Abstract
Competitive exclusion of Salmonella enterica serovar Enteritidis by a mixed culture of Lactobacillus crispatus and Clostridium lactatifermentans was studied in a sequencing fed-batch reactor mimicking the cecal ecophysiology of broiler chickens. Growth of serovar Enteritidis was inhibited by a mixed culture of L. crispatus and C. lactatifermentans at pH 5.8 but not by a monoculture of L. crispatus at the same pH. Moreover, experiments performed at pH 7.0 did not show growth inhibition of serovar Enteritidis. L. crispatus fermented lactose to lactate, and C. lactatifermentans fermented the lactate to acetate and propionate in a mixed culture of L. crispatus and C. lactatifermentans growing on lactose. In contrast, only lactate was produced from lactose by a monoculture of L. crispatus. At pH 5.8 considerable concentrations of acetate and propionate were present as undissociated acids, whereas only trace levels of undissociated lactate were present at pH 5.8 due to the low pKa of lactate. At pH 7.0 all three acids were present in their dissociated forms. We conclude that a mixed culture of L. crispatus and C. lactatifermentans inhibits growth of serovar Enteritidis under cecal growth conditions. The undissociated forms of acetate and propionate produced in the mixed culture inhibited the growth of serovar Enteritidis.
Salmonella enterica serovar Enteritidis has been reported to be an important cause of human salmonellosis in western countries, including the United States and The Netherlands (1, 26). Chicken and chicken products are the main reservoirs for human serovar Enteritidis infections. Young broiler chickens are especially susceptible to Salmonella infection (7, 9), probably because of the lack of a protective microflora in their gastrointestinal tracts. It was observed previously that 1-day-old broilers that were treated with a cecal microflora from Salmonella-free adult chickens were protected against colonization of their ceca by Salmonella (20). Since these observations were made, scientists have tried to develop a defined mixture of cecal bacteria that provide the same protective effect (25). Only mixtures containing large numbers of cecal bacterial species (25 to 35 species) provided protection similar to that provided by undefined cecal cultures (6, 25). In contrast, treatment with defined mixtures containing low numbers of bacterial species (one to three species) did not result in reduction of numbers of Salmonella cells in the ceca of chickens (24, 25) or resulted in only slight reduction (21).
Defined protective bacterial mixtures have always been developed empirically because the mechanism behind the reduction in Salmonella numbers resulting from the protective microflora is unknown. Correlation studies have indicated that volatile fatty acids, particularly propionic acid, might be important in the reduction of Salmonella viable counts (19, 32). However, it is not known whether the relationship between volatile fatty acids and reduction in numbers of salmonellae is causal or coincidental. Recently, using a sequencing fed-batch reactor mimicking cecal growth conditions, we have shown that volatile fatty acids are indeed responsible for the reduction in serovar Enteritidis biomass (27). Therefore, attention can be directed towards the development of a defined mixture containing a few bacterial species which are capable of increasing volatile fatty acid concentrations in the ceca of young chickens. Lactose can play an important role in achieving this goal. Lactose added to the feed or water of chickens can reach the ceca (15), because chickens have only a trace of lactase activity in their intestinal tissues (22, 23). In the cecum, lactose is fermented by the microflora (15).
Therefore, it would be particularly interesting to select cecal bacteria on the basis of their capacity to ferment lactose, alone or in a mixed culture, to acetate, propionate, or butyrate. For the study described in this paper, two bacteria were isolated from the ceca of chickens. These two bacteria were a Lactobacillus crispatus strain capable of fermenting lactose to lactate and a Clostridium lactatifermentans strain capable of fermenting lactate to acetate and propionate. Competitive exclusion of serovar Enteritidis was studied when this Salmonella serovar was grown in mixed cultures with L. crispatus and C. lactatifermentans. The objective was to study the effect of this mixed culture on the growth of serovar Enteritidis. Furthermore, the mechanism behind changes in the growth of serovar Enteritidis was determined. The experiments were performed in a sequencing fed-batch reactor (27). The ceca of chickens are filled continuously with material from the ileum and empty every 12 to 24 h (5). Furthermore, cecal contents are anoxic, remain at pH 5.8 (28), and have the body temperature of chickens (41°C). These conditions were mimicked in the sequencing fed-batch reactor (27).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. enterica serovar Enteritidis strain CVI-1 (phage type 4) was originally isolated from chickens (30). L. crispatus strain C33 was isolated in our lab from the cecal contents of a 28-day-old chicken and was typed on the basis of its 16S ribosomal DNA sequence (1,527 bp; 99.8% sequence similarity). C. lactatifermentans strain G17 (= DSM 14214) was isolated from the cecal contents of a 31-day-old chicken (29). A mineral carbonate-buffered medium described by van der Wielen et al. (27) was used; this medium was slightly modified by adding (per liter of Milli-Q water) 0.75 g of yeast extract and 1.5 ml of Tween 80. Lactose and arabinose were added from filter-sterilized stock solutions to obtain final concentrations of 9 and 30 mM, respectively. This medium was used to test growth of the three bacterial strains on lactose, arabinose, and lactate, for overnight cultures, and for the sequencing fed-batch reactor. Cecal growth conditions were mimicked in a sequencing fed-batch reactor, which was described by van der Wielen et al. (27). Briefly, medium was continuously pumped into the culture vessel. Every 12 h the volume of the medium in the culture vessel was reduced from 500 to 100 ml. The pH of the culture vessel was kept at 5.8 ± 0.1 with 1 N NaOH and 1 N HCl, the temperature was kept at 41°C, and the conditions were kept anaerobic by flushing the headspace of the culture vessel with nitrogen-carbon dioxide (80:20) at a flow rate of 4.2 liters h−1.
Experiments.
Five different experiments were conducted in the sequencing fed-batch reactor to study the competitive exclusion of serovar Enteritidis by L. crispatus and C. lactatifermentans. In the first experiment the sequencing fed-batch reactor was inoculated with an overnight culture of L. crispatus and C. lactatifermentans. Transient states were obtained 24 h after lactose became the substrate-limiting nutrient for L. crispatus. Subsequently, after the volume of medium in the sequencing fed-batch reactor was reduced to 100 ml, 50 ml of the transient-state culture of L. crispatus and C. lactatifermentans was replaced with 50 ml of an overnight culture of serovar Enteritidis. The second experiment was quite similar to the first experiment, except that serovar Enteritidis was in a transient state with arabinose as the substrate-limiting nutrient. In addition, 50 ml of the transient-state culture of serovar Enteritidis was replaced with 25 ml of an overnight culture of L. crispatus and 25 ml of an overnight culture of C. lactatifermentans. In the third experiment, L. crispatus grown as a monoculture was in a transient state with lactose as the substrate-limiting nutrient. As in the first experiment, 50 ml of the 100-ml transient-state culture was replaced with 50 ml of an overnight culture of serovar Enteritidis. The conditions of the fourth experiment were the same as those of the first experiment, except that the lactose concentration was doubled to 18 mM. The last experiment was also similar to the first experiment, except that the pH was kept constant at 7.0 ± 0.1 instead of 5.8 ± 0.1. In all experiments the cultures were sampled intensively for 72 h. Thereafter samples were taken every 24 h just before the volume in the sequencing fed-batch reactor was decreased from 500 to 100 ml. For all samples the optical density at 600 nm (OD600) was determined immediately, and the remainder of each sample was stored at −20°C pending further analysis.
Analytical procedures.
The biomass of the three strains was estimated by determining the OD600 and by determining the total cell protein content (17). Lactose, arabinose, lactate, acetate, and propionate concentrations were determined by high-performance liquid chromatography. After thawing, 997.5 μl of a sample was acidified with 2.5 μl of a 25% HCl solution and then centrifuged (10 min, 18,500 × g). Seventy microliters of a 0.5 M crotonic acid solution (as an internal standard) was added to 630 μl of supernatant, and the contents of samples were subsequently determined by high-performance liquid chromatography as described previously (28).
RESULTS
Growth tests performed with lactose, arabinose, and lactate showed that L. crispatus could grow only on lactose, serovar Enteritidis could grow only on arabinose, and C. lactatifermentans could grow only on lactate (data not shown). Therefore, each bacterium had its own specific carbon substrate for growth in the sequencing fed-batch reactor. As a result, interactions among the three bacteria could be monitored by determining the fate of the specific substrates (lactose, arabinose, and lactate). OD600 and protein analyses produced similar results; therefore, only OD600 data are used below to describe the total biomass of the three bacteria.
Growth inhibition of serovar Enteritidis.
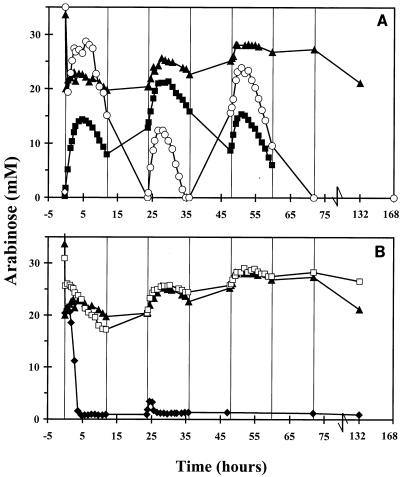
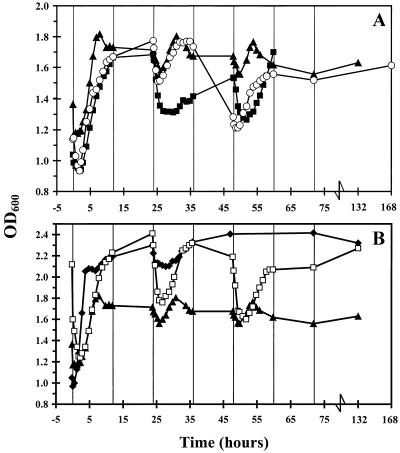
An initial decrease in the arabinose concentration was observed after 50 ml of a transient-state culture of L. crispatus and C. lactatifermentans was replaced with an overnight culture of serovar Enteritidis (at time zero in Fig. 1). This decrease in the arabinose concentration occurred because the remaining 50 ml of the transient-state culture of L. crispatus and C. lactatifermentans, which contained 30 mM arabinose (since these two bacteria cannot use arabinose for growth), was diluted with 50 ml of an overnight culture of serovar Enteritidis in which all of the arabinose had been consumed during growth. After serovar Enteritidis was added to the transient-state culture of L. crispatus and C. lactatifermentans, the concentration of arabinose did not decrease. This suggests that growth of serovar Enteritidis was inhibited (Fig. 1A). Furthermore, the arabinose concentration increased when L. crispatus and C. lactatifermentans were added to a transient-state culture of serovar Enteritidis (Fig. 1A). This indicates that growth of serovar Enteritidis was inhibited by L. crispatus and C. lactatifermentans. However, in the second experiment the inhibition of serovar Enteritidis growth was not as pronounced as in the first experiment. After 36 h the concentration of arabinose decreased (Fig. 1A), and there was a simultaneous increase in the OD600 (Fig. 2A). This indicates that serovar Enteritidis became adapted to the new conditions in the mixed culture in this experiment.
FIG. 1.
Changes in arabinose concentrations in the sequencing fed-batch reactor. Symbols: ▴, serovar Enteritidis added at time zero to a transient-state culture of L. crispatus and C. lactatifermentans growing on 9 mM lactose at pH 5.8; ♦, serovar Enteritidis added at time zero to a transient-state culture of L. crispatus and C. lactatifermentans growing on 9 mM lactose at pH 7.0; □, serovar Enteritidis added at time zero to a transient-state culture of L. crispatus and C. lactatifermentans growing on 18 mM lactose at pH 5.8; ○, serovar Enteritidis added at time zero to a transient-state culture of L. crispatus alone; ▪, L. crisptatus and C. lactatifermentans added at time zero to a transient-state culture of serovar Enteritidis.
FIG. 2.
Changes in OD600 in the sequencing fed-batch reactor. For an explanation of the symbols see the legend to Fig. 1.
To study whether both L. crispatus and C. lactatifermentans are necessary for inhibition of serovar Enteritidis growth, serovar Enteritidis was added to a transient-state culture of L. crispatus alone. Initially, the arabinose concentration decreased, and within 24 h arabinose became the growth-limiting substrate for serovar Enteritidis (Fig. 1A). Simultaneously, the OD600 increased (Fig. 2A). From 36 to 50 h, the arabinose concentration increased, but thereafter the concentration decreased again to zero (Fig. 1A). This indicates that initially no inhibition of serovar Enteritidis growth occurred but that after 36 h the growth of serovar Enteritidis was temporarily inhibited. Overall, the results show that an L. crispatus monoculture was not able to inhibit growth of serovar Enteritidis substantially.
The consumption of arabinose by serovar Enteritidis was not different when L. crispatus and C. lactatifermentans were in a transient state with 9 and 18 mM lactose except after 132 h (Fig. 1B). After 132 h the arabinose concentration remained high with 18 mM lactose, while it decreased slightly with 9 mM lactose. This slight decrease might indicate that growth of serovar Enteritidis was no longer inhibited and that serovar Enteritidis became adapted when L. crispatus and C. lactatifermentans were grown on 9 mM lactose, whereas this was not the case if the two bacteria were grown on 18 mM lactose.
Undissociated acetate and propionate were thought to play a role in the inhibition of serovar Enteritidis growth observed. Therefore, serovar Enteritidis was added to a mixed culture of L. crispatus and C. lactatifermentans growing at pH 7.0. At this pH, only trace amounts of undissociated acetate and propionate were present in the culture vessel. The arabinose concentration decreased rapidly in this experiment at pH 7.0 (Fig. 1B). The decrease was accompanied by an increase in the OD600 (Fig. 2B). This shows that the growth of serovar Enteritidis was not inhibited at neutral pH by L. crispatus and C. lactatifermentans.
Acetate and propionate production.
It has been shown in a previous study that volatile fatty acids play a pivotal role in inhibition of serovar Enteritidis growth (27). Therefore, in the present study the concentrations of volatile fatty acids and lactate in the sequencing fed-batch reactor were determined. We observed that L. crispatus produced lactate from lactose, serovar Enteritidis produced lactate and acetate from arabinose, and C. lactatifermentans produced acetate and propionate from lactate.
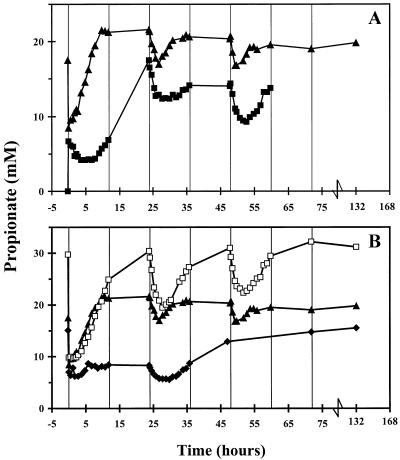
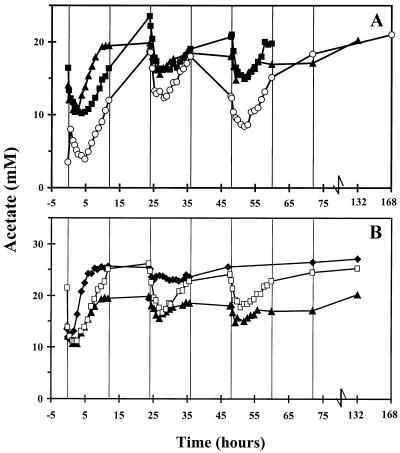
The propionate concentration was lower when L. crispatus and C. lactatifermentans were added to a transient-state culture of serovar Enteritidis than when serovar Enteritidis was added to a coculture of L. crispatus and C. lactatifermentans (Fig. 3A). Moreover, an increase in the concentration of lactose was observed after 24 h (data not shown). This indicates that there was some inhibition of L. crispatus and C. lactatifermentans growth on lactose and hence reduced production of propionate and acetate. However, the acetate concentrations were similar in the two experiments (Fig. 4A). Apparently, the production of acetate by C. lactatifermentans was diminished by acetate production of serovar Enteritidis. A coculture of L. crispatus and serovar Enteritidis resulted in production of acetate (Fig. 4A) and a substantial concentration of lactate (∼45 mM) (data not shown).
FIG. 3.
Changes in total propionate concentrations (undissociated propionate and dissociated propionate) in the sequencing fed-batch reactor. For an explanation of the symbols see the legend to Fig. 1.
FIG. 4.
Changes in total acetate concentrations (undissociated acetate and dissociated acetate) in the sequencing fed-batch reactor. For an explanation of the symbols see the legend to Fig. 1.
The propionate and acetate concentrations were the same after serovar Enteritidis was added to transient-state cultures of L. crispatus and C. lactatifermentans growing on 9 and 18 mM lactose. After 24 h, the acetate and propionate concentrations in the mixed culture growing on 18 mM lactose were higher (Fig. 3B and 4B); this was due to the higher substrate concentration.
The propionate concentration was slightly higher when serovar Enteritidis was added to a transient-state culture of L. crispatus and C. lactatifermentans at pH 5.8 than when serovar Enteritidis was added to a transient-state culture of L. crispatus and C. lactatifermentans at pH 7.0 (Fig. 3B). At pH 7.0, lactate was not completely degraded, which indicates that growth of C. lactatifermentans was inhibited to some extent. This resulted in production of less propionate and acetate by C. lactatifermentans. Still, acetate was produced by serovar Enteritidis at pH 7.0, and this production resulted in high acetate concentrations (Fig. 4B).
DISCUSSION
The results of this study show that growth of serovar Enteritidis in a sequencing fed-batch reactor was inhibited by a mixed culture of L. crispatus and C. lactatifermentans growing anaerobically on lactose at pH 5.8. Growth of L. crispatus alone at pH 5.8 resulted in only slight inhibition of serovar Enteritidis growth. The difference between these two conditions is that growth of L. crispatus alone resulted in production of lactate, whereas the coculture produced mainly acetate and propionate. We found that no inhibition of serovar Enteritidis growth occurred in the sequencing fed-batch reactor at neutral pH. It should be stressed that except for the pH, all conditions in the culture vessel were the same in the two experiments. Still, pH itself was not responsible for the growth inhibition since the initial growth of serovar Enteritidis was not inhibited at pH 5.8 after the organism was added to a transient-state culture of L. crispatus. Acetate, propionate, and lactate can inhibit Escherichia coli and Salmonella growth when they are present as undissociated acids (2-4, 28). The concentration of undissociated acetate, propionate, and lactate can be calculated by using the Henderson-Hasselbach equation [pH = pKa + 10log(A−)/(HA), where A− is the concentration of dissociated acids and HA is the concentration of undissociated acids]. The pKa for propionate is 4.87, the pKa for acetate 4.75, and the pKa for lactate 3.08 (31). It can be calculated that at pH 7.0, 0.7% of the total propionate and 0.55% of the total acetate are present as undissociated acids. In contrast, at pH 5.8, 10.5% of the total propionate and 8.2% of the total acetate are present as undissociated acids. The trace amounts of undissociated acetate and propionate at pH 7.0 did not affect the growth of serovar Enteritidis, while at pH 5.8 the concentrations of undissociated acetate and propionate inhibited serovar Enteritidis growth. Due to the low pKa of lactic acid only trace amounts of undissociated lactate (0.2% of the total lactate) were present when L. crispatus was grown without C. lactatifermentans at pH 5.8. Therefore, no inhibition of serovar Enteritidis growth was observed after serovar Enteritidis was added to a transient-state culture of L. crispatus alone. In conclusion, at pH 5.8 the concentrations of undissociated acetate and propionate produced by C. lactatifermentans (growing on lactate produced by L. crispatus) are responsible for the inhibition of serovar Enteritidis growth.
Inhibition of growth of S. enterica serovar Typhimurium and serovar Enteritidis was also observed with pure cultures of Veillonella sp. growing on lactate and Streptococcus sp. growing on lactose by an agar overlay method (10, 12, 13). In contrast to our results, Veillonella sp. inhibited Salmonella growth only when it produced high concentrations of propionate (>125 mM). Furthermore, a mixed culture of Enterococcus sp. and Veillonella sp. fermenting sugars to acetate and propionate at pH 5.0 under batch conditions did not inhibit serovar Typhimurium growth (8). These findings are in contrast to our findings which showed that relatively low concentrations of propionate (∼20 mM) inhibit growth of serovar Enteritidis. The most important difference between the experiments described in this paper and previous in vitro studies is that in this study we aimed to determine inhibition of serovar Enteritidis growth under cecal ecophysiological conditions. Previous experiments in which this system was used showed that volatile fatty acids representative of the acids in the ceca of broilers during growth were responsible for decreases in the biomass of serovar Enteritidis (27). Furthermore, those experiments showed that results obtained with the sequencing fed-batch reactor were comparable to observations made in vivo. This indicates that the sequencing fed-batch reactor might be a good model for studying bacterial interactions as they could occur in the ceca of broilers. In contrast, other in vitro experiments were not performed under cecal ecophysiological conditions (8, 10, 12, 13).
Results obtained in this study are in agreement with observations made with competitive exclusion cultures in vivo. In this study, inhibition of serovar Enteritidis growth was most dramatic when L. crispatus and C. lactatifermentans were established in the sequencing fed-batch reactor before serovar Enteritidis was added. If competitive exclusion cultures were administered to broiler chickens 2 days before Salmonella was administered, a dramatic reduction in the number of salmonellae was observed. In contrast, when Salmonella was administered to broiler chickens before or within 24 h after the competitive exclusion culture was administered, the reduction in the number of salmonellae was less pronounced (14, 16, 33). The concentration of propionate produced in the reactor by C. lactatifermentans was similar to concentrations that could be found in the ceca after broilers were treated with competitive exclusion cultures (6, 11, 19). The concentrations of acetate produced in the reactor were slightly lower than the concentrations observed in the cecal contents of competitive exclusion culture-treated birds (11). Nisbet and coworkers showed that the effectiveness of a competitive exclusion culture for reducing the viable counts of salmonellae in the ceca of broilers depended upon the production of propionate in the ceca of broilers 2 days after administration of the mixed culture (6, 18, 19). However, those authors concluded that propionate does not have to be responsible for this reduction. They suggested that the greater production of propionate in the ceca of competitive exclusion culture-treated broilers showed that the bacteria administered became established in the ceca of the broilers but that these bacteria could inhibit Salmonella growth by another mechanism.
We have shown for the first time that acetate and propionate produced by two cecal bacterial strains grown on lactose inhibited growth of serovar Enteritidis when these three organisms grew in a mixed culture under cecal ecophysiological conditions. Our results are an important step in the development of defined competitive exclusion mixtures containing low numbers of bacterial strains for which the mechanism of inhibition of Salmonella growth is known.
REFERENCES
- 1.Angulo, F. J., and D. L. Swerdlow. 1999. Epidemiology of human Salmonella enterica serovar Enteritidis infections in the United States, p. 33-41. In A. M. Saeed (ed.), Salmonella enterica serovar Enteritidis in humans and animals. Iowa State University Press, Ames.
- 2.Bearson, S., B. Bearson, and J. W. Foster. 1997. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147:173-180. [DOI] [PubMed] [Google Scholar]
- 3.Cherrington, C. A., M. Hinton, and I. Chopra. 1990. Effects of short-chain organic acids on macromolecular synthesis in Escherichia coli. J. Appl. Bacteriol. 68:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Cherrington, C. A., M. Hinton, G. R. Pearson, and I. Chopra. 1991. Short-chain organic acids at pH 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J. Appl. Bacteriol. 70:161-165. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, P. L. 1979. Coccidial infection with Eimeria tenella and caecal defaecation in chicks. Br. Poult. Sci. 20:317-322. [DOI] [PubMed] [Google Scholar]
- 6.Corrier, D. E., D. J. Nisbet, C. M. Scanlan, A. G. Hollister, and J. R. DeLoach. 1995. Control of Salmonella typhimurium colonization in broiler chicks with a continuous-flow characterized mixed culture of cecal bacteria. Poult. Sci. 74:916-924. [DOI] [PubMed] [Google Scholar]
- 7.Duchet-Suchaux, M., P. Lechopier, J. Marly, P. Bernardet, R. Delaunay, and P. Pardon. 1995. Quantification of experimental Salmonella enteritidis carrier state in B13 leghorn chicks. Avian Dis. 39:796-803. [PubMed] [Google Scholar]
- 8.Durant, J. A., D. J. Nisbet, and S. C. Ricke. 2000. Response of selected poultry cecal probiotic bacteria and a primary poultry Salmonella typhimurium isolate grown with or without glucose in liquid batch culture. J. Environ. Sci. Health B 35:503-516. [DOI] [PubMed] [Google Scholar]
- 9.Gorham, S. L., K. Kadavil, H. Lambert, E. Vaughan, B. Pert, and J. Abel. 1991. Persistence of Salmonella enteritidis in young chickens. Avian Pathol. 20:433-437. [DOI] [PubMed] [Google Scholar]
- 10.Hinton, A., Jr., D. E. Corrier, and J. R. DeLoach. 1992. In vitro inhibition of Salmonella typhimurium and Escherichia coli O157:H7 by an anaerobic gram-positive coccus isolated from the cecal contents of adult chickens. J. Food Prot. 55:162-166. [DOI] [PubMed] [Google Scholar]
- 11.Hinton, A., Jr., D. E. Corrier, G. E. Spates, J. O. Norman, R. L. Ziprin, R. C. Beier, and J. R. DeLoach. 1990. Biological control of Salmonella typhimurium in young chickens. Avian Dis. 34:626-633. [PubMed] [Google Scholar]
- 12.Hinton, A., Jr., and M. E. Hume. 1995. Synergism of lactate and succinate as metabolites utilized by Veillonella to inhibit the growth of Salmonella typhimurium and Salmonella enteritidis in vitro. Avian Dis. 39:309-316. [PubMed] [Google Scholar]
- 13.Hinton, A., Jr., M. E. Hume, and J. R. DeLoach. 1993. Role of metabolic intermediates in the inhibition of Salmonella typhimurium and Salmonella enteritidis by Veillonella. J. Food Prot. 56:932-937. [DOI] [PubMed] [Google Scholar]
- 14.Hume, M. E., D. E. Corrier, D. J. Nisbet, and J. R. DeLoach. 1998. Early Salmonella challenge time and reduction in chick cecal colonization following treatment with a characterized competitive exclusion culture. J. Food Prot. 61:673-676. [DOI] [PubMed] [Google Scholar]
- 15.Hume, M. E., L. F. Kubena, R. C. Beier, A. Hinton, Jr., D. E. Corrier, and J. R. DeLoach. 1992. Fermentation of [14C]lactose in broiler chicks by cecal anaerobes. Poult. Sci. 71:1464-1470. [DOI] [PubMed] [Google Scholar]
- 16.Impey, C. S., G. C. Mead, and M. Hinton. 1987. Influence of continuous challenge via the feed on competitive exclusion of salmonellas from broiler chickens. J. Appl. Bacteriol. 63:139-146. [DOI] [PubMed] [Google Scholar]
- 17.Lowry, O. H., N. H. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 18.Nisbet, D. J., D. E. Corrier, and J. R. DeLoach. 1993. Effect of mixed cecal microflora maintained in continuous culture and of dietary lactose on Salmonella typhimurium colonization in broiler chicks. Avian Dis. 37:528-535. [PubMed] [Google Scholar]
- 19.Nisbet, D. J., D. E. Corrier, S. C. Ricke, M. E. Hume, J. A. Byrd II, and J. R. DeLoach. 1996. Cecal propionic acid as a biological indicator of the early establishment of a microbial ecosystem inhibitory to Salmonella in chicks. Anaerobe 2:345-350. [Google Scholar]
- 20.Nurmi, E., and M. Rantale. 1973. New aspects of Salmonella infection in broiler production. Nature 241:210-211. [DOI] [PubMed] [Google Scholar]
- 21.Pascual, M., M. Hugas, J. I. Badiola, J. M. Monfort, and M. Garriga. 1999. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl. Environ. Microbiol. 65:4981-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddons, R. C. 1969. Intestinal disaccharidase activities in the chick. Biochem. J. 112:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddons, R. C., and M. E. Coates. 1972. The influence of the intestinal microflora on disaccharidase acitivities in the chick. Br. J. Nutr. 27:101-112. [DOI] [PubMed] [Google Scholar]
- 24.Soerjadi, A. S., S. M. Stehman, G. H. Snoeyenbos, O. M. Weinack, and C. F. Smyser. 1981. The influence of lactobacilli on the competitive exclusion of parathypoid salmonellae in chickens. Avian Dis. 25:1027-1033. [PubMed] [Google Scholar]
- 25.Stavric, S. 1992. Defined cultures and prospects. Int. J. Food Microbiol. 15:245-263. [DOI] [PubMed] [Google Scholar]
- 26.van de Giessen, A. W., W. J. van Leeuwen, and W. van Pelt. 1999. Salmonella enterica serovar Enteritidis in the Netherlands: epidemiology, prevention and control, p. 71-80. In A. M. Saeed (ed.), Salmonella enterica serovar Enteritidis in humans and animals. Iowa State University Press, Ames.
- 27.van der Wielen, P. W. J. J., S. Biesterveld, L. J. A. Lipman, and F. van Knapen. 2001. Inhibition of a glucose-limited sequencing fed-batch culture of Salmonella enterica serovar Enteritidis by volatile fatty acids representative of the ceca of broiler chickens. Appl. Environ. Microbiol. 67:1979-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Wielen, P. W. J. J., S. Biesterveld, S. Notermans, H. Hofstra, B. A. P. Urlings, and F. van Knapen. 2000. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 66:2536-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Wielen, P. W. J. J., G. M. L. L. Rovers, J. M. A. Scheepens, and S. Biesterveld. Clostridium lactatifermentans sp. nov., a lactate fermenting anaerobe isolated from the caeca of chickens. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 30.van Zijderveld, F. G., A. M. van Zijderveld-van Bemmel, and J. Anakotta. 1992. Comparison of four different enzyme-linked immunosorbent assays for serological diagnosis of Salmonella enteritidis infections in experimentally infected chicken. J. Clin. Microbiol. 30:2560-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weast, R. C. (ed.). 1971. CRC handbook of chemistry and physics, 52nd ed. Chemical Rubber Co., Cleveland, Ohio.
- 32.Ziprin, R. L., D. E. Corrier, A. Hinton, Jr., R. C. Beier, G. E. Spates, J. R. DeLoach, and M. H. Elissalde. 1990. Intracloacal Salmonella typhimurium infection of broiler chickens: reduction of colonization with anaerobic organisms and dietary lactose. Avian Dis. 34:749-753. [PubMed] [Google Scholar]
- 33.Ziprin, R. L., and J. R. DeLoach. 1993. Comparison of probiotics maintained by in vivo passage through laying hens and broilers. Poult. Sci. 72:628-635. [DOI] [PubMed] [Google Scholar]