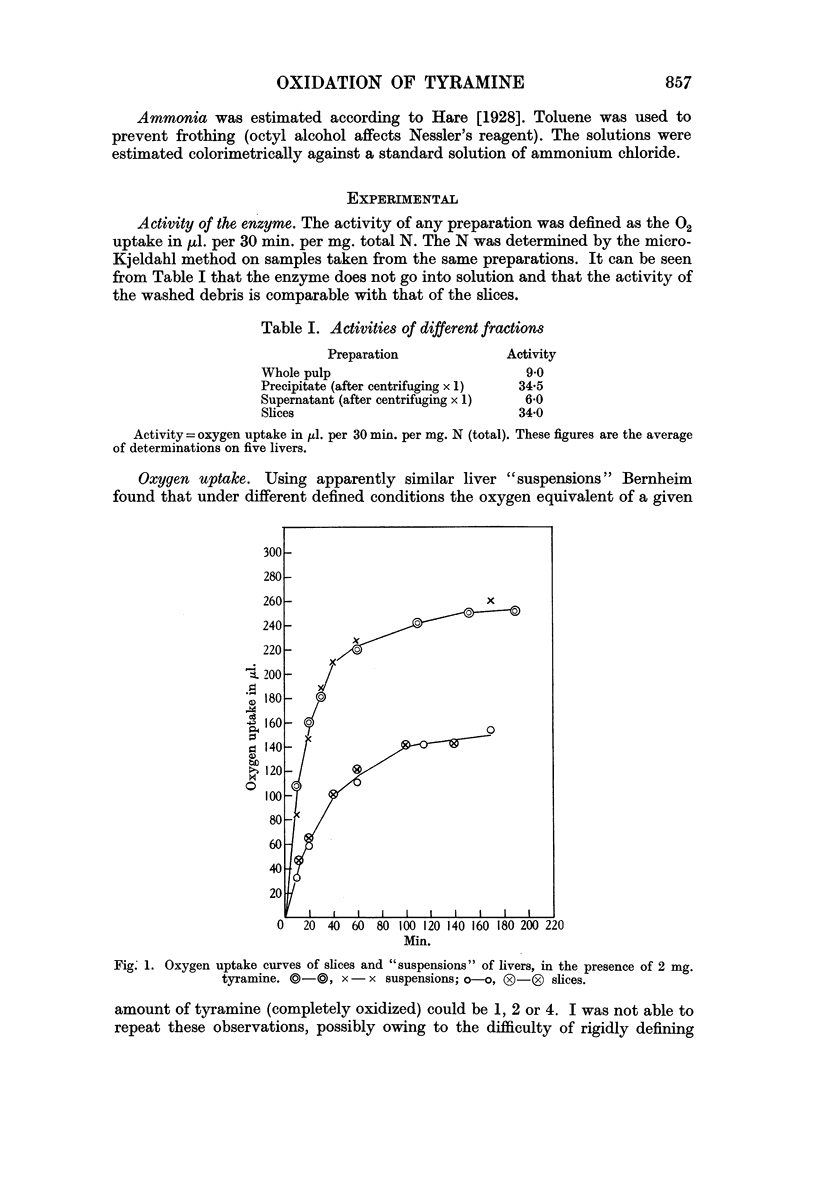
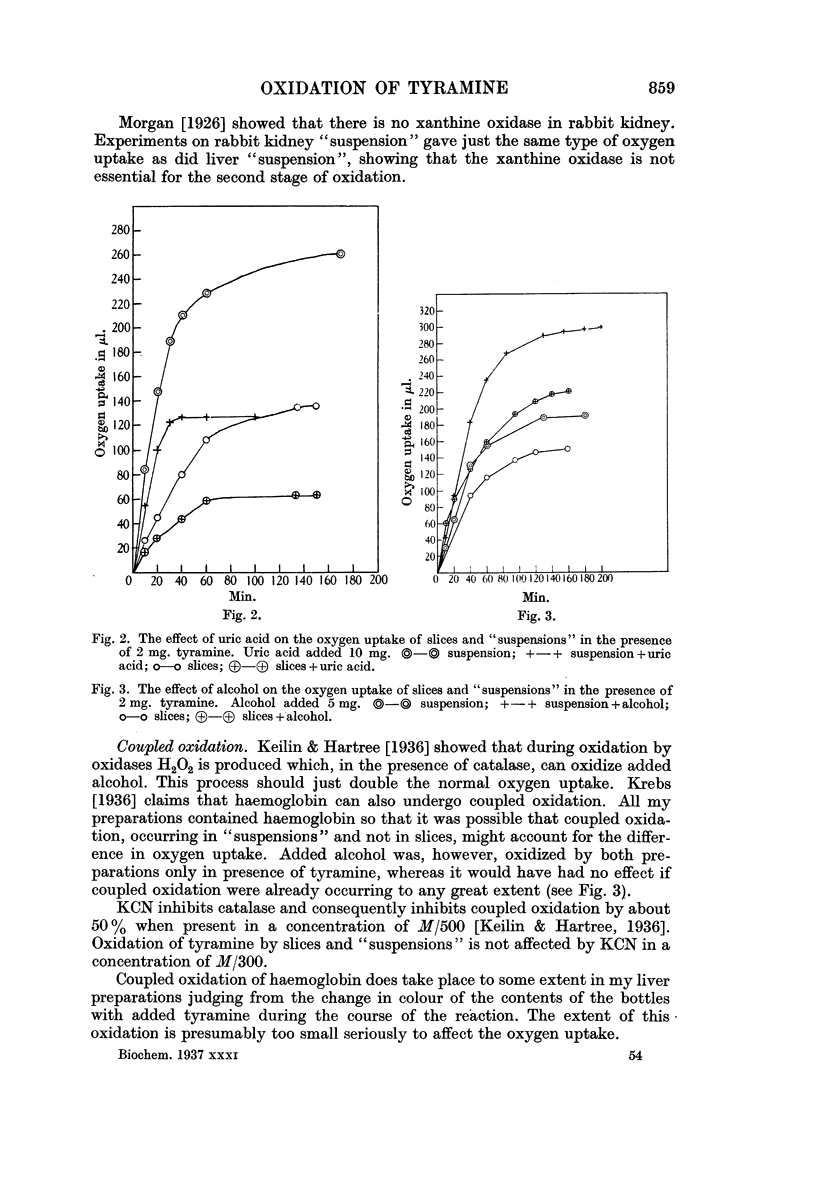
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dickens F. Metabolism of normal and tumour tissue: Action of some oxidation-reduction systems. Biochem J. 1936 Jun;30(6):1064–1074. doi: 10.1042/bj0301064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M., Thurlow S. Studies on Xanthine Oxidase: The Dynamics of the Oxidase System. Biochem J. 1924;18(5):976–988. doi: 10.1042/bj0180976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. A., Baker Z. The effects of oxidation-reduction potential indicator dyes on the metabolism of tumour and normal tissues. Biochem J. 1935 Oct;29(10):2396–2404. doi: 10.1042/bj0292396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. A., Schroeder E. F. The metabolism of lactic and pyruvic acids in normal and tumour tissue: Methods and results with kidney cortex. Biochem J. 1934;28(5):1920–1939. doi: 10.1042/bj0281920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Brosteaux J. The lactic dehydrogenase of animal tissues. Biochem J. 1936 Aug;30(8):1489–1508. doi: 10.1042/bj0301489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. J. The Distribution of Xanthine Oxidase I. Biochem J. 1926;20(6):1282–1291. doi: 10.1042/bj0201282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston F. J., Green D. E. The mechanism of the reaction of substrates with molecular oxygen. I. Biochem J. 1935 Aug;29(8):1983–2004. doi: 10.1042/bj0291983. [DOI] [PMC free article] [PubMed] [Google Scholar]


