Abstract
Tetrachloroethene (PCE) dehalorespiration was investigated in a continuous coculture of the sulfate-reducing bacterium Desulfovibrio fructosivorans and the dehalorespiring Desulfitobacterium frappieri TCE1 at different sulfate concentrations and in the absence of sulfate. Fructose (2.5 mM) was the single electron donor, which could be used only by the sulfate reducer. With 2.5 mM sulfate, the dehalogenating strain was outnumbered by the sulfate-reducing bacterium, sulfate reduction was the dominating process, and only trace amounts of PCE were dehalogenated by strain TCE1. With 1 mM sulfate in the medium, complete sulfate reduction and complete PCE dehalogenation to cis-dichloroethene (cis-DCE) occurred. In the absence of sulfate, PCE was also completely dehalogenated to cis-DCE, and the population size of strain TCE1 increased significantly. The results presented here describe for the first time dehalogenation of PCE by a dehalorespiring anaerobe in strict dependence on the activity of a sulfate-reducing bacterium with a substrate that is exclusively used by the sulfate reducer. This interaction was studied under strictly controlled and quantifiable conditions in continuous culture and shown to depend on interspecies hydrogen transfer under sulfate-depleted conditions. Interspecies hydrogen transfer was demonstrated by direct H2 measurements of the gas phase and by the production of methane after the addition of a third organism, Methanobacterium formicicum.
Some published results seem to indicate that dehalogenating and/or dehalorespiring bacteria and sulfate-reducing bacteria often share biotopes that are contaminated with chlorinated compounds. In this context for example, Gerritse et al. (23) reported on the isolation of a Desulfovibrio species (strain SULF1) and a Desulfitobacterium species (strain TCE1; now identified and deposited as Desulfitobacterium frappieri TCE1 [24]) from the same reactor, which was inoculated with a tetrachloroethene (PCE)-contaminated soil slurry. Both strains grew well in the presence of high concentrations of PCE (up to 30 mM) and sulfate (up to 20 mM) as electron acceptors. In another example, Drzyzga et al. (16) demonstrated dehalogenation of 2- and 4-fluorobenzoate by a freshwater coculture under sulfate-reducing conditions. One member of this coculture was identified as a Desulfotomaculum species, which was able to mineralize the aromatic intermediates, whereas the other unidentified strain was proposed to be responsible for the dehalogenation reaction. Because the authors of that study were not successful in separating both strains (only the spore-forming Desulfotomaculum species was obtained after pasteurization), they suggested that a symbiotic relationship between these two strains might have been essential when grown with halogenated compounds as the sole carbon source. Difficulties in separating dehalogenating and sulfate-reducing strains from a coculture were also reported by Mägli et al. (32), who isolated the dichloromethane-degrading Dehalobacterium formicoaceticum from a Desulfovibrio species (strain DMB) in a two-member coculture. These authors concluded that there might be a strong dependence on unknown growth factors for the dechlorinating bacterium released by the sulfate reducer. Indeed, by using spent medium from Desulfovibrio sp. strain DMB as a source of growth factors, they were successful in isolating Dehalobacterium formicoaceticum in pure culture.
In model coculture bioreactor experiments conducted by Eisenbeis (18), the successful cocultivation of Dehalospirillum multivorans and Desulfovibrio desulfuricans was demonstrated with sulfate and PCE as electron acceptors and with ethanol as electron donor. More recently, in a defined chemostat coculture study of Drzyzga et al. (15), the combined presence of sulfate and sulfate-reducing bacteria was reported to affect dechlorination of PCE mediated by a dehalorespiring anaerobe. Competition for shared electron donors (e.g., lactate) occurred and, particularly under sulfate-limiting and sulfate-depleted conditions, hydrogen equivalents from substrates used by the sulfate reducer were channelled to the dechlorinating anaerobe, which used these electron donors (hydrogen equivalents) along with the shared substrate (lactate) for PCE dehalorespiration and growth.
On-site studies by Chapelle (7) showed that reductive dehalogenation was mostly favored in methanogenic and sulfate-reducing zones and Drzyzga (unpublished results) also found that sulfate-reducing activity took place first in anoxic soil enrichments, soil slurries, and soil column experiments before dehalogenation could be detected. Finally, in addition to the aforementioned studies, a few further reports on co-metabolically mediated dehalogenation of halogenated organic compounds under sulfate-reducing conditions, but mostly with undefined mixed cultures and enrichments, have been described in the literature (see, for example, references 1, 3, 6, 8, 16, 24, 34, and 39). In many of these studies, significant dehalogenation of halo-organics has been observed (only) under very reduced anoxic conditions. These findings are supported further by the recently published calculations of Haas and Shock (28). These authors concluded that anaerobic microbially mediated degradation of various chloroethenes is especially favored under conditions at least sufficiently reducing to also promote sulfate reduction. Thus, syntrophic associations between sulfate-reducing bacteria and dehalogenating or dehalorespiring bacteria may be of great significance in anoxic, halo-organic-contaminated habitats but still require further investigation if effective clean-up strategies for polluted sites via bioremediation techniques are to be developed.
Much like various other dehalorespiring bacteria (e.g., Desulfomonile tiedje, Desulfuromonas chloroethenica, Dehalobacter restrictus, Dehalospirillum multivorans, and Dehalococcoides ethenogenes [12, 19, 22, 24, 26, 29, 34, 42]), the desulfitobacteria have attracted a great deal of interest over the past few years because they have considerable potential for the degradation of polyhalogenated soil and groundwater pollutants such as PCE, trichloroethene (TCE), carbon tetrachloride, and pentachlorophenol (1, 13, 14, 17, 19, 25, 30, 33, 38, 43). However, very little is known to date about biotic interactions between these dehalorespiring and sulfate-reducing bacterial species, which may interfere with the dehalogenation during anoxic degradation of chlorinated pollutants. For this reason we investigated the nature and details of the interaction between these two types of anaerobic organisms. We examined the possibility of PCE dehalorespiration of Desulfitobacterium frappieri TCE1 in strict dependence on the activity of sulfate-reducing bacterium Desulfovibrio fructosivorans at different sulfate concentrations in the presence of a substrate (fructose), which could exclusively be used by the sulfate reducer. Sulfate cannot be used as an alternative electron acceptor by Desulfitobacterium frappieri TCE1 and has no negative influence on PCE or TCE dehalorespiration when added in relatively high concentrations (up to 20 mM) to pure cultures of this anaerobe (23).
MATERIALS AND METHODS
Organisms, medium composition, and anoxic cultivation.
Desulfitobacterium frappieri TCE1 was isolated previously from an anoxic PCE-dechlorinating continuous mixed culture obtained with lactate as the primary carbon and electron source (24). Desulfitobacterium frappieri TCE1 has been deposited in the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) under the collection number DSM 12704. Desulfovibrio fructosivorans (35) and Methanobacterium formicicum (4) were obtained from the DSMZ (deposit numbers 3604 and 1535, respectively). Anoxic freshwater mineral medium was prepared under an N2-CO2 atmosphere (80:20 [vol/vol]) and contained the following components (in grams/liter): NaCl, 1.0; KH2PO4, 0.2; NH4Cl, 0.3; MgCl2 · 6H2O, 0.15; KCl, 0.5; and CaCl2 · 2H2O, 0.15. The medium also contained the redox indicator resazurin (1%) at 100 μl/liter. After autoclaving, tungsten-selenium solution, vitamin solution, and trace element solution were added from separately sterilized stocks as described elsewhere (16). The hydrogen carbonate-buffered medium (30 mM NaHCO3) was reduced to a negative redox potential by the addition of Na2S (1 mM). Filter-sterilized fructose and autoclaved sodium sulfate were added to the reservoir medium vessel from separate stock solutions. PCE was filter sterilized (0.2-μm [pore-size] PTFE filters; Alltech, Breda, The Netherlands) and was directly supplied to the reservoir medium at its maximum water solubility (ca. 600 μM) at room temperature, resulting in a PCE concentration in the liquid of the reactor of 170 ± 20 μM only. This means that more than two-thirds of this volatile compound was removed via the gas flush of the headspace of the bioreactor. This value was determined during the first 2 days of the study, when the reactor was driven under sulfate-reducing conditions with the sulfate reducer present as the single organism in the culture liquid (see also next paragraph). The final pH of the medium was adjusted to 7.1 ± 0.1 with 2.5 N HCl.
For the continuous-culture studies, a bioreactor, which was constructed of glass, stainless steel, and viton tubing with a working volume of 1,000 ml, was operated at 34.5°C. The dilution rate was set to 0.02 h−1. The pH was measured daily and was constant during the 50-day incubation period due to the presence of the carbonate buffer used. The culture was continuously stirred at 250 rpm. To avoid accumulation of toxic hydrogen sulfide and volatile cis-dichloroethene (cis-DCE), the gas phase of the chemostat was flushed with N2-CO2 (80:20 [vol/vol]) at a flow rate of ca. 50 ml h−1.
Reactor setup.
Initially, the bioreactor was started under sulfate-reducing conditions with Desulfovibrio fructosivorans inoculated into the reactor. Two days later, a PCE pregrown culture of Desulfitobacterium frappieri TCE1 (taken from the exponential growth phase) was added to the bioreactor to give a coculture. Approximately 1 week later, the first of four different stable conditions was achieved with fructose (2.5 mM in the reservoir medium), which was exclusively consumed by Desulfovibrio fructosivorans as a source of carbons and electrons. The first stable condition was achieved with 1 mM sulfate as the electron donor for the sulfate reducer, which was stoichiometrically limiting relative to the fructose concentration. After an increase of the sulfate concentration to 2.5 mM from day 9 onward, the second stable condition was achieved. Sulfate was now offered in an equimolar concentration to carry out incomplete oxidation of the entire amount of fructose according to the following equation (9): C6H12O6 + SO42− → 2CH3COO− + HS− + 2HCO3− + 3H+. In the third stable condition, sulfate was omitted from the reservoir medium (from day 19 onward) to allow fructose fermentation only. During these three stable conditions, PCE was present in the medium as electron acceptor for Desulfitobacterium frappieri TCE1. At day 32, PCE was omitted from the medium and at day 34, the methanogenic strain Methanobacterium formicicum was introduced to the culture liquid to replace the dehalorespiring strain and to demonstrate likely interspecies hydrogen transfer. After these changes of parameters, the fourth stable condition was obtained from day 42 onward. All stable conditions were kept for 6 to 8 days, which (according to the dilution rate of 0.02 h−1) corresponded to three to four complete volume changes of the liquid of the reactor.
Chemical determinations and further methods.
Organic acids and gases were analyzed by gas chromatography (with a flame ionization detector [FID] and a thermal conductivity detector [TCD], respectively) by using equipment and conditions described earlier (26, 27). Samples for gas measurements (0.5 ml) were taken directly from the gas phase of the bioreactor. Fructose concentrations were measured with a Pharmacia high-pressure liquid chromatograph equipped with a differential refractometer detector. The following conditions and materials were used: a OA HY Polyspher Column (Merck, Darmstadt, Germany) in a 65°C oven, an isocratic eluent (0.01 N H2SO4), a flow rate of 0.6 ml/min, and an injection volume of 25 μl. Chlorinated ethenes in the culture liquid were determined via triplicate headspace analyses by FID capillary gas chromatography (25). Bacterial optical densities were measured with a Biotron 101 colorimeter (Meyvis, Bergen op Zoom, The Netherlands) at a wavelength of 660 nm. Sulfide in the culture liquid was analyzed colorimetrically as described by Trüper and Schlegel (41). Sulfate was determined by precipitation with barium chloride (11). Cell numbers of the morphologically different strains were determined by counting individual cells directly with a light microscope and by using a Bürker-Türk counting chamber.
Chemicals.
All chemicals were obtained from commercial sources, and the highest purity available (>98%) was used.
RESULTS
Continuous culture with fructose and two different sulfate concentrations.
To study the interactions and dependences between the dehalorespiring strain Desulfitobacterium frappieri TCE1 and the sulfate-reducing strain Desulfovibrio fructosivorans, continuous cocultures of both types of bacteria were grown under sulfate-limiting (1 mM sulfate) and sulfate-sufficient (2.5 mM sulfate) conditions. The changes in coculture composition (by means of total cell numbers) and the dehalogenating activity of strain TCE1 were investigated with fructose (2.5 mM) serving as the single electron donor substrate, which exclusively was used by the sulfate reducer. Figure 1 depicts the various parameters measured during the four different stable situations, which were achieved in the bioreactor. Sulfate, when present, was always consumed completely, and the sulfide amount balance never gave 100%, because ca. 0.6 mM of (hydrogen) sulfide was removed via the gas flow of the bioreactor headspace.
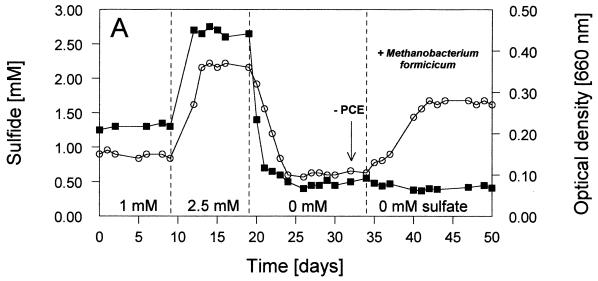
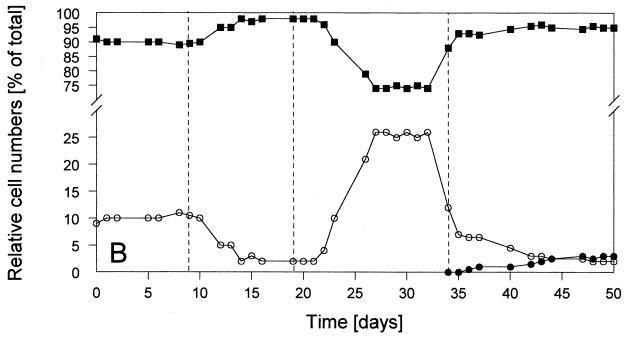
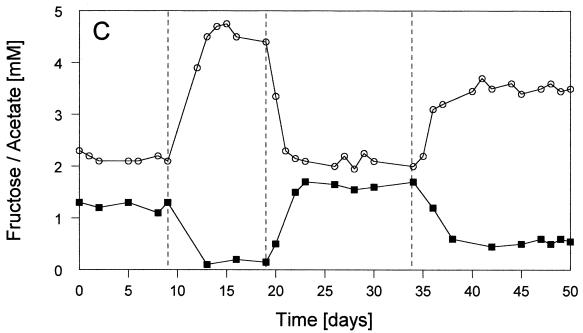
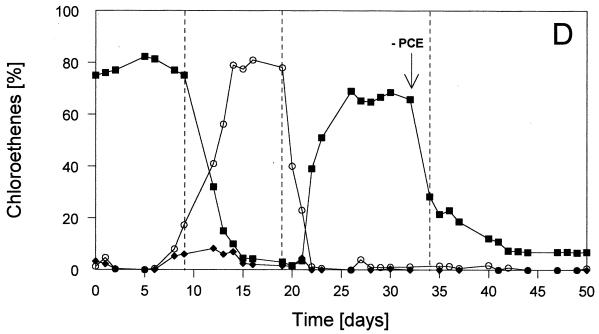
FIG. 1.
Time courses of relevant parameters of the defined continuous cultures during the experiment with various sulfate and PCE concentrations in the reservoir medium. Culture conditions: stable condition 1 with 1 mM SO42− plus PCE; stable condition 2 with 2.5 mM SO42− plus PCE; stable condition 3 without SO42− and with PCE; stable condition 4 without SO42− and without PCE; pH 7.1 (±0.1); 34.5°C; dilution rate, 0.02 h−1; electron donor, 2.5 mM fructose. PCE was present at 170 (±20) μM (=100%). PCE was omitted from the reservoir medium at day 32 (as indicated by the arrow). Methanobacterium formicicum was added at day 34. (A) Optical densities (○) and sulfide concentrations (▪) in the liquid. (B) Relative cell numbers in the percentages of Desulfitobacterium frappieri TCE1 (○), Desulfovibrio fructosivorans (▪), and Methanobacterium formicicum (•) (for 100%, see total values in Table 2). (C) Concentrations of fructose (▪) and acetate (○) in the culture liquid during sulfate reduction and/or fermentation by Desulfovibrio fructosivorans. (D) Concentrations of PCE (○), TCE (⧫), and cis-DCE (▪) in the culture liquid.
The first stable condition (condition 1 with 1 mM SO42−) was dominated by the sulfate reducer population (∼90% of total cell numbers; Fig. 1B), leading to complete reduction of sulfate to sulfide (Fig. 1A). The optical density of the coculture at 660 nm was relatively low (i.e., ∼0.15) because growth was limited by SO42−and fructose was not completely consumed (Fig. 1C). In contrast, dehalorespiration of all of the available PCE with concomitant conversion to cis-DCE was accomplished (see Fig. 1D). The use of PCE as an electron acceptor for the growth of strain Desulfitobacterium frappieri TCE1 resulted in a small but stable population of this anaerobe (∼10% of the total cells; Fig. 1B). Table 1 (condition 1) shows that the main part of the consumed fructose (1 mM) was oxidized during sulfate reduction and that a less significant part (0.3 mM) was used fermentatively with the release of H2 equivalents that stimulated PCE dehalorespiration and growth of Desulfitobacterium frappieri TCE1. Obviously, a combination of both sulfate reduction and fermentation was carried out by the sulfate-reducing bacterium during this first stable condition in the coculture. Nevertheless, no hydrogen was detectable in the gas phase of the bioreactor when we conducted a gas stop-flow experiment for 9 h during this stable condition (i.e., condition 1 [data not shown]).
TABLE 1.
Carbon balances of fructose (2.5 mM) and acetate during sulfate reduction and fermentation by Desulfovibrio fructosivorans in mixed cultures under the four different stable conditions
| Conditiona | Carbon fluxes of fructose and acetate (mM)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Fructose totally consumed | Fructose unused | Acetate totally detected | Fructoseb used for SRc | Acetateb produced by SRc | Fructoseb used for fermentation | Acetateb produced by fermentation | Acetate balance gapd | |
| 1 | 1.3 | 1.2 | 2.1 | 1.0 | 2.0 | 0.3 | 0.6 | −0.5 |
| 2 | 2.4 | 0.1 | 4.4 | 2.4 | 4.8 | 0 | 0 | −0.4 |
| 3 | 0.8 | 1.7 | 2.1 | 0 | 0 | 0.8 | 1.6 | 0.5 |
| 4 | 1.9 | 0.6 | 3.5 | 0 | 0 | 1.9 | 3.8 | −0.3 |
The different stable conditions are described in the text and in the legend of Fig. 1.
The theoretical values of fructose used and acetate produced were calculated according to the following stoichiometric equations: C6H12O6 + SO42− → 2CH3 − COO− + 2HCO3− + HS− + 3H+ for incomplete fructose oxidation during sulfate reduction (9) and C6H12O6 + 4H2O → 2CH3 − COO− + 2HCO3− + 4H++ 4H2 for hexose fermentation (10).
SR, sulfate reduction.
These values express the lacking acetate amounts likely used for biomass formation (except for condition 3, where the detected acetate concentration was too high).
After the transition to an increased supply of sulfate (2.5 mM SO42− from day 9 onward), the second stable condition, condition 2, was achieved. Sulfate reduction was the dominant process leading to the complete conversion of all sulfate to sulfide (Fig. 1A), and the sulfate reducer outnumbered the dehalorespiring strain almost completely (∼98% of the Desulfovibrio fructosivorans cells versus ∼2% of the Desulfitobacterium frappieri TCE1 cells; Fig. 1B). The highest optical density during the entire experiment was noted under stable condition 2 (A660 ∼0.37; Fig. 1A). Fructose was almost completely consumed, leading to the highest acetate production compared to the other stable conditions (Fig. 1C). The results presented in Table 1 (condition 2) clearly show that the fermentation of fructose was not carried out by the sulfate reducer and, as a consequence of almost no production of H2 equivalents, unused PCE accumulated, and only trace amounts of TCE and cis-DCE were detected in the culture liquid (Fig. 1D). As under condition 1, hydrogen in the gas phase of the reactor was not detected with this stable condition 2 (data not presented).
Continuous culture with fructose in the absence of sulfate.
At day 19, sulfate, as the electron acceptor for the Desulfovibrio species, was omitted and, 1 week later, stable condition 3 was obtained (Fig. 1A). PCE was now the single available electron acceptor remaining in the coculture. Under this most interesting stable condition, the ratio between the two populations changed significantly compared with that of the two earlier ones. As shown in Fig. 1B, the relative contribution of the sulfate reducer decreased to 74% of the coculture, and the cell numbers of the dehalorespiring species increased to a total of 26% (see also Table 2). The decrease of the total cell numbers of the sulfate reducer resulted in a decrease of the optical density of the coculture (A660 ∼0.11; Fig. 1A). This was compensated for only to some extent by the increased total cell numbers of the dehalorespiring species. Table 2 summarizes the actual cell numbers of the individual populations in the mixed cultures under stable conditions 1 to 4. In the absence of sulfate, all PCE was dechlorinated to cis-DCE without the occurrence of further intermediates (Fig. 1D). Similar to stable condition 1, fructose was not completely consumed, and the acetate production was in an identical amount (∼2.1 mM; Fig. 1C). Table 1 (condition 3) clearly shows that fermentation of ca. 0.8 mM fructose was the only metabolic process carried out in the coculture. Under stable condition 3 hydrogen was detected in the gas phase of the reactor for the first time. This gas accumulated during a 9-h gas stop-flow experiment (data not presented).
TABLE 2.
Culture cell counts of Desulfitobacterium frappieri TCE1, Desulfovibrio fructosivorans, and Methanobacterium formicicum at different optical densities during different stable conditions
| Conditiona | OD660b | Relative cell no. (cells/ml)
|
||
|---|---|---|---|---|
| D. frappieri TCE1 | D. fructosivorans | M. formicicum | ||
| 1 | 0.15 | l3.4 × 108 | 3.2 × 109 | None |
| 2 | 0.37 | 1.9 × 108 | 9.2 × 109 | None |
| 3 | 0.11 | 4.9 × 108 | 1.6 × 109 | None |
| 4 | 0.28 | 5.9 × 107 | 6.2 × 109 | 3.3 × 108 |
The different stable conditions are described in the text and in the legend of Fig. 1.
OD660, the optical densities of the culture were determined at a wavelength of 660 nm.
Continuous culture with fructose in the absence of sulfate and PCE.
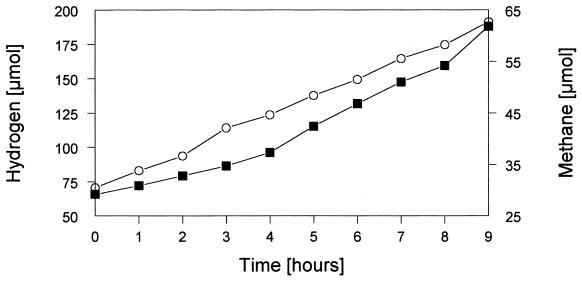
To demonstrate that hydrogen production was carried out by the sulfate reducer during fructose fermentation, which formed the basis for interspecies hydrogen transfer, a new organism was introduced to the reactor. Instead of Methanospirillum hungatei, which was used as a syntrophic partner in a similar coculture experiment by Cord-Ruwisch et al. (9), we inoculated the culture with Methanobacterium formicicum. Therefore, PCE was omitted from the reservoir medium at day 32 onward to wash out the dehalogenating species from the bioreactor, and a pure culture of Methanobacterium formicicum was added at day 34. Eight days later, the final stable condition (i.e., condition 4) was obtained without an electron acceptor that could be used by sulfate reducers and/or dehalorespirers (and thus without sulfate and PCE; Fig. 1A and D). Only the methanogenic bacterium was expected to grow by using H2 via interspecies hydrogen transfer and CO2 that was available in the carbonate-buffered medium and via the gas flow (N2-CO2, 80:20 [vol/vol]). The growth of this strain (visible by fluorescence microscopy) and the production of methane were the growth parameters measured. One day after the addition of the methanogenic strain, low concentrations of methane were detected in the gas phase of the bioreactor. The methane concentration increased during the next few days and reached a constant level from day 42 onward (data not shown), when the optical density of the culture had also reached a constant level (Fig. 1). The accumulation of methane and hydrogen during stable condition 4 is illustrated in Fig. 2, representing a 9-h gas stop-flow experiment. Within these few hours, the methane concentration (from ∼30 to ∼62 μmol/liter) and the hydrogen concentration (from ∼70 to ∼190 μmol/liter) increased significantly. Obviously, H2 was made available in excess by the fermentative activity of the sulfate reducer, because it was not used completely by the methanogenic strain, resulting in the accumulation of this gas. During this fourth stable condition a few interesting additional observations were made. The optical density of the complete culture increased again significantly to an A660 of ∼0.28 (Fig. 1A) and the sulfate-reducing species regained a very high percentage of total cell numbers (∼94%; Fig. 1B and Table 1). As shown in Fig. 1C and as summarized in Table 1, ∼80% of fructose was consumed by fermentation (ca. 0.6 mM remained), and the concentration of acetate increased to ca. 3.5 mM. We suppose that the strong stimulation of growth of Desulfovibrio fructosivorans was due to the presence of two hydrogen-consuming strains (Methanobacterium formicicum and remaining cells of Desulfitobacterium frappieri TCE1; see Table 2) as syntrophic partners. However, the accumulation of hydrogen (as presented in the gas stop-flow experiment; Fig. 2) clearly showed that much more H2 was produced than consumed by the syntrophic partners. Obviously, hydrogen was kept at a sufficiently low level that fructose could be fermented by Desulfovibrio fructosivorans with the release of H2 equivalents subsequently used by the methanogenic and the dehalorespiring species, resulting in a stable triculture (Fig. 1B). Interestingly, the microscopic picture revealed an intimate contact between the sulfate reducer and the methanogen since nearly every cell of Methanobacterium formicicum was surrounded by high numbers of Desulfovibrio fructosivorans cells, indicating a tight syntrophic association between the two strains. The possibility that part of the transfer of the reducing equivalents was in the form of formate cannot be excluded completely, especially if it was consumed very rapidly and so never detected in the bioreactor liquid by the methods used here.
FIG. 2.
Accumulation of hydrogen (○) and methane (▪) in the reactor gas phase of the continuous triculture during a 9-h gas stop-flow experiment in stable condition 4 (which was with Methanobacterium formicicum). Conditions are as described in the legend of Fig. 1.
An unexpected finding was the occurrence of stable cell numbers of the dehalorespiring species in spite of the fact that PCE was omitted from the reservoir medium. As shown in Fig. 1D, cis-DCE was continuously quantifiable in the culture liquid in low but constant concentrations (ca. 7% of cis-DCE resulting from ∼12 μM PCE). The reason for that might be a thin biofilm, which had been developed on the inner glass wall of the bioreactor during the long incubation time of the entire study. This biofilm was observed for the first time at the end of the second stable condition (at day 20) and may have functioned as a depository of PCE and therefore as a continuous source for available electron acceptor for the dehalorespiring strain after the exclusion of PCE from the reservoir medium. As a consequence of the development of this biofilm, the cell counts of all strains as listed in Table 2 may be slightly underestimated.
DISCUSSION
In this study we demonstrated that growth and PCE dehalorespiration are carried out by Desulfitobacterium frappieri TCE1 in strict dependence on the activity of a sulfate-reducing bacterium at the expense of an electron donor used exclusively by the sulfate reducer. Because of the hydrogen formation by the sulfate reducer during fructose fermentation (verified by direct hydrogen measurement and by the methane production through Methanobacterium formicicum), we propose that interspecies hydrogen transfer occurred in the coculture, leading to dehalorespiration and growth of Desulfitobacterium frappieri TCE1 with PCE as the electron acceptor. Interspecies hydrogen transfer to a methanogen (Methanospirillum hungatei) by Desulfovibrio fructosivorans in the absence of sulfate during fructose fermentation was demonstrated earlier by Cord-Ruwisch et al. (9). However, this is the first detailed report on such a syntrophic association between a sulfate-reducing bacterium and a dehalorespiring bacterium grown under defined conditions in continuous culture.
The production of hydrogen by the sulfate-reducing strain occurred only at low sulfate concentrations, under sulfate-depleted conditions, or in the absence of sulfate and excess of fructose. Various studies in the past have shown that sulfate-reducing bacteria (for example, Desulfovibrio vulgaris, Desulfovibrio gigas, and Desulfovibrio desulfuricans) are able to metabolize substrates such as lactate to acetate, CO2, and H2 when grown in the absence of sulfate or in media with low sulfate concentrations, in syntrophic association with H2-consuming bacteria keeping the hydrogen partial pressure low (for example, Desulfovibrio vulgaris with Methanosarcina barkeri or Desulfovibrio fructosivorans with Methanospirillum hungatei [5, 6, 9, 40]). It was also found that a few Desulfovibrio species can either utilize or produce hydrogen (mediated by reversible hydrogenases or two different hydrogenases) during the degradation of organic matter (for example, Desulfovibrio vulgaris, Desulfovibrio gigas, Desulfovibrio salexigens, and Desulfovibrio baculatus [20]). It is thus interesting that Pankhania et al. (36) found, with Desulfovibrio vulgaris (strain Marburg) grown on lactate, that in the presence of sulfate, the H2-evolving system was not completely suppressed and led to a minor loss of reducing power as H2.
We therefore suggest that Desulfovibrio fructosivorans used the substrate fructose fermentatively according to the equation C6H12O6 + 4H2O → 2CH3-COO− + 2HCO3− + 4H+ + 4H2 as described by Cord-Ruwisch et al. (10). This pathway of hexose fermentation via acetate, carbon dioxide, and hydrogen also appears in homoacetogenic bacteria (10). Desulfovibrio fructosivorans used the hexose fermentatively, thereby producing hydrogen and growing in a syntrophic association together with the dehalorespiring Desulfitobacterium frappieri strain TCE1. Our findings here and a those of a few earlier reports on the occurrence of sulfate-reducing bacteria in coculture with dehalogenating bacteria (see above and references 1, 6-8, 15, 16, 23, and 32) may indicate the possible ecological function of sulfate reducers as hydrogen-providing organisms in strictly anoxic environments that are polluted by halo-organic compounds. Therefore, it is important to note that bacterial dehalogenation and/or dehalorespiration of halo-organic compounds may be indirectly stimulated by the activity of (fermenting) sulfate reducers under sulfate-limiting or sulfate-depleted conditions that often appear in freshwater and soil environments.
The ecological importance of hydrogen as electron donor for dehalorespiration and/or dehalogenation of chloroorganic compounds, as well as the competition for hydrogen in anoxic habitats are frequently described in the literature (2, 10, 21, 37, 44). Some of these earlier studies and the recently published results of Löffler et al. (31) indicate that organochlorine-reducing bacteria can be excellent competitors for H2 in anoxic environments and may be the predominant hydrogenotrophic organisms in organochlorine-contaminated sites. Apparently, these bacteria are able to maintain H2 concentrations below the threshold H2 concentrations that would allow sulfate reduction or methanogenesis. Hence, chlororespiring bacteria may very well abound in anoxic environments as long as reductive dechlorination is not limited by the concentration and bioavailability of chlorinated compounds serving as their terminal electron acceptors (31). Our findings indicate that hydrogen was indeed the electron donor used for dehalorespiration of PCE mediated by Desulfitobacterium frappieri TCE1, which was channeled from a readily used substrate such as fructose as the primary electron donor for other bacterial species.
Slowly degradable hydrogen-releasing compounds such as polylactate esters, paper or wood chips, compost extract, or other natural organic matter (especially propionic acid) which deliver H2 continuously over long time periods had already been shown in some studies to be excellent substrates for regulating the supply rate of the electron donors for bioremediation purposes (21, 37, 44). This is in agreement with results obtained with coculture studies of Desulfitobacterium frappieri TCE1 with Desulfovibrio gigas on propanol or with Desulfobulbus propionicus on propionate, which also showed significant PCE dehalorespiration by strain TCE1 via substrates (propanol and propionate in these cases) not directly used by the dehalorespiring strain (O. Drzyzga et al., unpublished results). These results demonstrate that possibly a wide range of substrates (sugars, fatty acids, alcohols, and perhaps also aromatic compounds) not directly used by a dehalorespiring bacterium can be used indirectly for dehalorespiration and growth via interspecies hydrogen and acetate transfer mediated by sulfate-reducing bacteria. Environmentally, a consortium of sulfate-reducing and dehalogenating bacteria may therefore be important with respect to the cleanup of organochlorine-contaminated ecosystems, especially at sites with low sulfate concentrations (e.g., freshwater and soils) that are polluted by both halogenated compounds and heavy metals due to the simultaneous precipitation of these metals as metal sulfides.
Acknowledgments
This work was partially financed by a grant from the European Union (contract number BIO4-CT98-0303).
REFERENCES
- 1.Bagley, D. M., and J. M. Gossett. 1990. Tetrachloroethene transformation to tri-chloroethene and cis-1,2-dichloroethene by sulfate-reducing enrichment cultures. Appl. Environ. Microbiol. 56:2511-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballapragada, B. S., H. D. Stensel, J. A. Puhakka, and J. F. Ferguson. 1997. Effect of hydrogen on reductive dechlorination of chlorinated ethenes. Environ. Sci. Technol. 31:1728-1734. [Google Scholar]
- 3.Boyle, A. W., C. D. Phelps, and L. Y. Young. 1999. Isolation from estuarine sediments of a Desulfovibrio strain which can grow on lactate coupled to the reductive dehalogenation of 2,4,6-tribromophenol. Appl. Environ. Microbiol. 65:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant, M. P., and D. R. Boone. 1987. Isolation and characterization of Methanobacterium formicicum MF. Int. J. Syst. Bacteriol. 37:171. [Google Scholar]
- 5.Bryant, M. P., L. L. Campbell, C. A. Reddy, and M. R. Crabill. 1977. Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl. Environ. Microbiol. 33:1162-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabirol, N., F. Jacob, J. Perrier, B. Fouillet, and P. Chambon. 1998. Interaction between methanogenic and sulfate-reducing microorganisms during dechlorination of a high concentration of tetrachloroethylene. J. Gen. Appl. Microbiol. 44:297-301. [DOI] [PubMed] [Google Scholar]
- 7.Chapelle, F. H. 1996. Technical report of the Environmental Protection Agency, p. 19-20. Report EPA/540/R-96/509. U.S. Environmental Protection Agency, Dallas, Tex.
- 8.Colberg, P. J. S. 1990. Role of sulfate in microbial transformations of environmental contaminants: chlorinated aromatic compounds. Geomicrobiol. J. 8:147-165. [Google Scholar]
- 9.Cord-Ruwisch, R., B. Ollivier, and J.-L. Garcia. 1986. Fructose degradation by Desulfovibrio sp. in pure culture and in coculture with Methanospirillum hungatei. Curr. Microbiol. 13:285-289. [Google Scholar]
- 10.Cord-Ruwisch, R., H. J. Seitz, and R. Conrad. 1988. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch. Microbiol. 149:350-357. [Google Scholar]
- 11.Cypionka, H., and N. Pfennig. 1986. Growth yields of Desulfotomaculum orientis with hydrogen in chemostat culture. Arch. Microbiol. 143:396-399. [Google Scholar]
- 12.Damborsky, J. 1999. Tetrachloroethene-dehalogenating bacteria. Folia Microbiol. 44:247-262. [DOI] [PubMed] [Google Scholar]
- 13.DeWeerd, K. A., W. P. Flanagan, M. J. Brennan, J. M. Principe, and J. L. Spivack. 1998. Biodegradation of trichloroethylene and dichloromethane in contaminated soil and groundwater. Bioremed. J. 2:29-42. [Google Scholar]
- 14.Dolfing, J., and J. E. M. Beurskens. 1995. The microbial logic and environmental significance of reductive dehalogenation. Adv. Microb. Ecol. 14:143-206. [Google Scholar]
- 15.Drzyzga, O., J. Gerritse, J. A. Dijk, H. Elissen, and J. C. Gottschal. 2001. Coexistence of a sulphate-reducing Desulfovibrio species and the dehalo-respiring Desulfitobacterium frappieri TCE1 in defined chemostat cultures grown with various combinations of sulphate and tetrachloroethene. Environ. Microbiol. 3:92-99. [DOI] [PubMed] [Google Scholar]
- 16.Drzyzga, O., S. Jannsen, and K.-H. Blotevogel. 1994. Mineralization of monofluorobenzoate by a diculture under sulfate-reducing conditions. FEMS Microbiol. Lett. 116:215-219. [DOI] [PubMed] [Google Scholar]
- 17.Eekert, M. H. A. van, T. J. Schröder, A. J. M. Stams, G. Schraa, and J. A. Field. 1998. Degradation and fate of carbon tetrachloride in unadapted methanogenic granular sludge. Appl. Environ. Microbiol. 64:2350-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenbeis, M. 1998. Kinetische Untersuchungen zur reduktiven Dechlorierung von Tetrachlorethen durch Dehalospirillum multivorans in Zellsuspensionen und im Biofilm. Ph.D. thesis. University of Stuttgart, Stuttgart, Germany.
- 19.El Fantroussi, S., H. Naveau, and S. N. Agathos. 1998. Anaerobic dechlorinating bacteria. Biotechnol. Prog. 14:167-188. [DOI] [PubMed] [Google Scholar]
- 20.Fauque, G. D., Y. M. Berlier, M. H. Czechowski, B. Dimon, P. A. Lespinat, and J. LeGall. 1987. A proton-deuterium exchange study of three types of Desulfovibrio hydrogenases. J. Ind. Microbiol. 2:15-23. [Google Scholar]
- 21.Fennell, D. E., J. M. Gossett, and S. H. Zinder. 1997. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ. Sci. Technol. 31:918-926. [Google Scholar]
- 22.Fetzner, S. 1998. Bacterial dehalogenation. Appl. Microbiol. Biotechnol. 50:633-657. [DOI] [PubMed] [Google Scholar]
- 23.Gerritse, J., A. Alphenaar, and J. C. Gottschal. 1998. Ecophysiology and application of dechlorinating anaerobes, p. 227-232. In T. E. Wilson (ed.), Water resources and the urban environment '98. Proceedings of the 1998 National Conference on Environmental Engineering. American Society of Civil Engineers, Reston, Va.
- 24.Gerritse, J., O. Drzyzga, G. Kloetstra, M. Keijmel, L. P. Wiersum, R. Hutson, M. D. Collins, and J. C. Gottschal. 1999. Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl. Environ. Microbiol. 65:5212-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerritse, J., G. Kloetstra, A. Borger, G. Dalstra, A. Alphenaar, and J. C. Gottschal. 1997. Complete degradation of tetrachloroethene in coupled anoxic and oxic chemostats. Appl. Microbiol. Biotechnol. 48:553-562. [DOI] [PubMed] [Google Scholar]
- 26.Gerritse, J., V. Renard, T. M. Pedro Gomes, P. A. Lawson, M. D. Collins, and J. C. Gottschal. 1996. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch. Microbiol. 165:132-140. [DOI] [PubMed] [Google Scholar]
- 27.Gerritse, J., V. Renard, J. Visser, and J. C. Gottschal. 1995. Complete degradation of tetrachloroethene by combining anaerobic dechlorinating and aerobic methanotrophic enrichment cultures. Appl. Microbiol. Biotechnol. 43:920-928. [DOI] [PubMed] [Google Scholar]
- 28.Haas, J. R., and E. L. Shock. 1999. Halocarbons in the environment: estimates of thermodynamic properties for aqueous chloroethylene species and their stabilities in natural settings. Geochim. Cosmochim. Acta 63:3429-3441. [Google Scholar]
- 29.Holliger, C., G. Wohlfarth, and G. Diekert. 1999. Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol. Rev. 22:383-398. [Google Scholar]
- 30.Lee, M. D., J. M. Odom, and R. J. Buchanan, Jr. 1998. New perspectives on microbial dehalogenation of chlorinated solvents: insights from the field. Annu. Rev. Microbiol. 52:423-452. [DOI] [PubMed] [Google Scholar]
- 31.Löffler, F. E., J. M. Tiedje, and R. A. Sanford. 1999. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl. Environ. Microbiol. 65:4049-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mägli, A., M. Wendt, and T. Leisinger. 1996. Isolation and characterization of Dehalobacterium formicoaceticum gen. nov. sp. nov., a strictly anaerobic bacterium utilizing dichloromethane as source of carbon and energy. Arch. Microbiol. 166:101-108. [Google Scholar]
- 33.Meyer, O., R. I. Refae, J. Warrelmann, and H. von Reis. 1993. Development of techniques for the bioremediation of soil, air and groundwater polluted with chlorinated hydrocarbons: the demonstration project at the model site in Eppelheim. Microb. Releases 2:11-22. [Google Scholar]
- 34.Mohn, W. W., and J. M. Tiedje. 1992. Microbial reductive dehalogenation. Microbiol. Rev. 56:482-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ollivier, B., R. Cord-Ruwisch, E. C. Hatchikian, and J. L. Garcia. 1988. Characterization of Desulfovibrio fructosovorans sp. nov. Arch. Microbiol. 149:447-450. [Google Scholar]
- 36.Pankhania, I. P., A. M. Spormann, W. A. Hamilton, and R. K. Thauer. 1988. Lactate conversion to acetate, CO2, and H2 in cell suspensions of Desulfovibrio vulgaris (Marburg): indications for the involvement of an energy driven reaction. Arch. Microbiol. 150:26-31. [Google Scholar]
- 37.Smatlak, C. R., J. M. Gossett, and S. H. Zinder. 1996. Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environ. Sci. Technol. 30:2850-2858. [Google Scholar]
- 38.Spuij, F., A. Alphenaar, H. de Wit, R. Lubbers, K. van de Brink, J. Gerritse, J. C. Gottschal, and S. Houtman. 1997. Full-scale application of in situ bioremediation of PCE-contaminated soil, p. 431-437. In B. C. Alleman and A. Leeson (ed.), In situ and on site bioremediation, vol. 5. Batelle Press, Columbus, Ohio. [Google Scholar]
- 39.Townsend, G. T., and J. M. Suflita. 1997. Influence of sulfur oxyanions on reductive dehalogenation activities in Desulfomonile tiedjei. Appl. Environ. Microbiol. 63:3594-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traore, A. S., M.-L. Fardeau, C. E. Hatchikian, J. Le Gall, and J.-P. Belaich. 1983. Energetics of growth of a defined mixed culture of Desulfovibrio vulgaris and Methanosarcina barkeri: interspecies hydrogen transfer in batch and continuous cultures. Appl. Environ. Microbiol. 46:1152-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trüper, H. G., and H. G. Schlegel. 1964. Sulfur metabolism in Thiorodaceae. 1. Quantitative measurements on growing cells of Chromatium okenii. Antonie Leeuwenhoek 30:225-238. [DOI] [PubMed] [Google Scholar]
- 42.Wohlfarth, G., and G. Diekert. 1997. Anaerobic dehalogenases. Curr. Opin. Biotechnol. 8:290-295. [DOI] [PubMed] [Google Scholar]
- 43.Yager, R. M., S. E. Bilotta, C. L. Mann, and E. L. Madsen. 1997. Metabolic adaptation and in situ attenuation of chlorinated ethenes by naturally occurring microorganisms in a fractured dolomite aquifer near Niagara Falls, New York. Environ. Sci. Technol. 31:3138-3147. [Google Scholar]
- 44.Yang, Y., and P. L. McCarty. 1998. Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ. Sci. Technol. 32:3591-3597. [Google Scholar]