Abstract
Sourdough lactic acid bacteria were preliminarily screened for proteolytic activity by using a digest of albumin and globulin polypeptides as a substrate. Based on their hydrolysis profile patterns, Lactobacillus alimentarius 15M, Lactobacillus brevis 14G, Lactobacillus sanfranciscensis 7A, and Lactobacillus hilgardii 51B were selected and used in sourdough fermentation. A fractionated method of protein extraction and subsequent two-dimensional electrophoresis were used to estimate proteolysis in sourdoughs. Compared to a chemically acidified (pH 4.4) dough, 37 to 42 polypeptides, distributed over a wide range of pIs and molecular masses, were hydrolyzed by L. alimentarius 15M, L. brevis 14G, and L. sanfranciscensis 7A. Albumin, globulin, and gliadin fractions were hydrolyzed, while glutenins were not degraded. The concentrations of free amino acids, especially proline and glutamic and aspartic acids, also increased in sourdoughs. Compared to the chemically acidified dough, proteolysis by lactobacilli positively influenced the softening of the dough during fermentation, as determined by rheological analyses. Enzyme preparations of the selected lactobacilli which contained proteinase or peptidase enzymes showed hydrolysis of the 31-43 fragment of A-gliadin, a toxic peptide for celiac patients. A toxic peptic-tryptic (PT) digest of gliadins was used for in vitro agglutination tests on K 562 (S) subclone cells of human myelagenous leukemia origin. The lowest concentration of PT digest that agglutinated 100% of the total cells was 0.218 g/liter. Hydrolysis of the PT digest by proteolytic enzymes of L. alimentarius 15M and L. brevis 14G completely prevented agglutination of the K 562 (S) cells by the PT digest at a concentration of 0.875 g/liter. Considerable inhibitory effects by other strains and at higher concentrations of the PT digest were also found. The mixture of peptides produced by enzyme preparations of selected lactobacilli showed a decreased agglutination of K 562 (S) cells with respect to the whole 31-43 fragment of A-gliadin.
Panettone, Colomba, Pandoro, and different types of wheat and rye breads are made using sourdough (23). Sourdough is defined as a dough whose microorganisms, mainly lactic acid bacteria and yeasts, originate from sourdough or a sourdough starter and are metabolically active or need to be reactivated (1). Numerous genera and species of lactic acid bacteria have been identified in sourdough (33), but its propagation under specific environmental conditions usually promotes natural selection leading to one to three species at numbers 3 or 4 orders of magnitude above those of the fortuitous microflora (24). The predominant lactic acid bacteria belong to the genus Lactobacillus, and their key role in sourdough is well recognized.
The use of sourdough offers a number of advantages in baked goods technology. A great part of these advantages is promoted by the decrease in pH during fermentation: gas retention and resistance of the gluten network, inhibition of flour amylases, water binding of gluten and starch granules, swelling of pentosans, solubilization of the phytate complex by endogenous phytases, and prevention of malfermentation and spoilage (19, 23). The decrease in pH does not necessarily require fermentative activity; it can also be achieved by addition of acetic, lactic, tartaric, phosphoric, or citric acid to the dough. Nevertheless, sourdough lactic acid bacteria exert a complex of other biochemical activities which do not consist of only lactic acid fermentation (19).
Proteolysis by lactic acid bacteria during sourdough fermentation has been poorly investigated. It may have repercussions on rheology and staleness (12); free amino acids and small peptides are important for rapid microbial growth and acidification during fermentation and as precursors for flavor development of leavened baked goods (19). Proteolysis has been studied indirectly by determining the accumulation of free amino acids and peptides after fermentation (22). The main proteolytic activity was first attributed to endogenous flour enzymes, such as aminopeptidase, carboxypeptidase, and endopeptidase (39); later, enzymes of fortuitous microorganisms and lactic acid bacteria were also presumed to have a role (23). Proteolysis in sourdoughs has been found to be higher than in yeasted and unstarted doughs (22). The proteolytic system of Lactobacillus sanfranciscensis, a key sourdough lactic acid bacterium, has been characterized, and aminopeptidase, dipeptidase, and a cell-wall-associated serine proteinase have been purified to homogeneity (20). The activity of the last-named enzyme was in agreement with the adaptation of the microorganism to the dough environment, as it exhibited a higher activity on synthetic gliadin than on αs1- and β-casein substrates and maintained a relatively high activity under conditions prevailing in dough fermentation. Recently, the same aptitude to specifically hydrolyze gluten was shown when sourdough lactic acid bacterial cultures were compared with starters for meat fermentation (43). Some fundamental questions still remain unanswered: they concern the capacity of lactic acid bacteria to hydrolyze water-insoluble proteins, such as gliadins and glutenins; the influence of dough acidification in modifying the wheat protein pattern and network; and the capacity of lactic acid bacteria to interfere with the generation of biologically active peptides which adversely affect the human intestinal mucosa, resulting in cereal intolerance.
In 1953, it was first recognized that ingestion of wheat gluten causes celiac disease in sensitive individuals (42). Celiac disease is one of the most frequent genetically based diseases, occurring in 1 out of every 130 to 300 persons in the European population (18). The exact mechanism of the damaging effect in celiac patients is still unknown; however, all the major gliadin subgroups (α-, β-, γ-, and ω-gliadins) from some cereals (e.g., wheat, barley, and rye) have been shown to give rise under proteolytic digestion to peptide sequences which specifically bind with human leukocyte antigen class II molecules, such as a DQ α-β heterodimer, thus initiating the immune response and the disease process (38, 40). To facilitate identification of the toxic peptides and to estimate their damaging activities on the celiac intestine, in vitro methods based on cultured celiac jejunal mucosa, murine T cells, fetal rat intestine, and human myelogenous leukemia K 562 (S) cells are currently used (6, 34). Besides plant breeding to remove toxic peptides and/or the development of agents capable of blocking binding in the groove between human leukocyte molecules and gliadin-derived peptides (H. N. Marsh, S. Morgan, A. Ensari, I. Wardle, R. Lobley, and S. Auricchio, abstract from Digestive Disease Week and the 95th Annual Meeting of the American Gastroenterological Association, Gut 36:210A, 1995), it is worthwhile to evaluate the effect of sourdough lactic acid bacterial proteolysis on toxic peptide sequences that are liberated from or encrypted in cereal proteins.
This article describes the proteolytic activity of sourdough lactic acid bacteria. After an initial screening, selected strains were shown to hydrolyze albumins, globulins, and gliadins during sourdough fermentation, and their enzyme preparations were used to hydrolyze the toxic 31-43 fragment of A-gliadin and to inhibit the in vitro agglutination activity of a gliadin digest towards K 562 (S) cells.
MATERIALS AND METHODS
Microorganisms and culture conditions.
Fifty-five strains of lactic acid bacteria, previously isolated from sourdoughs from southern Italy, were used in this study (13). The species used were those commonly identified in sourdoughs: L. sanfranciscensis (1O, 2A, 5D, 5Q, 6G, 7A, 7H, 9F, 9M, 13A, 13R, 14C, 20C, 22E, and 22Z), Lactobacillus alimentarius (1A, 1B, 2B, 8D, 10α, 15A, 15F, 15M, 15R, 16B, 16I, and 16M), Lactobacillus brevis (1F, 1D, 6M, 10A, 14G, 17D, and 18B), Lactobacillus fermentum (6E, 18F, and 18I), Lactobacillus hilgardii (51B and 52B), Lactobacillus acidophilus (16A and 16α), Lactobacillus plantarum (17N, 18E, 19A, 20B, and 21B), Lactobacillus farciminis (I2), Lactobacillus fructivorans (DA106), Lactococcus lactis subsp. lactis (11M, 10γ, and 17C), Weissella confusa (14A) (11), and Leuconostoc citreum (10M, 11C, and 23B) (17).
The strains were routinely propagated for 24 h at 30 or 37°C in modified MRS broth (Oxoid, Basingstoke, Hampshire, England) with the addition of fresh yeast extract (5% [vol/vol]) and 28 mM maltose at a final pH of 5.6. When used for enzyme assays, sourdough fermentation, and subcellular fractionation, lactic acid bacterial cells were incubated until the late exponential phase of growth was reached (ca. 12 h).
Preliminary screening of proteolytic activity.
Twelve-hour-old cells of lactic acid bacteria cultivated in modified MRS broth were harvested by centrifugation at 9,000 × g for 10 min at 4°C, washed twice with 20 mM sterile phosphate buffer (pH 7.0), and resuspended in the same buffer at a concentration of ca. 109 CFU/ml.
A wheat flour hydrolysate was produced by stirring a suspension of wheat flour (30% [wt/vol] in 20 mM phosphate buffer, pH 7.0) for 1 h at 30°C. After incubation, the suspension was filtered (Whatman International Ltd. [Maidstone, England] no. 4), heated at 80°C for 5 min, centrifuged (10,000 × g for 15 min at 4°C), sterilized by filtration (0.45-μm-pore-size Millex-HA; Millipore S.A., Saint Quentin, France), and treated with 0.1% (wt/vol) protease from Bacillus licheniformis (ca. 3 U/mg) (Sigma Chemical Co., St. Louis, Mo.) having an optimum pH of 7.0. After 3 h of incubation at 30°C, the wheat flour hydrolysate treated with protease (WFHP) was heated at 80°C for 5 min to inactivate enzymes, sterilized again by filtration, and used as a substrate. The assay mixture, containing 0.8 ml of WFHP and 0.2 ml of the cellular suspension, was incubated at 30°C for 6 h, and the supernatant was recovered by centrifugation and used for electrophoresis. A preliminary assay conducted with undigested wheat flour hydrolysate and other non-cereal protein substrates gave unsatisfactory results. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted according to the Laemmli procedure (26); the gels contained 12.5% acrylamide (separation distance, 10 cm; gel thickness, 1.5 mm) and were stained with B10 Bio-Safe Coomassie blue (Bio-Rad Laboratories, Hercules, Calif.). Low-range SDS-PAGE molecular mass standards (Bio-Rad) were used. Three gels from each assay were analyzed for the protein band intensities in WFHP (piWFHP) and in WFHP treated with lactic acid bacteria (piWFHPL) with the Quantity One software package (Bio-Rad). The hydrolysis factors for individual protein bands were calculated as [(piWFHP − piWFHPL)/piWFHP] × 100.
Sourdough fermentation.
The characteristics of the wheat flour used were as follows: moisture, 12.8%; protein (N × 5.70), 10.7% of dry matter (d.m.); fat, 1.8% of d.m.; ash, 0.6% of d.m.; and total soluble carbohydrates, 1.5% of d.m.
Two hundred grams of wheat flour, 70 ml of tap water, and 30 ml of cellular suspension containing 109 CFU of each lactic acid bacterial strain/ml (final concentration in the dough, 5 × 107 CFU/g) were used to produce 300 g of dough (dough yield: 150) with a continuous high-speed mixer (60 × g; dough mixing time, 5 min). Sourdough fermentations were carried out at 37°C for 4 and 8 h, which corresponded to the most typical times of fermentation used for Italian wheat sourdoughs (13, 36). All the sourdoughs started with selected lactic acid bacteria had pHs ranging from 4.3 to 4.6, depending on the time of incubation. A dough produced with 200 g of wheat flour and 100 g of tap water, without bacterial inoculum and containing 0.15 g of NaN3 (wt/wt), was incubated under the same conditions and was used as the control. After incubation, the dough was acidified to pH 4.4 with a mixture of lactic and acetic acids in a molar ratio of 4:1, which corresponds to that usually found after sourdough fermentation (19). Under our experimental conditions, the total bacterial count of this unstarted dough varied from 2 × 104 to 7 × 104 CFU/g after incubation without appreciable variation in pH and dough rheology and taste. The chemical acidification was also carried out directly, without addition of NaN3, during dough mixing or gradually during dough incubation.
Protein extraction and sample preparation for 2D electrophoresis.
Wheat flour protein fractions were extracted from sourdough and control samples following the method originally described by Osborne (32) and further modified by Weiss et al. (44). One gram of dough was diluted with 4 ml of 50 mM Tris-HCl (pH 8.8), held at 4°C for 1 h with vortexing at 15-min intervals, and centrifuged at 20,000 × g for 20 min. The supernatant contained albumins and globulins. In order to minimize cross contamination among albumins, globulins, and gliadins, the pellets were further extracted twice with 50 mM Tris-HCl (pH 8.8), and the supernatants were discarded. After being washed with distilled water to remove buffer ions, the pellets were diluted with 4 ml of ethanol (75% [vol/vol]), stirred at 25°C for 2 h, and centrifuged as described above. The supernatant contained gliadins. The extraction by ethanol was also repeated twice. Residual ethanol was eliminated by resuspending the pellets with distilled water and centrifugation. Finally, the pellets were diluted with 4 ml of a urea-dithiothreitol (DTT) mixture (6 M urea, 1% [vol/vol] Triton X-100, 0.5% [wt/vol] DTT, and 0.5% [vol/vol] 2-D Pharmalyte [pH 3 to 10]), held for 2 h at room temperature with occasional vortexing, and centrifuged. The supernatant contained glutenins. All extracts were stored at −80°C until they were used. To compare proteins with different solubilities, all the extracts were diluted with equal volumes of 6 M urea containing 1% (vol/vol) Triton X-100, 0.5% (wt/vol) DTT, and 0.5% (vol/vol) 2-D Pharmalyte (pH 3 to 10) before two-dimensional (2D) electrophoresis.
2D gel electrophoresis.
2D gel electrophoresis was performed using the immobiline-polyacrylamide system, essentially as described by Bjellqvist et al. (10). Aliquots (30 μl) were used for each electrophoretic run. Isoelectric focusing was carried out on immobiline strips providing a nonlinear 3-to-10 pH gradient (IPG strips; Amersham Pharmacia Biotech) by IPG-phore at 15°C. The voltage was as follows: 0 to 300 V for 1 h, 300 to 500 V for 3 h, 500 to 2,000 V for 4 h, and a constant 8,000 V for 4 h. After electrophoresis, the IPG strips were equilibrated for 12 min against 6 M urea, 30% (vol/vol) glycerol, 2% (wt/vol) SDS, 0.05 M Tris-HCl (pH 6.8), and 2% (wt/vol) dithioerythritol and for 5 min against 6 M urea, 30% (vol/vol) glycerol, 2% (wt/vol) SDS, 0.05 M Tris-HCl (pH 6.8), 2.5% (wt/vol) iodioacetamide, and 0.5% bromophenol blue. The second dimension was carried out in a Laemmli system (26) on 12% polyacrylamide gels (13 cm by 20 cm by 1.5 mm) at a constant current of 40 mA/gel and at 10°C for approximately 5 h until the dye front reached the bottom of the gel. The gels were calibrated with two molecular mass markers: comigration of the extracts with human serum proteins for a molecular mass range of 200 to 10 kDa and markers for two-dimensional electrophoresis (pI range, 7.6 to 3.8; molecular mass range, 17 to 89 kDa) from Sigma Chemical Co. The electrophoretic coordinates used for serum proteins were according to the method Bjellqvist et al. (10). The gels were silver stained as described by Hochstrasser et al. (25). The protein maps were scanned with an Image Scanner and analyzed with the Image Master 2D version 3.01 computer software (Amersham Pharmacia Biotech). Three gels were analyzed, and spot intensities of chemically acidified dough (siCAD) and sourdough (siSD) were normalized as reported by Bini et al. (9). In particular, the spot quantification for each gel was calculated as relative volume (percent volume); the relative volume was the volume of each spot divided by the total volume over the whole image. In this way, differences in color intensities among the gels were eliminated (2). The hydrolysis factor for individual proteins was expressed as [(siCAD − siSD)/siCAD] × 100. All the hydrolysis factors were calculated based on the average of the spot intensities of each of the three gels, and standard deviation was calculated. Only hydrolysis factors with statistical significances where the P value was <0.05 were reported (see Table 2).
TABLE 2.
Properties of wheat flour polypeptides hydrolyzed by sourdough lactobacilli during sourdough fermentationa
| Spot designationb | Estimated pI | Estimated molecular mass (kDa) | Hydrolysis factor
|
|||
|---|---|---|---|---|---|---|
| L. alimentarius 15M | L. brevis 14G | L. sanfranciscensis 7A | L. hilgardii 51B | |||
| 1c | 5.58 | 84.0 | 9.7 ± 0.2 | 50.3 ± 1.3 | 76.3 ± 2.6 | 60.8 ± 4.2 |
| 2c | 6.70 | 68.0 | 97.3 ± 5.0 | 58.4 ± 2.2 | 95.0 ± 8.1 | 67.2 ± 4.6 |
| 3c | 5.39 | 64.5 | 31.8 ± 2.5 | 0 | 81 ± 4.6 | 0 |
| 4c | 5.58 | 64.0 | 83.3 ± 1.9 | 91 ± 7.4 | 65.7 ± 3.4 | 0 |
| 5c | 5.77 | 64.0 | 50 ± 3.6 | 50 ± 0.6 | 90.1 ± 5.3 | 0 |
| 6d | 5.75 | 62.0 | 54.2 ± 2.9 | 56.8 ± 4.7 | 93.6 ± 5.4 | 0 |
| 7d | 6.9 | 59.0 | 0 | 0 | 5.81 ± 0.4 | 0 |
| 8d | 5.13 | 53.8 | 0 | 0 | 94.8 ± 4.7 | 0 |
| 9d | 5.70 | 53.8 | 10 ± 0.4 | 50.7 ± 1.9 | 73.0 ± 5.6 | 0 |
| 10d | 6.61 | 53.3 | 86.6 ± 7.1 | 0 | 86.0 ± 3.1 | 89.1 ± 4.6 |
| 11d | 6.9 | 52.4 | 17.8 ± 1.4 | 0 | 50 ± 1.3 | 0 |
| 12d | 5.85 | 51.6 | 94.2 ± 7.5 | 47.5 ± 2.7 | 88.7 ± 7.5 | 0 |
| 13d | 8.01 | 50.8 | 0 | 98.2 ± 4.5 | 16.0 ± 0.9 | 0 |
| 14d | 6.70 | 50.0 | 14.9 ± 0.6 | 0 | 81.2 ± 2.1 | 0 |
| 15d | 7.23 | 48.0 | 15 ± 0.7 | 96.5 ± 0.8 | 13.7 ± 0.5 | 0 |
| 16d | 6.35 | 46.0 | 0 | 10 ± 0.4 | 0 | 0 |
| 17d | 7.20 | 43.8 | 0 | 60.8 ± 4.5 | 54.6 ± 1.8 | 0 |
| 18d | 7.50 | 43.0 | 0 | 52.2 ± 4.8 | 63.5 ± 2.7 | 0 |
| 19c | 5.50 | 42.5 | 50 ± 1.0 | 60 ± 2.1 | 90 ± 5.6 | 50 ± 2.6 |
| 20d | 7.00 | 40 | 11.4 ± 0.5 | 73.5 ± 1.3 | 0 | 50 ± 1.0 |
| 21d | 6.64 | 38.7 | 0 | 30 ± 1.4 | 0 | 20 ± 1.4 |
| 22d | 7.80 | 38.0 | 5.2 ± 0.1 | 0 | 37.4 ± 0.7 | 0 |
| 23d | 7.61 | 33.9 | 54.8 ± 4.5 | 97.2 ± 1.0 | 4.3 ± 0.1 | 0 |
| 24c | 5.76 | 32.0 | 94.7 ± 4.8 | 97.1 ± 1.9 | 93 ± 4.6 | 98 ± 6.1 |
| 25d | 7.61 | 31.3 | 89.9 ± 4.1 | 98.3 ± 1.8 | 11.7 ± 0.6 | 0 |
| 26d | 7.80 | 30.0 | 0 | 97.1 ± 8.9 | 91.2 ± 2.7 | 80 ± 6.8 |
| 27c | 6.60 | 29.1 | 65 ± 1.0 | 70 ± 4.2 | 65 ± 5.4 | 10 ± 0.2 |
| 28c | 7.40 | 29.0 | 92.7 ± 1.5 | 98.4 ± 8.5 | 93.8 ± 5.7 | 0 |
| 29c | 6.35 | 28.2 | 75 ± 5.2 | 87.1 ± 1.4 | 84.5 ± 0.8 | 0 |
| 30c | 6.70 | 28.5 | 63 ± 2.0 | 61 ± 1.0 | 59 ± 3.7 | 0 |
| 31d | 7.80 | 28 | 91.6 ± 7.2 | 98.4 ± 4.2 | 94.5 ± 4.1 | 51 ± 2.4 |
| 32d | 7.35 | 27.5 | 90.4 ± 4.2 | 97.2 ± 4.1 | 97 ± 2.5 | 50 ± 1.1 |
| 33c | 6.68 | 26.2 | 51.6 ± 4.6 | 50 ± 1.1 | 50 ± 1.8 | 0 |
| 34c | 7.30 | 21.5 | 74.6 ± 4.3 | 90.3 ± 4.1 | 69.1 ± 4.8 | 0 |
| 35c | 7.60 | 19.9 | 60.2 ± 4.5 | 95 ± 4.7 | 88.7 ± 4.2 | 0 |
| 36c | 7.70 | 18.8 | 51.8 ± 4.0 | 97.7 ± 4.3 | 96 ± 5.2 | 0 |
| 37c | 6.05 | 17.4 | 97.5 ± 7.6 | 98 ± 7.0 | 97.3 ± 8.5 | 97.4 ± 1 |
| 38c | 5.31 | 17.0 | 92.7 ± 1.5 | 90 ± 1.1 | 90 ± 1.3 | 50 ± 1.6 |
| 39c | 6.18 | 16.9 | 0 | 90.2 ± 4.5 | 30 ± 1.7 | 0 |
| 40c | 6.75 | 16.6 | 57.3 ± 4.8 | 73.8 ± 2.0 | 40 ± 2.5 | 0 |
| 41c | 7.21 | 16.6 | 98 ± 4.6 | 96.8 ± 4.2 | 90.4 ± 7.8 | 0 |
| 42c | 5.07 | 16.6 | 95 ± 5.7 | 90 ± 2.1 | 0 | 43 ± 1.9 |
| 43c | 6.05 | 15.3 | 97.3 ± 6.2 | 91.2 ± 7.5 | 96 ± 8.4 | 0 |
| 44c | 6.61 | 15.3 | 83 ± 4.6 | 70 ± 1.7 | 70 ± 5.1 | 0 |
| 45c | 5.90 | 15.2 | 96 ± 6.4 | 84.3 ± 3.5 | 97 ± 0.9 | 0 |
| 46c | 6.74 | 15.0 | 54 ± 2.1 | 98.4 ± 4.6 | 60.8 ± 2.7 | 0 |
Analyses were performed with Image Master software (Pharmacia). Three gels of independent replicates were analyzed. For spot quantification and hydrolysis factor calculation, see Materials and Methods. All of the hydrolysis factors were calculated based on the average of the spot intensities of each of three gels, and standard deviations were calculated. Only hydrolysis factors with P values of <0.05 are reported.
Polypeptide numbers correspond to those of the gels.
Albumin or globulin fraction.
Gliadin fraction.
Determination of free amino acids.
The concentrations of free amino acids in the water extracts of chemically acidified dough and sourdoughs were determined. Ten grams of dough were diluted with 50 ml of distilled water, homogenized with a Classic Blender (PBI International, Milan, Italy), and incubated with stirred conditions (100 rpm) at 30°C for 30 min. After centrifugation at 12,000 × g for 15 min, the supernatant was freeze-dried. Twenty milligrams of extract was resuspended in 6 ml of distilled water and filtered through a membrane having a 500-Da cutoff. The permeate was previously derivatized in a 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate precolumn and then used for high-performance liquid chromatography analysis (AccQ-Tag method; Waters Associates, Milford, Mass.). The chromatographic separation was carried out on a Waters AccQ-Tag column at 37°C, and elution was at a flow rate of 1 ml/min with a ternary gradient composed of 50 mM acetate buffer, pH 5.0, containing phosphoric acid (A), acetonitrile (B), and water (C). A fluorescence detector was used at a 250-nm excitation wavelength and a 395-nm emission wavelength. Identification and quantification of amino acids were carried out by comparison with a standard mixture of amino acids (Sigma Chemical Co.).
Rheological analyses.
For rheological analyses, the total weight of the dough was increased to 1.2 kg, keeping a constant dough yield of 150, and the time of incubation was varied from 1.5 to 4 h. NaCl at a concentration of 0.8% (wt/wt of dough), as normally used in Italian breadmaking (13), was also added to the dough. For rheological analyses, the chemical acidification of the dough was carried out directly only during mixing.
The maximum resistance (maximum height of the curve in extensographic units) and extensibility (length of the curve in millimeters) of sourdoughs fermented for 1.5 h were determined by the Brabender OHG (Duisburg, Germany) extensograph. The resistance to deformation (P; height of the curve in millimeters), the extensibility (L; length of the curve in millimeters), and the P/L ratio of sourdoughs fermented for 4 h were determined with the Chopin (Chopin, France) alveograph. The resistance to mixing was estimated by the Brabender farinograph on sourdoughs fermented for 4 h. The degree of softening of the dough, expressed in Brabender units, was defined as the difference between the starting and final resistance values during one run (20 min) in the farinograph.
Subcellular fractionation and enzyme preparations.
Twelve-hour-old cells of lactic acid bacteria cultivated in modified MRS broth were used for subcellular fractionation by lysozyme treatment in 50 mM Tris-HCl buffer, pH 7.5, containing 24% (wt/vol) sucrose, as described by Gobbetti et al. (20, 21). The only modification was that spheroplasts resuspended in isotonic buffer were sonicated by four cycles (10 each) (Sony Prep model 150; Sanyo, Tokyo, Japan) to recover the cell cytoplasm. Two cellular fractions were used: cell wall and cytoplasm. Both fractions were dialyzed for 24 h at 4°C against 20 mM phosphate buffer, pH 7.0, and concentrated ca. 20-fold by freeze-drying (MOD E1PTB; Edwards, Milan, Italy). The protein profile of the cell wall fraction was checked by SDS-PAGE in five repeated assays, as described by De Angelis et al. (15).
Proteinase activity was measured in 20 mM phosphate buffer, pH 7.0, by the method of Twinning (41) with fluorescent casein (1.0% [wt/vol]) as the substrate. Peptidase activity was measured on Leu-p-nitroanilide by the method of Gobbetti et al. (20). Proteinase activity on fluorescent casein was mainly detected in the cell wall fraction, while as expected, peptidase activity was found only in the cytoplasmic preparation. Both cellular fractions showed activity towards the nonapeptide bradykinin (Sigma Chemical Co.), which was used to standardize the enzyme activities.
Hydrolysis of the 31-43 fragment of A-gliadin.
The 31-43 fragment of A-gliadin was chemically synthesized by the Neosystem Laboratoire (Strasbourg, France). The peptide has the following sequence: L-G-Q-Q-Q-P-F-P-P-Q-Q-P-Y. All of the enzyme preparations used in the assays showed ca. 80% hydrolysis on bradykinin substrate. The reaction mixture contained 160 μl of 20 mM phosphate buffer (pH 7.0), 75 μl of 4 mM fragment 31-43, 4 μl of NaN3 (0.05% final concentration), and 100 μl of the enzyme preparation. The enzyme activity was stopped by addition of 0.1% (vol/vol) (final concentration) trifluoroacetic acid. Peptides were separated from the mixture by reversed-phase-fast performance liquid chromatography (RP-FPLC) using a PepRPC HR 5/5 column and FPLC equipment with a UV detector operating at 210 nm (Pharmacia Biotech). Elution was at a flow rate of 0.5 ml/min with a linear gradient (0 to 100%) of acetonitrile in 0.1% trifluoroacetic acid.
The 31-43 fragment of A-gliadin and the hydrolyzed mixtures produced by enzyme treatments were freeze-dried and used for agglutination tests.
Agglutination test.
Ethanol-extractable proteins (gliadins) from wheat flour (S. Pastore variety) were submitted to peptic-tryptic (PT) sequential digestion to produce the corresponding PT digest (4). After production, the PT digest was heated at 100°C for 30 min to inactivate enzymes. This peptide preparation was used directly for the agglutination test, or it was further digested with enzyme preparations from lactic acid bacteria before the agglutination test. A mixture containing 160 μl of 20 mM phosphate buffer (pH 7.0), 75 μl of PT digest (21 mg/ml), 4 μl of NaN3 (0.05% final concentration), and 100 μl of enzyme preparation, which consisted of equal volumes of cell wall and cytoplasmic fractions, was incubated at 30°C for 24 h and then immediately assayed.
K 562 (S) subclone cells of human myelagenous leukemia origin from the European Collection of Cell Culture (Salisbury, United Kingdom) were used for the agglutination test (6). The cells were grown in RPMI medium (HyClone, Cramlington, United Kingdom) supplemented with 0.2 mM l-glutamine, 50 U of penicillin/ml, 50 mg of streptomycin/ml, and 10% (vol/vol) fetal calf serum (Flow Laboratories, Irvine, Scotland) at 37°C in a humidified atmosphere of 5% CO2 in air for 96 h. After cultivation, the human cells were harvested by centrifugation at 900 × g for 5 min, washed twice with 0.1 M phosphate-buffered saline solution (Ca2+ and Mg2+ free; pH 7.4), and resuspended at a concentration of 108/ml in the same buffer. Twenty-five microliters of this cell suspension was added to wells of a microtiter plate containing serial dilutions (0.218 to 7.0 g/liter) of PT digest, 4 mM 31-43 fragment of A-gliadin, or 4 mM mixtures of the 31-43 fragment hydrolyzed by lactobacillus enzyme preparations in phosphate-buffered saline. The total volume in the well was 100 μl, and the mixture was held for 30 min at room temperature. After incubation, a drop of the suspension was applied to a microscope slide to count clumped and single cells. Agglutination tests were carried out in triplicate, and photographs were taken with a Diaphot-TMD inverted microscope (Nikon Corp., Tokyo, Japan).
RESULTS
Preliminary screening of proteolytic activity.
Fifty-five strains belonging to 12 species of lactic acid bacteria were preliminarily screened for proteolytic activity using the WFHP substrate, which contained a digest of albumin and globulin polypeptides with masses of 10.0 to 61.6 kDa (data not shown). Based on SDS-PAGE analysis, the strains were differentiated according to the hydrolysis of eight polypeptides. Strains belonging to the same species mainly grouped together, and all the strains fell into four main groups (Table 1). L. alimentarius, L. plantarum, L. farciminis, and L. lactis subsp. lactis strains had the same proteolysis profile as L. alimentarius 15M, which, compared to the WFHP substrate, completely degraded polypeptides 1, 5, and 7; gave rise to a new polypeptide band of ca. 53.4 kDa, probably by hydrolysis of polypeptide 1; and slightly hydrolyzed protein bands designated 2, 3, 4, and 6. L. brevis 14G did not hydrolyzed polypeptide 7, did not produce the ca. 53.4-kDa polypeptide, and had a lower activity on protein band 1. The other strains of L. brevis and those of L. citreum and L. fructivorans behaved similarly. All the strains of L. sanfranciscensis, including 7A, differed from L. brevis 14G mainly due to the presence of the new polypeptide. L. hilgardii 51B and L. acidophilus and W. confusa strains were characterized by a moderate activity on all the polypeptides considered.
TABLE 1.
Proteolytic activity of sourdough lactobacilli on wheat flour hydrolysate treated with protease (WFHP) substratea
| Spot designationb | Estimated molecular mass (kDa) | Rfd | Hydrolysis factorc
|
|||
|---|---|---|---|---|---|---|
| L. alimentarius 15M | L. brevis 14G | L. sanfranciscensis 7A | L. hilgardii 51B | |||
| 1 | 61.6 | 0.167 | 100 | 43.3 ± 6.4 | 56 ± 2.8 | 100 |
| NP | 53.4 | 0.235 | + | − | + | − |
| 2 | 44.8 | 0.273 | 14.7 ± 2.1 | 10.3 ± 0.8 | 0 | 12 ± 0.5 |
| 3 | 44.4 | 0.290 | 3 ± 0.1 | 11 ± 0.6 | 8 ± 0.3 | 46.5 ± 0.7 |
| 4 | 42.4 | 0.328 | 4 ± 0.2 | 12 ± 0.3 | 7.5 ± 0.2 | 39.5 ± 3.4 |
| 5 | 38.5 | 0.379 | 100 | 100 | 100 | 100 |
| 6 | 30.8 | 0.488 | 5 ± 0.1 | 0 | 0 | 6 ± 0.2 |
| 7 | 17.9 | 0.766 | 100 | 0 | 0 | 9 ± 0.3 |
| 8 | 10.1 | 0.987 | 0 | 0 | 0 | 9 ± 0.3 |
Analyses were performed with the Quantity One software package (Bio-Rad). Three gels of independent replicates were analyzed. For protein band intensity quantification and hydrolysis factor calculation, see Materials and Methods. All of the hydrolysis factors were calculated based on the average of the protein band intensities of each of three gels, and standard deviations were calculated. Only hydrolysis factors with P values of <0.05 are reported.
NP, new polypeptide not present in the WFHP substrate.
+, presence;−, absence.
Relative mobility.
L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B were used for further studies, since they were representative of the selected groups and are widely isolated from sourdoughs (19).
Proteolysis during sourdough fermentation.
The selected lactobacilli were used to study proteolysis during sourdough fermentation. All the sourdoughs contained ca. 109 CFU of each lactic acid bacterial strain/g and had final pH values ranging from 4.3 to 4.6, depending on the time of fermentation. In agreement with previous findings (21, 22), proteolysis at 4 h, expressed in terms of the free amino acid concentration, was 80 to 85% of that reached after 8 h. Results from sourdough fermented for 8 h were further described.
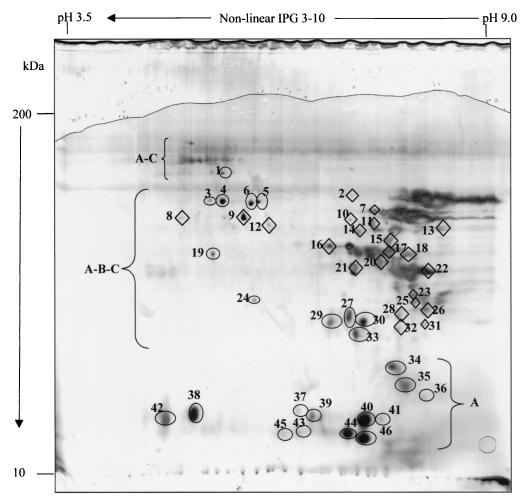
Wheat flour proteins were selectively extracted from sourdoughs and further analyzed by 2D electrophoresis. Figure 1 shows the albumin, globulin, gliadin, and glutenin fractions in the chemically acidified (pH 4.4) dough. In agreement with previous electrophoretic separations (35, 44), albumin and globulin polypeptides were distributed over the entire range of pIs (4.4. to 8.7) and molecular masses (15 to 100 kDa), while gliadins were located in well-defined regions: α, β, and γ fractions (28 to 50 kDa) formed a cluster in the alkaline zone of the gel (pIs, 6.5 to 8.5), and ω fractions (55 to 70 kDa) were confined to the pI area of 4.0 to 7.0. The high-molecular-mass glutenin subunits were positioned in the pI range of 5.1 to 5.4 with a mass of ca. 100 kDa, while low-molecular-mass subunits (35 to 50 kDa) exhibited pIs of 6.0 to 9.0 and partially overlapped the α-, β-, and γ-gliadins. The possibility of slight contamination among protein fractions during selective extraction cannot be excluded.
FIG. 1.
2D electrophoresis analysis of wheat flour protein fractions in chemically acidified dough. A, albumins and globulins; B, gliadins; C, glutenins. The numbered ovals and diamonds refer to hydrolyzed albumins and globulins and to gliadins, respectively.
Since biological or chemical acidification caused a marked modification of the 2D polypeptide pattern with respect to the nonacidified dough (data not shown), the sourdoughs fermented by selected lactobacilli were compared to the chemically acidified dough. A total of 46 polypeptides belonging to albumin, globulin, and gliadin fractions were hydrolyzed by the selected lactic acid bacteria (Table 2). No hydrolysis of glutenins was detected under these conditions. L. alimentarius 15 M showed hydrolysis of 37 polypeptides; 24 of them were located in the region of the albumin and globulin fractions. Almost all the albumin and globulin polypeptides (22 of 24) were degraded by hydrolysis factors of ≥50%. Only 7 of the 13 gliadins were degraded to the same extent. Except for L. hilgardii 51B, the proteolytic activities of the lactobacilli affected polypeptides with a wide range of pIs and molecular masses. Hydrolysis was particularly intense towards protein spots with masses of less than 34 kDa. L. brevis 14G hydrolyzed 39 polypeptides, 24 of which were albumins and globulins. Compared to that of L. alimentarius 15M, the hydrolysis was more pronounced, since all of the albumin and globulin polypeptides and 12 of the 15 gliadins were degraded by hydrolysis factors of ≥50%. L. sanfranciscensis 7A showed proteolysis towards the largest number (41) of polypeptides, 24 albumins and globulins and 18 gliadins. L. hilgardii 51B showed slight proteolysis; only 14 polypeptides were degraded, 8 of them being albumins and globulins.
Proteolysis during sourdough fermentation was also estimated by the concentration of free amino acids (Table 3). The unstarted wheat flour dough incubated for 8 h at 37°C had a final pH of 5.6 and did not show an appreciable increase in total free amino acids with respect to the wheat flour. Also, the addition of the cell suspension to the dough did not increase the free amino acids (data not shown). Chemical acidification to pH 4.4 produced an increase in the total free amino acids (1,278 mg/kg), which reflected a nonspecific increase in individual amino acids. Fermentation by selected lactobacilli resulted in a markedly higher total concentration (1,767 to 2,020 mg/kg) with some differences in the amino acid patterns. Compared to the chemically acidified dough, all the lactobacilli caused a marked increase in Pro, Leu, and Phe concentrations, and L. alimentarius 15M, L. brevis 14G, and L. sanfranciscensis 7A also favored an increase in Asp and Glu. L. hilgardii 51B had the lowest capacity to liberate amino acids.
TABLE 3.
Concentrations of free amino acids in chemically acidified and fermented doughs
| Amino acid | Unstarted dough batch no. | Chemically acidified dough batch no. | Concn (mg/kg)a
|
|||
|---|---|---|---|---|---|---|
| L. alimentarius 15M | L. brevis 14G | L. sanfranciscensis 7A | L. hilgardii 51B | |||
| Asp | 69 | 61 | 241 | 178 | 174 | 50 |
| Ser | 78 | 103 | 105 | 116 | 156 | 118 |
| Glu | 20 | 44 | 129 | 126 | 155 | 97 |
| Gly | 41 | 49 | 62 | 108 | 101 | 100 |
| His | 87 | 72 | 66 | 45 | 61 | 88 |
| Arg | 83 | 102 | 166 | 138 | 153 | 130 |
| Thr | 41 | 58 | 88 | 68 | 94 | 82 |
| Ala | 30 | 34 | 30 | 22 | 30 | 25 |
| Pro | 82 | 111 | 205 | 259 | 209 | 263 |
| Tyr | 52 | 68 | 117 | 127 | 81 | 80 |
| Val | 50 | 57 | 100 | 104 | 125 | 96 |
| Met | 26 | 32 | 85 | 149 | 79 | 60 |
| Lys | 122 | 127 | 157 | 138 | 170 | 218 |
| Ile | 28 | 46 | 73 | 74 | 79 | 63 |
| Leu | 53 | 145 | 238 | 235 | 224 | 191 |
| Phe | 45 | 69 | 131 | 133 | 122 | 106 |
| Total | 907 | 1,278 | 1,993 | 2,020 | 2,013 | 1,767 |
Each value is the average of three doughs independently analyzed. The coefficient of variation of the individual amino acid concentrations was always less than 2%.
Effect of proteolysis on dough rheology.
The sourdoughs used for proteolysis analyses were also evaluated for some rheological properties according to the method proposed by Martinez-Anaya et al. (31). Compared to the chemically acidified dough, the sourdough produced by L. brevis 14G had the lowest maximum resistance (560 versus 1,000 extensographic units) and the highest extensibility (97 versus 60 mm), as analyzed by the Brabender extensograph (Table 4). The sourdoughs started with L. alimentarius 15M and L. sanfranciscensis 7A behaved similarly, while that from L. hilgardii 51B had higher resistance and lower extensibility. These findings were confirmed by the P/L ratios of the Chopin alveograph, which decreased from 10.80 to 1.52 going from the chemically acidified dough to the L. brevis 14G dough. The greatest softening of the dough caused by the four lactobacilli was also demonstrated by the Brabender farinograph, which pointed out the hardness of the chemically acidified dough, characterized by a very low degree of softening (380 versus 430 to 500 Brabender units). An unacidified dough, having the same dough yield of 150, showed a degree of softening which approached that of the chemically acidified dough, as determined by the Brabender farinograph (data not shown).
TABLE 4.
Brabender extensograph and farinograph and Chopin alveograph parameters of chemically acidified and fermented doughs
| Parameterb | Valuea
|
||
|---|---|---|---|
| Chemically acidified dough | L. brevis 14G | L. hilgardii 51B | |
| R | 1,000 | 560 | 900 |
| E | 60 | 97 | 75 |
| P/Lc | 10.80 | 1.52 | 3.12 |
| S | 1,000 | 900 | 900 |
| F | 620 | 400 | 470 |
| Degree of softening (S − F) | 380 | 500 | 430 |
Each value is the average of three doughs independently analyzed.
R, maximum resistance (in extensographic units); E, extensibility (in millimeters); S, starting resistance (in Brabender units) [BU]; F, final resistance (in BU).
As determined by the Chopin alveograph (see Materials and Methods).
Hydrolysis of the 31-43 fragment of A-gliadin.
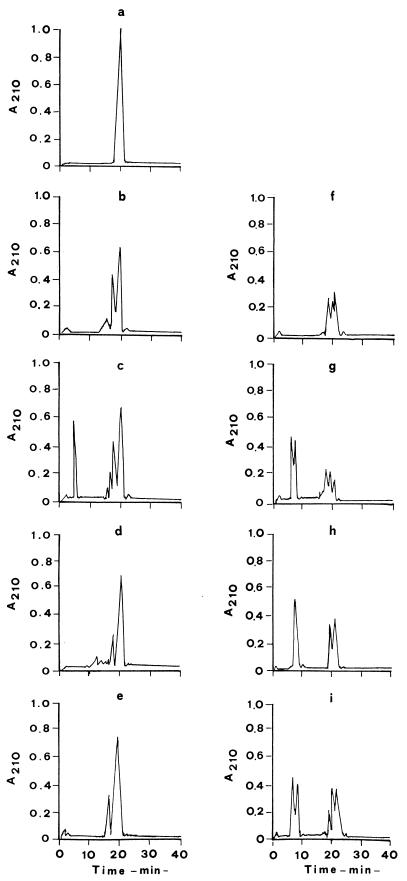
The four selected lactobacilli were further used to hydrolyze the chemically synthesized 31-43 fragment of A-gliadin, which was found to be toxic to celiac patients by the most common in vitro systems (7, 34). A-gliadin is usually obtained as a sediment by ultracentrifugation (133,000 × g) of the acetic extract of wheat flour; its electrophoretic pattern in a dissociating buffer is identical to that of highly purified α-gliadin fractions. The hydrolysis of the peptide was assayed by using two bacterial cell fractions, cell wall and cytoplasm, which contained the greatest part of the proteinase and peptidase activity, respectively. The cell fractions were used in the enzyme assay at a concentration corresponding to 109 CFU/ml. After 4 h of incubation, hydrolysis of the 31-34 peptide of A-gliadin by proteinases, expressed as percentage reduction of the peak area of the untreated substrate, was ca. 54 to 50% for L. alimentarius 15M and L. brevis 14G, 43% for L. sanfranciscenis 7A, and 35% for L. hilgardii 51B (Fig. 2b, c, d, and e). All the strains showed a common product of hydrolysis, which eluted after 18 min of the acetonitrile gradient; the most complex profile was for L. brevis 14G, which produced four main peptides. Compared to the cell wall, the activity of the cytoplasmic preparation was higher, always resulting in a percentage of hydrolysis greater than 50% (Fig. 2f, g, h, and i). The hydrolysis pattern was only partly similar to that found in the presence of the proteinase activity.
FIG. 2.
RP-FPLC chromatograms of A-gliadin 31-43 fragment (a) by cell wall and cytoplasmic enzyme preparations of L. alimentarius 15M (b and f), L. brevis 14G (c and g), L. sanfranciscensis 7A (d and h), and L. hilgardii 51B (e and i).
Agglutination test.
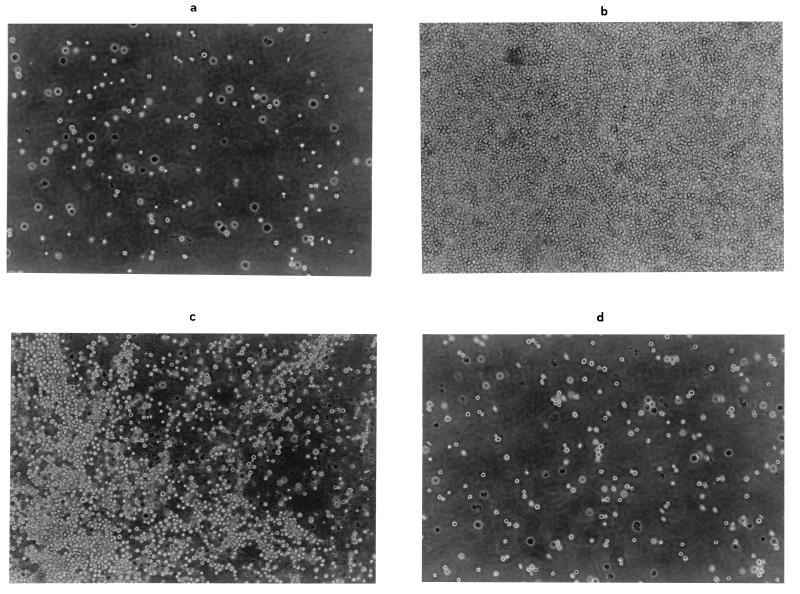
A PT digest of gliadins, obtained by simulating in vivo protein digestion, was used for the agglutination test on K 562 (S) cells. According to previous findings (6), no significant evidence of cell clustering was found when the undifferentiated K 562 (S) cells were not treated with the PT digest (Fig. 3a). On the contrary, the PT digest agglutinated 100% of the undifferentiated cells at the lowest concentration of 0.218 g/liter (Fig. 3b and Table 5). The agglutinated cells had a peculiar appearance, i.e., a tendency to form a continuous cell layer with high resistance to shearing and whirling forces. Before being used, the PT digest was further digested for 24 h with the cell wall and cytoplasmic preparations of selected lactic acid bacteria. When assayed alone, the enzyme preparations were ineffective in causing cell agglutination (data not shown). In the presence of the highest concentration of PT digest (7.0 g/liter), further hydrolysis by L. alimentarius 15M, L. brevis 14G, and L. sanfranciscensis 7A exerted a 50 to 60% inhibition of cell agglutination. L. hilgardii 51B had no effect (Table 5). At an intermediate concentration of 0.875 g/liter, the agglutination activity of the PT digest was completely inhibited by treatment with the L. alimentarius 15M and L. brevis 14G enzymes, while L. sanfranciscensis 7A gave complete inhibition only at 0.218 g/liter.
FIG. 3.
Agglutination test on K 562 (S) cells. (a) Untreated cells. (b) Cells treated with PT-gliaden digest at a concentration of 0.875 g/liter. (c and d) Cells treated with PT-gliadin digest at a concentration of 0.875 g/liter with further treatment by enzyme preparations of L. hilgardii 51B (c) and L. brevis 14G (d).
TABLE 5.
Inhibition of PT-gliadin digest agglutination activity on K 562 (S) cells by proteolysis from sourdough lactobacillia
| Strain | % Inhibition for PT gliadin digest concn (g/liter) of:
|
|||||
|---|---|---|---|---|---|---|
| 7.0 | 3.5 | 1.75 | 0.875 | 0.437 | 0.218 | |
| L. alimentarius 15 M | 60 | 60 | 75 | 100 | 100 | 100 |
| L. brevis 14G | 60 | 70 | 80 | 100 | 100 | 100 |
| L. sanfranciscensis 7A | 50 | 50 | 50 | 75 | 80 | 100 |
| L. hilgardii 51B | 0 | 30 | 30 | 50 | 50 | 60 |
Each value is the average of three independent agglutination tests. The coefficient of variation of the individual percentages of inhibition was always less than 4%. For PT-gliadin digest preparation, see Materials and Methods.
The agglutination test was also used to assay the activity of the 31-43 fragment of A-gliadin hydrolyzed by the enzyme preparations of lactobacilli. Compared to the whole fragment, all the peptide mixtures gave decreases of K 562 (S) cell agglutination which ranged from 40 to 85%.
DISCUSSION
Proteolysis during sourdough fermentation is one of the activities which is still unclear, because of the confused borderline between the effects of acidification and enzyme activity and because of the various endogenous microbial and wheat flour proteolytic enzymes which could be active in the dough. The few reports which have dealt with this subject have shown only the accumulation of free amino acids during fermentation and the adaptation of the lactic acid bacterial enzymes to the dough environment (39). This report has highlighted the role of lactic acid bacteria in sourdough proteolysis, showing their technological usefulness and some new potentialities regarding nutrition.
A rather large number of sourdough lactic acid bacteria (55 strains belonging to 12 species) was preliminarily screened. Proteolysis was assayed by use of a wheat flour hydrolysate, further treated with a microbial protease (WFHP), as a substrate. Hemoglobin and casein were initially used, but the need for a more specific substrate was evident (20). Recently, proteolysis by sourdough lactic acid bacteria was tested directly on gluten by determining the clearing zones in agar medium, the increase of trichloroacetic acid-soluble polypeptides, and the release of free amino acids (43). The WFHP substrate used contained a digest of albumin and globulin polypeptides and was useful for a preliminary differentiation of four main groups of lactic acid bacteria based on their SDS-PAGE hydrolysis profiles.
Four strains, one from each of the above-mentioned groups, were further investigated: L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B. They were used in a traditional sourdough fermentation process, after which proteolysis was estimated by a fractionated method of protein extraction (44) and subsequent 2D electrophoresis. Cereal proteins have been studied by a number of analytical techniques over the years, but high-resolution 2D electrophoresis can be considered one of the most powerful methodologies utilized (8). Since the acidification and related redox potential were found to affect the solubility, polymerization, and hydrolysis of the polypeptides, all the results were compared to those of a chemically acidified dough (pH 4.4). The chemical acidification by lactic and acetic acids was performed directly during dough mixing, gradually during dough incubation to simulate the microbial production of organic acids, or at the end of dough incubation. No significant differences were found in the patterns of extractable protein fractions. This comparison also allowed us to exclude interference due to the activity of the fortuitous microflora and of exo- and endoproteolytic enzymes of the wheat flour. To our knowledge, this is the first demonstration of the hydrolysis of albumin, globulin, and especially gliadin fractions by sourdough lactic acid bacteria. Glutenins were not hydrolyzed. Compared to the chemically acidified dough, 37 to 42 polypeptides, distributed over a large range of pIs and molecular masses, were hydrolyzed, especially by L. alimentarius 15M, L. brevis 14G, and L. sanfranciscensis 7A. Overall, hydrolysis factors greater than 50% were found, and the hydrolysis of gliadin fractions was consistent, although it was lower than that found for the albumin and globulin fractions. The great liberation of free amino acids during fermentation further supported these findings. All of the strains produced a marked increase in Pro, Leu, and Phe. One of the typical features of the amino acid composition of wheat gliadin is, together with glutamine, the high content of proline (on average, one proline residue for every seven residues). L. alimentarius 15M, L. brevis 14G, and L. sanfranciscensis 7A, especially, also caused an increase in dicarboxylic (Asp and Glu) amino acids, which, having the capacity to ionize in water solutions, are mainly present in the albumin and globulin fractions (45).
The grain protein concentration and protein quality are among the major factors which influence extensibility, resistance to extension (elasticity), and workability of the dough. Furthermore, the lengths of the gliadin and glutenin subunit chains influence their tendency to polymerize as well as their aggregative behavior in forming the gluten mass (31, 35). The partial hydrolysis of gliadin by lactic acid bacteria also seemed to positively influence the degree of softening of the dough and its stability during fermentation. Compared to the performance exhibited by the chemically acidified dough, all the parameters determined with the Brabender extensograph and farinograph and the Chopin alveograph indicated an improvement by lactic acid bacteria, which varied slightly depending on the strain. The effect was particularly evident in terms of the P/L ratio and degree of softening, which expressed the workability of the dough. The combined effect of lactobacillus proteolysis and acidification may produce a shorter and harder gluten, which may account for a more elastic structure (30).
The above findings encouraged the use of lactic acid bacteria for hydrolyzing gliadin-derived peptides, which are involved in celiac disease. Toxic peptides derived from the proteolytic digestion of the alcohol-soluble endosperm proteins (prolamins) of some cereals (e.g., wheat, barley, and rye) in vivo adversely affect the intestinal mucosa of celiac patients. They interact with undifferentiated cells, either agglutinating them or affecting their proliferation and metabolism (4, 6). Studies with fragments of A-gliadin clearly indicated that a few short sequences very rich in glutamine and proline residues (e.g., P-S-Q-Q and Q-Q-Q-P sequences) are toxic to celiac patients (Marsh et al., Gut 36:210A, 1995). The infusion of the 31-43 fragment of A-gliadin, which contains the sequence Q-Q-Q-P, directly into the jejunum of treated celiac patients was shown to be toxic by mucosal biopsies (Marsh et al., Gut 36:210A, 1995). Fragment 31-43 of A-gliadin was hydrolyzed after 4 h of treatment by enzyme preparations of lactobacilli which separately contained proteinase and peptidase activities. Enzyme preparations were used to clearly demonstrate the hydrolytic activity of cell wall-associated proteinases, which may have easier access to the peptide substrates during sourdough fermentation. Differences in hydrolysis patterns were found among the strains, and the cytoplasmic preparation showed the highest activity. Similar results were found with viable cells. Nevertheless, autolysis of lactic acid bacteria during sourdough preparation and fermentation is not a rare event and may favor the release of intracellular peptidases. Microbial cells may be disrupted during mixing and/or the release of intracellular peptidase may be enhanced under less acidic conditions or when additives such as citrate are used (14).
Because of the many ethical and practical constraints of in vivo studies, some investigations aimed at identifying and characterizing cereal peptides deemed to be toxic under pathological conditions have been performed with biopsy specimens of intestinal mucosa from celiac patients and to a larger extent with a variety of in vitro systems, including isolated organs, tissues, cells, and subcellular fractions from a variety of sources (37). The digestion of cereal protein fractions has been mimicked in vitro by means of a sequential digestion with pepsin and trypsin (PT digest). A number of investigations (4, 16, 46) have shown the ability of wheat gliadin peptide activity, including PT digest, to prevent the in vitro recovery of active celiac mucosa biopsy specimens, thus causing disorganization of crypt architecture, reduced height, irregularities of enterocytes and crypt cells, and even tissue necrosis. We assayed the effect of lactic acid bacterial proteolysis on the agglutination activity of a PT digest towards undifferentiated K 562 (S) cells. A high correlation was found between the agglutination activities of cereal components against K 562 (S) cells and their toxicities in clinical and in vitro trials based on the biopsy of intestinal mucosa from celiac patients (3-7). Under our experimental conditions, the PT digest caused 100% agglutination of K 562 (S) cells at a minimum concentration of 0.218 g/liter. Before use, the PT digest was also further digested (24 h) with enzyme preparations of lactic acid bacteria which corresponded to the cell concentration (109/g) usually found during sourdough fermentation (19). The treatment with proteolytic enzymes of L. alimentarius 15M and L. brevis 14G completely prevented the agglutination of K 562 (S) cells by the PT digest used at a concentration of 0.875 g/liter. Lower percentages of inhibition were found in the presence of higher PT digest concentrations and with the other two selected lactobacilli. Compared to the whole 31-43 fragment of A-gliadin, all the peptide mixtures produced by the enzyme preparations of selected lactobacilli showed considerably lower agglutination activities, thus indicating a suitable enzyme substrate specificity which excluded the generation of more toxic peptides.
Sourdough affects the nutritional value of baked goods, especially bread. It has been reported that the glycemic response to baked goods made from sourdough is lower (28, 29) and that the availability of minerals in sourdough bread is increased (27). Currently, the use of some protective substances (e.g., mannan and oligomers of N-acetylglucosamine, such as N,N",N""-triacetylchitotriose and N,N"-diacetylchitobiose) is the best choice to prevent the effects of the prolamin toxic peptides (37). A long time (24 h) was allowed for PT digest hydrolysis, and sourdough cannot be used as the only component of the baking dough in the traditional technology; nevertheless, this study is the first to show that selected sourdough lactic acid bacteria have hydrolyzing activities towards prolamin peptides involved in human cereal intolerance. These activities could be easily improved under more suitable technological conditions and/or addressed to the production of special sourdough-type breads with low contents of gliadin toxic peptides.
Acknowledgments
This work was supported by the Italian Ministry of University and Scientific and Technological Research (MURST), Development of Research Networks no. 488/92, Cluster C06+07, Project 6-2.2.
The valuable technical assistance of S. L. Lonigro is gratefully acknowledged.
REFERENCES
- 1.Anonymous. 1994. Bekanntmachung von weiteren Leitsatzen des Deutschen Lebensmittelbuches. Bundesanzeiger 46:7-8. [Google Scholar]
- 2.Appel, D., and D. F. Hochstrasser. 1999. Computer analysis of 2-D images, p. 431-443. In A. J. Link (ed.), Methods in molecular biology, vol. 11. 2-D proteome analysis protocols. Humana Press Inc., Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 3.Auricchio, S., G. De Ritis, M. De Vincenzi, G. Magazzù, L. Maiuri, E. Mancini, O. Sapora, and V. Silano. 1990. Mannan and oligomers of N-acetylglucosamine protect intestinal mucosa of coeliacs with active disease from in vitro toxicity of gliadin peptides. Gastroenterology 99:973-978. [DOI] [PubMed] [Google Scholar]
- 4.Auricchio, S., G. De Ritis, M. De Vincenzi, P. Occorsio, and V. Silano. 1982. Effect of gliadin peptides prepared from hexaploid and tetraploid wheat on cultures of intestine from rat fetuses and coeliac children. Pediatr. Res. 16:1004-1010. [DOI] [PubMed] [Google Scholar]
- 5.Auricchio, S., G. De Ritis, M. De Vincenzi, and V. Silano. 1985. Toxicity mechanisms of wheat and other cereals in celiac disease and related enteropathies. J. Pediatr. Gastroenterol. Nutr. 4:923-930. [DOI] [PubMed] [Google Scholar]
- 6.Auricchio, S., G. De Ritis, M. De Vincenzi, M. Minetti, O. Sapora, and V. Silano. 1984. Agglutination activity of gliadin-derived peptides from bread wheat: implications for coeliac disease pathogenesis. Biochem. Biophys. Res. Commun. 21:428-433. [DOI] [PubMed] [Google Scholar]
- 7.Auricchio, S., L. Maiuri, A. Picarelli, M. De Vincenzi, R. Troncone, V. Pavone, and M. J. Mayer. 1996. In vitro activities of A-gliadin-related synthetic peptides. Damaging effect on the atrophic coeliac mucosa and activation of mucosal immune response in the treated coeliac mucosa. Scand. J. Gastroenterol. 31:247-253. [DOI] [PubMed] [Google Scholar]
- 8.Bean, S. R., and G. L. Lookart. 2000. Electrophoresis of cereal storage proteins. J. Chromatogr. 881:23-36. [DOI] [PubMed] [Google Scholar]
- 9.Bini, L., B. Magi, B. Marzocchi, F. Arcuri, S. Tripodi, M. Cintorino, J. C. Sanchez, S. Frutiger, G. Hughes, V. Pallini, D. F. Hochstrasser, and P. Tosi. 1997. Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis 18:2832-2841. [DOI] [PubMed] [Google Scholar]
- 10.Bjellqvist, B., G. J. Hughes, C. Pasquali, N. Paquet, F. Ravier, J. C. Sanchez, S. Frutiger, and D. Hochstrasser. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14:1023-1031. [DOI] [PubMed] [Google Scholar]
- 11.Collins, M. D., J. Samelis, J. Metaxopoulos, and S. Walbanks. 1993. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissela for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 75:595-603. [DOI] [PubMed] [Google Scholar]
- 12.Corsetti, A., M. Gobbetti, B. De Marco, F. Balestrieri, F. Paoletti, L. Russi, and J. Rossi. 2000. Combined effect of sourdough lactic acid bacteria and additives on bread firmness and staling. J. Agric. Food Chem. 48:3044-3051. [DOI] [PubMed] [Google Scholar]
- 13.Corsetti, A., P. Lavermicocca, M. Morea, F. Baruzzi, N. Tosti, and M. Gobbetti. 2001. Phenotypic and molecular identification and clustering of lactic acid bacteria and yeast from wheat (species Triticum durum and Triticum aestivum) sourdoughs of Southern Italy. Int. J. Food Microbiol. 64:95-104. [DOI] [PubMed] [Google Scholar]
- 14.De Angelis, M., P. Pollacci, and M. Gobbetti. 1999. Autolysis of Lactobacillus sanfranciscensis. Eur. Food Res. Technol. 210:57-61. [Google Scholar]
- 15.De Angelis, M., A. Corsetti, N. Tosti, J. Rossi, M. R. Corbo, and M. Gobbetti. 2001. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl. Environ. Microbiol. 67:2011-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falchuk, Z. M., R. L. Gebhard, C. Sessoms, and W. Strober. 1974. An in vitro model of gluten sensitive enteropathy: effect of gliadin on intestinal epithelial cells of patients with gluten sensitive enteropathy in organ culture. J. Clin. Investig. 53:487-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrow, J. A. E., R. R. Facklam, and M. D. Collins. 1989. Nucleic acid homologies of some vancomycin-resistant leuconostocs and description of Leuconostoc citreum sp. nov. and Leuconostoc pseudomesenteroides sp. nov. Int. J. Syst. Bacteriol. 39:279-283. [Google Scholar]
- 18.Fasano, A., and C. Catassi. 2001. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 120:636-651. [DOI] [PubMed] [Google Scholar]
- 19.Gobbetti, M. 1998. The sourdough microflora: interactions between lactic acid bacteria and yeast. Trends Food Sci. Technol. 9:267-274. [Google Scholar]
- 20.Gobbetti, M., E. Smacchi, and A. Corsetti. 1996. The proteolytic system of Lactobacillus sanfrancisco CB1: purification and characterization of a proteinase, a dipeptidase, and an aminopeptidase. Appl. Environ. Microbiol. 62:3220-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobbetti, M., E. Smacchi, P. F. Fox, L. Stepaniak, and A. Corsetti. 1996. The sourdough microflora. Cellular localization and characterization of proteolytic enzymes in lactic acid bacteria. Lebensm. Wiss. U. Technol. 29:561-569. [Google Scholar]
- 22.Gobbetti, M., M. S. Simonetti, J. Rossi, L. Cossignani, A. Corsetti, and P. Damiani. 1994. Free D- and L- amino acid evolution during sourdough fermentation and baking. J. Food Sci. 59:881-884. [Google Scholar]
- 23.Hammes, W. P., and M. G. Ganzle. 1998. Sourdough breads and related products, p. 199-216. In B. J. B. Wood (ed.), Microbiology of fermented foods. Blackie Academic & Professional, London, United Kingdom.
- 24.Hammes, W. P., and R. F. Vogel. 1997. Sauerteig, p. 201-285. In W. Holzapfel and H. Weber (ed.), Mikrobiologie der Lebensmittel, Lebensmittel pflanlicher Herkunft. Behr's Verlag, Hamburg, Germany.
- 25.Hochstrasser, D. F., M. G. Harrington, A. C. Hochstrasser, M. J. Miller, and C. R. Merril. 1988. Methods for increasing the resolution of two dimensional protein electrophoresis. Anal. Biochem. 173:424-435. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Larsson, M., and A. S. Sandberg. 1991. Phytate reduction in bread containing oat flour, oat bran or rye bran. J. Cereal Sci. 14:141-149. [Google Scholar]
- 28.Liljeberg, H., and I. Bjorck. 1994. Bioavailibility of starch in bread products. Postprandial glucose and insulin responses in healthy subjects and in vitro resistant starch content. Eur. J. Clin. Nutr. 48:151-163. [PubMed] [Google Scholar]
- 29.Liljeberg, H. G. M., C. H. Lonner, and I. M. E. Bjorck. 1995. Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J. Nutr. 125:1503-1511. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz, K. 1983. Sourdough processes. Methodology and biochemistry. Baker's Dig. 55:32-36. [Google Scholar]
- 31.Martinez-Anaya, M. A., B. Pitarch, and C. B. de Barber. 1993. Biochemical characteristics and breadmaking performance of freeze-dried wheat sour dough starters. Z. Lebensem. Unters. Forsch. 196:360-365. [Google Scholar]
- 32.Osborne, T. B. 1907. The proteins of the wheat kernel. Carnegie Institute of Washington publication 84. Judd and Detweiler, Washington, D.C.
- 33.Ottogalli, G., A. Galli, and R. Foschino. 1996. Italian bakery products attained with sourdough: characterization of the typical sourdough flora. Adv. Food Sci. 18:131-144. [Google Scholar]
- 34.Picarelli, A., L. Di Tola, M. Sabbatella, R. Greco, M. Silano, and M. De Vincenzi. 1999. 31-43 Amino acid sequence of the α-gliadin induces anti-endomysial antibody production during in vitro challenge. Scand. J. Gastroenterol. 34:1099-1102. [DOI] [PubMed] [Google Scholar]
- 35.Pogna, N., P. Tusa, and G. Boggini. 1996. Genetic and biochemical aspects of dough quality in wheat. Adv. Food Sci. 18:145-151. [Google Scholar]
- 36.Quaglia, G. 1984. La fermentazione, p. 295-296. In G. Quaglia (ed.), Scienza e tecnologia della panificazione. Chiriotti, Pinerolo, Italy.
- 37.Silano, M., and M. De Vicenzi. 1999. Bioactive antinutritional peptides derived from cereal prolamins: a review. Nahrung 42:175-184. [DOI] [PubMed] [Google Scholar]
- 38.Sollid, L. M., G. Markussen, J. E. K., H. Gjerde, F. Vartdal, and E. Thorsby. 1989. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J. Exp. Med. 169:345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spicher, G., and W. Nierle. 1988. Proteolytic activity of sourdough bacteria. Appl. Microbiol. Biotechnol. 28:487-492. [Google Scholar]
- 40.Tosi, R., D. Vismara, and N. Tanigaki. 1983. Evidence that celiac disease is primarily associated with a DC locus allelic specificity. Clin. Immunol. Immunopathol. 28:395-404. [DOI] [PubMed] [Google Scholar]
- 41.Twinning, S. 1984. Fluorescein isothiocynate-labeled casein assay for proteolytic enzymes. Anal. Biochem. 143:30-34. [DOI] [PubMed] [Google Scholar]
- 42.Van de Kamer, J. H., H. A. Weijers, and W. K. Dicke. 1953. An investigation into the injurious constituents of wheat in connection with their action on patients with coeliac disease. Acta Paediatr. Scand. 42:223-231. [DOI] [PubMed] [Google Scholar]
- 43.Wehrle, K., N. Crowe, I. van Boeijen, and E. K. Arendt. 1999. Screening methods for the proteolytic breakdown of gluten by lactic acid bacteria and enzyme preparations. Eur. Food Res. Technol. 209:428-433. [Google Scholar]
- 44.Weiss, W., C. Vogelmeier, and A. Gorg. 1993. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers' asthma. Electrophoresis 14:805-816. [DOI] [PubMed] [Google Scholar]
- 45.Wieser, H. 1996. Relation between gliadin structure and coeliac toxicity. Acta Pediatr. Suppl. 412:3-9. [DOI] [PubMed] [Google Scholar]
- 46.Wieser, H., H. D. Belitz, A. Ashkenazi, and D. Idar. 1983. Isolation of coeliac active peptide fractions from gliadin. Z. Lebensm. Unters. Forsch. 176:85-94. [DOI] [PubMed] [Google Scholar]