Abstract
Heme-containing peroxidases from white rot basidiomycetes, in contrast to most proteins of fungal origin, are poorly produced in industrial filamentous fungal strains. Factors limiting peroxidase production are believed to operate at the posttranslational level. In particular, insufficient availability of the prosthetic group which is required for peroxidase biosynthesis has been proposed to be an important bottleneck. In this work, we analyzed the role of two components of the secretion pathway, the chaperones calnexin and binding protein (BiP), in the production of a fungal peroxidase. Expression of the Phanerochaete chrysosporium manganese peroxidase (MnP) in Aspergillus niger resulted in an increase in the expression level of the clxA and bipA genes. In a heme-supplemented medium, where MnP was shown to be overproduced to higher levels, induction of clxA and bipA was also higher. Overexpression of these two chaperones in an MnP-producing strain was analyzed for its effect on MnP production. Whereas bipA overexpression seriously reduced MnP production, overexpression of calnexin resulted in a four- to fivefold increase in the extracellular MnP levels. However, when additional heme was provided in the culture medium, calnexin overexpression had no synergistic effect on MnP production. The possible function of these two chaperones in MnP maturation and production is discussed.
Filamentous fungi have a large protein secretion capacity and are therefore exploited for the industrial production of endogenous and recombinant proteins (3). However, whereas secreted homologous protein yields can reach several grams per liter, production of proteins of mammalian or avian origin usually remains in the range of milligrams per liter (18, 34). Fungal metalloproteins, such as laccases, heme peroxidases, and other oxidases, although of fungal origin, are also normally produced in limited amounts (1, 5, 22, 24, 36, 38, 40).
Recently, we reported the expression of two heme-containing peroxidases from the white rot basidiomycete Phanerochaete chrysosporium in Aspergillus niger (10). We showed that production of the P. chrysosporium manganese peroxidase (MnP) could be significantly increased by heme supplementation of the culture medium of the producing strains. Similar observations have been reported previously (2, 39). This suggests that limitation at the level of cofactor availability is a bottleneck for the overproduction of this type of protein in filamentous fungi. In other examples of heterologous protein production in filamentous fungi as well, limitations at posttranscriptional stages are believed to be responsible for the low secretion yields (3).
During passage through the endoplasmic reticulum (ER), secretory proteins are assisted by an array of proteins, i.e., chaperones and foldases, which modulate protein folding and maturation. These proteins are also involved in processes such as translocation, intramolecular bonding, quality control, and ER-associated degradation (reviewed in reference 9). The expression of chaperone- and foldase-encoding genes is induced by situations of stress in the ER that result in the accumulation of unfolded proteins through the so-called unfolded protein response (UPR) pathway (for reviews, see references 7 and 49). In filamentous fungi, UPR-inducing agents have been shown to provoke a rapid and strong increase in the expression of the major ER-resident chaperone binding protein (BiP) (28, 43), whereas induction of the expression of foldases of the protein disulfide isomerase (PDI) family occurred in a delayed and/or less intense fashion (27, 28, 37).
As with stress-causing agents, the expression of heterologous proteins often correlates with an enhanced transcript level of chaperones and foldases (reviewed in reference 9). This may indicate that heterologous proteins cause a UPR through folding restrictions in the ER. This UPR stress could possibly be the cause for the low secretion yields.
We and other groups have analyzed the possibility of increasing heterologous protein production in fungi by overexpressing chaperones and foldases. In other expression systems, this approach has in some cases been successful (19, 41), although examples can also be found where overexpression of chaperones and/or foldases had no or a negative effect on protein secretion (8, 13, 21, 35). Overexpression of chaperones or foldases in filamentous fungi has so far failed to increase the extracellular levels of the heterologous proteins analyzed (11, 28, 33, 48). This variety of results suggests that a positive effect on heterologous protein secretion may be specific to the chaperone-protein system analyzed.
In this paper we report the differential effect of increasing the expression level of BiP and the recently isolated calnexin on the production of manganese peroxidase (MnP) in Aspergillus niger and discuss the possible interactions of these chaperones with the maturation of this metalloprotein in the ER.
MATERIALS AND METHODS
Strains.
A. niger MGG029 (10) was used as a recipient strain in fungal transformations. Escherichia coli DH5α was used for construction and propagation of vector molecules.
Construction of expression vectors and other molecular methods.
Molecular methods were carried out essentially as described (39). Vector pgpdMnP1.I-AmdS carries the P. chrysosporium MnP (isozyme H4)-encoding gene, mnp1, under the control of the Aspergillus nidulans glyceraldehyde-3-phosphate dehydrogenase (gpdA) promoter and was constructed by inserting an NcoI-HindIII fragment from pMnp1.I (10), comprising the complete mnp1 coding region, into expression vector pAN52-5Not (containing the A. nidulans gpdA promoter; accession number Z32750). The A. nidulans amdS gene from pAmdSNot (P. Punt, unpublished) was introduced as a 5.5-kb NotI fragment in the resulting vector.
Vector pGLACLX was constructed by amplifying the A. niger calnexin gene, clxA, from pCLX2.5 (GenBank accession number AJ299945) with a vector primer and CALN1201 (5′-GCTATCCATCATGAGGTTCAACGCTGCTTTGAC-3′). The resulting amplification fragment was digested with BspHI and EcoRV and cloned into pAN52-12Not (a derivative of pAN52-7Not carrying the A. niger glucoamylase [glaA] promoter; Punt, unpublished) at the NcoI and EcoRV sites. The sequence of the amplification product was confirmed by sequencing. Construction of pGLABIP/hph, carrying the A. niger bipA gene under the control of the A. niger glaA promoter and the hygromycin selection marker, was described elsewhere (33).
Total fungal RNA was isolated as described (25) using the RNAzol kit from CINNA/BIOTECX. Probes used for Northern analysis were a 1.6-kb EcoRI-HindIII fragment from pABiP1-22, a pABiP1 subclone (43), containing the A. niger bipA gene; a 2-kb BspHI-EcoRV fragment from pGLACLX (calnexin probe); a 1.1-kb NcoI-HindIII fragment from pMnP1.I (mnp1 probe) (10); and a 1.5-kb HindIII fragment from pAB5-2 (gpdA probe) (47). Northern hybridization signals were measured with Cyclone Storage Phosphor (Packard) and quantified with OptiQuant 3.0 software (Packard Instruments Co.).
Fungal transformations.
Fungal cotransformation was basically carried out as described (32) using pgpdMnp1.I-AmdS and pAB4-1 (44) containing the A. niger pyrG selection marker. Multicopy cotransformants were selected for growth on uridine and on acetamide-containing plates (46). Transformants were screened for MnP production by halo formation in o-anisidine-containing plates as previously described (10). An efficient MnP-producing strain, MGG029[pgpdMnP1.I]#13, was selected for supertransformation with pGLABiP/hph (33) or with pGLACLN in combination with pAN7-1 (32). Transformants were selected for growth on hygromycin-containing plates.
Protein methods.
MnP activity was measured by monitoring the oxidation of diammonium 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) in the presence of 20 μM sodium oxalate at 415 nm (17). MnP protein levels were also analyzed by Western blotting as described previously (10). Total protein was determined according to the method of Bradford (6) using bovine serum albumin as the standard.
Culturing conditions.
MnP production was analyzed in shake-flask cultures. Flasks (500 ml) containing 100 ml of Aspergillus maltodextrin minimal medium (AMM-maltodextrin) (10) were inoculated with 5 × 107 fresh conidia and grown at 30°C with agitation at 300 rpm. After 24 h of growth, mycelium was harvested by filtering through a Miracloth and washed with physiological salt. Equal amounts of mycelium were then transferred to 300-ml flasks containing either 50 ml of AMM-maltodextrin or 50 ml of AMM-maltodextrin supplemented with hemin (500 mg/liter). Strains were grown in the same conditions for another 24 h, and medium and mycelium were sampled at various intervals.
RESULTS
Induction of clxA and bipA transcript levels upon MnP production.
The levels of clxA and bipA transcripts were analyzed in strain MGG029[pgpdMnp1.I]#13, which contains multiple copies of the P. chrysosporium mnp1 gene under the control of the strong and constitutively expressed gpdA promoter. Transcriptional analysis was performed with samples taken from shake-flask cultures grown in the presence and absence of additional heme. Addition of heme to the culture medium increases extracellular MnP production (10), while the mnp1 transcript levels remain unchanged (data not shown).
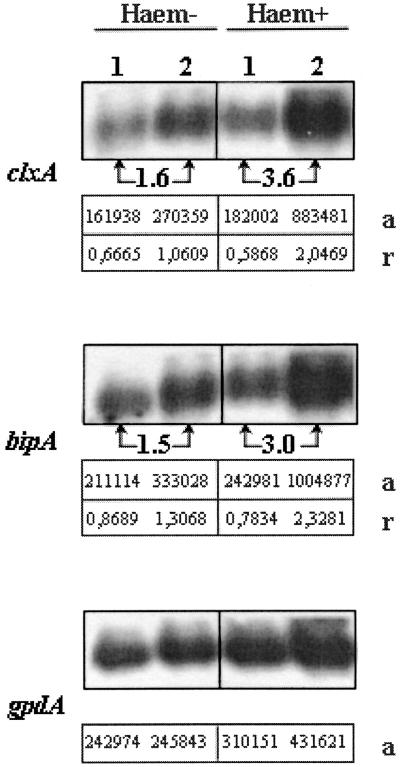
Transcriptional analysis showed similar induction patterns for both chaperones. When the cultures were grown in the inducing medium AMM-maltodextrin, the clxA and bipA steady-state mRNA levels were approximately 50% higher in the MGG029[pgpdMnp1.I]#13 than in the wild-type strain transformed with the selection marker pAB4-1. Under conditions of exogenous heme addition to the culture medium, the expression of the clxA and bipA genes in the MnP-producing strain was increased by factors of 3.6 and 3.0, respectively, compared to that of the MGG029[pAB4-1] strain. In the wild-type strain, heme supplementation had no significant effect on the transcript level of either chaperone (Fig. 1).
FIG. 1.
Induction of clxA and bipA gene expression by MnP production. The Northern blot was prepared with RNA from the wild-type strain MGG029 (lane 1) and the MnP-producing strain MGG029[pgpdMnp1.I]#13 (lane 2). Strains were grown for 24 h in AMM-maltodextrin medium and then transferred to either AMM-maltodextrin (Haem−) or AMM-maltodextrin supplemented with hemin (500 mg/liter) (Haem+). After 24 h of additional growth, samples were harvested. The signal for gpdA was used as a loading control. a, PhosphorImager measurements (arbitrary units) of the RNA intensities; r, transcript abundance corrected for gpdA loading control signal. Induction factors, calculated after correction for loading differences, are indicated between arrows.
Effect of calnexin and BiP overexpression on MnP production.
To study the effect of calnexin and BiP overexpression on the production of MnP by A. niger, we used expression cassettes in which the clxA and bipA genes were placed under the control of the strong and regulated glucoamylase promoter (P glaA). This approach has been shown to be successful in providing increased intracellular chaperone levels (42). As a different promoter, PgpdA, controls the expression of the mnp1 gene in strain MGG029[pgpdMnp1.I]#13, no interfering transcriptional titration effects are expected between expression of the chaperone and mnp1.
Strain MGG029[pgpdMnp1.I]#13 was supertransformed either with pGLABiP/hph, containing the A. niger bipA and the hygromycin (hph) selection marker in one vector, or with pGLACLX in combination with pAN7-1, containing the A. niger clxA and hph genes, respectively. pGLACLX cotransformants were identified by colony PCR (45), and 14 pGLACLX/pAN7-1 and 6 pGLABiP/hph transformants were screened for MnP production using the o-anisidine plate assay (10).
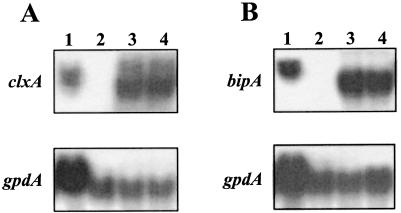
All pGLABiP/hph transformants showed reductions in halo formation similar to that of MGG029[pgpdMnp1.I]#13 or to this strain transformed only with pAN7-1, indicating a reduction in MnP secretion (data not shown). In contrast, 10 of the pGLACLX/pAN7-1 cotransformants developed a larger halo than the control strains, whereas the remaining four showed a wild-type halo. Northern blot analysis of the pGLABiP/hph and the MnP-overproducing pGLACLX/pAN7-1 strains confirmed the overexpression of the corresponding chaperone genes (data not shown). Two clxA-overexpressing strains, MGG029-pgpdMnp1.I#13[pGLACLX]#1 and MGG029-pgpdMnp1.I#13[pGLACLX]#4, showed intense halo formation and a high level of clxA overexpression (6.5- and 8-fold, respectively), and two bipA-overexpressing strains, MGG029-pgpdMnp1.I#13[pGLABIPA]#1 (5-fold bipA overexpression) and MGG029-pgpdMnp1.I#13[pGLABIPA]#2 (5.5-fold bipA overexpression) (Fig. 2), were selected to study MnP production in shake-flask cultures.
FIG. 2.
Northern blot analysis of clxA and bipA overexpression. RNA was extracted after 24 h of culture in AMM-maltodextrin medium. (A) Overexpression of clxA. Lane 1, MGG029[pgpdMnp1.I]#13, concentrated RNA sample; lane 2, MGG029[pgpdMnp1.I]#13; lane 3, MGG029-pgpdMnp1.I#13[pGLACLX]#1; lane 4, MGG029-pgpdMnp1.I#13[pGLACLX]#4. (B) Overexpression of bipA. Lane 1, MGG029[pgpdMnp1.I]#13, concentrated RNA sample; lane 2, MGG029[pgpdMnp1.I]#13; lane 3, MGG029-pgpdMnp1.I#13[pGLABIPA]#1; lane 4, MGG029-pgpdMnp1.I#13[pGLABIPA]#2. The signal for gpdA was used as a loading control.
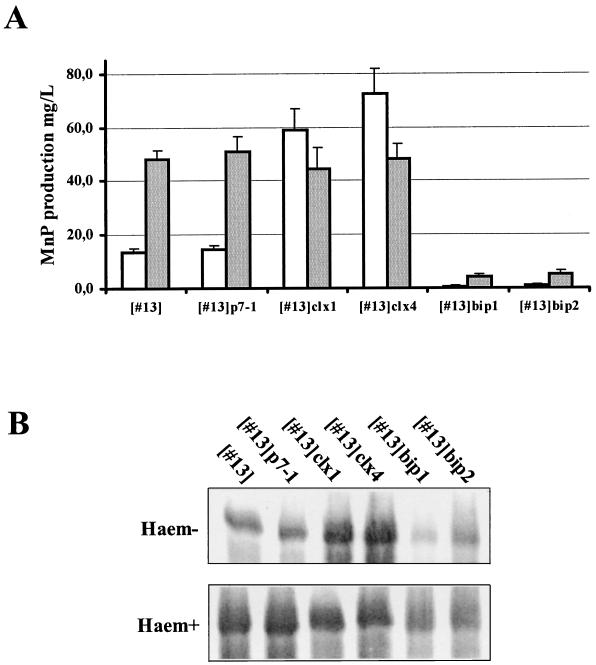
MnP production was monitored in AMM-maltodextrin medium. Cultures were started from equal amounts of mycelium obtained from a 24-h preculture. MnP activity was measured after 10 and 24 h of additional growth. An increase in extracellular MnP activity was observed in both clxA-overexpressing strains compared to the MGG029[pgpdMnp1.I]#13 strain or this strain transformed only with the hygromycin selection marker. This increase was weak but already visible after 10 h of induction (data not shown) and reached a 4- to 5-fold value after 24 h (Fig. 3A, empty bars).
FIG. 3.
Effect of clxA and bipA overexpression on MnP production by A. niger. Cultures were inoculated with equal amounts of mycelium obtained from a 24-h preculture in AMM-maltodextrin medium. Strains: [#13], MGG029[pgpdMnp1.I]#13; [#13]p7-1, MGG029-pgpdMnp1.I#13[pAN7-1]; [#13]clx1, MGG029-pgpdMnp1.I#13[pgpdGLACLX]#1; [#13]clx4, MGG029-pgpdMnp1.I#13[pgpdGLACLX]#4; [#13]bip1, MGG029-pgpdMnp1.I#13[pgpdGLABIPA]#1; [#13]bip2, MGG029-pgpdMnp1.I#13[pGLABIPA]#2. (A) MnP activity determined in the culture broth of strains grown in AMM-maltodextrin (open bars) or in AMM-maltodextrin supplemented with hemin (500 mg/liter) (solid bars) (n = 2). (B) Detection of MnP production by Western blotting. Haem− and Haem+ are defined in the legend to Fig. 1.
In contrast, both bipA-overexpressing strains showed a considerable reduction in extracellular MnP activity to almost undetectable levels at 24 h after induction (Fig. 3A, empty bars). At each time point, the amounts of total extracellular protein were similar among the six strains (data not shown). Western blot analysis showed that the observed differences in MnP activity corresponded with similar differences in the extracellular levels of the MnP protein (Fig. 3B). These results indicate that overexpression of clxA results in an increase in the production of MnP, whereas bipA overexpression reduces MnP secretion.
Growth in the presence of excess heme eliminates the effect of calnexin overexpression.
We have previously shown that addition of a heme source to the culture medium of MnP-producing A. niger strains results in an improvement in the production level of MnP (10). To analyze whether this effect was additive to or could counteract the observed effects of clxA and bipA overexpression, we studied MnP production in the various strains grown under conditions of heme supplementation. Strains were cultivated from mycelial inocula in maltodextrin medium supplemented with hemin (500 mg/liter), and MnP activity was measured after 24 h growth.
Under these conditions, a 3.5-fold increase in MnP extracellular activity was observed in the MGG029[pgpdMnp1.I]#13 strain compared to the level observed in the unsupplemented medium (Fig. 3A, solid bars). MnP production in both bipA-overexpressing strains also increased but still reached less than 10% of the value obtained for MGG029[pgpdMnp1.I]#13. In contrast, MnP production in both clxA-overexpressing strains did not increase further upon hemin supplementation and stayed at the level obtained for MGG029[pgpdMnp1.I]#13 in the heme-supplemented medium (Fig. 3A, solid bars). As with the results obtained in nonsupplemented medium, Western blot analysis confirmed the increase in extracellular MnP protein levels (Fig. 3B).
DISCUSSION
In previous studies, we and other groups have shown that limited heme availability is a bottleneck for the overproduction of heme-containing peroxidases in filamentous fungi (2, 10, 40). In this paper we present our continuing work on the analysis of the factors affecting the secretion levels of this type of protein. We have studied the role of two ER-resident chaperones, the lectin-like calnexin and the binding protein BiP, in the secretion of the P. chrysosporium MnP in A. niger. Calnexin is involved in the folding of glycosylated proteins and is a major component of the ER quality control system (20). BiP is a well-studied member of the heat shock protein family with multiple functions in the secretory pathway (for reviews, see references 16 and 30).
Overexpression of MnP in A. niger resulted in a moderate (1.5- to 1.6-fold) increase in the transcript levels of both clxA and bipA. Induction was higher (≈3-fold) in the heme-supplemented condition, which, in this case, corresponds to a 3.5-fold higher MnP production level than that in nonsupplemented medium. Many studies on chaperone induction by expression of heterologous proteins in filamentous fungi report induction values of the same magnitude. Expression of the hen egg lysozyme in A. niger resulted in a 1.8- to 2-fold increase in the bipA mRNA level and a 2- to 3-fold increase in the levels of pdiA and the PDI-related tigA transcripts (28). Likewise, tissue plasminogen activator expression in A. niger increased bipA, pdiA, and the cyclophilin cypB transcript levels by a factor of 1.5 to 2 (11).
In Trichoderma reesei, a two- to threefold enhancement in pdiA mRNA level was observed in an Fab antibody fragment-overexpressing strain. BiP is considered a major component of the primary stress response to the accumulation of infolded proteins in the ER (16). The induction of BiP expression in the MnP-producing strain may therefore indicate a limitation at the stage of folding for the biogenesis of this hemoprotein in A. niger. The clxA steady-state mRNA levels were increased by MnP overexpression and also by overexpression of chymosin (H. Wanf, J. Lambert, E. Mortin, D. Archer, J. Peberdy, M. Ward, and D. J. Jeenes, submitted for publication), indicating that clxA responds to signals generated by the secretion of heterologous proteins.
Compared to the nonsupplemented medium, induction of the bipA and clxA transcripts in MGG029[pgpdMnp1.I]#13 was higher in the heme-supplemented condition. This increase was not due to the additional heme, since the clxA and bipA mRNA levels were not changed in the wild-type strain. More likely, the higher bipA and clxA induction could be related to the higher MnP production level reached upon heme addition.
Although MnP production induced bipA expression, when a high expression level of this chaperone was provided by introduction of multiple bipA gene copies under the control of a strong promoter, the production of MnP was severely reduced. A similar inverse correlation of BiP transcript levels with heterologous protein production was observed by Dorner and colleges in CHO cells (12, 13). In contrast, BiP overexpression increased the production levels of chymosin and the cocaine-sensitive serotonin transporter (SERT) in yeast and insect cells, respectively (19, 41). In other studies where the role of BiP in heterologous protein production has been addressed, BiP overexpression increased the intracellular but not secreted levels of the proteins analyzed (21, 33). On the other hand, overexpression of the bipA gene in A. niger failed to improve production levels of a fungal cutinase (42).
The reason for this variety of results is not clear but could be related to the multifunctionality of the BiP chaperone. BiP is involved in several functions in the ER (16). Some processes, such as translocation into the ER and protein folding, could be classified as favorable for protein production, whereas others, such as the ER-associated degradation of misfolded proteins, can be considered unfavorable. The participation of each process in the secretion efficiency of heterologous proteins may be protein specific and lead to different results when BiP is overproduced. Also, the level of BiP overexpression can influence its effect on the production level of heterologous protein (19). We suggest that, under the conditions of BiP overexpression as described here, the unfavorable interactions predominate for MnP, increasing degradation and diminishing the production level of this hemoprotein.
In contrast to the results obtained with the BiP-overproducing strains, overexpression of clxA in strain MGG029[pgpdMnp1.I]#13 resulted in a four- to fivefold increase in MnP production after 24 h. Calnexin is a lectin-like chaperone involved in the folding of glycosylated protein and is part of the ER quality control system (20, 23). MnP is glycosylated (29) and therefore a potential substrate for calnexin. Calnexin cooverexpression also enhanced the levels of functional serotonin transporter produced using the baculovirus expression system, whereas cooverexpression of other chaperones (BiP, calreticulin, and Erp57) had a lesser or no effect (41). In contrast, cooverexpression of calnexin did not increase the formation of hepatitis C virus envelope protein complexes in mammalian cells (8). On the other hand, calnexin disruption in Saccharomyces cerevisiae resulted in a 2.5-fold increase in the secretion of unstable lysozyme mutants (4). Also for calnexin, this variety of results suggests different specificities for different protein-chaperone combinations.
The positive effect of calnexin cooverexpression in MnP production suggests its participation in the maturation of MnP. Interestingly, Naussef and coworkers, studying the biosynthesis of another heme-containing protein, the human myeloperoxidase (MPO), observed a specialized role for calnexin in MPO maturation (26). They showed that two ER-resident lectins, calreticulin and calnexin, associated with apopro-MPO, but only calnexin interacted with holopro-MPO, and heme insertion was required to release holopro-MPO from calnexin binding. This suggests a function for calnexin at the stage of incorporation of the prosthetic group into this hemoprotein. Furthermore, Fayadat and coworkers showed that calnexin overexpression increased the initial folding steps of the human thyroperoxidase (15) and that heme insertion was required for secretion of this hemoprotein (14).
Considering all these results, we postulate a role for calnexin during heme incorporation into MnP. We have shown that heme is limiting for MnP production and apo-forms of MnP do not accumulate extracellularly (10). Under these heme-limiting conditions, calnexin overexpression may help MnP maturation, e.g., by facilitating or providing sufficient time for heme insertion and therefore increasing MnP secretion. However, under conditions of heme supplementation, the limitation at this maturation step is alleviated, and consequently calnexin overexpression would not have a further positive effect.
In conclusion, our results suggest differential roles of calnexin and BiP in the maturation of MnP. Although both chaperones are induced by mnp1 overexpression, indicating a folding limitation for MnP in the ER, calnexin has a positive role, possibly by facilitating proper folding and heme incorporation, whereas BiP may act mainly in the degradation of misfolded MnP molecules. Additional work is required to elucidate the kinetics and specificity of these interactions.
This is, to our best knowledge, the first report on a successful modulation of chaperone expression for increasing heterologous protein production in filamentous fungi and contrast with previous studies where chaperone or foldase overexpression had no or little effect on heterologous protein production. Our results suggest that manipulation of the secretion pathway for industrial strain improvement requires a profound understanding of the complexity and specificity of the molecular interactions among its components.
Acknowledgments
We thank N. van Luijk and V. Joosten for technical assistance.
REFERENCES
- 1.Aifa, M. S., S. Sayadi, and A. Gargouri. 1999. Heterologous expression of lignin peroxidase of Phanerochaete chrysosporium in Aspergillus niger. Biotechnol. Lett. 21:849-853. [Google Scholar]
- 2.Andersen, H. D., E. B. Jensen, and K. G. Welinder. 1992. A method to produce heme proteins. European patent application 0505311A2.
- 3.Archer, D. B., and J. F. Peberdy. 1997. The molecular biology of secreted enzyme production by fungi. Crit. Rev. Biotechnol. 17:273-306. [DOI] [PubMed] [Google Scholar]
- 4.Arima, H., T. Kinoshita, H. R. Ibrahim, H. Azakami, and A. Kato. 1998. Enhanced secretion of hydrophobic peptide fused lysozyme by the introduction of N-glycosylation signal and the disruption of calnexin gene in Saccharomyces cerevisiae. FEBS Lett. 440:89-92. [DOI] [PubMed] [Google Scholar]
- 5.Berka, R. M., P. Schneider, E. J. Golightly, S. H. Brown, M. Madden, K. M. Brown, T. Halkier, K. Mondorf, and F. Xu. 1997. Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl. Environ. Microbiol. 63:3151-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive assay for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Chapman, R., C. Sidrauski, and P. Walter. 1998. Intracellular signalling from the endoplasmic reticulum to the nucleus. Annu. Rev. Cell Dev. Biol. 14:459-485. [DOI] [PubMed] [Google Scholar]
- 8.Choukhi, A., S. Ung, C. Wychowski, and J. Dubuisson. 1998. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J. Virol. 72:3851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conesa, A., P. J. Punt, and C. A. M. J. J. van den Hondel. 2001. The secretion pathway in filamentous fungi: a biotechnological view. Fung. Gen. Biol. 33:155-171. [DOI] [PubMed] [Google Scholar]
- 10.Conesa, A., C. A. van Den Hondel, and P. J. Punt. 2000. Studies on the production of fungal peroxidases in Aspergillus niger. Appl. Environ. Microbiol. 66:3016-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derkx, P. M. F. 2000. Ph.D. thesis. University of Copenhagen, Copenhagen, Denmark.
- 12.Dorner, A. J., M. G. Krane, and R. J. Kaufman. 1988. Reduction of endogenous GRP78 levels improves secretion of a heterologous protein in CHO cells. Mol. Cell. Biol. 8:4063-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorner, A. J., L. C. Wasley, and R. J. Kaufman. 1992. Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J. 11:1563-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayadat, L., P. Niccoli-Sire, J. Lanet, and J. L. Franc. 1999. Role of heme in intracellular trafficking of thyroperoxidase and involvement of H2O2 generated at the apical surface of thyroid cells in autocatalytic covalent heme binding. J. Biol. Chem. 274:10533-10538. [DOI] [PubMed] [Google Scholar]
- 15.Fayadat, L., S. Siffroi-Fernandez, J. Lanet, and J. L. Franc. 2000. Calnexin and calreticulin binding to human thyroperoxidase is required for its first folding step(s) but is not sufficient to promote efficient cell surface expression. Endocrinology 141:959-966. [DOI] [PubMed] [Google Scholar]
- 16.Gething, M. J. 1999. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10:465-472. [DOI] [PubMed] [Google Scholar]
- 17.Glenn, J. K., and M. H. Gold. 1985. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Arch. Biochem. Biophys. 242:329-341. [DOI] [PubMed] [Google Scholar]
- 18.Gouka, R. J., P. J. Punt, and C. A. van den Hondel. 1997. Efficient production of secreted proteins by Aspergillus: progress, limitations and prospects. Appl. Microbiol. Biotechnol. 47:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Harmsen, M. M., M. I. Bruyne, H. A. Raue, and J. Maat. 1996. Overexpression of binding protein and disruption of the PMR1 gene synergistically stimulate secretion of bovine prochymosin but not plant thaumatin in yeast. Appl. Microbiol. Biotechnol. 46:365-370. [DOI] [PubMed] [Google Scholar]
- 20.Helenius, A., S. Trombetta, D. N. Hebert, and J. F. Simons. 1997. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 7:193-200. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, T. A., J. J. Eiden, P. Bourgarel, T. Meo, and M. J. Betenbaugh. 1994. Effects of co-expressing chaperone BiP on functional antibody production in the baculovirus system. Protein Express. Purif. 5:595-603. [DOI] [PubMed] [Google Scholar]
- 22.Huang, K. X., I. Fujii, Y. Ebizuka, K. Gomi, and U. Sankawa. 1995. Molecular cloning and heterologous expression of the gene encoding dihydrogeodin oxidase, a multicopper blue enzyme from Aspergillus terreus. J. Biol. Chem. 270:21495-21502. [DOI] [PubMed] [Google Scholar]
- 23.Jakob, C. A., and P. Burda. 1999. Quality control in biosynthetic pathways of N-linked glycoproteins in the yeast endoplasmic reticulum. Protoplasma 207:1-7. [Google Scholar]
- 24.Kersten, P., C. Witkek, A. V. Wymelenberg, and D. Cullen. 1995. Phanerochaete chrysosporium glyoxal oxidase is encoded by two allelic variants: structure, genomic organization, and heterologous expression of glx1 and glx2. J. Bacteriol. 177:6106-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolar, M., P. J. Punt, C. A. van den Hondel, and H. Schwab. 1988. Transformation of Penicillium chrysogenum using dominant selection markers and expression of an Escherichia coli lacZ fusion gene. Gene 62:127-134. [DOI] [PubMed] [Google Scholar]
- 26.Nauseef, W. M., S. J. McCormick, and M. Goedken. 1998. Coordinated participation of calreticulin and calnexin in the biosynthesis of myeloperoxidase. J. Biol. Chem. 273:7107-7111. [DOI] [PubMed] [Google Scholar]
- 27.Ngiam, C., D. J. Jeenes, and D. B. Archer. 1997. Isolation and characterisation of a gene encoding protein disulphide isomerase, pdiA, from Aspergillus niger. Curr. Genet. 31:133-138. [DOI] [PubMed] [Google Scholar]
- 28.Ngiam, C., D. J. Jeenes, P. J. Punt, C. A. van den Hondel, and D. B. Archer. 2000. Characterization of a foldase, protein disulfide isomerase A, in the protein secretory pathway of Aspergillus niger. Appl. Environ. Microbiol. 66:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paszczynski, A., V. B. Huynh, and R. Crawford. 1986. Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch. Biochem. Biophys. 244:750-765. [DOI] [PubMed] [Google Scholar]
- 30.Pedrazzini, E., and A. Vitale. 1996. The binding protein (BiP) and the synthesis of secretory proteins. Plant Physiol. Biochem. 34:207-216. [Google Scholar]
- 31.Punt, P. J., R. P. Oliver, M. A. Dingemanse, P. H. Pouwels, and C. A. van den Hondel. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117-124. [DOI] [PubMed] [Google Scholar]
- 32.Punt, P. J., and C. A. van den Hondel. 1992. Transformation of filamentous fungi based on hygromycin B and phleomycin resistance markers. Methods Enzymol. 216:447-457. [DOI] [PubMed] [Google Scholar]
- 33.Punt, P. J., I. A. van Gemeren, J. Drint-Kuijvenhoven, J. G. Hessing, G. M. van Muijlwijk-Harteveld, A. Beijersbergen, C. T. Verrips, and C. A. van den Hondel. 1998. Analysis of the role of the gene bipA, encoding the major endoplasmic reticulum chaperone protein in the secretion of homologous and heterologous proteins in black aspergilli. Appl. Microbiol. Biotechnol. 50:447-454. [DOI] [PubMed] [Google Scholar]
- 34.Radzio, R., and U. Kurk. 1997. Synthesis of biotechnologically relevant heterologous proteins in filamentous fungi. Process Biochem. 32:529-539. [Google Scholar]
- 35.Robinson, A. S., J. A. Bockhaus, A. C. Voegler, and K. D. Wittrup. 1996. Reduction of BiP levels decreases heterologous protein secretion in Saccharomyces cerevisiae. J. Biol. Chem. 271:10017-10022. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Dueñas, F. J., M. J. Martinez, and A. T. Martinez. 1999. Heterologous expression of Pleurotus eryngii peroxidase confirms its ability to oxidize Mn2+ and different aromatic substrates. Appl. Environ. Microbiol. 65:4705-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saloheimo, M., M. Lund, and M. E. Penttilä. 1999. The protein disulphide isomerase gene of the fungus Trichoderma reesei is induced by endoplasmic reticulum stress and regulated by the carbon source. Mol. Gen. Genet. 262:35-45. [DOI] [PubMed] [Google Scholar]
- 38.Saloheimo, M., and M.-L. Niku-Paavola. 1991. Heterologous production of a ligninolytic enzyme: expression of the Phlebia radiata laccase gene in Trichoderma reesei. Bio/Technology 9:987-990. [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Stewart, P., R. E. Whitwam, P. J. Kersten, D. Cullen, and M. Tien. 1996. Efficient expression of a Phanerochaete chrysosporium manganese peroxidase gene in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 62:860-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tate, C. G., E. Whiteley, and M. J. Betenbaugh. 1999. Molecular chaperones stimulate the functional expression of the cocaine-sensitive serotonin transporter. J. Biol. Chem. 274:17551-17558. [DOI] [PubMed] [Google Scholar]
- 42.van Gemeren, I. A., A. Beijersbergen, C. A. van den Hondel, and C. T. Verrips. 1998. Expression and secretion of defined cutinase variants by Aspergillus awamori. Appl. Environ. Microbiol. 64:2794-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gemeren, I. A., P. J. Punt, A. Drint-Kuyvenhoven, M. P. Broekhuijsen, A. van't Hoog, A. Beijersbergen, C. T. Verrips, and C. A. van den Hondel. 1997. The ER chaperone encoding bipA gene of black Aspergilli is induced by heat shock and unfolded proteins. Gene 198:43-52. [DOI] [PubMed] [Google Scholar]
- 44.van Hartingsveldt, W., I. E. Mattern, C. M. van Zeijl, P. H. Pouwels, and C. A. van den Hondel. 1987. Development of a homologous transformation system for Aspergillus niger based on the pyrG gene. Mol. Gen. Genet. 206:71-75. [DOI] [PubMed] [Google Scholar]
- 45.van Zeijl, C. M., E. H. van de Kamp, P. J. Punt, G. C. Selten, B. Hauer, R. F. van Gorcom, and C. A. van den Hondel. 1997. An improved colony-PCR method for filamentous fungi for amplification of PCR-fragments of several kilobases. J. Biotechnol. 59:221-224. [DOI] [PubMed] [Google Scholar]
- 46.Verdoes, J. C., P. J. Punt, J. M. Schrickx, H. W. van Verseveld, A. H. Stouthamer, and C. A. van den Hondel. 1993. Glucoamylase overexpression in Aspergillus niger: molecular genetic analysis of strains containing multiple copies of the glaA gene. Transgenic Res. 2:84-92. [DOI] [PubMed] [Google Scholar]
- 47.Verdoes, J. C., P. J. Punt, A. H. Stouthamer, and C. A. van den Hondel. 1994. The effect of multiple copies of the upstream region on expression of the Aspergillus niger glucoamylase-encoding gene. Gene 145:179-187. [DOI] [PubMed] [Google Scholar]
- 48.Wang, H., and M. Ward. 2000. Molecular characterization of a PDI-related gene prpA in Aspergillus niger var. awamori. Curr. Genet. 37:57-64. [DOI] [PubMed] [Google Scholar]
- 49.Welihinda, A. A., W. Tirasophon, and R. J. Kaufman. 1999. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Express. 7:293-300. [PMC free article] [PubMed] [Google Scholar]