Abstract
Bacteriocin-producing starter cultures have been suggested as natural food preservatives; however, development of resistance in the target organism is a major concern. We investigated the development of resistance in Listeria monocytogenes to the two major bacteriocins pediocin PA-1 and nisin A, with a focus on the variations between strains and the influence of environmental conditions. While considerable strain-specific variations in the frequency of resistance development and associated fitness costs were observed, the influence of environmental stress seemed to be bacteriocin specific. Pediocin resistance frequencies were determined for 20 strains and were in most cases ca. 10−6. However, two strains with intermediate pediocin sensitivity had 100-fold-higher pediocin resistance frequencies. Nisin resistance frequencies (14 strains) were in the range of 10−7 to 10−2. Strains with intermediate nisin sensitivity were among those with the highest frequencies. Environmental stress in the form of low temperature (10°C), reduced pH (5.5), or the presence of NaCl (6.5%) did not influence the frequency of pediocin resistance development; in contrast, the nisin resistance frequency was considerably reduced (<5 × 10−8). Pediocin resistance in all spontaneous mutants was very stable, but the stability of nisin resistance varied. Pediocin-resistant mutants had fitness costs in the form of reduction down to 44% of the maximum specific growth rate of the wild-type strain. Nisin-resistant mutants had fewer and less-pronounced growth rate reductions. The fitness costs were not increased upon applying environmental stress (5°C, 6.5% NaCl, or pH 5.5), indicating that the bacteriocin-resistant mutants were not more stress sensitive than the wild-type strains. In a saveloy-type meat model at 5°C, however, the growth differences seemed to be negligible. The applicational perspectives of the results are discussed.
One of the objectives of using bacteriocins produced by lactic acid bacteria for the biopreservation of food products is to eliminate or control Listeria monocytogenes. The main focus so far has been on nisin, which is the only bacteriocin to date approved as food preservative (36), and the pediocin-like or class IIa bacteriocins. Accumulating evidence suggests that there is an abundance and diversity of class IIa bacteriocin-producing organisms present in various food products (12), representing a natural reservoir of protective cultures.
A main concern regarding biopreservation is the possibility of impediment by natural or developed resistance. Natural resistance to class IIa bacteriocins has been reported in 1 to 8% of tested wild-type strains (11, 20, 32), and variations in natural sensitivity to nisin have also been observed (13, 32, 38).
Nisin resistance in L. monocytogenes has been observed to develop at frequencies ranging from <10−9 to 10−5 (3, 8, 27) and from 10−8 to 10−2 in other gram-positive organisms (24, 27). The spontaneous resistance was stable in L. monocytogenes (3, 26, 39) and in other organisms (4, 14, 22, 34). In one report, however, sequential exposure to nisin was required to attain stable resistance to the bacteriocin in L. monocytogenes (27). Resistance to class IIa and other bacteriocins occurred at frequencies of 10−6 to 10−3 in L. monocytogenes (10, 33, 41), and the developed resistance was stable in some mutants (9, 33) but unstable in others (10). Pediocin resistance has been reported to develop at very high levels (see, for example, reference 9), ostensibly by an on-off type mechanism, but nisin resistance occurs by small and gradual increases in MIC (15).
Various physiological changes in the cell envelope of Listeria following nisin resistance development have been described (5, 7, 21, 26, 28, 39) and suggest that the mutants could have impaired growth or adaptation to environmental stresses such as salt and low temperature. In concordance with this, bacteriocin resistance development has in most, but not all, mutants been associated with fitness costs. Nisin or class IIa bacteriocin resistance conferred various degrees of growth inhibition in some spontaneous mutants of Listeria and other organisms (9, 10, 14, 21, 26, 27), while others had growth characteristics similar to those of the wild-type strain (3, 39).
Since most studies have employed one or only a few strains, it seems likely that some of these confusing results are due to strain- or isolate-specific variation in the frequency of bacteriocin resistance development, the stability of the arising phenotype, and the subsequent fitness costs. The observed wide range of resistance frequencies indicates the possibility of a critically high level of arising resistance; however, there is not enough information available to determine the probable consequences for the efficacy of biopreservation of food products. In particular, data are lacking on the biodiversity of the response of wild-type isolates.
We present here the first comprehensive investigation of bacteriocin resistance development under various food-relevant conditions in a broad selection of wild-type strains representing the observed levels of pediocin and nisin sensitivity. We determined the frequencies of pediocin and nisin resistance development at relevant pH, NaCl, and temperature levels, and we assessed the fitness costs and the stability of the mutants under similar conditions. The conclusions regarding fitness costs and stability were examined in a saveloy-type meat model system. Additionally, the frequency of simultaneous pediocin and nisin resistance development was investigated. The results give a general impression of the natural variation to be expected in food systems, and in this context the perspectives regarding application are discussed.
(Part of this work was published in the Proceedings of the 17th International Conference of the International Committee on Food Microbiology and Health in 1999.)
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The L. monocytogenes strains used in this study (Table 1) were from the strain collections at the Department of Dairy and Food Science and the Danish Meat Research Institute (Roskilde, Denmark). The susceptibility of wild-type strains to nisin and pediocin was described by Rasch and Knøchel (32). Most of the tested wild-type strains were bacteriocin sensitive, being inhibited by 500 IU of nisin or 640 arbitrary units (AU) of pediocin/ml at pH 7.3 (regarding pediocin activity units, see below). A few strains had intermediately enhanced resistance to either nisin or pediocin and exhibited limited growth at these concentrations. Wild-type strains with complete resistance to pediocin, i.e., showing uninhibited growth at the maximum concentration employed (see below), were also included in the study.
TABLE 1.
L. monocytogenes wild-type strains and derived spontaneous bacteriocin-resistant mutants
| L. monocytogenes strain | Origin or reference | Bacteriocin phenotypea |
|---|---|---|
| 11 | Human | Pedr Niss |
| 22 | Human | Pedi Niss |
| 31 | Human | Peds Nisi |
| 31P | This work | Pedr mutant of 31d |
| 31N2 | This work | Nisr mutant of 31b |
| 33 | Human | Pedi Niss |
| 59 | Cattle | Pedi Niss |
| 114 | Cattle | Pedr Niss |
| 124 | Swine | Pedi Niss |
| 128 | Swine | Pedi Niss |
| 201 | Swine | Peds Niss |
| 272 | Swine | Peds Niss |
| 272P | This work | Pedr mutant of 272d |
| 288 | Wastewater | Pedi Niss |
| 290 | Sewage water | Pedi Niss |
| 317 | Swine | Pedr Niss |
| 319 | Swine | Pedr Niss |
| 322 | Swine | Pedr Niss |
| 322N2 | Gravesen et al. (15) | Nisr mutant of 322b |
| 340 | Swine | Pedi Niss |
| 358 | Swine | Pedi Nisi |
| 358N | Gravesen et al. (15) | Nisr mutant of 358c |
| 386 | Heat-treated pork | Peds Niss |
| 387 | Heat-treated pork | Peds Nisi |
| 393 | Heat-treated pork | Peds Nisi |
| 412 | Raw, salted pork | Peds Niss |
| 412P | Gravesen et al. (16) | Pedr mutant of 412f |
| 412N | Gravesen et al. (15) | Nisr mutant of 412e |
| 412NP | Gravesen et al. (16) | Pedr mutant of 412Nf |
| Scott A | Clinical | Peds Niss |
| ScottA-Ln | This work | Pedr mutant of Scott Ag |
| VDL144 | Reference strain | Peds Niss |
| GR9 | This work | Pedr mutant of VDL144g |
| GR10 | This work | Pedr mutant of VDL144g |
Ped, pediocin; Nis, nisin. The superscripts “s,” “r,” and “i” indicate sensitive, resistant, and intermediate, respectively. The remaining superscript letters indicate the following. Spontaneous resistant mutants were isolated after 10 (“b”) or 27 (“c”) sequential passages on nisin-containing TSA or after 8 (“d”) passages on pediocin-containing TSA (pH 7.3, 30°C). Other mutants were isolated after a single exposure to 500 IU of nisin/ml (“e”) or 2,560 AU of pediocin/ml (“f”) (pH 6.5, 30°C) or from an agar diffusion assay, with Leuconostoc carnosum 4010 as an inhibitor, as single colonies in the inhibition zone in BHI soft agar (“g”).
Spontaneous bacteriocin-resistant mutants were isolated after single or sequential exposure to the relevant compound (16, 15) as described in Table 1. For the nisin-resistant mutants there was typically a two- to fourfold increase in the nisin MIC compared to the respective parental strain (15), whereas the pediocin-resistant mutants all exhibited the same complete pediocin-resistance phenotype as the wild-type strains described above. Mutants isolated in the agar diffusion assay employing Leuconostoc carnosum 4010 as inhibitor were cross-resistant to pediocin, indicating that Leuconostoc carnosum 4010 produces class IIa bacteriocin(s).
Cultures were grown in brain heart infusion broth (BHI broth; Difco, Detroit, Mich.) without agitation or on tryptone soy agar (TSA; Oxoid, Basingstoke, United Kingdom), both at 30°C. When indicated, NaCl was added at 3 or 6% (wt/vol), and the pH was adjusted to 6.5, 6.0, or 5.5 with 2 M HCl. Preinoculum was cultured either at 30°C for 19 h (standard preinoculum) or at 5°C for 7 days, followed by 2 days at 10°C (low-temperature-adapted preinoculum).
A stock solution of 4 × 104 IU of pure nisin A (supplied by Aplin and Barrett Ltd. [Danisco Ingredients], Beaminster, United Kingdom)/ml in 0.02 M HCl was added to TSA (pH 6.5) to a final concentration of 100 or 500 IU/ml. Pediocin PA-1 fermentate was supplied by B. B. Budde (Frederiksberg, Denmark). The pediocin activity was determined by an automated turbidimetric screening method with Lactobacillus sake NCFB 2714 as an indicator organism (modified after an earlier study [30]). The obtained AU (19) are equal to 0.4 × the units determined by Rasch and Knøchel (32) using a well diffusion assay. The fermentate was added to TSA at a maximal final concentration of 25%, a value corresponding to 2,560 AU/ml.
Spot method for bacteriocin resistance frequency determination.
A 5-μl portion of a 10-fold serial dilution of either standard or low-temperature-adapted preinoculum in peptone saline was spotted onto TSA plates. From each dilution, four aliquots were spotted onto selective TSA agar at the relevant pH and NaCl levels. Another four spots were made on similar plates without the bacteriocin. After incubation of the plates, the CFU/ml values were calculated from dilutions giving up to 25 colonies per spot (in most cases 5 to 15). The resistance frequency (i.e., the ratio between the CFU/ml values with and without selection) was determined as the average of two independent experiments. The statistical significance of observed differences between methods was assessed by analysis of variance conducted in SAS 6.12 (SAS Institute, Cary, N.C.). Statistically significant differences between the log10 of resistance frequencies of individual strains were estimated by the least significant difference (LSD) as an option under the statement MEANS in SAS.
Stability of bacteriocin resistance.
The stability of the acquired resistance was monitored for at least 100 generations of exponential growth in BHI broth (pH 6.5) without selection. Exponential growth was maintained by twice-daily dilutions (10−3 to 10−5) of the cultures into fresh broth, which kept the optical density at 600 nm (OD600) under ca. 0.2. At regular intervals the cultures were tested for bacteriocin resistance, i.e., ability to grow on TSA plates containing either 500 IU of nisin or 1,720 AU of pediocin (both at pH 6.5)/ml. The corresponding wild-type strains were included as sensitive controls.
Fitness costs of bacteriocin resistance at 30°C.
The growth of strains having developed resistance was compared to the parental wild-type strain under different conditions. Growth was monitored in BHI broth with the pH adjusted to 6.5, 6.0, or 5.5 combined with NaCl added at 0, 3, or 6%. Each broth was inoculated to 105 CFU/ml with standard preinoculum, and 200 μl was loaded in triplicate in honeycomb microtiter plates. The OD600 was measured every 10 min for 27 h by using a Bioscreen C unit (Labsystems, Helsinki, Suomi Finland). For each well the OD600 as a function of time was fitted to a four-parameter logistic model by using the method of least squares. The maximum specific growth rate, μmax, and the time, T, to reach μmax were estimated and compared pairwise by using the statement LSMEANS and the option TDiff in SAS. After the run, bacteriocin resistance was verified on selective plates (TSA with 2,000 IU of nisin or 2,560 AU of pediocin [both at pH 7.3]/ml).
Fitness costs in BHI broth at 5°C.
Growth was monitored in BHI broth with the pH adjusted to 6.25 and 1.5% added NaCl. Low-temperature-adapted preinoculum was inoculated in duplicate at 103 CFU/ml. The OD600 was measured every second day for 20 days. Growth curves were fitted to a logistic model, the T and μmax values were determined, and these values were compared by LSMEANS. Bacteriocin resistance was verified on selective plates on days 0, 4, 8, 12, and 16.
Fitness costs in a meat model at 5°C.
The meat model was a saveloy-type standard sausage (diameter, 7 cm; pH 6.25; 2% added NaCl corresponding to 3.1% NaCl in the water phase; 14.6% fat; initial content, 60 ppm of nitrite resulting in 40 ppm of nitrite in the final product), freshly produced and kindly supplied by the Danish Meat Research Institute. One-centimeter slices were inoculated with ca. 103 CFU of low-temperature-adapted preinoculum per slice and vacuum packed. The number of CFU cm−2 was determined in duplicate after 0, 3, 7, 14, 21, and 28 days at 5°C. Two randomly chosen packages were opened, and the meat was homogenized in 50 ml of peptone saline for 30 s at medium intensity in a Stomacher 400 lab blender (Seward Medical, London, United Kingdom). The CFU cm−2 was determined on Listeria selective agar plates (Oxford Formula; Oxoid), and growth curves were determined from the averages of two results. The bacteriocin resistance was tested after 28 days by parallel enumeration on selective plates (TSA with 2,000 IU of nisin or 2,560 AU of pediocin [both at pH 7.3]/ml). The natural flora, normally dominated by lactic acid bacteria, was assessed on days 14 and 21 by parallel enumeration on de Man, Rogosa, Sharpe agar (MRS; Oxoid), TSA, and Listeria selective agar, and the results verified that L. monocytogenes was the dominating species.
RESULTS
Strain variations in frequency of resistance development.
Initial experiments showed no significant difference (P > 0.05) between resistance frequencies determined by the described spot method and traditional plating (results not shown). Use of the spot method considerably reduced the required number of plates and the number of colonies to be counted.
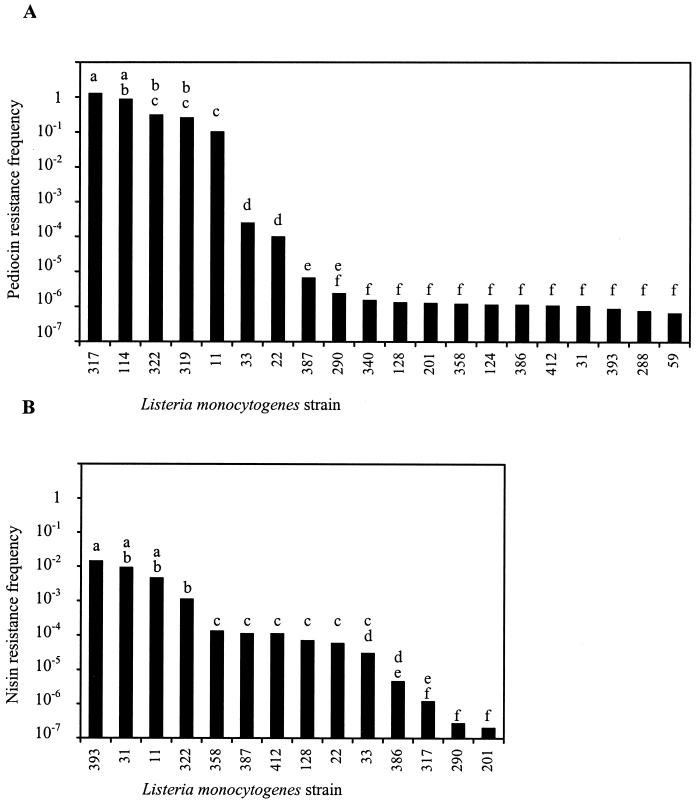
The frequency of the development of pediocin resistance (1,720 AU/ml) was determined for 20 strains (Fig. 1A). Of these, five were resistant, six were sensitive, and nine had intermediately enhanced resistance to pediocin. Analysis by LSD divided the strains into three statistically distinguishable groups. Two of the nine strains with intermediate pediocin sensitivity had frequencies of ca. 10−4. The remaining nonresistant strains, i.e., of the nine strains with intermediately enhanced resistance and the six sensitive strains, developed pediocin resistance at frequencies of ca. 10−6 (6 × 10−7 to 7 × 10−6). The resistant strains comprised a group with frequencies of 0.1 to 1, as expected.
FIG. 1.
Frequency of development of resistance to 1,720 AU of pediocin/ml (A) or 500 IU of nisin/ml (B) in TSA, at pH 6.5 and 30°C, in strains of L. monocytogenes. Differences between strains with the same letter designation above the columns were not statistically significant.
The frequency of nisin resistance development (500 IU/ml) was determined for 14 strains (Fig. 1B), of which four had intermediately enhanced nisin resistance and the remaining ones were sensitive to nisin. Here, the strains did not form clearly discernible groups, which was corroborated by the LSD test. Four strains comprised a group having frequencies of 10−3 to 10−2, with two of the intermediately nisin sensitive strains (L. monocytogenes 31 and 393) having the highest frequencies. The remaining strains could not be subdivided further and had nisin resistance frequencies in the range 10−7 to 10−4; the intermediately sensitive strains (L. monocytogenes 358 and 387) were among those with the highest frequencies.
Influence of pH, salt, and temperature on the frequency of resistance development.
The frequency of resistance development was determined for the four strains L. monocytogenes 22, 322, 358, and 412, representing the observed natural variations in bacteriocin sensitivity, in a full factorial experimental design with two levels of pH (5.5 and 6.5), added NaCl (0 and 6%), and temperature (10 and 30°C), by using low-temperature-adapted preinoculum. L. monocytogenes 22 turned out to be pH sensitive, preventing meaningful determinations at pH 5.5 for this strain.
The frequency of development of resistance to pediocin (1,720 AU/ml) was not appreciably affected by any of the applied conditions. The observed frequencies for the four strains were 2 × 10−4 to 4 × 10−4, 0.7 to 1.2, 3 × 10−6 to 9 × 10−6, and 3 × 10−6 to 6 × 10−6, respectively, compared to 1 × 10−4, 0.3, 1 × 10−6, and 1 × 10−6 in the previous experiment (Fig. 1A).
In contrast, the development of resistance to 500 IU of nisin/ml was strongly affected by the employed conditions. The frequencies for all four strains were below the detection limit of 5 × 10−8 except for plates with pH 6.5 and no added NaCl incubated at 30°C. These plates corresponded to the standard conditions in the previous experiment (Fig. 1B), the only difference being that in the current experiment a low-temperature-adapted preinoculum was employed. Here, the nisin resistance frequency for L. monocytogenes 322 decreased 100-fold and for L. monocytogenes 412 it increased 10-fold, whereas there were only minor effects for the two remaining strains compared to the experiment in Fig. 1B.
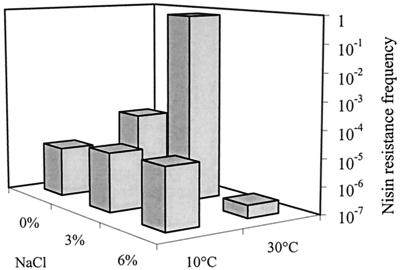
The effect of salt and incubation temperature was further investigated for L. monocytogenes 412 by reducing the nisin concentration to 100 IU/ml at pH 6.5 with low-temperature-adapted preinoculum (Fig. 2). An intermediate salt level (3% added NaCl) was included in the experiment. At 10°C, the nisin resistance frequency was 6 × 10−6 to 2 × 10−5, showing a minor effect of salt content at this temperature. At 30°C, there was a pronounced effect. With 3% added NaCl, the frequency was 0.7, indicating that L. monocytogenes 412 was practically insensitive to the applied nisin. Without added salt, the frequency (6 × 10−5) corresponded to values observed at 10°C. With 6% NaCl, the frequency was reduced to 3 × 10−7. The effects of NaCl and temperature were statistically significant (P < 0.001 and P < 0.01, respectively).
FIG. 2.
Effect of added NaCl and temperature on the frequency of development of resistance to 100 IU of nisin/ml in TSA at pH 6.5 in L. monocytogenes 412.
Frequency of simultaneous pediocin and nisin resistance development.
The resistance frequency was determined for two strains, L. monocytogenes 412 and 22, with plates containing 1,720 AU of pediocin/ml, 500 IU of nisin/ml, or the combination of 1,720 AU of pediocin and 500 IU of nisin/ml (standard preinoculum, TSA [pH 6.5], 30°C). For both strains, simultaneous exposure to pediocin and nisin reduced the frequency of resistance development. L. monocytogenes 22 developed resistance to pediocin and nisin at frequencies of 1 × 10−4 and 7 × 10−5, respectively. If the two effects were independent, resistance to the two bacteriocins applied simultaneously would be expected to occur additively, at a frequency of ca. 7 × 10−9; however, resistance to the bacteriocin combination developed at 8 × 10−7. L. monocytogenes 412 had resistance frequencies of 1 × 10−5, 5 × 10−4, and 9 × 10−8 for pediocin, nisin, and the combination, respectively (additive effect, 5 × 10−9).
Stability of developed resistance.
The stability of pediocin or nisin resistance was determined for the mutants listed in Table 1 and for the L. monocytogenes 412 mutant isolated with the combination of pediocin and nisin. The pediocin-resistant mutants all retained this phenotype during 100 generations of exponential growth without selection in BHI broth at 30°C. The stability of the nisin-resistant mutants varied. L. monocytogenes 412N, 412NP, and 322N2 were stable, whereas L. monocytogenes 358N and the L. monocytogenes 412 derivative isolated by simultaneous exposure to nisin and pediocin both reverted to nisin sensitivity after 50 to 100 generations. L. monocytogenes 31N2 was not tested.
The stability of the pediocin mutants of L. monocytogenes 412 isolated in the factorial experiment described above was tested. All eight were stable for over 100 generations.
Additionally, all of the mutants tested in the fitness cost experiments (see below) retained their resistance phenotypes during growth in BHI broth at the various levels of salt, pH, and temperature and in the meat model (all corresponding to ca. 20 generations).
Fitness costs of developed resistance.
Growth in BHI broth at 30°C was determined for six pediocin-resistant, four nisin-resistant, and one “doubly resistant” mutant (i.e., resistant to both pediocin and nisin). In order to assess the effect of salt and pH, nine different broths were used (0, 3, and 6% added NaCl and pH at 5.5, 6.0, and 6.5). Table 2 shows the maximum specific growth rates, μmax, with 0 and 3% added NaCl (six different broths). A significantly lower μmax (P < 0.05) was frequently observed in the bacteriocin-resistant mutants compared to the parental strain, indicating that there was a fitness cost associated with resistance development. The effect was more pronounced for the pediocin-resistant mutants than for the nisin-resistant mutants. L. monocytogenes 412N and 31N2 did not have significantly different μmax values (P < 0.05) in any of the six media. For L. monocytogenes 322N2 and 358N, μmax was reduced to 59 to 80% of the rate of the wild-type strains in two and three of the tested media, respectively. The pediocin-resistant mutants had a significantly lower μmax value (P < 0.05) in three to five of the six broths. Additionally, the reductions in μmax were larger for the pediocin-resistant mutants than for the nisin-resistant mutants. For example, at pH 6.5 without added NaCl, the μmax of the nisin-resistant mutants was 71 to 100% of the rate of the corresponding wild-type strains, while the μmax of the pediocin-resistant mutants was reduced to 44 to 57% of the wild-type rate. The doubly resistant strain L. monocytogenes 412NP generally had slightly larger fitness costs than the corresponding pediocin-resistant mutant.
TABLE 2.
Maximum specific growth rate in BHI broth at 30°C of wild-type L. monocytogenes strains and derived pediocin-resistant and nisin-resistant mutantsa
| L. monocytogenes strain | μmax (h−1) at:
|
||||||
|---|---|---|---|---|---|---|---|
| pH 6.5 with:
|
pH 6.0 with:
|
pH 5.5 with:
|
|||||
| 0% NaCl | 3% NaCl | 0% NaCl | 3% NaCl | 0% NaCl | 3% NaCl | ||
| 31 | 1.10 | 0.82 | 0.84 | ND | 0.66 | 0.34 | |
| 31N2 | 1.06 | 0.74 | 0.86 | 0.62 | 0.70 | 0.52 | |
| 31P | 0.60∗ | 0.44∗ | 0.50∗ | 0.52 | 0.50 | 0.40 | |
| 272 | 1.00 | 0.86 | 0.80 | 0.48 | 0.74 | 0.50 | |
| 272P | 0.54∗ | 0.48∗ | 0.48∗ | 0.36 | 0.44∗ | 0.30∗ | |
| 322 | 0.82 | 0.46 | 0.58 | 0.40 | 0.54 | 0.40 | |
| 322N2 | 0.58∗ | 0.44 | 0.48 | 0.28 | 0.32∗ | 0.30 | |
| 358 | 0.90 | 0.76 | 0.78 | 0.52 | 0.60 | 0.46 | |
| 358N | 0.72∗ | 0.60 | 0.58∗ | 0.44 | 0.44∗ | 0.36 | |
| 412 | 0.94 | 0.76 | 0.80 | 0.64 | 0.58 | 0.42 | |
| 412N | 0.96 | 0.72 | 0.72 | 0.58 | 0.50 | 0.38 | |
| 412P | 0.54∗ | 0.46∗ | 0.54∗ | 0.44∗ | 0.46∗ | 0.46 | |
| 412NP | 0.62∗ | 0.42∗ | 0.52∗ | 0.36∗ | 0.42∗ | 0.38 | |
| ScottA | 1.04 | 0.68 | 0.86 | 0.56 | 0.54 | 0.46 | |
| ScottA-Ln | 0.52∗ | 0.42∗ | 0.50∗ | 0.44 | 0.40 | ND | |
| VDL144 | 1.08 | 0.74 | 0.90 | 0.64 | 0.66 | 0.52 | |
| GR9 | 0.54∗ | 0.50∗ | 0.42∗ | 0.44∗ | 0.38∗ | 0.48 | |
| GR10 | 0.48∗ | 0.50∗ | 0.42∗ | 0.44∗ | 0.38∗ | 0.48 | |
Pediocin-resistant strains are designated “P,” “Ln,” or “GR”. Nisin-resistant strains are designated “N.” Mutants with a significantly different (P < 0.05) maximum growth rate compared to the corresponding wild-type strain are indicated with an asterisk. ND, not determined.
At 6% added NaCl, the growth curves were incomplete, which prevented calculation of the μmax. However, the growth curves likewise indicated larger fitness costs in the pediocin-resistant mutants than in the nisin-resistant mutants (results not shown).
Similar general observations were made for the time, T, to reach maximum growth rate (results not shown). Significant increases in T (P < 0.05) were observed especially for the pediocin-resistant mutants. The doubly resistant strain and the three mutants isolated after exposure to L. carnosum 4010 showed the largest increases in T.
Fitness costs in BHI broth at 5°C.
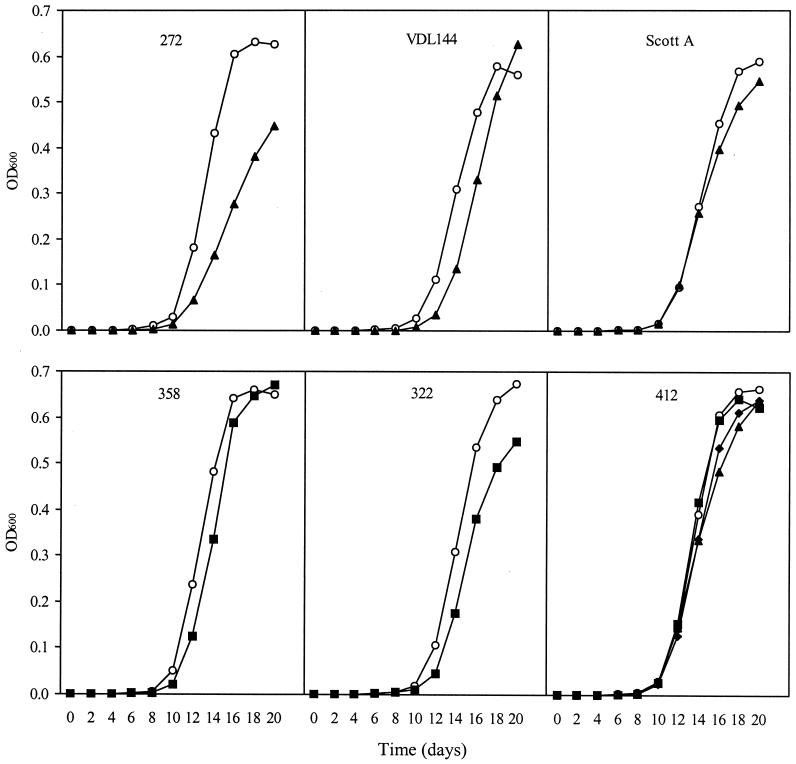
The growth of wild-type and resistant strains at 5°C was determined in BHI broth at pH 6.25 with 1.5% added NaCl (Fig. 3). These levels were chosen in order to mimic the conditions in meat products. The growth of the nisin-resistant mutants was virtually identical to that of the corresponding wild-type strains. The pediocin-resistant mutants had significant (P < 0.05) reductions of μmax to 59 to 87% of the rate of the corresponding wild-type strain.
FIG. 3.
Fitness costs during growth in BHI broth (pH 6.25 and 1.5% added NaCl) at 5°C of spontaneous bacteriocin-resistant mutants of L. monocytogenes 412, 272, 322, 358, Scott A, and VDL 144. Symbols: ○, wild-type strain; ▴, pediocin-resistant mutant; ▪, nisin-resistant mutant; ⧫, pediocin- and nisin-resistant mutant (412NP).
Fitness costs in a meat model.
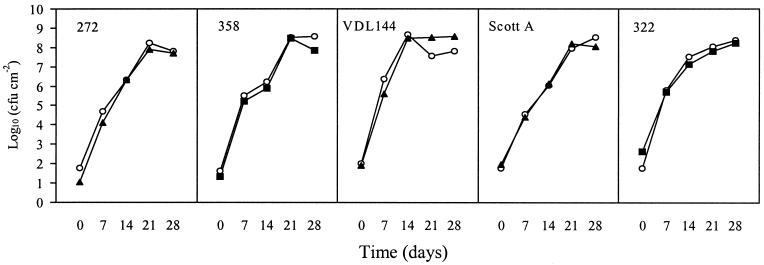
In this experiment, growth at 5°C of three pediocin-resistant and two nisin-resistant mutants was compared to the respective wild-type strains (Fig. 4). The observed growth of the resistant mutants was indistinguishable from that of the wild-type strains, indicating that bacteriocin resistance development did not confer measurable fitness costs in the meat model system based on CFU counts.
FIG. 4.
Fitness costs during growth in a meat model at 5°C of spontaneous pediocin-resistant mutants of L. monocytogenes 272, Scott A, and VDL 144 and spontaneous nisin-resistant mutants of L. monocytogenes 322 and 358. Symbols: ○, wild-type strain; ▴, pediocin-resistant mutant; ▪, nisin-resistant mutant.
DISCUSSION
Frequency of resistance development.
The results of this study demonstrate a substantial natural variation in the frequency of bacteriocin resistance development in L. monocytogenes, reflecting the effects of strain, bacteriocin, culture preadaptation, and environmental factors such as pH, NaCl, and temperature.
In addition to being practically invariable to specific strain and environmental conditions, the frequency of pediocin resistance development was not affected by varying the pediocin concentration from 1,200 to 2,560 AU/ml in two strains (results not shown). Similar results for strain, condition, and concentration were obtained in our laboratory when we used the fermentate of another class IIa bacteriocin producer, L. carnosum 4010 (results not shown), suggesting that the seemingly invariable resistance frequency could be a common property of this class. A subgroup of the intermediately pediocin sensitive strains had a ∼100-fold-higher frequency of pediocin resistance development. If this observation were extrapolated with respect to the prevalence of intermediately susceptible strains reported by Rasch and Knøchel (32), the higher resistance frequency would be expected in ca. 2% of natural L. monocytogenes isolates. Compared to our results, most previous studies employing various class IIa bacteriocins have reported relatively high resistance frequencies of 10−4 to 10−3 in one or two tested strains (33, 41), although in one strain frequencies of 10−6 to 10−4 were observed (10).
We observed very substantial variations in nisin resistance development, with pronounced effects of all tested parameters. In concordance with these findings, previous studies of one to three strains have mentioned frequencies ranging from <10−9 to 10−3 (3, 6, 8, 18, 26, 27, 34). Also in agreement with our results, increasing the nisin concentration or lowering the incubation temperature reduced the nisin resistance frequency (26). However, the results on the effect of salt are somewhat contradictory. Nisin resistance development in L. monocytogenes Scott A was enhanced when the salt concentration was increased from 0.5 to 3.5% during incubation at 10°C but not at 20 or 30°C (8), while we saw a similar protective effect in L. monocytogenes 412 at 30 but not at 10°C. This protective effect occurred at 3.5% but not at 6.5% NaCl. An increased nisin resistance frequency upon addition of 0.5% salt (37°C) has also been reported (3). Protection from nisin inactivation at certain pH levels was observed by a gradient plate method, but a similar effect was not seen for salt (37). The observed responsiveness to the specific conditions emphasizes the importance of caution when results obtained under different experimental procedures are compared and suggests a complex interaction between salt and nisin resistance development.
A strategy envisioned to circumvent problems resulting from bacteriocin resistance is the application of two bacteriocins with different antibacterial activity mechanisms (17, 34). In the two strains we tested, the frequency of the simultaneous development of resistance to pediocin and nisin was 10- to 100-fold higher than what would be expected from a combination of the two bacteriocins applied separately. Similar results have previously been obtained for nisin and enterocin CRL35, another class IIa bacteriocin (41).
Distinct differences between nisin and pediocin were observed. While the employed environmental conditions had no effect on pediocin resistance, a considerable reduction in the development of nisin resistance was observed during salt or low-temperature stress. Additionally, preadaptation of the inoculum to low temperature did not affect the development of pediocin resistance, whereas strain-specific effects on the development of nisin resistance were observed. The observed differences probably reflect differences in resistance mechanisms for the two bacteriocins.
Resistance to various antibiotics has been determined to develop at frequencies of 10−9 to 10−7 in L. monocytogenes by procedures similar to those in this study (2, 29, 31, 40). Thus, bacteriocin resistance seems to arise at relatively high frequencies. The reason for this is not known.
Fitness cost and stability of developed resistance.
The mutations conferring bacteriocin resistance could possibly reduce the growth potential of the cells or render them more sensitive to preservation parameters such as salt, low pH, or low temperature. We observed that bacteriocin-resistant mutants, and pediocin-resistant mutants in particular, frequently exhibited fitness costs, having a reduced maximum specific growth rate as well as an increased lag phase in broth. Previous reports on fitness costs in Listeria have varied greatly.
The findings on the influence of environmental factors also apparently conflict. The mutants we characterized were not more sensitive to salt or low pH than their parental strains, since the fitness costs in broth did not increase with increasing salt or low pH stress. Studies on different nisin-resistant mutants of L. monocytogenes Scott A have reported a larger growth rate reduction at 20°C than at 37°C (27) but similar growth rate reductions in the range 10 to 20°C (26). We observed comparable, isolate-specific growth rate reductions at 10°C and at various NaCl and pH levels at 30°C, indicating that the mutants did not have altered sensitivity to these stress factors. Various degrees of increased sensitivity to NaCl and low pH have been described for two different mutants (25).
It would thus seem that the actual fitness cost and sensitivity to environmental conditions are isolate specific, although variations in the applied methods may also be part of the explanation. Our results additionally show that fitness costs following pediocin resistance development are more severe than those imposed by the level of nisin resistance observed here.
In a saveloy-type meat model system, no fitness costs were observed. This seeming discrepancy could be due to the inherently lower accuracy of the latter procedure, i.e., the fitness costs in broth were not large enough to be detectable in the meat model, and therefore are probably not of any practical importance. Alternatively, currently unknown factors in the anaerobic meat system could favor the bacteriocin-resistant mutants and thereby improve their growth potential.
A fitness cost as described above would be expected to influence the stability of a bacteriocin-resistant culture, since a bacteriocin-sensitive revertant with a higher growth rate would invade the resistant culture, as described previously (10). It is therefore surprising that bacteriocin resistance in most cases was very stable and that the pediocin-resistant mutants, in spite of having more pronounced fitness costs, were more stable than their nisin-resistant counterparts. Additionally, there was no apparent correlation between reductions in the maximum specific growth rate and the stability of the nisin-resistant mutants in this study. Most other studies have also reported bacteriocin resistance in L. monocytogenes to be stable (26, 33). Ming and Daeschel (27) reported that resistance following a single exposure to nisin was unstable, whereas serial transfer in nisin-containing media resulted in stable resistance. We did not observe such a correlation.
Conclusions and perspectives with respect to application.
The presented results allow some general considerations regarding the likelihood of development and the persistence of resistant strains.
Resistance to class IIa bacteriocins may be expected to arise at a frequency of ca. 10−6, irrespective of the prevailing conditions. Therefore, the number of listerial cells (i.e., the contamination level) can be expected to be a determining factor. The observation that one strain developed simultaneous resistance to pediocin and nisin at a frequency of 10−6 underlines the importance of maintaining a low contamination level.
The frequency of development of increased resistance to nisin cannot be predicted, since it will be a function of the specific strain and conditions. Since food systems inherently are heterogeneous, it is conceivable that gradients (especially with respect to salt) will create local areas where increases in resistance can occur at relatively high frequencies. This will presumably only result in a low level of enhanced nisin resistance. However, since the nisin resistance phenotype can be very stable, it is conceivable that it may subsequently constitute the basis for further increases and thereby have an important role for the final emergence of high nisin resistance levels, similar to observations for some types of antibiotics (23).
If bacteriocin-resistant strains do arise, they must be expected to proliferate in meat and possibly also other food systems. Additionally, the high stability often observed could infer that secondary mutations subsequently would arise, ameliorating the fitness cost, as seen previously in antibiotic-resistant mutants (1, 35).
It is clear that many interacting factors will influence the probability of bacteriocin resistance development during biopreservation of food. To a certain extent, the outcome may be predicted through mathematical modeling, provided that sufficient and suitable data are available. We are currently investigating such an approach. Ultimately, the answer must be acquired through realistic food trials, and such verification is urgently required.
Acknowledgments
The technical assistance of Lene Gertman and Susanne Emborg Thorsen is greatly appreciated. We thank K. B. Rechinger for critically reading the manuscript and B. B. Budde and Aplin and Barrett Ltd. (Danisco Ingredients) for supplying pediocin and nisin, respectively.
This work was supported by the Danish Food Technology program FØTEK 2 (93s-2469-å95-00064), the Danish Bacon and Meat Council, and the Food Biotechnology Program of the Danish Ministry for Food, Agriculture, and Fisheries (BIOT99-8).
REFERENCES
- 1.Andersson, D. I., and B. R. Levin. 1999. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 2.Boisivon, A., C. Guiomar, and C. Carbon. 1990. In vitro bactericidal activity of amoxicillin, gentamicin, rifampicin, ciprofloxacin and trimethoprim-sulfamethoxazole alone or in combination against Listeria monocytogenes. Eur. J. Clin. Microbiol. Infect. Dis. 9:206-209. [DOI] [PubMed] [Google Scholar]
- 3.Bouttefroy, A., and J. B. Milliere. 2000. Nisin-curvaticin 13 combinations for avoiding the regrowth of bacteriocin resistant cells of Listeria monocytogenes ATCC 15313. Int. J. Food Microbiol. 62:65-75. [DOI] [PubMed] [Google Scholar]
- 4.Breidt, F., K. A. Crowley, and H. P. Fleming. 1993. Isolation and characterization of nisin-resistant Leuconostoc mesenteroides for use in cabbage fermentations. Appl. Environ. Microbiol. 59:3778-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall, A. D., and T. J. Montville. 1998. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl. Environ. Microbiol. 64:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, E. A., and M. R. Adams. 1994. Resistance of Listeria monocytogenes to the bacteriocin nisin. Int. J. Food Microbiol. 21:341-347. [DOI] [PubMed] [Google Scholar]
- 7.Davies, E. A., M. B. Falahee, and M. R. Adams. 1996. Involvement of the cell envelope of Listeria monocytogenes in the acquisition of nisin resistance. J. Appl. Bacteriol. 81:139-146. [DOI] [PubMed] [Google Scholar]
- 8.de Martinis, E. C. P., A. D. Crandall, A. S. Mazzotta, and T. J. Montville. 1997. Influence of pH, salt, and temperature on nisin resistance in Listeria monocytogenes. J. Food Prot. 60:420-423. [DOI] [PubMed] [Google Scholar]
- 9.Duffes, F., P. Jenoe, and P. Boyaval. 2000. Use of two-dimensional electrophoresis to study differential protein expression in divercin V41-resistant and wild-type strains of Listeria monocytogenes. Appl. Environ. Microbiol. 66:4318-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dykes, G. A., and J. W. Hastings. 1998. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett. Appl. Microbiol. 26:5-8. [DOI] [PubMed] [Google Scholar]
- 11.Ennahar, S., N. Deschamps, and J. Richard. 2000. Natural variation in susceptibility of Listeria strains to class IIa bacteriocins. Curr. Microbiol. 41:1-4. [DOI] [PubMed] [Google Scholar]
- 12.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira, M. A., and B. M. Lund. 1996. The effect of nisin on Listeria monocytogenes in culture medium and long-life cottage cheese. Lett. Appl. Microbiol. 22:433-438. [DOI] [PubMed] [Google Scholar]
- 14.Goulhen, F., J. Meghrous, and C. Lacroix. 1998. Characterization of nisin-resistant variants of Pediococcus acidilactici UL5, a producer of pediocin. J. Appl. Microbiol. 85:387-397. [Google Scholar]
- 15.Gravesen, A., K. Sørensen, F. M. Aarestrup, and S. Knøchel. 2001. Spontaneous nisin-resistant Listeria monocytogenes mutants with increased expression of a putative penicillin-binding protein and their sensitivity to various antibiotics. Microb. Drug Resist. 7:127-135. [DOI] [PubMed] [Google Scholar]
- 16.Gravesen, A., P. Warthoe, S. Knøchel, and K. Thirstrup. 2000. Restriction fragment differential display of pediocin-resistant Listeria monocytogenes 412 mutants shows consistent overexpression of a putative beta-glucoside-specific PTS system. Microbiology 146:1381-1389. [DOI] [PubMed] [Google Scholar]
- 17.Hanlin, M. B., N. Kalchayanand, P. Ray, and B. Rak. 1993. Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity. J. Food Prot. 56:252-255. [DOI] [PubMed] [Google Scholar]
- 18.Harris, L. J., H. P. Fleming, and T. R. Klaenhammer. 1991. Sensitivity and resistance of Listeria monocytogenes ATCC 19115, Scott A and UAL500 to nisin. J. Food Prot. 54:836-840. [DOI] [PubMed] [Google Scholar]
- 19.Jydegaard, A. M., A. Gravesen, and S. Knøchel. 2000. Growth condition-related response of Listeria monocytogenes 412 to bacteriocin inactivation. Lett. Appl. Microbiol. 31:68-72. [DOI] [PubMed] [Google Scholar]
- 20.Larsen, A. G., and B. Nørrung. 1993. Inhibition of Listeria monocytogenes by bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. Lett. Appl. Microbiol. 17:132-134. [Google Scholar]
- 21.Maisnier-Patin, S., and J. Richard. 1996. Cell wall changes in nisin-resistant variants of Listeria innocua grown in the presence of high nisin concentrations. FEMS Microbiol. Lett. 140:29-35. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani, H. C., and J. B. Russell. 2001. Nisin resistance of Streptococcus bovis. Appl. Environ. Microbiol. 67:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez, J. L., and F. Baquero. 2000. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 44:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzotta, A. S., A. D. Crandall, and T. J. Montville. 1997. Nisin resistance in Clostridium botulinum spores and vegetative cells. Appl. Environ. Microbiol. 63:2654-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzotta, A. S., K. D. Modi, and T. J. Montville. 2000. Nisin-resistant (Nisr) Listeria monocytogenes and Nisr Clostridium botulinum are not resistant to common food preservatives. J. Food Safety 65:888-890. [Google Scholar]
- 26.Mazzotta, A. S., and T. J. Montville. 1997. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10°C and 30°C. J. Appl. Microbiol. 82:32-38. [DOI] [PubMed] [Google Scholar]
- 27.Ming, X., and M. A. Daeschel. 1993. Nisin resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes Scott A. J. Food Prot. 56:944-948. [DOI] [PubMed] [Google Scholar]
- 28.Ming, X., and M. A. Daeschel. 1995. Correlation of cellular phospholipid content with nisin resistance of Listeria monocytogenes Scott A. J. Food Prot. 58:416-420. [DOI] [PubMed] [Google Scholar]
- 29.Morse, R., K. O'Hanlon, M. Virji, and M. D. Collins. 1999. Isolation of rifampin-resistant mutants of Listeria monocytogenes and their characterization by rpoB gene sequencing, temperature sensitivity for growth, and interaction with an epithelial cell line. J. Clin. Microbiol. 37:2913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mørtved, C. I., and I. Nes. 1990. Plasmid-associated bacteriocin production by a Lactococcus sake strain. J. Gen. Microbiol. 136:1601-1607. [Google Scholar]
- 31.Pierre, J., A. Boisivon, and L. Gutmann. 1990. Alteration of PBP 3 entails resistance to imipenem in Listeria monocytogenes. Antimicrob. Agents Chemother. 34:1695-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasch, M., and S. Knøchel. 1998. Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA-1 and bavaricin A. Lett. Appl. Microbiol. 27:275-278. [DOI] [PubMed] [Google Scholar]
- 33.Rekhif, N., A. Atrih, and G. Lefebvre. 1994. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr. Microbiol. 28:237-241. [Google Scholar]
- 34.Schillinger, U., H. S. Chung, K. Keppler, and W. H. Holzapfel. 1998. Use of bacteriocinogenic lactic acid bacteria to inhibit spontaneous nisin-resistant mutants of Listeria monocytogenes Scott A. J. Appl. Microbiol. 85:657-663. [DOI] [PubMed] [Google Scholar]
- 35.Spratt, B. G. 1996. Antibiotic resistance: counting the cost. Curr. Biol. 6:1219-1221. [DOI] [PubMed] [Google Scholar]
- 36.Thomas, L., M. R. Clarkson, and J. Delves-Broughton. 2000. Nisin, p. 463-524. In A. S. Naidu (ed.), Natural food antimicrobial systems. CRC Press, Boca Raton, Fla.
- 37.Thomas, L. V., and J. W. Wimpenny. 1996. Investigation of the effect of combined variations in temperature, pH, and NaCl concentration on nisin inhibition of Listeria monocytogenes and Staphylococcus aureus. Appl. Environ. Microbiol. 62:2006-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ukuku, D. O., and L. A. Shelef. 1997. Sensitivity of six strains of Listeria monocytogenes to nisin. J. Food Prot. 60:867-869. [DOI] [PubMed] [Google Scholar]
- 39.Verheul, A., N. J. Russell, H. R. Van't, F. M. Rombouts, and T. Abee. 1997. Modifications of membrane phospholipid composition in nisin-resistant Listeria monocytogenes Scott A. Appl. Environ. Microbiol. 63:3451-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicente, M. F., J. C. Perez-Daz, F. Baquero, D. P. Angel, and J. Berenguer. 1990. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob. Agents Chemother. 34:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vignolo, G., J. Palacios, M. E. Farias, F. Sesma, U. Schillinger, W. Holzapfel, and G. Oliver. 2000. Combined effect of bacteriocins on the survival of various Listeria species in broth and meat systems. Curr. Microbiol. 41:410-416. [DOI] [PubMed] [Google Scholar]