez, S., V. Pavon, I. Esteve, R. Guerrero, and N. Gaju. 1995. Analysis of bacterial genomes by pulsed field gel electrophoresis. Microbiol. Semin. 11:323-336. [PubMed] [Google Scholar]
- 27.Mora, D., M. G. Fortina, C. Parini, and P. L. Manachini. 1997. Identification of Pediococcus acidilactici and Pediococcus pentosaceus based on 16S rRNA and ldhD gene-targeted multiplex PCR analysis. FEMS Microbiol. Lett. 151:231-236. [DOI] [PubMed] [Google Scholar]
- 28.Mora, D., C. Parini, M. G. Fortina, and P. L. Manachini. 1998. Discrimination among pediocin AcH/PA-1 producer strains by comparison of pedB and pedD amplified genes and multiplex PCR assay. Syst. Appl. Microbiol. 21:454-460. [DOI] [PubMed] [Google Scholar]
- 29.Mora, D., M. G. Fortina, C. Parini, D. Daffonchio, and P. L. Manachini. 2000. Genomic sub-populations within the species Pediococcus acidilactici detected by multilocus typing analysis: relationships between pediocin Ach/PA-1 producing and non-producing strains. Microbiology 146:2027-2038. [DOI] [PubMed] [Google Scholar]
- 30.Mora, D., C. Parini, M. G. Fortina, and P. L. Manachini. 2000. Development of molecular RAPD marker for the identification of Pediococcus acidilactici strains. Syst. Appl. Microbiol. 23:400-408. [DOI] [PubMed] [Google Scholar]
- 31.Nettles, C. G., and S. F. Barefoot. 1993. Biochemical and genetic characteristics of bacteriocins of food-associated lactic acid bacteria. J. Food Prot. 56:338-356. [DOI] [PubMed] [Google Scholar]
- 32.Nigatu, A., S. Ahrne, B. A. Gashe, and G. Molin. 1998. Randomly amplified polymorphic DNA (RAPD) for discrimination of Pediococcus pentosaceus and Ped. acidilactici and rapid grouping of Pediococcus isolates. Lett. Appl. Microbiol. 26:412-416. [Google Scholar]
- 33.Omar, N. B., F. Ampe, M. Raimbault, J.-P. Guyot, and P. Tailliez. 2000. Molecular diversity of lactic acid bacteria from cassava sour starch (Colombia). Syst. Appl. Microbiol. 23:285-291. [DOI] [PubMed] [Google Scholar]
- 34.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p 493-521. In M. Goodfellow and A. G. O'Donnell (ed.), Chemical methods in prokaryotic systematics. J. Wiley & Sons, Chichester, United Kingdom.
- 35.Priest, F. G. 1996. Gram-positive brewery bacteria, p. 127-161. In F. G. Priest and I. Campbell (ed.), Brewing microbiology, 2nd ed. Elsevier, London, United Kingdom.
- 36.Riebel, W. J., and J. A. Washington. 1990. Clinical and microbiologic characteristics of pediococci. J. Clin. Microbiol. 28:1348-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez, J. M., L. M. Cintas, P. Casaus, M. I. Suarez, and P. E. Hernandez. 1997. Detection of pediocin PA-1-producing pediococci by rapid molecular biology techniques. Food Microbiol. 14:363-371. [Google Scholar]
- 38.Satokari, R., T. Mattila-Sandholm, and M. L. Suihko. 2000. Identification of pediococci by ribotyping. J. Appl. Microbiol. 88:260-265. [DOI] [PubMed] [Google Scholar]
- 39.Tannock, G. W. 1997. Probiotic properties of lactic-acid bacteria: plenty of scope for R. & D. Trends Biotechnol. 15:270-274. [DOI] [PubMed] [Google Scholar]
- 40.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. A. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tynkkynen, S., R. Satokari, M. Saarela, T. Mattila-Sandholm, and M. Saxelin. 1999. Comparison of ribotyping, randomly amplified polymorphic DNA analysis, and PFGE in typing of Lactobacillus rhamnosus and L. casei strains. Appl. Environ. Microbiol. 65:3908-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanbelle, M., E. Teller, and M. Focant. 1990. Probiotics in animal nutrition: a review. Arch. Anim. Nutr. Berlin 40:543-567. [DOI] [PubMed] [Google Scholar]
- 43.Welsh, J., and M. McClelland. 1990. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 18:7213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiting, M., M. Crichlow, W. M. Ingledew, and B. Ziola. 1992. Detection of Pediococcus spp. in brewing yeast by a rapid immunoassay. Appl. Environ. Microbiol. 58:713-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams, J. G. K., A. R. Kubelik, K. J. Livak, J. A. Rafalski, and S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acid Res. 18:6531-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]