Abstract
Due to concerns about a link between variant Creutzfeldt-Jakob disease in humans and similar prion protein-induced disease in cattle, i.e., bovine spongiform encephalopathy (BSE), strict controls are in place to exclude BSE-positive animals and/or specified risk materials including bovine central nervous system (CNS) tissue from the human food chain. However, current slaughter practice, using captive bolt guns, may induce disruption of brain tissues and mobilize CNS tissues into the bovine circulatory system, leading to the dispersion of CNS tissues (including prion proteins) throughout the derived carcass. This project used a marker (antibiotic-resistant) strain of Pseudomonas fluorescens to model the effects of commercial captive bolt stunning procedures on the movement of mobilized CNS material within slaughtered animals and the abattoir environment. The marker organism, introduced by injection through the bolt entry aperture or directly using a cartridge-fired captive bolt, was detected in the slaughter environment immediately after stunning and in the abattoir environment at each subsequent stage of the slaughter-dressing process. The marker organism was also detected on the hands of operatives; on slaughter equipment; and in samples of blood, organs, and musculature of inoculated animals. There were no significant differences between the results obtained by the two inoculation methods (P < 0.05). This study demonstrates that material present in, or introduced into, the CNS of cattle during commercial captive bolt stunning may become widely dispersed across the many animate and inanimate elements of the slaughter-dressing environment and within derived carcasses including meat entering the human food chain.
Physical disruption of the mammalian central nervous system (CNS) can release tissue fragments into the blood system and cause subsequent distribution of these tissues into distal organs and tissues. For example, brain emboli in the lungs have been reported previously for humans following severe head injury (19) or birth trauma (12), as well as for cattle following stunning (2, 9). Such distribution of CNS tissues may have important food safety implications, as it means that brain tissue of stunned cattle infected with the prion responsible for bovine spongiform encephalopathy (BSE) (22, 26) may be distributed into other tissues within the derived carcass.
Soon after the first case of BSE and the discovery of the link between BSE in cattle and variant Creutzfeldt-Jakob disease in humans (5), concerns were expressed about transmission of the implicated prion into the human population by consumption of beef and beef products contaminated with prion containing CNS tissues (3, 8, 25). Thus, slaughter processes were modified to exclude visible intact specified risk material tissues from the food chain (6, 7). Much of this attention focused on preventing prion transfer from contaminated tissue, transfer of brain or spinal cord tissue by saws and tools used during slaughter, inclusion of paraspinal ganglia in cuts of meat (e.g., T-bone steaks), and most importantly the presence of residual spinal cord and paraspinal tissue in the paste of mechanically recovered meat added to various beef products (4).
More recently, concerns have also emerged in relation to the role of animal stunning methods which invade the skull cavity and induce disruption of brain (CNS) tissues. Such methods include pneumatically operated penetrative captive bolt stunning (23) and procedures combining initial cartridge-fired penetrative captive bolt stunning with subsequent pithing (to restrict activities of the autonomic nervous system) (18). As pneumatic-bolt-based systems and bolt stunning followed by pithing have been shown elsewhere to mobilize CNS tissue within the circulatory systems of beef cattle, leading to dissemination of specified risk material into such organs as the lung and spleen, these specific procedures have been banned within the European Union since January 2001 (European Commission Decision, 00/418/EC [http:europa.eu.int/eur-lex/en/lif/dat/2000/en__300D0418.html]). However, cartridge-fired captive bolt stunning without subsequent pithing is still widely used. Concerns remain that such processes may disperse infected brain and spinal cord material within the carcass of BSE-infected cattle and from infected cattle into the immediate stunning area, contaminating slaughterhouse workers, equipment, etc., and directly and indirectly disseminating prions onto meat during subsequent processing.
This study modelled the dispersion of CNS tissues within the internal organs and musculature of cattle and in the abattoir environment by tracking the spread of a marker organism inoculated during and directly after stunning with a cartridge-fired penetrative captive bolt gun.
MATERIALS AND METHODS
Marker organism and inoculum preparation.
A nonpathogenic Pseudomonas fluorescens strain (ATCC 13525) was obtained from the American Type Culture Collection and maintained on Protect beads (Technical Service Consultants Ltd., Lancaster, United Kingdom) at −18°C. This culture, made resistant to 10 μg of nalidixic acid (Sigma) ml−1 by the method of Park (21), was used throughout this investigation. Its antibiotic resistance characteristics were checked by culture on Pseudomonas CFC selective agar plates (CFC) (Pseudomonas agar base, Oxoid CM559, plus Pseudomonas CFC supplement, Oxoid SR103E) containing a final concentration of 10 μg of nalidixic acid ml−1.
The P. fluorescens culture, prepared by incubating one Protect bead in 100 ml of tryptone soya broth (TSB; Oxoid CM129) at 30°C for 24 h, was enumerated using the acridine orange direct count technique described by Kerr et al. (13) and adjusted with maximum recovery diluent (MRD; Oxoid CM733) to contain 10.0 log10 CFU ml−1. This suspension was diluted 1:10 (vol/vol) with MRD to produce inocula containing 6.0 or 9.0 log10 CFU ml−1. One-milliliter volumes of these inocula were used in syringe inoculation experiments. A captive bolt inoculum pellet was prepared by centrifugation at 2,800 × g for 10 min at 4oC (centrifuge 5403; Eppendorf, Hamburg, Germany) of 1 ml of the 9.0-log10-CFU-ml−1 dilution of the marker organism.
Abattoir.
The experimental abattoir used in this study, located at The National Food Centre, was designed for low-throughput slaughter and processing of cattle, sheep, and pigs. The online dressing procedure in the abattoir follows a serpentine design with the major elements of the slaughter and dressing operations being as follows: (i) stunning, (ii) exsanguination, (iii) dehiding, (iv) evisceration, (v) back splitting, and (vi) carcass weighing and washing undertaken in different zones of the abattoir. During this study, the abattoir was cleaned the day before, and immediately after, the slaughter of each inoculated animal. The floors, walls, and stainless steel work surfaces were foamed with a 3% solution of a liquid detergent (Chemical Solutions Ltd.) which was a proprietary blend of surfactants, sodium metasilicate, and caustic soda in an aqueous solution. The foam was left on surfaces for 30 min and then washed off with cold potable water.
Equipment preparation and swabbing.
Knives, scabbards, and steels were sterilized by autoclaving at 121°C for 15 min before use. The saw used to split carcasses was sterilized by being sprayed with 10% sodium hypochlorite (BDH, Poole, United Kingdom). The knife used to take spinal cord samples was sterilized in a water bath at 82°C for 30 s between samples. The operatives' hands were washed with antibacterial soap (Leverline) directly before the slaughter of each animal. Bacteria present on equipment and operatives' hands were sampled using wet and dry swabs (14). Briefly, an MRD-moistened cotton swab was rubbed across the surface to be sampled. The sample was rotated through 90° and swabbed again using the same swab. The handle of the swab was broken off, leaving the swab tip in a 30-ml screw-cap tube containing 10 ml of MRD. The sample area was swabbed again using a second, dry swab. Both swab samples were transported to the laboratory and analyzed. Samples were taken by this method from the captive bolt gun (1 cm2), the exsanguination knife (10 cm2), the carcass splitting saw (40 cm2), and the hands of operatives (20 cm2) before and after slaughter of syringe-inoculated, captive bolt-inoculated, or control animals.
Abattoir surface sampling.
The larger surface areas of the abattoir, such as the floor and walls, were sampled before and after each slaughter event using the inverted bag technique of Lasta et al. (16). Briefly, sterilized polyurethane sponges (10 by 10 by 1 cm) were prepared inside a sterile plastic bag. Samples were taken by grasping the sponge through the bag, inverting the bag over the hand, and rubbing the exposed sponge once over the entire area to be sampled. After sampling, the bag was returned to its original configuration to enclose the sponge, sealed, and transported to the laboratory. Areas sampled by this method included the floor (17 m2), left wall (100 m2), right wall (46 m2), and back wall (115 m2) of the area directly in front of the stunning box where the head of the animal protruded from the stunning box.
Detection of aerosolized, suspended, and/or dispersed bacteria in the abattoir environment.
The abattoir environment was sampled after abattoir cleaning and before, during, and after each slaughter event by timed exposure of plates of CFC agar containing 10 μg of nalidixic acid ml−1. Plates were placed on the floor around the online dressing system at the location of each major slaughter-dressing event, i.e., exsanguination, dehiding, evisceration, and back splitting. Seventy-six CFC sedimentation plates, placed in two concentric rings 0.5 and 0.5 m from the center of each slaughter-dressing location, were exposed to the abattoir environment for 10 min during each slaughter-dressing event. After exposure, these plates were incubated overnight at 30°C and examined for the presence of the marker organism.
Shooting procedures.
Animals were placed in a stunning box and shot at the intersection between two imaginary lines extending from the back of the eye to the contralateral horn bud (1) with a cartridge-fired captive bolt pistol (Cash special 0.22-caliber pistol; Accles and Shelvoke Ltd.). In all, 24 inoculated animals and 3 control animals were used in this study.
Inoculation methods.
In initial experiments the marker organism was introduced to the bovine CNS system by syringe inoculation at 6.0 or 9.0 log10 CFU (experiment 1). Based on those results, subsequent experiments involved introduction of the marker organism by captive bolt inoculation at 9.0 log10 CFU (experiment 2).
Syringe inoculation.
In this procedure, 1 ml of inoculum containing 6.0 or 9.0 log10 CFU was delivered into the brains of cattle (n = 18) through the captive bolt aperture formed in the skull by captive bolt stunning. The inoculum was introduced within 30 to 60 s of stunning, using a sterile 1-ml syringe (Becton Dickinson) with a 16-gauge long-bevel 10-cm sterile needle (Labchem).
Captive bolt inoculation.
In this procedure, an 0.1-ml pellet of inoculum containing 9.0 log10 CFU was delivered into the brain of each of six test animals during slaughter. Each pellet was aseptically placed within a 5-ml solidified plug of tryptone soya agar (Oxoid CM131) formed within the concave end of the bolt of the captive bolt gun and inoculated into the bovine brain using the shooting procedures described above.
Bolt aperture sealing and swabbing.
After inoculation by either of the above methods, the aperture caused by the captive bolt was plugged with a sterile rubber bung (British Standard no. 9). A 5-cm2 surface area of the head of each animal surrounding the plugged aperture was swabbed using wet and dry swabs as described above. The area was sterilized by spraying it with 10% sodium hypochlorite (BDH) and immediately reswabbed.
Inoculated animals and uninoculated control animals were exsanguinated, dehided, eviscerated, split into sides, washed, and weighed according to normal commercial dressing procedures.
Tracking of contamination.
For all inoculated and control animals, movement of the marker organism within the blood and internal organs and contamination of slaughter equipment, abattoir surfaces, and operatives' hands were tracked by collection and analysis of samples as follows.
Blood and tissue collection.
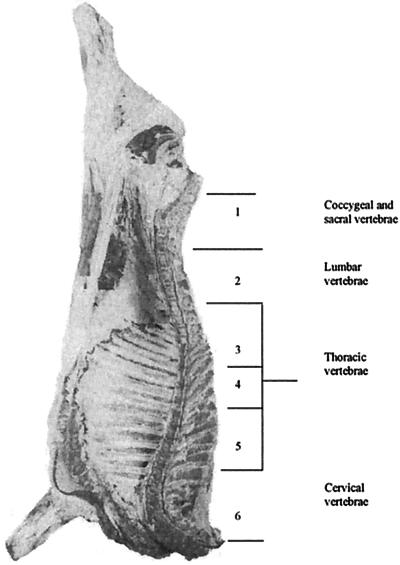
The time interval between stunning and the commencement of exsanguination was between 1 and 2 min. Immediately after the commencement of exsanguination, four 30-ml samples of blood were collected by placing sterile heparinized 30-ml plastic screw-cap tubes (Bibby Sterilin Ltd.) into the blood flow. Each tube filled with blood within 10 s. During carcass dressing a number of tissues and organs were removed, i.e., trapezius cervicis (neck) muscle, lymph node, lungs, spleen, liver, kidney, and spinal cord (see Table 1), and seared by flaming in 70% (vol/vol) alcohol (18); subsamples of spinal cord were taken from six different regions within the vertebral column as outlined in Fig. 1.
TABLE 1.
Tissue type, sample site, total weight of muscle or organ, and total weight of tissue sampled from each animal for determination of the presence of P. fluorescens along with number of plates used and detection limit of the sampling method
| Tissue | Sample site | Total wt (kg) | Total wt sampled (kg) | No. of plates used | Limit of detection (CFU g−1) |
|---|---|---|---|---|---|
| Trapezius cervicis | Neck | 3.00 | 0.40 | 48 | 0.21 |
| Lymph node | Prescapular | 0.03 | 0.03 | 3 | 1.66 |
| Lungs | Left and right | 2.65 | 0.30 | 36 | 0.27 |
| Spleen | Entire | 0.75 | 0.20 | 24 | 0.42 |
| Liver | Entire | 5.90 | 0.30 | 36 | 0.27 |
| Kidney | Left and right | 0.90 | 0.10 | 12 | 0.83 |
| Spinal cord | Entire | 0.37 | 0.03 | 3 | 1.37 |
FIG. 1.
The median sagittal plane of a bovine carcass showing the six sampling sections for the spinal cord and each of the five vertebral divisions.
Blood and tissue sample analysis.
Blood samples were serially diluted (1:10 [vol/vol]) in 9 ml of MRD. One-milliliter aliquots of the bulk suspensions and members of the dilution series were directly spread plated onto CFC plates and dried in a laminar flow hood for 10 min. The plates were incubated for 2 h at 30°C and then overlaid with 0.21 ml of a filter-sterilized stock solution of nalidixic acid at 1 mg ml−1 to achieve a final concentration of 10 μg of nalidixic acid ml−1 within the CFC agar. These plates were incubated for 48 h at 30°C and examined (detection limit, 0.25 CFU ml−1). Undiluted blood samples were enriched by incubation at 30°C overnight, spread plated (0.1-ml aliquots) onto CFC plates containing 10 μg of nalidixic acid ml−1, and incubated overnight at 30°C.
A section of the surface of each organ or muscle was aseptically removed. In each case a 25-g sample of interior tissue was aseptically transferred into sterile stomacher bags (Seward, London, United Kingdom) and stomached for 120 s with 225 ml of TSB. The total weight of tissue sampled from each organ or muscle and the limit of detection of the method used are outlined in Table 1. After direct plating the samples were enriched overnight at 30°C. One-milliliter aliquots of each stomached suspension were directly spread plated in triplicate onto CFC plates and incubated for 48 h at 30°C. The enriched samples were treated as detailed above.
The identity of presumptive isolates of the marker organism was confirmed by biochemical tests (20).
Examination of swab and sponge samples of slaughter equipment, hides, the hands of operatives, and the abattoir environment.
Wet and dry swabs from the captive bolt gun, the exsanguination knife, the carcass splitting saw, the hides of animals, and the hands of operatives were mixed thoroughly by vortexing them for 10 s. The limit of detection for each sample was 5, 0.5, 0.12, 1.0, and 0.25 CFU cm−2, respectively. Sponge samples from the abattoir floor and walls were individually stomached in 100-ml volumes of TSB. One-milliliter aliquots of the bulk swab and sponge suspensions together with 1:10 (vol/vol) dilutions in 9 ml of MRD were directly spread plated in duplicate onto CFC plates containing 10 μg of nalidixic acid ml−1 and examined as described above.
Sedimentation plates.
The dispersal of the marker organism during four stages of carcass dressing, that is, exsanguination, dehiding, evisceration, and carcass splitting, was estimated as the number of CFU recovered on agar plates during exposure for a 10-min period at each stage. These were enumerated as CFU m−2 10 min−1.
Statistical analysis.
Since the log transformation did not satisfactorily normalize the data, the nonparametric Mann-Whitney U test was used to compare the numbers of the marker organism following syringe inoculation of 6.0 and 9.0 log10 CFU among each of the following sample types: the blood and tissues of cattle, the hide of the animal after inoculation, the exsanguination knife, operatives' hands, and the carcass splitting saw. Statistical analysis of the syringe and captive bolt inoculations of 9.0 CFU was carried out using analysis of variance, and all statistical tests for bacterial counts were performed with log transformations. For all data, 0.001 was added to the original values prior to log transformation. The purpose of this was to cater for zero values (in the original data) which could not be log transformed. The latter values became −3.00 on the log scale while no appreciable difference was made to the nonzero values. This allows all data to be reported while in no way biasing the results (10, 11). The statistical package used was Genstat 5 (Rothamsted Experimental Station, Harpenden, United Kingdom).
RESULTS
An antibiotic-resistant mutant of P. fluorescens was not detected by direct plating or enrichment either (i) during swab, sponge, or exposed sedimentation plate analysis of the abattoir environment, equipment, or personnel after initial abattoir cleaning or after abattoir cleaning between slaughter events or (ii) in tissue and blood samples obtained from control animals.
There was no significant difference between the numbers of the marker organism detected in blood samples following syringe inoculation at 6.0 and 9.0 log10 CFU and those detected following syringe and bolt inoculation at 9.0 log10 CFU. Following syringe inoculation the marker organism was detected in the blood at mean values ranging from 0.66 to 8.00 CFU ml−1 at 6.0 log10 CFU and 3.50 to 7.16 CFU ml−1 at 9.0 log10 CFU (Table 2). Following inoculation of 9.0 log10 CFU by both methods the marker organism was detected in the blood at mean values ranging from 0.67 to 1.00 log10 CFU ml−1 following syringe inoculation and −0.88 to 0.12 log10 CFU ml−1 following bolt inoculation (see Table 5).
TABLE 2.
Mean numbers of P. fluorescens organisms from blood, tissue, and equipment and personnel samples following syringe inoculation
| Sample | No. of organismsa for inoculum (log10 CFU)b
|
|
|---|---|---|
| 6.0 | 9.0 | |
| Exsanguination blood, 10 | 0.66 | 7.16 |
| Exsanguination blood, 20 | 1.30 | 5.30 |
| Exsanguination blood, 30 | 8.00 | 3.50 |
| Exsanguination blood, 40 | 3.83 | 4.50 |
| Lymph node | 0.00 | 7.67* |
| Lung (left) | 0.33 | 5.67** |
| Lung (right) | 0.00 | 1.33* |
| Spleen | 19.50 | 12.33 |
| Liver | 0.60 | 14.00*** |
| Kidney | 2.60 | 44.17 |
| Spinal cord section 6 (anterior end) | 0.00 | 6.50** |
| Hide after 10% hypochlorite wash | 125.67 | 141.67 |
| Stick knife after exsanguination | 12.50 | 3.67 |
| Operatives' hands after exsanguination | 0.25 | 0.42 |
| Carcass splitting saw | 7.54 | 2.83 |
Values are shown as CFU per milliliter of blood samples, CFU per gram of tissue samples, and CFU per square centimeter of equipment and personnel samples.
The significance of differences was determined by the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 5.
Blood samples from animals (n = 6) showing the presence of P. fluorescens determined by direct plating or enrichment after syringe or bolt inoculation using a 6.0- or 9.0-log10 CFU P. fluorescens inoculum
| Time from start of exsanguination (s) | Detection method | No. of positive animals/total no. of animals (log10 CFU of bacteria ml−1) for inoculation type and inoculum (log10 CFU)
|
|||||
|---|---|---|---|---|---|---|---|
| Syringe
|
Bolt | ||||||
| 6.0 | 9.0 (expt 1) | 9.0 (expt 2) | |||||
| 10 | Direct plating | 1/6 (−0.18) | 2/6 (0.85) | 3/6 (0.67) | 2/6 (0.00) | ||
| Enrichment | 1/6 | 2/6 | 3/6 | 2/6 | |||
| 20 | Direct plating | 2/6 (0.11) | 2/6 (0.72) | 3/6 (0.79) | 2/6 (−0.88) | ||
| Enrichment | 2/6 | 2/6 | 3/6 | 2/6 | |||
| 30 | Direct plating | 3/6 (0.90) | 2/6 (0.54) | 4/6 (0.76) | 4/6 (0.12) | ||
| Enrichment | 3/6 | 2/6 | 4/6 | 4/6 | |||
| 40 | Direct plating | 1/6 (0.58) | 2/6 (0.65) | 4/6 (1.00) | 2/6 (−0.60) | ||
| Enrichment | 1/6 | 2/6 | 4/6 | 2/6 | |||
The numbers of bacteria detected by direct plating following syringe inoculation at 6.0 log10 CFU in animal tissues such as the lymph node, lungs, spleen, liver, kidney, and spinal cord extended from 0.0 CFU g−1 (that is, below detectable levels [Table 1]) in the lymph node and spinal cord to 19.50 CFU g−1 in the spleen (Table 2). At 9.0 log10 CFU the marker organism was detected by direct plating in tissues at values ranging from 1.33 CFU g−1 in the right lung to 44.17 CFU g−1 in the kidney. There were significant differences among counts in the lymph node, lung, liver, and spinal cord tissue of inoculated animals (Table 2). The numbers of the marker organism in animals inoculated with 9.0 log10 CFU were significantly higher (P < 0.05) than the numbers in equivalent tissues inoculated at 6.0 log10 CFU. There was no significant difference between the numbers of the organism at 6.0 log10 CFU and those at 9.0 log10 CFU in the spleen or kidney.
The numbers of the marker organisms in muscle and organ tissue samples from animals inoculated with 9.0 log10 CFU by syringe or bolt methods are also presented in Table 3. For muscle samples a mean value of −1.11 log10 CFU g−1 was detected by direct plating. There was no significant difference among counts from specific tissue types from animals inoculated by the two methods. However, there were significant differences among the counts obtained from different tissues when analyzed separately, with significantly higher numbers in the liver, kidney, and spleen than in other tissues (P < 0.05).
TABLE 3.
P. fluorescens numbers in tissue samples from animals inoculated at 9.0 log10 CFU by syringe and bolta
| Inoculation method | Log10 CFU of bacteria g of tissue type−1
|
SED (df = 30) | ||||||
|---|---|---|---|---|---|---|---|---|
| Trapezius cervicis (neck) | Lymph node | Lungs | Spleen | Liver | Kidney | Spinal cord | ||
| Syringe | −1.11 | −2.45 | −0.91 | −0.34 | 0.77 | 0.43 | −0.85 | 1.03 |
| Bolt | NS | −2.44 | −0.36 | 0.73 | 1.16 | 0.48 | −1.64 | 0.84 |
| SED (df = 10) | 0.78 | 0.92 | 0.91 | 0.25 | 1.03 | 1.29 | ||
Abbreviations: SED, standard error of differences between means; df, degree of freedom; NS, not sampled.
Table 4 shows the numbers of the marker organism on swab samples taken from equipment, the hides of animals, and personnel following inoculation of cattle by syringe or bolt inoculation. In relation to each specific sample location, no significant differences were observed between the numbers of the organism detected after syringe inoculation and those detected after bolt inoculation. However, significant differences in contamination of operatives' hands, animal hide, and equipment were noted when the data from each inoculation method were analyzed separately. Following syringe inoculation the highest counts were on the hide. The effectiveness of the hypochlorite wash in decontaminating this site was significant following syringe inoculation only (P < 0.01). The bacterium was detected on the knife after exsanguination, the operatives' hands, and the carcass splitting saw at lower levels than on the hide (P < 0.05).
TABLE 4.
P. fluorescens numbers on equipment, personnel, and animal hide following syringe and bolt inoculation at 9.0 log10 CFUa
| Sample type | Log10 CFU of bacteria m−2
|
SED (10 df) | |
|---|---|---|---|
| Syringe inoculation | Bolt inoculation | ||
| Captive bolt gun after stunning | NS | 2.39 | |
| Hide after inoculation | 3.19 | 3.49 | 0.44 |
| Hide after 10% hypochlorite wash | −0.11 | 2.47 | 1.33 |
| Knife after exsanguination | −0.01 | −1.38 | 1.0 |
| Operatives' hands after exsanguination | −0.62 | −0.47 | 1.28 |
| Carcass splitting saw | −0.30 | −0.19 | 0.85 |
| SED (25 df) | 1.17 | 0.83 | |
Abbreviations: SED, standard error of differences between means; df, degree of freedom; NS, not sampled.
Following bolt inoculation there were significant differences among the levels of the marker organism on the captive bolt gun, the knife after exsanguination, the operatives' hands, and the carcass splitting saw (P < 0.01). There were also significant differences among the levels of the organism on the hide after inoculation, the knife after exsanguination, the operatives' hands, and the carcass splitting saw (P < 0.001).
Following syringe inoculation, at the two inoculum levels, there was no significant difference between the numbers of the organism on the animal hide after inoculation, the equipment used, or operatives' hands (Table 2).
The numbers of animals with blood samples showing the presence of the marker organism following inoculation determined by direct plating and enrichment are shown in Table 5. Table 5 also presents the arithmetic mean numbers of the organism in blood at different sampling times, determined by direct plating. When direct plating was used to detect bacteria in the blood of animals inoculated by syringe, the numbers of positive animals were higher at 9.0 than at 6.0 log10 CFU at time 10 and 40 s but were variable between the two inoculum levels at 20 and 30 s. Enriched samples did not increase the numbers of animals showing the presence of the organism in the blood.
Table 6 presents the numbers of animals with each tissue type showing the presence of the marker organism following inoculation determined by direct plating and enrichment. It also presents the arithmetic mean numbers of the marker organism in such tissue determined by direct plating methods. In the majority of samples involving animals inoculated by syringe with 6.0 log10 CFU, the detection method used affected the number of samples found to contain the marker organism. Generally, the organism was detected in more samples by enrichment than by direct plating. For lymph node samples, the choice of enrichment method did not affect the number of samples in which the marker organism was detected. In animals inoculated with 9.0 log10 CFU by both methods, enrichment was not needed for detection of the organism. However, there were some cases in which enrichment was required for detection in all inoculated animals, that is, in the examination of neck muscle, lung tissue, and kidney, following syringe inoculation. For example, direct plating detected the marker organism in only three of six neck muscle samples, while enrichment detected the organism in six of six samples.
TABLE 6.
Tissue samples from animals (n = 6) showing the presence of P. fluorescens determined by direct plating or enrichment after syringe or bolt inoculation using a 6.0- or 9.0-log10 CFU P. fluorescens inoculum
| Sample tissue | Detection method | No. of positive animals/total no. of animals (log10 CFU of bacteria g−1) for inoculation type and inoculum (log10 CFU)a
|
|||||
|---|---|---|---|---|---|---|---|
| Syringe
|
Bolt (9.0) | ||||||
| 6.0 | 9.0 (expt 1) | 9.0 (expt 2) | |||||
| Trapezius cervicis | Direct plating | NS | NS | 3/6 (0.58) | NS | ||
| Enrichment | NS | NS | 6/6 | NS | |||
| Lymph node | Direct plating | 0/6 (ND) | 4/6 (0.88) | 1/6 (−0.46) | 1/6 (−0.44) | ||
| Enrichment | 0/6 | 4/6 | 1/6 | 1/6 | |||
| Lung | Direct plating | 1/6 (−0.78) | 6/6 (0.54) | 4/6 (0.14) | 5/6 (0.95) | ||
| Enrichment | 3/6 | 6/6 | 6/6 | 5/6 | |||
| Spleen | Direct plating | 5/6 (1.29) | 6/6 (1.09) | 4/6 (1.05) | 6/6 (1.21) | ||
| Enrichment | 6/6 | 6/6 | 4/6 | 6/6 | |||
| Liver | Direct plating | 3/6 (−0.22) | 6/6 (1.15) | 6/6 (0.93) | 6/6 (1.31) | ||
| Enrichment | 4/6 | 6/6 | 6/6 | 6/6 | |||
| Kidney | Direct plating | 3/6 (0.41) | 5/6 (1.65) | 5/6 (1.28) | 5/6 (1.44) | ||
| Enrichment | 5/6 | 6/6 | 5/6 | 5/6 | |||
| Spinal cord | Direct plating | 0/6 (ND) | 5/6 (0.81) | 3/6 (1.05) | 2/6 (0.77) | ||
| Enrichment | 3/6 | 5/6 | 3/6 | 2/6 | |||
Abbreviations: NS, not sampled; ND, not detected.
Table 7 presents the numbers of surface swabs of equipment, hide, and operatives' hands that were found to contain the marker organism by direct plating and enrichment methods. It also presents the arithmetic mean numbers of the organism present in such swabs, determined by direct plating. In the case of animals syringe inoculated with 6.0 log10 CFU, swab samples from the animal hide and the carcass splitting saw had higher numbers of swabs positive following enrichment than by direct plating. However, the choice of detection method did not affect the numbers of positive swabs from the exsanguination knife or operatives' hands. At the higher inoculation level, both inoculation methods gave the same numbers of positive swab samples in most cases. Exceptions to this were observed, following syringe inoculation, on the knife and hands after exsanguination and the carcass splitting saw and, after bolt inoculation, on the captive bolt gun.
TABLE 7.
Swab samples from equipment, animal hide, and personnel (n = 6) showing the presence of P. fluorescens determined by direct plating or enrichment after syringe or bolt inoculation using a 6.0- or 9.0-log10 CFU P. fluorescens inoculum
| Sample type | Detection method | No. of positive swab samples/total no. of samples (log10 CFU of bacteria cm−2) for inoculation type and inoculum (log10 CFU)
|
|||||
|---|---|---|---|---|---|---|---|
| Syringe
|
Bolt (9.0) | ||||||
| 6.0 | 9.0 (expt 1) | 9.0 (expt 2) | |||||
| Captive bolt gun | Direct plating | NSa | NS | NS | 5/6 (4.20) | ||
| Enrichment | NS | NS | NS | 6/6 | |||
| Animal hide after inoculation | Direct plating | 3/6 (2.09) | 3/6 (2.15) | 6/6 (3.63) | 6/6 (3.76) | ||
| Enrichment | 4/6 | 3/6 | 6/6 | 6/6 | |||
| Knife after exsanguination | Direct plating | 4/6 (1.09) | 3/6 (0.56) | 5/6 (0.83) | 3/6 (5.8) | ||
| Enrichment | 4/6 | 4/6 | 5/6 | 3/6 | |||
| Operatives' hands after exsanguination | Direct plating | 1/6 (−0.60) | 1/6 (−0.38) | 4/6 (0.97) | 4/6 (0.62) | ||
| Enrichment | 1/6 | 3/6 | 4/6 | 4/6 | |||
| Carcass splitting saw | Direct plating | 3/6 (0.87) | 4/6 (0.45) | 5/6 (0.86) | 5/6 (0.41) | ||
| Enrichment | 5/6 | 4/6 | 6/6 | 5/6 | |||
NS, not sampled.
Following bolt inoculation P. fluorescens was detected on the left wall, back wall, right wall, and floor of the area directly in front of the stunning box, at values of 0.67, 0.90, 2.05, and 1.12 log10 CFU m−2, respectively. The highest levels of surface contamination were noted on the right wall, i.e., the area closest to the shooting position, and on the floor.
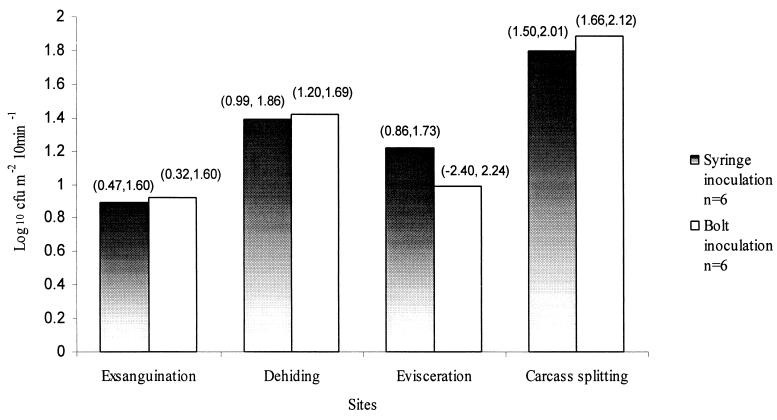
Figure 2 shows numbers of the marker organism settling on agar plates placed around animals during dressing at exsanguination, dehiding, evisceration, and carcass splitting. The organism was recovered from all four process sites related to syringe-inoculated animals and all but one process site, evisceration, related to bolt-inoculated animals. While the highest mean counts were at carcass splitting, significant differences among the process sites were observed following syringe inoculation only, among exsanguination, dehiding, and carcass splitting and between evisceration and carcass splitting (P < 0.05).
FIG. 2.
Numbers (log10 CFU m−2 10 min−1) of P. fluorescens on sedimentation plates exposed for 10 min during beef carcass dressing at exsanguination, dehiding, evisceration, and carcass splitting. The 95% confidence intervals for each data set are presented in parentheses.
DISCUSSION
The data obtained in the present study indicated that a marker organism inoculated into the brain of cattle during or after stunning could subsequently be detected in the blood, musculature, and a number of organs, including the spinal cord, throughout the body. The method of inoculation did not appear to be significant, but the concentration of inoculum used did have an effect.
The spread of brain tissue as a result of stunning is now well established and has been demonstrated elsewhere for cattle by a number of workers (1, 2, 9). All these studies report the presence of emboli in the lungs of cattle after stunning, using a variety of captive bolt guns. In the present study the marker organism was found in the lungs of some of the animals tested and was dependent on the inoculum level used and the method of inoculation. It is generally acknowledged that the brain emboli are transferred to the lungs via the bloodstream (1, 2, 18). The marker organism was also detected in the blood of some animals sampled during exsanguination, over a period of 40 s. In studies by Love et al. (17) and Anil et al. (1) the presence of brain emboli and CNS tissue proteins was demonstrated in jugular venous blood 30 s after stunning. Blood clots located in the hearts of cattle after stunning were also found to contain spinal cord segments (23).
The above data suggest that a marker organism inoculated into the brain of cattle could be used to model the transfer of CNS tissue in the bloodstream and the deposition of such material in the lungs.
It was also demonstrated that the organism could be found in a number of other organs throughout the body and in muscle. Similar observations have been made previously from experiments using a captive bolt gun inoculated with a marked strain of Escherichia coli (18). These workers showed that the spleen was contaminated in cattle after stunning, with and without pithing, but that both flank and neck muscles were contaminated only after pithing.
At present there is no evidence for the transfer of CNS material to these organs as a result of stunning. In order for CNS tissue to be deposited in the musculature or visceral organs, such as the spleen, it would have to be capable of passing through the pulmonary capillary vessels and entering the systemic circulation via the heart. In the present study the marker organism appeared to have been capable of following this route.
An important factor in determining the spread of the marker organism to multiple organs and muscle tissue is that the method used permitted the detection of very low levels of the organism. This was dependent on the inoculum level used and the organ examined. Because the organism produces a visible colony on a plate, its presence even at low levels is easily determined.
The numbers of bacteria in the blood and organs of inoculated animals have been determined and compared to the initial inoculum used. This shows that there is a very large discrepancy between the numbers inoculated and those recovered. This result was not unexpected, given the bactericidal properties of the blood, in particular against gram-negative organisms (15). The recovery assumes that each colony on a plate arises from the growth of a single bacterium.
It is doubtful if proteins released into the bloodstream as a result of stunning would be depleted in this way, so detecting their presence would be dependent on the initial quantities present and the method of detection. While methods to detect specific brain proteins such as syntaxin do exist (1, 17, 24), their sensitivity in detecting the levels of proteins released as a result of stunning or inoculation in organs other than the lungs remains to be established.
The process of stunning the animals had a significant influence in contaminating the immediate environment. This would be expected given the impact of the inoculated captive bolt on the head of the animal. The contamination of the exsanguination knife, the operatives' hands, and the saw was in keeping with the observed contamination of the blood and the spinal column of the animal. The contamination of these is of special importance, since they all have the potential to spread contamination from one carcass to the next.
The presence of the marker organism as an aerial contaminant in the abattoir indicated that it was present in the environment throughout the slaughter process. The organism was probably present as an aerosol, and this probably came about (i) from the shooting of the animals with the contaminated captive bolt, (ii) during exsanguination from contaminated blood, and (iii) during the splitting of the carcass. It was observed that at carcass splitting there was a slight increase in the aerial contamination, suggesting that the spinal cord was making an increased contribution to the levels of contamination present. It was not possible to distinguish which of these sources was directly responsible for the aerial contamination observed, since any one of them alone or in combination could have been responsible for the observed effects. It seems reasonable to suggest that carcasses being produced in this environment and indeed personnel could be contaminated from this source.
Whether this aerosolization could be demonstrated for brain proteins, such as syntaxin, produced as a result of shooting or in the blood is not known.
The marker organism appeared to mimic the effect of stunning in cattle in relation to the subsequent appearance of CNS materials in the lungs and blood of animals. It did show a much more widespread distribution to other organs which has not previously been observed for CNS tissue or proteins. This could be a reflection of the inability of this material to pass through the pulmonary capillaries or the lack of a sufficiently sensitive test to detect the presence of the material. These issues need to be resolved and will be the subject of future experiments where a prion will be inoculated into the brain of cattle after stunning and the dispersion will be monitored, as in the present experiments. This will then determine the effectiveness of using a bacterium, which may not behave in exactly the same manner, to model the spread of a prion as a result of stunning with a captive bolt gun.
Acknowledgments
This research was financially supported by the European Commission under the FAIR PL97/3301.
Sincere thanks are due to Dermot Harrington and to Paula Reid, Teagasc Headquarters, Sandymount, Dublin 4, Ireland, for their assistance with the statistical analysis.
REFERENCES
- 1.Anil, M. H., S. Love, S. Williams, A. Shand, J. L. McKinstry, C. R. Helps, A. Waterman-Pearson, J. Seghatchian, and D. A. Harbour. 1999. Potential contamination of beef carcases with brain tissue at slaughter. Vet. Rec. 145:460-462. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, N. E., T. Garland, and J. F. Edwards. 1996. Brain emboli in slaughtered cattle. Vet. Pathol. 33:600. [Google Scholar]
- 3.Brown, P. 1998. On the origins of BSE. Lancet 352:252-253. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P., R. G. Will, R. Bradley, D. M. Asher, and L. Detwiler. 2001. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease: background, evolution, and current concerns. Emerg. Infect. Dis. 7:6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttie, L. McCardle, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that "new variant' CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 6.Collee, J. G., and R. Bradley. 1997. BSE: a decade on--part 1. Lancet 349:636-642. [DOI] [PubMed] [Google Scholar]
- 7.Collee, J. G., and R. Bradley. 1997. BSE: a decade on--part 2. Lancet 349:715-721. [DOI] [PubMed] [Google Scholar]
- 8.Dealler, S. F., and R. Lacey. 1990. Transmissible spongiform encephalopathies: the threat of BSE to man. Food Microbiol. 7:253-279. [Google Scholar]
- 9.Garland, T., N. Bauer, and M. Bailey. 1996. Brain emboli in the lungs of cattle after stunning. Lancet 348:610.. [DOI] [PubMed] [Google Scholar]
- 10.Gill, C. O., J. C. McGinnis, and M. Badoni. 1996. Assessment of the hygienic characteristics of a beef carcass dressing process. J. Food Prot. 59:136-140. [DOI] [PubMed] [Google Scholar]
- 11.Gill, C. O., and J. Bryant. 1997. Decontamination of carcasses by vacuum-hot water cleaning and steam pasteurisation during routine operations at a beef packing plant. Meat Sci. 47:267-276. [DOI] [PubMed] [Google Scholar]
- 12.Hauck, A. J., J. F. Bambara, and W. D. Edwards. 1990. Embolism of brain tissue to the lung in a neonate. Arch. Pathol. Lab. Med. 114:217-218. [PubMed] [Google Scholar]
- 13.Kerr, M., M. Fitzgerald, J. J. Sheridan, D. A. McDowell, and I. S. Blair. 1999. Survival of Escherichia coli O157:H7 in bottled natural mineral water. J. Appl. Microbiol. 87:833-841. [DOI] [PubMed] [Google Scholar]
- 14.Kitchell, A. G., G. C. Ingram, and W. R. Hudson. 1973. Microbiological sampling in abattoirs, p. 43-61. In R. G. Board and D. W. Lovelock (ed.), Sampling--microbiological monitoring of environments. Academic Press, London, United Kingdom.
- 15.Labadie, J., P. Gouet, and J. Fournaud. 1977. Blood poisonings at slaughter and their consequences. Zentbl. Bakteriol. Hyg. I Abt. Orig. B 164:390-396. [PubMed] [Google Scholar]
- 16.Lasta, J. A., R. Rodriguez, M. Zanelli, and C. M. Margaria. 1992. Bacterial count from bovine carcasses as an indicator of hygiene at slaughtering places: a proposal for sampling. J. Food Prot. 54:271-278. [DOI] [PubMed] [Google Scholar]
- 17.Love, S., C. R. Helps, S. Williams, A. Shand, J. L. McKinstry, S. N. Brown, D. A. Harbour, and M. H. Anil. 2000. Methods for detection of haematogenous dissemination of brain tissue after stunning of cattle with captive bolt guns. J. Neurosci. Methods 99:53-58. [DOI] [PubMed] [Google Scholar]
- 18.Mackey, B. M., and C. M. Derrick. 1979. Contamination of the deep tissues of carcasses by bacteria present on the slaughter instruments or in the gut. J. Appl. Bacteriol. 46:355-366. [DOI] [PubMed] [Google Scholar]
- 19.McMillan, J. B. 1956. Emboli of cerebral tissue in the lungs following severe head injury. Am. J. Pathol. 32:405-415. [PMC free article] [PubMed] [Google Scholar]
- 20.Molin, G., and A. Ternström. 1982. Numerical taxonomy of psychrothropic pseudomonads. J. Gen. Microbiol. 128:1249-1264. [DOI] [PubMed] [Google Scholar]
- 21.Park, R. W. 1978. The isolation and use of streptomycin resistant mutants for following development of bacteria in mixed populations, p. 107-112. In D. Lovelock and R. Davies (ed.), Techniques for the study of mixed populations. Academic Press, London, United Kingdom.
- 22.Prusiner, S. B. 1991. Molecular biology of prion diseases. Science 252:1515-1522. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt, G. R., K. L. Hossner, R. S. Yemm, and D. H. Gould. 1999. Potential for disruption of central nervous system tissue in beef cattle by different types of captive bolt stunners. J. Food Prot. 62:390-393. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt, G. R., K. L. Hossner, R. S. Yemm, D. H. Gould, and J. P. O'Callaghan. 1999. An enzyme-linked immunosorbent assay for glial fibrillary acidic protein as an indicator of the presence of brain or spinal cord in meat. J. Food Prot. 62:394-397. [DOI] [PubMed] [Google Scholar]
- 25.Taylor, D. M. 1989. Bovine spongiform encephalopathy and human health. Vet. Rec. 125:413-415. [DOI] [PubMed] [Google Scholar]
- 26.Wells, G. A. H., A. C. Scott, C. T. Johnson, R. F. Gunning, R. D. Hancock, M. Jeffrey, M. Dawson, and R. Bradley. 1987. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 121:419-420. [DOI] [PubMed] [Google Scholar]