Abstract
We analyzed the occurrence of the naphthalene degradation upper-pathway (nah) genes in the western Mediterranean region. The amplification, restriction, and sequence analysis of internal fragments for several nah genes (nahAc, nahB, nahC, and nahE) from naphthalene-degrading strains isolated from this geographical area proved the coexistence of two distinct sets of nah genes.
Naphthalene degradation has been extensively studied in several Pseudomonas strains, such as the archetypes Pseudomonas putida G7 (11, 23, 25, 29) and P. putida NCIB9816 (5, 19). In both strains, the dissimilatory genes are organized into two operons: one coding for the enzymes involved in the conversion of naphthalene to salicylate (nahAaAbAcAdBFCED, naphthalene degradation upper pathway) and the second coding for the conversion of salicylate to tricarboxylic acid cycle intermediates (pyruvate and acetyl coenzyme A) through the meta-cleavage pathway (nahGTHINLOMKJ, naphthalene degradation lower pathway). Regulation of both operons is mediated by a single protein, NahR, that acts as a positive regulator for both promoters, with salicylate being the inducer of the system (23).
Nucleotide sequences coding for the entire naphthalene degradation upper pathway have been determined in several Pseudomonas species such as Pseudomonas sp. strain C18 (6), P. putida OUS82 (27), Ralstonia sp. strain U2 (7), P. stutzeri AN10 (4), P. putida BS202 (GenBank accession no. AF010471), and P. aeruginosa PaK1 (GenBank accession no. D84146). Interestingly, comparison of this last sequence with the sequences for P. stutzeri AN10 and for Pseudomonas sp. strain C18 revealed that the upper pathway of strain PaK1 could be the result of a recombination event between the upper pathways found in the other two strains (4). In this manner, it has been suggested that a P. aeruginosa PaK1 ancestor recruited two entire naphthalene degradation upper pathways and a mosaic upper pathway was selected for, probably due to environmental pressure, in the ancestral strain of PaK1 (4). Other mosaic patterns within individual degradative genes or within catabolic operons as a result of recombination between homologous sequences have been previously reported (1, 2, 10), and the same is true for the coexistence of nearly identical metabolic modules (i.e., pWW53 and related plasmids) (24, 28).
Our objectives in the present study were (i) to analyze the presence of two distinct sets of nah upper-pathway genes in the western Mediterranean region and (ii) to prove that these two distinct types of genes could physically coexist in the same host strain.
Amplification of nahAc homologues from naphthalene-degrading Pseudomonas strains
Naphthalene dioxygenase (NahA) catalyzes the first step in the degradation of naphthalene, the cis-dihydroxylation reaction (12), being a three-component class III oxygenase (ferredoxin [NahAb], ferredoxin reductase [NahAa], and terminal dioxygenase [NahAcAd, also NDO]) in which terminal dioxygenase is an α3β3 hexamer (13). Lloyd-Jones and coworkers (14, 15) affiliated the NDO large subunit (α, NahAc-like) gene sequences with three major branches: the nah-like group, the dnt/ntd group, and the phn-type group.
Sequences for NDO large subunit genes from Rhodococcus sp. strain NCIMB12038 (narAa [GenBank accession no. AF082663]), Burkholderia sp. strain RP007 (phnAc [AF061751]), Alcaligenes faecalis AFK2 (phnAc [AB024945]), Ralstonia sp. strain U2 (nagAc [AF036940]), Comamonas testosteroni H (pahAc [AF252550]), Burkholderia sp. strain DNT (dntAc [U62430]), Pseudomonas sp. strain JS42 (ntdAc [U49504]), Pseudomonas stutzeri AN10 (nahAc [AF039533]), P. aeruginosa PAK1 (pahA3 [D84146]), P. putida G7 (nahAc [M83949]), P. fluorescens ATCC 17483 (ndoC2 [AF004283]), P. putida OUS82 (pahAc [AB004059]), P. putida ATCC 17484 (ndoC2 [AF004284]), P. putida NCIB9816 (ndoB [M23914]), P. putida NCIB9816-4 (nahAc [M83950]), P. putida BS202 (nahA3 [AF010471]), and Pseudomonas sp. strain C18 (doxB [M60405]) were aligned and forward (Ac149f, 5"-CCCYGGCGACTATGT-3") and reverse (Ac1014r, 5"-CTCRGGCATGTCTTTTTC-3") degenerated primers were chosen in conserved regions among the genes belonging to the nah-like and dnt/ntd groups. In this regard, it was not possible to target the less related phn-type dioxygenases.
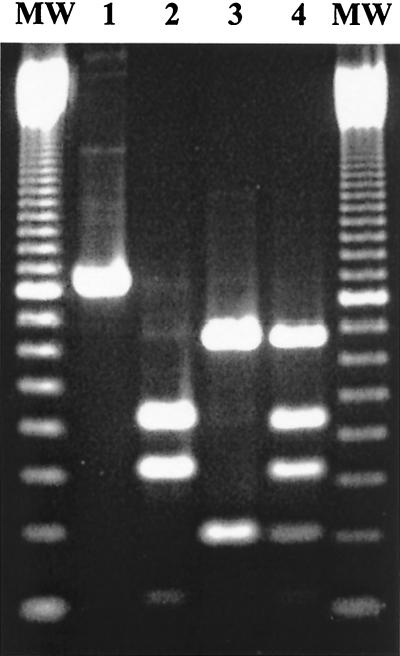
Primers were used on 66 naphthalene-degrading Pseudomonas strains isolated from the western Mediterranean region. These strains had been isolated in our laboratory over the last 20 years and were able to grow with naphthalene as the unique energy and carbon source (Table 1). A single PCR fragment of the predicted size (866 bp) was amplified in all of them (Fig. 1, lane 1). In order to evaluate the genetic diversity of the naphthalene dioxygenase genes, PCR fragments were digested with the restriction enzyme HaeIII. These strains clustered in three groups. The first group consisted of a nah-like AN10 group, with a restriction pattern similar to nahAc from P. stutzeri AN10 (4) and pahA3 from P. aeruginosa PaK1 (Fig. 1, line 3) (35 strains, 53% of the total). The second group was the nah-like C18 group, with a pattern similar to doxB from Pseudomonas sp. strain C18 (6) and nahAc from P. putida G7 (25) (Fig. 1, line 2) (25 strains, 38% of the total). The third group was composed of six strains (9% of the total) with identical restriction patterns (Fig. 1, lane 4), suggesting by the sum of the resulting fragments the coexistence of both AN10 and C18 naphthalene dioxygenase genes in each of these strains.
TABLE 1.
Naphthalene degradation strains used for this study
| Presence (+) or absence (−) of key physiologic markersa | nahActypeb | Strain(s)c | Origin, date of isolation (yr)d | Source or reference | |||
|---|---|---|---|---|---|---|---|
| GEL | STA | NAR | ARG | ||||
| − | + | + | − | AN10 | AN10, AN11 | Marine isolate, Barcelona, 1982 | 9 |
| LSMN2, ST27MN2, ST27MN3, LS401 | Marine isolate, Barcelona, 1988 | 21 | |||||
| SP1402 | Wastewater, Mallorca, 1988 | 21 | |||||
| B1SMN1, B2SMN, S1MN1 | Wastewater, Menorca, 1988 | 21 | |||||
| − | + | + | + | AN10 | LSMN7, ST27MN1, ST27401, ST27402, ST27403, 19SDN3, 19SMN5, 19SMN6 | Marine isolate, Barcelona, 1988 | 20 |
| + | + | + | + | AN10 | LSMN3 | Marine isolate, Barcelona, 1988 | 20 |
| − | − | + | − | AN10 | SP401 | Wastewater, Mallorca, 1988 | 20 |
| 19SDN2 | Marine isolate, Barcelona, 1988 | 20 | |||||
| + | − | + | + | AN10 | 5NH(5a), 5IIDSal, 9NH, 16NH, 16IDSal, 23IIIASal | Marine isolate, Barcelona, 1987 | 8 |
| LS402 | Marine isolate, Barcelona, 1988 | 20 | |||||
| SP1403 | Wastewater, Mallorca, 1988 | 20 | |||||
| + | − | + | + | C18 | 8IDINH, 14IDNH, 18ID2NH, 32IINH | Marine isolate, Barcelona, 1987 | 8 |
| − | − | − | − | AN10 | S1MN3 | Wastewater, Menorca, 1988 | 20 |
| − | − | − | + | AN10 | 3IIIA2NH, 4IASal, 6IIANH, 11IIIDNH | Marine isolate, Barcelona, 1987 | 8 |
| LSMN6 | Marine isolate, Barcelona, 1988 | 20 | |||||
| − | − | − | + | C18 | 1IIA2NH, 3IA2NH, 8IDNH, 15IDINH, 15ID2NH, 15IIID, 17IIDNH, 19IIDNH, 23IDNH, 24INH, 26INH, 27INH, 28I2NH, 30INH, 33IINH, 34JB, 34INH, 34IINH, | Marine isolate, Barcelona, 1987 | 8 |
| PR1MN1, PR2MN1, PR3MN2 | Wastewater, Mallorca, 1988 | 20 | |||||
| − | − | − | + | AN10 + C18 | 2IDINH, 5IIIASal, 7IIANH, 10IDINH, 11NH | Marine isolate, Barcelona, 1987 | 8 |
| S1MN2 | Wastewater, Menorca, 1988 | 20 | |||||
All strains were identified as members of the genus Pseudomonas, being catalase-positive, motile, gram-negative, rod-shaped bacteria with a strict aerobic respiratory metabolism (18). Supplementary key physiologic markers were as follows: GEL, hydrolysis of gelatin; STA, hydrolysis of starch; NAR, denitrification capability; and ARG, presence of arginine hydrolase.
Classification was performed according to the HaeIII restriction pattern observed in Fig. 2.
Strains in boldface were selected as representative strains and were used for 16S rRNA and nahAc-like phylogenetic studies. Single-underlined strains had been previously identified as P. stutzeri (21, 22). Double-underlined strains had been previously identified as P. balearica (3).
That is, Barcelona, Spain, Mallorca, Spain, and Menorca, Spain.
FIG. 1.
Agarose gel showing the HaeIII restriction products of the intra-nahAc amplicon. Lanes: 1, nondigested 866-bp intra-nahAc PCR product from P. stutzeri AN10; 2, digested PCR product from Pseudomonas sp. strain C18; 3, digested PCR product from P. stutzeri strain AN10; 4, digested PCR product from Pseudomonas sp. strain 5IIIASal. MW, 100-bp molecular mass marker (Gibco-BRL).
Grouping and selection of representative strains
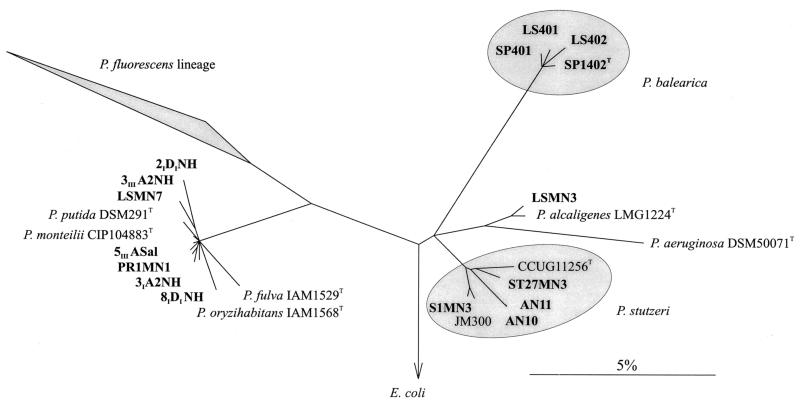
In order to select a reduced number of strains to be further studied, strains were grouped according to several key physiological characteristics that are discriminative among true pseudomonads (18). The results were compared with the nahAc restriction pattern type, and a tentative strain grouping was achieved (Table 1). One or two representatives in each group were selected, and their 16S rRNA was sequenced (indicated in boldface in Table 1). Almost complete sequences of strains 5IIIASal, 3IIIA2NH, 3IA2NH, PR1MN1, and 2ID1NH were aligned and their phylogeny reconstructed by using the current database of ca. 20.000 aligned sequences (17). Once a consensus tree was drawn (Fig. 2), aligned partial sequences of strains LSMN3, LSMN7, 8IDINH, S1MN3, LS402, and SP401 were added to this tree by using the parsimony tool of the ARB program (26) to determine their affiliation. As shown in Fig. 2, new sequences were affiliated with four different branches within the true members of the genus Pseudomonas. The phylogenetic affiliation did not match with the groups made after physiological similarities, showing a relatively poor value of the key characteristics chosen. Most of them (7 of 11) were affiliated with the P. putida-P. monteilii branch; thus, we could not assign them to any of the known species because of the extremely close relationships among the sequences of the members of this branch. One sequence, that of LSMN3, could be affiliated with P. alcaligenes. One sequence, that of S1MN3, could be affiliated with genomovar 8 of P. stutzeri, and two sequences, those of SP401 and LS402, could be affiliated with P. balearica.
FIG. 2.
16S rRNA-based tree reflecting the phylogenetic affiliation of the sequenced strains. The tree is based on the results of a parsimony analysis that included only complete or nearly complete 16S rRNA sequences of representative bacteria (17). The topology of the tree was corrected as described by Ludwig et al. (16). Multifurcations indicate where a topology could not be resolved unambiguously. The phylogenetic positions of partial sequences resulted from the insertion of the aligned sequences into the tree by using the parsimony ARB tool (26) without modifying its topology during the sequence positioning. Group names indicate the most representative organism of the phylogenetic branch. The bar indicates 5% estimated sequence divergence. Representative sequences shown in the tree and their accession numbers were as follows: P. putida DSM291T (Z76667), P. monteilii CIP104883T (AB021409), P. fulva IAM1529T (D84015), P. oryzihabitans IAM1568T (D84004), P. alcaligenes LMG1224T (Z76653), P. aeruginosa DSM50071 (X06684), P. balearica SP1402T (U26418), P. balearica LS401 (U26417), P. stutzeri CCUG11256T (U26262), P. stutzeri ST27MN3 (U26419), P. stutzeri AN11 (U25280), P. stutzeri AN10 (U22427), and P. stutzeri JM300 (X98607). The sequences were submitted to EMBL as follows (with the accession numbers in parentheses): Pseudomonas sp. strain 2IDINH (AF307868), Pseudomonas sp. strain 3IA2NH (AF307866), Pseudomonas sp. strain 3IIIA2NH (AF307865), Pseudomonas sp. strain LSMN7 (AF307871), Pseudomonas sp. strain 8IDINH (AF307872), Pseudomonas sp. strain 5IIIASal (AF307864), Pseudomonas sp. strain PR1MN1 (AF307867), P. alcaligenes LSMN3 (AF307870), P. stutzeri S1MN3 (AF307873), P. balearica LS402 (AF307874), and P. balearica SP401 (AF307875).
nahAc gene fragment analysis
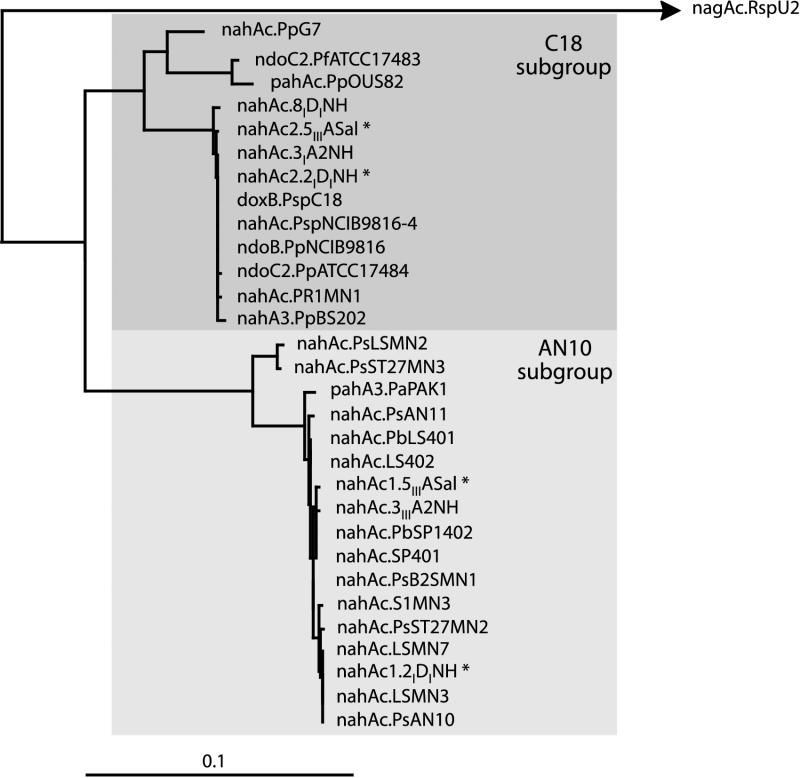
The internal nahAc gene fragments from all selected strains (Table 1) were cloned and sequenced. The nucleotide sequences were aligned, and their phylogenies were reconstructed by using the neighbor-joining algorithm (Fig. 3). All nahAc-like sequences were affiliated with both AN10 and C18 branches. This confirmed the previously observed clustering observed by amplification and restriction analysis.
FIG. 3.
Phylogenetic relationships between the intra-nahAc fragments obtained from the selected naphthalene-degrading strains and from strains retrieved from databases. The relative evolutionary distances were determined by using the program DNADist from the PHYLIPS package program. The tree has been drawn by using the program DrawTree from the same package based on pairwise similarity scores. The bar indicates 10% estimated sequence divergence. The name and GenBank-EMBL accession number of genes noted in plain characters are described in the text. The naphthalene-degrading strains analyzed in this study are noted in boldface type, with their names and nahAc-like gene GenBank-EMBL accession numbers (in parentheses) as follows: Pseudomonas sp. strain 8IDINH (AF306433), Pseudomonas sp. strain 3IA2NH (AF306436), Pseudomonas sp. strain PR1MN1 (AF306432), P. stutzeri LSMN2 (AF306423), P. stutzeri ST27MN3 (AF306425), P. stutzeri AN11 (AF306419), P. balearica LS401 (AF306420), P. balearica LS402 (AF306429), Pseudomonas sp. strain 3IIIA2NH (AF306430), P. balearica SP1402T (AF306421), P. balearica SP401 (AF306428), P. stutzeri B2SMN1 (AF306424), P. stutzeri S1MN3 (AF306435), P. stutzeri ST27MN2 (AF306422), Pseudomonas sp. LSMN7 (AF306431), P. alcaligenes LSMN3 (AF306427), Pseudomonas sp. strain 2IDINH (AF306437 [nahAc1] and AF306438 [nahAc2]), Pseudomonas sp. strain 5IIIASal (AF306439 [nahAc1] and AF306440 [nahAc2]). Asterisks denote strains containing two nahAc alleles.
Upon comparing reconstructed phylogenies of the 16S rRNA gene and nahAc (Fig. 2 and 3, respectively) we found that (i) marine bacteria with different affiliations (i.e., P. stutzeri ST27MN2, P. alcaligenes LSMN3, Pseudomonas sp. strain LSMN7, and P. balearica LS401) but isolated from the same geographical location and at the same time (i.e., Barcelona, Spain, in 1988) harbored nearly identical nahAc genes (AN10 type) and (ii) marine bacteria with a common origin (Barcelona, Spain, in 1987) such as those affiliated with the P. putida-P. monteilii branch may harbor one of the two nahAc type genes (strain 3IIIA2NH, AN10 type, and strain 8IDINH, C18 type). Additionally, all strains with the complex nahAc restriction pattern (Fig. 1, lane 4) were confirmed to harbor both types of genes. Thus, it can be concluded that in the western Mediterranean region at least two distinct naphthalene dioxygenase genes can be found and may coexist within the same host strain. This might also be true for the rest of the naphthalene degradation upper-pathway genes.
Coexistence of other nah upper-pathway genes
In order to study the coexistence of two copies of other nah upper-pathway genes, sequences for the nahB-, nahC-, and nahE-like genes from Pseudomonas sp. strain C18 (doxE, doxG, and doxI [GenBank accession number M60405]), P. stutzeri AN10 (nahB, nahC, and nahE [AF039533]), P. putida OUS82 (pahB, pahC, and pahE [AB004059]), P. putida G7 (nahB, nahC, and nahE [AF125184, J04994, and U09057, respectively]), P. aeruginosa PaK1 (pahB, pahC, and pahE [D84146]), and P. putida BS202 (nahB, nahC, and nahE [AF010471]) were aligned, and amplification primers were designed for the conserved regions. The primers and expected PCR products were as follows: B6f (5"-CAATCAACAAGTCGTTTC-3") and B778r (5"-ACTTGCGACCGAGCG-3") were used to amplify an internal nahB-like fragment of 773 bp, C118f (5"-GAGAAGGACCGTTTCTATC-3") and C814r (5"-CACCTCGCCAGCCGGG-3") were used to amplify an internal nahC-like fragment of 697 bp, and E207f (5"-CGCYACGTTGACCTGGG-3") and E826r (5"-CCGAAAAGTCGCCACGC-3") were used to amplify an internal nahE-like fragment of 620 bp. Degeneracy in primer E207f was needed to accommodate all nahE-like genes. The primers were used on all strains harboring two distinct nahAc-like genes. A single PCR product, equal in size to the predicted amplicons, was amplified in all of them. The PCR products were restricted with TaqI, AluI, or HaeIII endonucleases and, in all cases, their restriction pattern suggested the presence of two distinct copies of each nahB, nahC, and nahE gene (results not shown). Pseudomonas sp. strain 5IIIASal was selected to further clone and sequence the internal nahB-, nahC-, and nahE-like fragments. Sequence alignments confirmed the presence of two plausible functional copies for all genes. One of the alleles for each gene (nahB1, nahC1, and nahE1) was closer in sequence to its nah homologous gene present in P. stutzeri AN10 (4), while the other alleles (nahB2, nahC2, and nahE2) showed greater identity to their relative in the dox pathway of Pseudomonas sp. strain C18 (6) (nahB1 of Pseudomonas sp. strain 5IIIASal [AF320638], 99.2% identity to nahB, 86.9% identity to doxE; nahB2 [AF320639], 89.9% identity to nahB, 96.9% identity to doxE; nahC1 [AF320640], 98.5% identity to nahC, 85.5% identity to doxG; nahC2 [AF320641], 85.7% identity to nahC, 99.7% identity to doxG; nahE1 [AF320642], 95.9% identity to nahE, 94.8% identity to doxI; nahE2 [AF320643], 90.4% identity to nahE, 100.0% identity to doxI). Thus, strains harboring two distinct nahAc-like genes may also have two copies of all naphthalene-degradation upper pathway genes: one homologous to the nah genes from P. stutzeri AN10 and another homologous to the ones present in Pseudomonas sp. strain C18.
In summary, our results reveal that closely related naphthalene-degrading bacteria (Pseudomonas spp.) isolated from the western Mediterranean region may independently harbor two distinct nahAc-type genes: the AN10 and the C18 types (Fig. 3). Furthermore, we have shown that, in some cases, both naphthalene dioxygenase-encoding genes and other nah upper pathway genes coexist in the same host strain. Since assimilation of polyaromatic hydrocarbons such as naphthalene and its derivatives is due to the combined action of several enzymes and not only to the action of a unique enzyme, the initial dioxygenase, plausible recombination events between homologous but distinct gene copies could result in new hybrid alleles of these genes. The corresponding gene products might show small differences in their amino acid sequence that could improve the degradation of naphthalene (and its derivatives), thereby conferring a selective advantage on these strains. In this sense, strains harboring these two nearly identical but distinct copies of the nah upper-pathway genes, such as Pseudomonas sp. strain 5IIIASal and Pseudomonas sp. strain 2IDINH, can be excellent models for studying the role of natural recombination between homologous catabolic pathways in accelerating their evolution and improving their biochemical capabilities. Thus, experiments to improve naphthalene degradation capabilities and analysis of their resulting nah upper-pathway gene combinations are called for.
Acknowledgments
This work was supported by grants BIO97-0639 and COO1999-AX108 (Spanish CICYT). R. Bosch, J. Lalucat, and R. Rosselló-Mora were also supported by the program Acciones Integradas HA1999-0013 (CICYT-DAAD). A postdoctoral fellowship from CONICET (Argentina) to M. Ferrero is gratefully acknowledged.
REFERENCES
- 1.Aemprapa, S., and P. A. Williams. 1998. Implications of the xylQ gene of TOL plasmid pWW102 for the evolution of aromatic catabolic pathways. Microbiology 144:1387-1396. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin, R. C., J. A. Voss, and D. A. Kunz. 1991. Nucleotide sequence of xylE from the TOL pDK1 plasmid and structural comparison with isofunctional catechol-2,3-dioxygenase genes from TOL pWW0 and NAH7. J. Bacteriol. 173:2724-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennasar, A., R. Rosselló-Mora, J. Lalucat, and E. R. B. Moore. 1996. 16S rRNA sequence analysis relative to genomovars of Pseudomonas stutzeri and proposal of Pseudomonas balearica sp. nov. Int. J. Syst. Bacteriol. 46:200-205. [DOI] [PubMed] [Google Scholar]
- 4.Bosch, R., E. García-Valdés, and E. R. B. Moore. 1999. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper-pathway from Pseudomonas stutzeri AN10. Gene 236:149-157. [DOI] [PubMed] [Google Scholar]
- 5.Cane, P. A., and P. A. Williams. 1986. A restriction map of naphthalene catabolic plasmid pWW60-1 and the location of some of its catabolic genes. J. Gen. Microbiol. 132:2919-2929. [Google Scholar]
- 6.Denome, S. A., D. C. Stanley, E. S. Olson, and K. D. Young. 1993. Metabolism of dibenzothiophene and naphthalene in Pseudomonas strains: complete DNA sequence of an upper naphthalene catabolic pathway. J. Bacteriol. 175:6890-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuenmayor, S. L., M. Wild, A. L. Boyes, and P. A. Williams. 1998. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J. Bacteriol. 180:2522-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Valdés, E. 1987. Ph.D. thesis. Universitat de les Illes Balears, Palma de Mallorca, Spain.
- 9.García-Valdés, E., E. Cózar, R. Rotger, J. Lalucat, and J. Ursing. 1988. New naphthalene-degrading marine Pseudomonas strains. Appl. Environ. Microbiol. 54:2478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harayama, S., and M. Rekik. 1993. Comparison of the nucleotide sequences of the meta-cleavage pathway genes of TOL plasmid pWW0 from Pseudomonas putida with other meta-cleavage genes suggests that both single and multiple nucleotide substitutions contribute to enzyme evolution. Mol. Gen. Genet. 239:81-89. [DOI] [PubMed] [Google Scholar]
- 11.Harayama, S., M. Rekik, A. Wasserfallen, and A. Bairoch. 1987. Evolutionary relationships between catabolic pathways for aromatics: conservation of gene order and nucleotide sequences of catechol oxidation genes of pWW0 and NAH7 plasmids. Mol. Gen. Genet. 210:241-247. [DOI] [PubMed] [Google Scholar]
- 12.Jeffrey, A. M., A. J. C. Yeh, D. M. Jerina, T. R. Patel, J. F. Davey, and D. T. Gibson. 1984. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry 14:575-583. [DOI] [PubMed] [Google Scholar]
- 13.Kauppi, B., K. Lee, E. Carredano, R. E. Parales, D. T. Gibson, H. Eklund, and S. Ramaswamy. 1998. Structure of an aromatic ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure 6:571-586. [DOI] [PubMed] [Google Scholar]
- 14.Laurie, A. D., and G. Lloyd-Jones. 1999. The phn genes of Burkholderia sp. strain RP007 constitute a divergent gene cluster for polycyclic aromatic hydrocarbon catabolism. J. Bacteriol. 181:531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Jones, G., A. D. Laurie, D. W. F. Hunter, and R. Fraser. 1999. Analysis of catabolic genes for naphthalene and phenanthrene degradation in contaminated New Zealand soils. FEMS Microbiol. Ecol. 29:69-79. [Google Scholar]
- 16.Ludwig, W., O. Strunk, S. Klugbauer, N. Klugbauer, M. Weizenegger, J. Neumaier, M. Bachleitner, and K.-H. Schleifer. 1998. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554-568. [DOI] [PubMed] [Google Scholar]
- 17.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, J. M. Stredwick, G. M. Garrity, B. Li, G. J. Olsen, S. Pramanik, T. M. Schmidt, and J. M. Tiedje. 2000. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 28:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palleroni, N. J. 1984. Genus I Pseudomonas, p. 141-199. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md. [Google Scholar]
- 19.Platt, A., V. Shingler, S. C. Taylor, and P. A. Williams. 1995. The 4-hydroxy-2-oxovalerate aldolase and acetaldehyde dehydrogenase (acylating) encoded by the nahM and nahO genes of the naphthalene catabolic plasmid pWW60-22 provide further evidence of conservation of meta-cleavage pathway gene sequences. Microbiology 141:2223-2233. [DOI] [PubMed] [Google Scholar]
- 20.Rosselló, R. 1992. Ph.D. thesis. Universitat de les Illes Balears, Palma de Mallorca, Spain.
- 21.Rossello, R., E. García-Valdés, J. Lalucat, and J. Ursing. 1991. Genotypic and phenotypic diversity of Pseudomonas stutzeri. Syst. Appl. Microbiol. 14:150-157. [Google Scholar]
- 22.Rosselló-Mora, R. A., J. Lalucat, and E. García-Valdés. 1994. Comparative biochemical and genetic analysis of naphthalene degradation among Pseudomonas stutzeri strains. Appl. Environ. Microbiol. 60:966-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schell, M. A., and P. E. Wender. 1986. Identification of the nahR gene product and nucleotide sequences required for its activation of the sal operon. J. Bacteriol. 166:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sentchilo, V. S., A. N. Perebituk, A. J. B. Zehnder, and, J. R. van der Meer. 2000. Molecular diversity of plasmids bearing genes that encode toluene and xylene metabolism in Pseudomonas strains isolated from different contaminated sites in Belarus. Appl. Environ. Microbiol. 66:2842-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon, M. J., T. D. Osslund, R. Saunders, B. D. Ensley, S. Suggs, A. Harcourt, W. Suen, D. L. Cruden, D. T. Gibson, and G. J. Zylstra. 1993. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB9816-4. Gene 127:31-37. [DOI] [PubMed] [Google Scholar]
- 26.Strunk, O., and W. Ludwig. 1998. ARB: a software environment for sequence data. Department of Microbiology, Technische Universität München, Munich, Germany.
- 27.Takizawa, N., N. Kaida, S. Torigoe, T. Moritani, T. Sawada, S. Satoh, and H. Kiyohara. 1994. Identification and characterization of genes encoding polycyclic aromatic hydrocarbon dioxygenase and polycyclic aromatic hydrocarbon dihydrodiol dehydrogenase in Pseudomonas putida OUS82. J. Bacteriol. 176:2444-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams, P. A., S. J. Assinder, P. de Marco, K. J. O'Donell, C. L. Poh, L. E. Shaw, and M. K. Winson. 1992. Catabolic gene duplications in TOL plasmids, p. 341-352. In E. Galli, S. Silver, and B. Witholt (ed.), Pseudomonas: molecular biology and biotechnology. American Society for Microbiology, Washington, D.C.
- 29.Yen, K.-M., and I. C. Gunsalus. 1982. Plasmid gene organization: naphthalene/salicylate oxidation. Proc. Natl. Acad. Sci. USA 79:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]