Abstract
Comamonas sp. strain JS765 can grow with nitrobenzene as the sole source of carbon, nitrogen, and energy. We report here the sequence of the genes encoding nitrobenzene dioxygenase (NBDO), which catalyzes the first step in the degradation of nitrobenzene by strain JS765. The components of NBDO were designated ReductaseNBZ, FerredoxinNBZ, OxygenaseNBZα, and OxygenaseNBZβ, with the gene designations nbzAa, nbzAb, nbzAc, and nbzAd, respectively. Sequence analysis showed that the components of NBDO have a high level of homology with the naphthalene family of Rieske nonheme iron oxygenases, in particular, 2-nitrotoluene dioxygenase from Pseudomonas sp. strain JS42. The enzyme oxidizes a wide range of substrates, and relative reaction rates with partially purified OxygenaseNBZ revealed a preference for 3-nitrotoluene, which was shown to be a growth substrate for JS765. NBDO is the first member of the naphthalene family of Rieske nonheme iron oxygenases reported to oxidize all of the isomers of mono- and dinitrotoluenes with the concomitant release of nitrite.
Nitroaromatic compounds are used extensively as industrial feedstocks for many manufacturing processes, including the production of pesticides, dyes, and explosives (11). Due to improper storage, use, and disposal, nitroaromatic compounds have been released into the environment, where they are considered environmental pollutants. For example, nitrobenzene and 2,4- and 2,6-dinitrotoluene are included in the U.S. Environmental Protection Agency's list of priority pollutants (14).
The biodegradation of aromatic hydrocarbons and related compounds by aerobic bacteria is often initiated by multicomponent dioxygenase systems that catalyze the addition of both atoms of molecular oxygen to the substrate. Nitroaromatic compounds, in general, are resistant to oxidative attack due to the electron-withdrawing nature of the nitro groups and the stability of the benzene ring (29, 33). Only recently have aerobic bacteria been isolated that utilize nitroaromatic compounds as growth substrates (19, 32). One example is Comamonas sp. strain JS765, which can grow with nitrobenzene as the sole source of carbon, nitrogen, and energy. Previous experiments showed that JS765 uses an oxidative pathway for the degradation of nitrobenzene, with the initial reaction catalyzed by nitrobenzene 1,2-dioxygenase (NBDO; Fig. 1) (18). Other nitroarene dioxygenase genes from aerobic bacteria have been cloned and sequenced; these include genes encoding 2-nitrotoluene dioxygenase from Pseudomonas sp. strain JS42 (20) and 2,4-dinitrotoluene dioxygenases (DNTDOs) from Burkholderia sp. strain DNT (35) and Burkholderia cepacia R34 (G. R. Johnson, B. E. Haigler, R. K. Jain, and J. C. Spain, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. Q-83, p. 435, 1998). However, strains DNT and R34 are unable to grow with nitrobenzene and we have observed only slight growth of JS42 with nitrobenzene.
FIG. 1.
Dioxygenation of nitrobenzene catalyzed by NBDO.
The majority of nitroaromatic compounds are synthetic, and therefore they have been present in the environment for a relatively short time. Because of this, bacteria have had a brief time to evolve mechanisms to degrade these toxic compounds. To better understand the molecular basis of nitrobenzene degradation, we cloned and characterized the genes encoding NBDO from strain JS765. Results presented here show that NBDO belongs to the naphthalene family of Rieske nonheme iron oxygenases (8). In addition, we have extended preliminary studies that showed that NBDO can oxidize a wide range of substrates (18).
MATERIALS AND METHODS
Bacterial strains and plasmids.
Escherichia coli DH5α (Gibco-BRL Laboratories, Gaithersburg, Md.) was used for isolation and maintenance of recombinant derivatives of pUC18 (36). DH5α containing derivatives of pUC18 was maintained on agar plates containing minimal-salts medium (MSB) (34), 0.8% (wt/vol) agar, 10 mM glucose, 1 mM thiamine, and ampicillin at 150 μg/ml. Plasmid pDTG925 consists of an 8.9-kb EcoRI fragment from JS765 inserted into the EcoRI site of pUC18. Plasmid pDTG927 was generated by subcloning a 4.6-kb SacI DNA fragment containing the nbzAaAbAcAd genes from pDTG925 into the SacI site of pUC18. DH5α(pDTG927) was used for whole-cell biotransformation experiments. JS765 was maintained on MSB containing Balch's vitamin solution (without thiamine) (6) and either succinate (10 mM) or nitrobenzene (supplied as vapor) as the sole carbon source.
DNA manipulations, sequencing, and computer analysis.
Genomic DNA from JS765 was prepared as previously described (9). Standard protocols were used for DNA cloning and transformations (30). Plasmid DNA purification was performed with the QIAprep spin miniprep kit in accordance with the manufacturer's (Qiagen, Valencia, Calif.) instructions. Restriction endonuclease digestions and ligations with T4 ligase were conducted in accordance with the manufacturer's (New England Biolabs, Beverly, Mass.) instructions. The QIAquick gel extraction kit (Qiagen) was used for the recovery of DNA fragments from agarose gels. Fluorescent automated DNA sequencing was performed by the University of Iowa DNA Facility with an Applied Biosystems 373A automated DNA sequencer. Computer analysis of DNA sequences was carried out by using the Wisconsin Sequence Analysis package (Genetics Computer Group, Madison, Wis.).
Probe design and screening.
The probe used to screen for the genes encoding NBDO was obtained from the genome of strain JS765 by PCR. Comparison of the genes encoding the α subunit proteins for nitroarene dioxygenases from strains DNT (35), JS42 (20), and R34 (nucleotide sequence unpublished) (17) revealed that regions of the genes were well conserved. A PCR primer pair that allowed amplification of a 442-bp portion of the conserved region was devised. The primer sequences (5"ACACGAATTCAACCCACCTTCAAGCACTCTG3" and 5"ACAGGGATCCCGAWGGCATACGTCCAAWCC3") included restriction endonuclease recognition sequences (underlined) near the 5" termini to facilitate subcloning. The complementary sequences correspond to nucleotides 4371 to 4392 and 4853 to 4834 of the DNTDO gene sequence from strain DNT (GenBank accession no. U62430). After amplification of the gene fragment from the genome of JS765, the product was subcloned into vector pBluescript II KS+ (Stratagene, La Jolla, Calif.) and the plasmid was designated pJS1209. For Southern blot analysis, the insert was excised from the recombinant plasmid, purified by agarose gel electrophoresis, and subjected to random primed labeling with digoxigenin-dUTP by using DIG high prime labeling and detection starter kit I (Boehringer Mannheim, Indianapolis, Ind.) in accordance with the manufacturer's instructions. A partial genomic library was made by digesting JS765 DNA with the restriction enzyme EcoRI and ligating fragments (7 to 10 kb) into EcoRI-digested, bacterial alkaline phosphatase-treated pUC18 (Boehringer Mannheim). The library was introduced into DH5α cells by transformation. For subsequent colony blot screenings to identify transformants carrying the NBDO gene cluster, the 442-bp insert from pJS1209 was labeled by priming with [α-32P]dCTP using the Ready-to-go labeling kit (Pharmacia Biotech, Piscataway, N.J.). Colony hybridizations were performed as described previously (30).
Whole-cell biotransformations.
DH5α(pDTG927) and DH5α(pUC18) were grown at 30°C with shaking in Luria-Bertani medium (3) supplemented with 0.7% glycerol and ampicillin (150 μg/ml). When the turbidity (A600) of the culture reached 0.65 to 0.80, isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM) was added to induce synthesis of NBDO. After 4 h of incubation, cells were harvested by centrifugation, washed in phosphate buffer (20 mM, pH 7.5), and suspended in a minimal volume of phosphate buffer.
Whole-cell biotransformation experiments were conducted in triplicate, in 125-ml baffled shaker flasks containing cells (final A600, 2.0) suspended in 20 ml of phosphate buffer. Substrates were added to control reaction mixtures containing identically treated cells of DH5α(pUC18) to account for substrate transformation and products resulting from gratuitous reduction of the nitroarenes by the host strain. Cell suspensions were incubated with shaking (220 rpm, 26°C), and samples were collected at appropriate intervals for high-performance liquid chromatography (HPLC) and nitrite analysis to monitor the progress of the reaction. Samples for HPLC analysis were mixed with 0.5 volume of acetonitrile and centrifuged to remove cells. After a 60-min incubation, the triplicate reactions were pooled and prepared for gas chromatography-mass spectrometry (GC-MS) analysis (see below).
Partial purification of oxygenaseNBZ.
OxygenaseNBZ was purified from DH5α(pDTG927) as described for naphthalene dioxygenase (NDO) (16), with the following exceptions. NBDO activity was demonstrated by using a nitrite assay (see below). In addition, OxygenaseNBZ did not bind to a Q-Sepharose column. The wash fraction was applied to a butyl-Sepharose column, and oxygenaseNBZ was eluted with a linear gradient of (NH4)2SO4 (1.0 to 0.2 M). Fractions containing OxygenaseNBZ were pooled and concentrated, and the buffer was exchanged with 100 mM MES buffer (pH 6.8). This preparation was frozen immediately in 25-μl aliquots and maintained at −70°C until use.
Enzyme assay.
NBDO activity was determined by the measurement of nitrite released from nitroaromatic substrates and by HPLC analysis of the hydroxylated products formed. Nitrite was determined by a modification of the method described previously (1). A 0.75-ml reaction mixture contained 50 mM MES buffer (pH 6.8), 0.2 mM NADH, 0.1 mM substrate (added from a methanol stock solution), 19 μg of Reductase2NT, and 31 μg of Ferredoxin2NT purified from 2NTDO expressed by strain JS42 (F. K. N. Lee, J. V. Parales, and D. T. Gibson, unpublished data). The reaction was initiated by the addition of OxygenaseNBZ (52 μg), and incubation was carried out at 30°C and 220 rpm in a water bath shaker. After 2 min, 100 μl of the reaction mixture was added to 100 μl of 1% (wt/vol) sulfanilamide in 1.5 N HCl. After mixing, 100 μl of 0.02% (wt/vol) N-(1-naphthyl)ethylenediamine in 1.5 N HCl was added to the reaction mixture. The A540 of the pink complex was measured after 15 min. The amount of nitrite evolved was determined from a calibration curve of sodium nitrite. Nitrite formation was determined with saturating concentrations of substrates, and initial reaction rates were linear with respect to the concentration of OxygenaseNBZ. The hydroxylated product(s) formed after 2 min was determined by adding a 100-μl sample from the reaction mixture to 150 μl of acetonitrile. The amount of product(s) formed was determined by HPLC as described below based on a standard calibration curve for each product.
Analytical methods.
HPLC analysis was done with a model 1050 chromatography system equipped with a model 1040M diode array detector from Hewlett-Packard Inc., Santa Clarita, Calif.. Compounds were separated on a Supelcosil LC-ABZ+Plus column (25 by 4.6 mm; Supelco, Bellefonte, Pa.) with a mobile phase of acetonitrile-0.1% trifluoroacetic acid (1 ml min−1). The composition of the mobile phase was varied, depending on the properties of the substrates and products being analyzed. Reaction mixtures containing naphthalene were analyzed by using a mobile phase of acetonitrile-water to limit the decomposition of naphthalene cis-dihydrodiol. Product formation was monitored at 254 nm. The enantiomeric composition of naphthalene cis-dihydrodiol was determined by chiral stationary-phase HPLC using a Chiracel OJ column (Chiral Technologies Inc., Exton, Pa.) as described previously (28).
For GC-MS analyses, 50 ml of each culture supernatant was extracted with ethyl acetate (previously washed with 1 N NaOH). Reaction mixtures containing 2,4-dinitrotoluene or 2,6-dinitrotoluene were acidified to pH 2.5 prior to extraction of products. Following extraction, the solvent was evaporated under nitrogen and the residue was dissolved in N,N-dimethylformamide. Butyl boronate derivatives were prepared by standard methods (15). Trimethylsilyl derivatives were prepared with N,O-bis(trimethylsilyl)-trifluoroacetamide by methods provided by the distributor (Alltech Associates Inc., Deerfield, Ill.). GC-MS analyses were conducted with a Hewlett-Packard series 5971 mass spectrometer and a Hewlett-Packard model 5890 gas chromatograph with an HP-5 M.S. capillary column (30 m by 0.25 mm, 0.25-μm film thickness; Hewlett-Packard). Helium was used as the carrier gas at a constant flow-rate of 0.8 ml min−1. The injector and transfer line temperatures were 280 and 300°C, respectively. The chromatography program was as follows: initial column temperature of 120°C, isothermal for 2 min, temperature increase of 10°C min−1 to 280°C, and isothermal for 6 min. The ionization voltage and electron multiplier settings were 70 eV and 2,000 V, respectively. Product identities were confirmed by comparison of the GC-MS data with known standards. New products were identified by GC-MS retention times (Rt) and characteristic mass fragments as follows; 4-methyl-3-nitrocatechol, Rt 9.9 min, molecular ion [M+ (% relative intensity)] at m/z 313 (17) with major fragment ions at m/z 298 (50), 281 (12), 73 (100); 2,4-dinitrobenzyl alcohol, Rt 11.0 min., M+, m/z 270 (32), 255 (46), 253 (79), 238 (23), 73 (100); 3-methyl-6-nitrocatechol or 4-methyl-6-nitrocatechol, Rt 10.4 min, M+, m/z 302 (88), 179 (13), 73 (100); 4-chloro-3-methyl-6-nitrophenol or 3-chloro-2-methyl-5-nitrophenol, Rt 9.5 min, M+, m/z 259 (50), 244 (100), 197 (16), 73 (52); 4-chloro-3-methylcatechol, Rt 8.8 min, M+, m/z 302 (48), 73 (100); cis-(1,2)-dihydroxy-1,2-dihydro-8-nitronaphthalene, Rt 12.8 min, M+, m/z 351 (1), 246 (96), 233 (20), 218 (18), 216 (16), 203 (14), 202 (21), 192 (15), 191 (86), 147 (55), 73 (100); cis-(1,2)-dihydroxy-1,2-dihydro-8-nitronaphthalene (butyl boronate derivative), Rt 14.4 min, M+, m/z 273 (4), 257 (10), 256 (57), 200 (20), 189 (15), 170 (45), 160 (24), 144 (34), 115 (100), 114 (39).
Protein concentrations were determined with the bicinchoninic acid protein assay (Pierce, Rockford, Ill.) (31) with bovine serum albumin as the standard.
Chemicals.
Nitrobenzene was from Fisher Scientific (Pittsburgh, Pa.), and 1-nitronaphthalene, 1,2-dihydroxynaphthalene, and 2,3-dihydroxynaphthalene were from TCI America (Portland, Oreg.). 4-Methyl-5-nitrocatechol was a gift from Ronald Spanggord (SRI International, Menlo Park, Calif.). 3-Nitrocatechol, 3-methyl-4-nitrocatechol, toluene cis-2,3-dihydrodiol, and naphthalene cis-1,2-dihydrodiol were produced enzymatically as previously described (7, 10, 12). All other aromatic substrates and standards were from Aldrich Chemical Inc. (Milwaukee, Wis.).
Nucleotide sequence accession number.
The sequence of the DNA fragment containing the nbzAaAbAcAd genes has been deposited in the GenBank database under accession number AF379638.
RESULTS
Cloning and sequencing of genes encoding NBDO.
Southern hybridization experiments revealed that the oxygenase α subunit-specific DNA probe hybridized to an 8.9-kb EcoRI fragment of JS765 chromosomal DNA. The EcoRI fragment was cloned as described in Materials and Methods. NBDO activity (nitrite release from nitrobenzene) was detected with DH5α(pDTG925), indicating that all of the necessary genes were present on the EcoRI fragment. The 4.6-kb SacI fragment from pDTG925 was used to generate DH5α(pDTG927), which retained NBDO activity. Both strands of the 4.6-kb fragment in pDTG927 were sequenced, and five open reading frames (ORFs) with predicted amino acid sequences similar to known Rieske nonheme iron dioxygenase components were identified. The gene organization and calculated molecular weights of the five polypeptides are shown in Fig. 2. Predicted polypeptides from four of the ORFs were designated ReductaseNBZ, FerredoxinNBZ, OxygenaseNBZα, and OxygenaseNBZβ. The genes were designated nbzAa, nbzAb, nbzAc, and nbzAd (nbz for nitrobenzene degradation).
FIG. 2.
Partial restriction map and predicted nbzAaAbAcAd gene products from the 4.6-kb SacI chromosomal DNA fragment from JS765 in pDTG927. Protein designations are given in the text. MW, molecular weight.
Sequence comparisons.
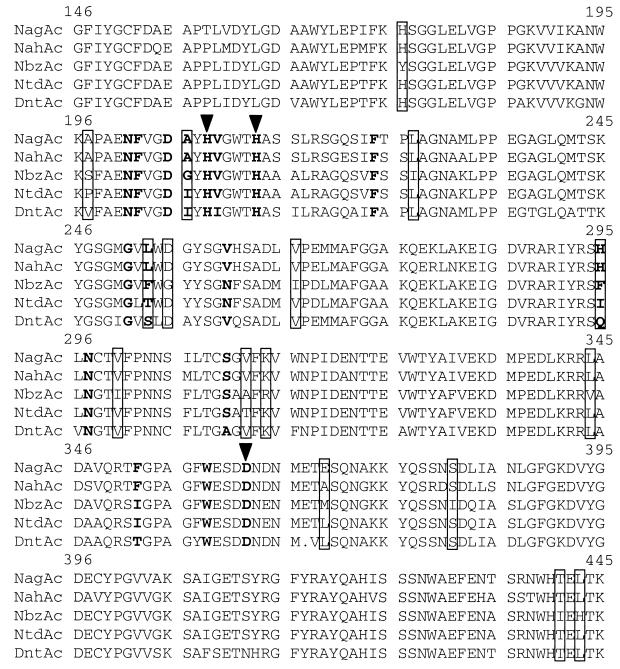
The predicted amino acid sequences of the polypeptides from four of the ORFs share identity with polypeptides from other known three-component dioxygenase systems. A comparison of these relationships is summarized in Table 1. Interestingly, ReductaseNBZ and FerredoxinNBZ are identical in amino acid sequence to the Reductase2NT and Ferredoxin2NT from 2-nitrotoluene 2,3-dioxygenase (2NTDO) from Pseudomonas sp. strain JS42. The amino acid sequences of OxygenaseNBZα and OxygenaseNBZβ are most similar to that of 2NTDO from JS42. Based on the crystal structure of NDO, the 2-His-1-carboxylate facial triad that coordinates the mononuclear iron was identified at the active site in OxygenaseNAPα (13). The 2-His-1-carboxylate facial triad is conserved in the C-terminal region of OxygenaseNBZα, as shown by the inverted triangles in Fig. 3. This catalytic domain is present in the oxygenase α subunits from 2NTDO, DNTDO, and NDO (21, 23, 24). As shown in Fig. 3, the C terminus of OxygenaseNBZα contains 16 unique amino acid residues compared with the α subunits of NDO, 2NTDO, and DNTDO. However, many of the residues that are near the active site of NDO are conserved in NBDO.
TABLE 1.
Comparison of proteins with similarities to the products of nbz ORFs
| Gene | Function | Deduced no. of amino acid residues | Protein with similar sequence | % Identity (no. of residues) | Organism | Accession no. |
|---|---|---|---|---|---|---|
| nbzAa | ReductaseNBZ | 328 | NtdAa | 100 (328) | Pseudomonas sp. strain JS42 | U49504 |
| NagAa | 99 (328) | Ralstonia sp. strain U2 | AF036940 | |||
| DntAa | 91 (346) | Burkholderia sp. strain DNT | U62430 | |||
| NahAa | 67 (328) | Pseudomonas sp. NCIB 9816-4 | M83950 | |||
| TodA | 21 (410) | Pseudomonas putida F1 | J04996 | |||
| orf2 | Unknown | 186 | Orf2 | 98 (186) | Pseudomonas sp. strain JS42 | U49504 |
| NagG | 96 (423) | Ralstonia sp. strain U2 | AF036940 | |||
| Orf2 | 88 (423) | Burkholderia sp. strain DNT | U62430 | |||
| BphA1 | 39 (458) | Pseudomonas pseudoalcaligenes KF707 | A42409 | |||
| TodC1 | 37 (450) | Pseudomonas putida F1 | J04996 | |||
| nbzAb | FerredoxinNBZ | 104 | NtdAb | 100 (104) | Pseudomonas sp. strain JS42 | U49504 |
| NagAb | 83 (104) | Ralstonia sp. strain U2 | AF036940 | |||
| DntAb | 79 (104) | Burkholderia sp. strain DNT | U62430 | |||
| NahAb | 72 (104) | Pseudomonas sp. NCIB 9816-4 | M83950 | |||
| TodB | 36 (107) | Pseudomonas putida F1 | J04996 | |||
| nbzAc | OxygenaseNBZα | 447 | NtdAc | 95 (447) | Pseudomonas sp. strain JS42 | U49504 |
| NagAc | 88 (447) | Ralstonia sp. strain U2 | AF036940 | |||
| DntAc | 87 (451) | Burkholderia sp. strain DNT | U62430 | |||
| NahAc | 82 (449) | Pseudomonas sp. NCIB 9816-4 | U49496 | |||
| TodC1 | 36 (450) | Pseudomonas putida F1 | J04996 | |||
| nbzAd | OxygenaseNBZβ | 194 | DntAd | 96 (194) | Burkholderia sp. strain DNT | U62430 |
| NtdAd | 95 (194) | Pseudomonas sp. strain JS42 | U49504 | |||
| NagAd | 92 (194) | Ralstonia sp. strain U2 | AF036940 | |||
| NahAd | 78 (194) | Pseudomonas sp. NCIB 9816-4 | U49496 | |||
| TodC2 | 26 (187) | Pseudomonas putida F1 | J04996 |
FIG. 3.
Alignment of deduced amino acid sequences of the C termini (residues 146 to 445, NDO numbering) from NbzAc (oxygenaseNBZα), NtdAc (oxygenase2NTα), DntAc (oxygenaseDNTα), NagAc (oxygenaseNAGα from strain U2), and NahAc (oxygenaseNAPα from strain NCIB 9816-4). Amino acid residues at the active site of NDO are in bold. Amino acids unique to the C terminus of oxygenaseNBZα are boxed. Conserved amino acids that coordinate mononuclear iron (2-His-1-carboxylate facial triad) are denoted by inverted triangles.
The predicted amino acid sequence of ORF2 in the NBDO gene cluster has the highest sequence identity to ORF2 in the 2NTDO cluster from strain JS42. ORF2 in both strains appears to encode a truncated form of NagG, the large subunit of salicylate 5-hydroxylase from Ralstonia sp. strain U2 (5). The function of ORF2, if any, is unknown.
Biotransformation of aromatic substrates by DH5α(pDTG927).
A number of substituted aromatic compounds were hydroxylated by NBDO. The results show that a nitro-substituted position was generally the preferred, but not exclusive, oxidation site (Table 2). All of the nitrotoluene and dinitrotoluene isomers tested were transformed by NBDO (Table 2). Oxidation at the 3,4 position of the ring, with respect to the methyl substituent, appeared to be favored, since 3-methylcatechol was not produced from 3-nitrotoluene, and 4-methyl-5-nitrocatechol was the predominant product from 2,4-dinitrotoluene oxidation. However, the nitro substituent appears to contribute significantly to specificity because 3-methyl-4-nitrocatchol was the exclusive product formed from 2,6-dinitrotoluene and 3-methylcatechol was the predominant product formed from 2-nitrotoluene.
TABLE 2.
Products formed from aromatic substrates by E. coli DH5α(pDTG927)
| Substrate | Product(s)a | Retention time(s) (min) | Relative yield (%) |
|---|---|---|---|
| Nitrobenzene | Catechol | 5.5 | 100 |
| 2-Nitrotoluene | 3-Methylcatechol | 6.5 | 55b |
| 2-Nitrobenzyl alcohol | 7.6 | 45b | |
| 3-Nitrotoluene | 4-Methylcatechol | 6.4 | 100 |
| 4-Nitrotoluene | 4-Methylcatechol | 6.4 | 85 |
| 4-Nitrobenzyl alcohol | 8.8 | 15 | |
| Naphthalene | Naphthalene cis-1,2-dihydrodiol | 9.6, 10.6c | 100 |
| 2,3-Dinitrotoluene | 4-Methyl-3-nitrocatechol | 9.9 | 100 |
| 2,4-Dinitrotoluene | 4-Methyl-3-nitrocatechol | 9.9 | 21 |
| 4-Methyl-5-nitrocatechol | 10.9 | 62 | |
| 2,4-Dinitrobenzyl alcohol | 11.0 | 17 | |
| 2,6-Dinitrotoluene | 3-Methyl-4-nitrocatechol | 11.4 | 100 |
| 3,4-Dinitrotoluene | 4-Methyl-6-nitrocatechol | 10.4 | 100 |
| 1,3-Dinitrobenzene | 4-Nitrocatechol | 10.5 | 100 |
| 1,4-Dinitrobenzene | 4-Nitrocatechol | 8.4 | 100 |
| 2-Chloro-4-nitrotoluene | 3-Chloro-4-methylcatechol | 8.4 | 68 |
| 4-Chloro-5-methylcatechol | 8.5 | 19 | |
| 4-Chloro-3-methyl-6-nitrophenold | 9.5 | 7 | |
| 3-Chloro-2-methyl-5-nitrophenold | 9.8 | 6 | |
| 2-Chloro-6-nitrotoluene | 4-Chloro-3-methylcatechol | 8.8 | 100 |
| 1-Nitronaphthalene | 1,2-Dihydroxynaphthalene | 10.9, 11.4c | 1 |
| cis-(1,2)-Dihydroxy-1,2-dihydro-8-nitronaphthalene | 12.8, 14.4c | 99 | |
| Toluene | Toluene cis-2,3-dihydrodiol | 5.8,5.9c | 100 |
Product identity based on GC-MS analysis of trimethylsilyl derivatives compared with standards (bold) or interpretation of GC-MS, HPLC, and absorption spectral data (normal type face).
Relative yield of products formed from 2-nitrotoluene determined without derivatization.
Derivatized with butylboronate.
Phenol formed from dehydration of putative unstable cis-2,3-dihydro-6-chloro-4-nitrotoluenediol.
Transformation of 1-nitronaphthalene by NBDO yielded two hydroxylated products. A small amount of 1,2-dihydroxynaphthalene was formed with the concomitant release of nitrite. The amount of 1,2-dihydroxynaphthalene detected by HPLC or GC analysis did not correspond well to the measured nitrite concentration. The discrepancy is probably due to the known instability of 1,2-dihydroxynaphthalene (4). The major product formed from 1-nitronaphthalene gave a mass spectrum consistent with a nitronaphthalene cis-dihydrodiol. Only one compound gave the appropriate molecular ion for a nitrodihydrodiol, indicating a highly regiospecific reaction with 1-nitronaphthalene. The product was tentatively identified as cis-1,2-dihydroxy-1,2-dihydro-8-nitronaphthalene (Table 2) based on analogy with the other products formed from the oxidation of 1-substituted naphthalenes by NDO (27).
Naphthalene and toluene were tested to determine the specificity of NBDO with non-nitro-containing aromatic compounds. Naphthalene was transformed to naphthalene cis-1,2-dihydrodiol, the same product formed by NDO (27). However, the diol formed by NBDO was not homochiral. The enantiomeric composition of this product was 57% with respect to the (+)-(1R,2S) enantiomer. This result is different from those obtained with NDO, which forms enantiomerically pure (+)- (1R,2S)-naphthalene cis-1,2-dihydrodiol (12), and with 2NTDO, which forms 70% of the (+)-(1R,2S) enantiomer (21). Like toluene dioxygenase, NBDO transformed toluene to toluene cis-2,3-dihydrodiol (7), in contrast to NDO, which oxidizes toluene to benzyl alcohol (27).
Determination of relative reaction rates with partially purified OxygenaseNBZ.
OxygenaseNBZ, purified to near homogeneity as determined by SDS-PAGE (data not shown), was used to analyze relative reaction rates with different substrates. The reaction rates obtained with the three nitrotoluene isomers gave further insight into the catalytic specificity of the enzyme (Table 3). NBDO demonstrated a preference for 3-nitrotoluene, as the rate of oxidative transformation was more than twofold greater than that observed with nitrobenzene.
TABLE 3.
Substrate specificity of NBDO
| Substrate | Product(s) | Relative activitya |
|---|---|---|
| Nitrobenzene | Catechol + NO2− | 100 |
| 2-Nitrotoluene | 3-Methylcatechol + NO2− | 59 |
| 2-Nitrobenzyl alcohol | 67 | |
| 3-Nitrotoluene | 4-Methylcatechol + NO2− | 221 |
| 4-Nitrotoluene | 4-Methylcatechol + NO2− | 42 |
| 4-Nitrobenzyl alcohol | 3 | |
| 1,3-Dinitrobenzene | 4-Nitrocatechol + NO2− | 89 |
| 1,4-Dinitrobenzene | 4-Nitrocatechol + NO2−b | BDc |
| 2,6-Dinitrotoluene | 3-Methyl-4-nitrocatechol + NO2− | 24 |
| 1-Nitronaphthalened | 1,2-Dihydroxynaphthalene + NO2− | 30 |
| Naphthalene | Naphthalene cis-1,2-dihydrodiol | 59 |
Reaction mixtures (final volume, 750 μl) contained 100 μM substrate, 200 μM NADH, 19 μg of Reductase2NT, 31 μg of Ferredoxin2NT, and 52 μg of oxygenaseNBZ in 50 mM MES buffer, pH 6.8. Samples were collected after 2 min of incubation. The amounts of nitrite evolved and hydroxylated transformation products that accumulated following incubation were determined as described in Materials and Methods. The values reported are the averages of duplicate reactions with respect to the specific activity with nitrobenzene as the substrate [302 nmol min−1 (mg of OxygenaseNBZ)−1].
No products were detected in this reconstituted enzyme assay. The products listed were identified in the whole-cell biotransformation assays (Table 2).
BD, below detection [<5 nmol min−1 (mg of OxygenaseNBZ)−1].
The rate of nitronaphthalene dihydrodiol (Table 2) production was not determined.
The experiments with dinitrobenzene isomers demonstrated clear differences in substrate preference. Oxidation of both the 1,3- and 1,4 isomers resulted in the production of 4-nitrocatechol. Oxidation of 1,4-dinitrobenzene was not measurable in the assays with reconstituted enzyme, while the rate of 4-nitrocatechol formation from 1,3-dinitrobenzene was comparable to the transformation rate with nitrobenzene (Table 3).
Growth of JS765 with other substrates.
Previous experiments demonstrated that catechol and nitrobenzene are the only aromatic substrates that serve as growth substrates for JS765 (18). However, since 3-nitrotoluene appeared to be a good substrate for NBDO, growth of JS765 was tested on MSB agar plates (supplemented with vitamins) with 3-nitrotoluene supplied as the sole carbon source. JS765 grew well with either nitrobenzene or 3-nitrotoluene. Slight growth was observed with 4-nitrotoluene. 2-Nitrotoluene, 1,3-dinitrobenzene, and naphthalene did not serve as growth substrates.
DISCUSSION
Based on gene order and degree of sequence similarity, NBDO clearly belongs to the naphthalene family of Rieske nonheme iron oxygenases (8). The components of NBDO share the highest degree of similarity with those of 2NTDO. There are no differences in the amino acid sequences of the reductase and ferredoxin components of the two enzymes. Diversity in the amino acid and nucleotide sequences between the nbz and ntd operons (encoding NBDO and 2NTDO from strains JS765 and JS42, respectively) only occurs in the oxygenase subunits. In Rieske nonheme iron oxygenases, in the naphthalene family in particular, it appears that the C-terminal portion of the α subunit is responsible for catalytic specificity (21). As shown in the alignment in Fig. 3, the amino acid residues that are unique to OxygenaseNBZα fall within the C terminus of the protein. Together, these results suggest that electron transfer between the enzyme components is conserved and only the catalytic specificities of the dioxygenase components differ.
The nbz genes (nbzAa, orf2, and nbzAbAcAd; Fig. 2) and the isofunctional ntd genes (20) from strain JS42 are identically organized. A similar organization is seen in the dnt genes, encoding DNTDO from strain DNT (35), and the nag genes, encoding NDO from Ralstonia sp. strain U2 (5). A comparison of gene order and amino acid sequences in these four operons has led to the hypothesis that nitroarene dioxygenase genes have evolved from a parental gene cluster similar to the nag genes present in strain U2 (5, 22). Strain U2 grows with naphthalene and metabolizes salicylate through the gentisate ring cleavage pathway (5) rather than the better-known catechol meta-cleavage pathway. The amino acid sequences of the four components of NBDO are more similar to those of NDO from strain U2 than to the analogous NDO components from strain NCIB 9816-4 (Table 1). The ORF2 sequences in the nbz and ntd operons appear to be truncated forms of nagG, which, in strain U2, encodes the large subunit of salicylate 5-hydroxylase. The relatively high rate of naphthalene oxidation by purified NBDO (Table 3) supports the hypothesis that nitroarene dioxygenases may have evolved from an NDO. Several nitroarene gene clusters have associated transposase genes and insertion sequence (IS) elements, and some of the nitroarene dioxygenase gene clusters have been localized to plasmids (22). The sequence upstream of the nbz operon revealed an IS element with similarity to IS 1631 from Bradyrhizobium japonicum (data not shown).
NDO from strain NCIB 9816-4 has a relaxed substrate specificity and is able to oxidize more than 70 substrates. Thus, it is of interest that NDO is unable to dihydroxylate nitroaromatic compounds with the concomitant release of nitrite (27). The crystal structure of NDO has been solved, and recent work has focused on identifying amino acids in the catalytic domain of the α subunit that control the regioselectivity and enantioselectivity of NDO (24, 26). The structure of NDO shows that 17 amino acids line the substrate binding pocket of the active site (2). Several of these amino acids are present in NBDO, as shown in Fig. 3. However, the residues at positions 206, 253, and 295 (NDO numbering) are unique to NBDO when compared to the related dioxygenases (Fig. 3). We have recently reported that mutations at these three positions in NDO cause changes in the regioselectivity and enantioselectivity of the reactions catalyzed by NDO (37). In addition, phenylalanine 352 in the α subunit of NDO has been shown to play an important role in the regioselectivity and enantioselectivity of NDO (24, 26). In NBDO, there is an isoleucine at position 352. Introduction of an isoleucine at this position in NDO resulted in an enzyme with substrate specificity significantly different from that of the wild-type enzyme (26). If the three-dimensional structures of these enzymes are conserved, it is plausible that a few changes in the amino acids at the active site could have substantial effects on enzyme specificity. There are few changes in the C-terminal portion of the α subunit of NBDO compared to NDO, DNTDO, and especially 2NTDO, yet the products formed from nitrobenzene and nitrotoluenes by these enzymes are very different. 2NTDO oxidizes nitrobenzene to catechol, although not as well as NBDO (21). NBDO appears to favor hydroxylation at the 3,4 positions with the nitrotoluene isomers. This is clearly seen with 3-nitrotoluene. NBDO produces 4-methylcatechol as the sole product, while 2NTDO preferentially produces 3-methylcatechol over 4-methylcatechol by a ratio of 2:1 (21). In addition, 2NTDO is unable to oxidize 2,4-dinitrotoluene, whereas NBDO oxidizes 2,4-dinitrotoluene to a mixture of products (Table 2).
NBDO shows a strong preference for 3-nitrotoluene as a substrate, with a twofold increase in the transformation rate compared to nitrobenzene. A catechol 2,3-dioxygenase (cdoE) gene has been cloned and sequenced from JS765 (25). CdoE oxidizes catechol and 3- and 4-methylcatechol, which explains why JS765 can utilize 3-nitrotoluene and 4-nitrotoluene, as well as nitrobenzene, as carbon sources. We have been unable, however, to demonstrate growth of JS765 with 2-nitrotoluene, despite the production of 3-methylcatechol by NBDO.
The amino acid residues that are unique to the C-terminal portion of NBDO most likely account for the enzyme specificity differences among NBDO, 2NTDO, DNTDO, and NDO. Work has begun to obtain pure NBDO for crystallization in order to determine structural differences between the active sites of NBDO and NDO. The data presented here and those obtained from future experiments will provide further information on the structure-function relationship of oxygenases that could lead to the engineering of oxygenases with new specificities.
Acknowledgments
This work was supported in part by funds from the Strategic Environmental Research and Development Program (to R.E.P. and J.C.S.), the Air Force Office of Scientific Research (to J.C.S.), and the U.S. Army Research Laboratory and the U.S. Army Research Office under grant DAAD19-99-1-0285 (to D.T.G.). D.J.L. has been supported by a National Science Foundation research training grant (DBI9602247), an Augmentation Award for Science and Engineering Research training grant (F49620-97-1-0402), and a National Institutes of Health training fellowship in biotechnology (T32GM8365).
We thank Juan Parales for assistance with the purification of NBDO. We also thank Juan Parales and Frank Lee for providing purified reductase2NT and ferredoxin2NT.
REFERENCES
- 1.An, D., D. T. Gibson, and J. C. Spain. 1994. Oxidative release of nitrite from 2-nitrotoluene by a three-component enzyme system from Pseudomonas sp. strain JS42. J. Bacteriol. 176:7462-7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carredano, E., A. Karlsson, B. Kauppi, D. Choudhury, R. E. Parales, J. V. Parales, K. Lee, D. T. Gibson, H. Eklund, and S. Ramaswamy. 1999. Substrate binding site of naphthalene 1,2-dioxygenase: functional implications of indole binding. J. Mol. Biol. 296:701-712. [DOI] [PubMed] [Google Scholar]
- 3.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 4.Eaton, R. W., and P. J. Chapman. 1992. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J. Bacteriol. 174:7542-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuenmayor, S. L., M. Wild, A. L. Boyles, and P. A. Williams. 1998. A gene cluster encoding steps in the conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J. Bacteriol. 180:2522-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.). 1994. Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 7.Gibson, D. T., M. Hensley, H. Yoshioka, and T. J. Mabry. 1970. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry 9:1626-1630. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, D. T., and R. E. Parales. 2000. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr. Opin. Biotechnol. 11:236-243. [DOI] [PubMed] [Google Scholar]
- 9.Gray, K. M., and E. P. Greenberg. 1992. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri strain isolated from a squid light organ. J. Bacteriol. 174:4384-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haigler, B. E., and J. C. Spain. 1991. Biotransformation of nitrobenzene by bacteria containing toluene degradative pathways. Appl. Environ. Microbiol. 57:3156-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartter, D. R. 1985. The use and importance of nitroaromatic chemicals in the chemical industry, p. 1-14. In D. E. Rickert (ed.), Toxicity of nitroaromatic compounds. Chemical Industry Institute of Toxicology, Washington, D.C.
- 12.Jeffrey, A. M., H. J. C. Yeh, D. M. Jerina, T. R. Patel, J. F. Davey, and D. T. Gibson. 1975. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry 14:575-583. [DOI] [PubMed] [Google Scholar]
- 13.Kauppi, B., K. Lee, E. Carredano, R. E. Parales, D. T. Gibson, H. Eklund, and S. Ramaswamy. 1998. Structure of an aromatic ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure 6:571-586. [DOI] [PubMed] [Google Scholar]
- 14.Keith, L. H., and W. A. Telliard. 1979. Priority pollutants I--a perspective view. Environ. Sci. Technol. 13:416-423. [Google Scholar]
- 15.Knapp, D. R. 1979. Handbook of analytical derivatization reactions. John Wiley & Sons, Inc., New York, N.Y.
- 16.Lee, K., B. Kauppi, R. E. Parales, D. T. Gibson, and S. Ramaswamy. 1997. Purification and crystallization of the oxygenase component of naphthalene dioxygenase in native and selenomethionine derivatized forms. Biochem. Biophys. Res. Commun. 241:553-557. [DOI] [PubMed] [Google Scholar]
- 17.Nishino, S. F., G. C. Paoli, and J. C. Spain. 2000. Aerobic degradation of dinitrotoluenes and the pathway for bacterial degradation of 2,6-dinitrotoluene. Appl. Environ. Microbiol. 66:2139-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino, S. F., and J. C. Spain. 1995. Oxidative pathway for the biodegradation of nitrobenzene by Comamonas sp. strain JS765. Appl. Environ. Microbiol. 61:2308-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino, S. F., J. C. Spain, and Z. He. 2000. Strategies for aerobic degradation of nitroaromatic compounds by bacteria: process discovery to field application, p. 7-61. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. CRC Press, Inc., Boca Raton, Fla.
- 20.Parales, J. V., A. Kumar, R. E. Parales, and D. T. Gibson. 1996. Cloning and sequencing of the genes encoding 2-nitrotoluene dioxygenase from Pseudomonas sp. JS42. Gene 181:57-61. [DOI] [PubMed] [Google Scholar]
- 21.Parales, J. V., R. E. Parales, S. M. Resnick, and D. T. Gibson. 1998. Enzyme specificity of 2-nitrotoluene 2,3-dioxygenase from Pseudomonas sp. strain JS42 is determined by the C-terminal region of the α subunit of the oxygenase component. J. Bacteriol. 180:1194-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parales, R. E. 2000. Molecular biology of nitroarene degradation, p. 63-89. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. CRC Press, Inc., Boca Raton, Fla.
- 23.Parales, R. E., M. D. Emig, N. A. Lynch, and D. T. Gibson. 1998. Substrate specificities of hybrid naphthalene and 2,4-dinitrotoluene dioxygenase enzyme systems. J. Bacteriol. 180:2337-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parales, R. E., K. Lee, S. M. Resnick, H. Jiang, D. J. Lessner, and D. T. Gibson. 2000. Substrate specificity of naphthalene dioxygenase: effect of specific amino acids at the active site of the enzyme. J. Bacteriol. 182:1641-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parales, R. E., T. A. Ontl, and D. T. Gibson. 1997. Cloning and sequence analysis of a catechol 2,3-dioxygenase gene from the nitrobenzene-degrading strain Comamonas sp. JS765. J. Ind. Microbiol. Biotechnol. 19:385-391. [DOI] [PubMed] [Google Scholar]
- 26.Parales, R. E., S. M. Resnick, C. L. Yu, D. R. Boyd, N. D. Sharma, and D. T. Gibson. 2000. Regioselectivity and enantioselectivity of naphthalene dioxygenase during arene cis-dihydroxylation: control by phenylalanine 352 in the α subunit. J. Bacteriol. 182:5495-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resnick, S. M., K. Lee, and D. T. Gibson. 1996. Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J. Ind. Microbiol. 17:438-457. [Google Scholar]
- 28.Resnick, S. M., D. S. Torok, K. Lee, J. M. Brand, and D. T. Gibson. 1994. Regiospecific and stereoselective hydroxylation of 1-indanone and 2-indanone by naphthalene dioxygenase and toluene dioxygenase. Appl. Environ. Microbiol. 60:3323-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieger, P.-G., and H.-J. Knackmuss. 1995. Basic knowledge and perspectives on biodegradation of 2,4,6-trinitrotoluene and related nitroaromatic compounds in contaminated soil, p. 1-18. In J. C. Spain (ed.), Biodegradation of nitroaromatic compounds. Plenum Press, New York, N.Y.
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 32.Spain, J. C. 1995. Bacterial biodegradation of nitroaromatic compounds under aerobic conditions, p. 19-35. In J. C. Spain (ed.), Biodegradation of nitroaromatic compounds. Plenum Press, New York. N.Y.
- 33.Spain, J. C. 1995. Biodegradation of nitroaromatic compounds. Annu. Rev. Microbiol. 49:523-555. [DOI] [PubMed] [Google Scholar]
- 34.Stanier, R. Y., N. J. Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads; a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 35.Suen, W.-C., B. E. Haigler, and J. C. Spain. 1996. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J. Bacteriol. 178:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 37.Yu, C.-L., R. E. Parales, and D. T. Gibson. 2001. Multiple mutations at the active site of naphthalene dioxygenase affect regioselectivity and enantioselectivity. J. Ind. Microbiol. Biotechnol. 27:24-103. [DOI] [PubMed] [Google Scholar]