Abstract
The mcyABCDEFGHIJ gene cluster of Microcystis aeruginosa encodes the mixed polyketide synthase/nonribosomal peptide synthetase (microcystin synthetase) which is responsible for biosynthesis of the potent liver toxin microcystin. The sequence and orientation of the mcy genes have previously been reported, but no transcriptional analysis had been performed prior to this study. The mcyABCDEFGHIJ genes are transcribed as two polycistronic operons, mcyABC and mcyDEFGHIJ, from a central bidirectional promoter between mcyA and mcyD. Two transcription start sites were detected for both mcyA and mcyD when cells were exposed to light intensities of 68 and 16 μmol of photons m−2 s−1. The start sites, located 206 and 254 bp upstream of the translational start for mcyD under high and low light conditions, respectively, indicate long untranslated leader regions. Putative transcription start sites were also identified for mcyE, mcyF, mcyG, mcyH, mcyI, and mcyJ but not for mcyB and mcyC. A combination of reverse transcription-PCR and rapid amplification of cDNA ends was employed throughout this work, which may have been one of the first transcriptional analyses of a large nonribosomal polyketide gene cluster.
Cyanobacteria are known to produce a wide range of bioactive compounds, including nonribosomally made peptides, polyketides, alkaloids, and lipopolysaccharides. Some of these compounds are neuro- or hepatotoxins, and their impact on human and animal health has resulted in concern worldwide. Microcystin is a hepatotoxic heptapeptide produced by several species of the genera Microcystis, Anabaena, Nostoc, and Oscillatoria. More than 65 structural isoforms of microcystins with various toxicities have been identified having the common structure cyclo(Adda–d-Glu–Mdha–d-Ala–l-X–d-MeAsp–l-Z), where X and Z are variable l amino acids, Adda is 3-amino-9-methoxy-2,6,8,-trimethyl-10-phenyl-4,6-decadienoic acid, d-MeAsp is 3-methylaspartic acid, and Mdha is N-methyl-dehydroalanine (27, 31).
Microcystin is synthesized nonribosomally via a mixed polyketide synthase/nonribosomal peptide synthetase system called microcystin synthetase (2, 12, 23). Recently, the gene cluster encoding the microcystin synthetase complex has been identified and sequenced (25, 34). This 55-kb gene cluster consists of six open reading frames (ORFs) with a mixed nonribosomal peptide synthetase/polyketide synthase nature (mcyA to mcyE and mcyG) and four smaller ORFs with putative precursor and tailoring functions (mcyF and mcyH to mcyJ). Catalytic domains in mcyA to mcyE and mcyG are responsible for incorporation of the precursors phenylacetate, malonyl coenzyme A, S-adenosyl-l-methionine, glutamate, serine, alanine, leucine, d-methyl-isoaspartate, and arginine. The smaller ORFs encode monofunctional proteins which are putatively involved in O-methylation (McyJ), epimerization (McyF), dehydration (McyI), and cellular localization (McyH) (24, 34).
The 10 ORFs are bidirectionally transcribed from a central 737-bp locus between mcyA and mcyD and are arranged in what until now has been classified as two putative operons, mcyABC and mcyDEFGHIJ. It has previously been reported that the mcyB and mcyD transcript levels are similar when they are determined under various light conditions (18). Nevertheless, the operon structure, the transcription start sites for mcyA and mcyD, and the presence and locations of putative promoters for individual genes within the cluster have not been investigated in detail (18, 24). Transcriptional analysis of the mcy cluster not only should increase our understanding of microcystin synthetase regulation and toxin biosynthesis but may also provide useful insights into other nonribosomal systems, some of which are involved in antibiotic production. Knowledge of the structure of the mcy gene cluster may therefore have important implications for further research to produce new antibiotics by combinational biosynthesis.
Transcriptional analysis of gene clusters has traditionally been carried out by Northern blotting, while transcription start sites are commonly mapped by using radioactive primer extension or S1 nuclease mapping (13). However, it has not been possible to detect the mcyABCDEFGHIJ genes on Northern blots, probably because of the very low levels and potential lengths of the transcripts. As a result, reverse transcription (RT)-PCR was used to detect mRNA transcripts in the mcyABCDEFGHIJ cluster. This method has previously been used for transcriptional analysis of cyanobacterial hydrogenase genes in Anabaena sp. (3, 5) and GTPase/ATPase genes in Synechocystis sp. (16).
Transcription initiation points can be mapped by rapid amplification of cDNA ends (RACE), also known as ligation-anchored PCR (30). This method has only recently been modified so that it can be used with prokaryotes (33). It involves production of the desired cDNA by RT, linking of a modified anchor oligonucleotide to the 5′ end of cDNA, and successive amplification with a primer complementary to the anchor and primers specific for the cDNA upstream of the reverse transcriptase primer sequence. The addition of the anchor places a known unique sequence at the unmapped 5′ end of the cDNA, which can be identified by DNA sequencing of the nested fragment.
Using RT-PCR and RACE, we performed a complete transcriptional analysis of the mcyABCDEFGHIJ cluster, including an analysis of putative transcription initiation points for the mcy genes and alternate transcription start sites for mcyA and mcyD under different light conditions. Our results indicate that complex gene regulation occurs in hybrid nonribosomal peptide-polyketide biosynthesis involving both multiple and alternate messages.
Materials and Methods
Cyanobacterial strains and culturing.
The axenic strain Microcystis aeruginosa PCC7806 (Braakman Reservoir, The Netherlands) was provided by the Pasteur Culture Collection, Paris, France (28). Cells were grown in BG11 (Sigma-Aldrich, Steinheim, Switzerland) in glass cylinders (diameter, 5.5 cm) with continuous aeration at 25°C and with continuous light (31 μmol of photons m−2 s−1). For the light experiments the cultures were exposed to 68 μmol of photons m−2 s−1 (high light conditions) or 16 μmol of photons m−2 s−1 (low light conditions) for 6 h before they were harvested.
Sampling, RNA extraction, and DNase treatment.
Both the sampling and RNA extraction procedures used have been described previously (18). After extraction with Trizol (Gibco BRL, Life Technologies, Rockville, Md.), RNA was column purified (SV total-RNA isolation system; Promega, Madison, Wis.). DNA was removed by treatment with DNase (Gibco BRL), followed by phenol-chloroform-isoamyl alcohol (25:24:1) extraction. The amount and purity of the RNA were determined from the optical densities at 260 and 280 nm and by electrophoresis on a 1% formaldehyde gel.
RT.
RT for production of cDNA for RACE or RT-PCR analysis (see below) was carried out by using Superscript II (Gibco BRL). RNA (0.5 to 1 μg) was added to 1× Superscript II buffer containing 10 pmol of reverse primer 2 (Table 1), each deoxynucleoside triphosphate at a concentration of 0.2 mM, 1 mM dithiothreitol, and enough H2O to bring the final volume to 45 μl. The Superscript II enzyme (200 U diluted in enough H2O to bring the final volume to 5 μl) was added only after stepwise primer annealing by a touchdown incubation procedure consisting of 70°C for 2 min, followed by 65°C for 1 min, 60°C for 1 min, 55°C for 1 min, 50°C for 1 min, and 45°C for 1 min, using a thermocycler (Perkin-Elmer). After the enzyme was added, the reaction mixture was incubated at 42°C for 30 min, which was followed by five cycles consisting of 50°C for 1 min, 53°C for 1 min, and 56°C for 1 min. The enzyme was omitted from reaction mixtures that were used as negative controls in the RT-PCR analysis. Untranscribed RNA was removed by alkaline cleavage; 1 μl of 0.5 M EDTA and 12.5 μl of 0.2 M NaOH were added directly to the RT reaction mixture, and then the preparation was incubated at 68°C for 5 min. The reaction was stopped by adding 12.5 μl of 1 M Tris-HCl (pH 7.4). cDNA was precipitated with 60 μl of isopropanol, 5 μl of 3 M sodium acetate, and 20 μg of glycogen. The pellet was dried, resuspended in 20 μl of TE (10 mM Tris-HCl [pH 7.4], 1 mM EDTA [pH 8.0]), and stored at −20°C.
Table 1.
Oligonucleotides used for RT and PCR
| Primer
|
Sequence (5′-3′) | ||
|---|---|---|---|
| Gene | Typea | Designation | |
| Primers used for RT and RACE-PCR | |||
| mcyA | R2 | PMR2 | CTA GAG TAG TTA CTG GAT CTA |
| mcyB | R2 | mcyBpR2 | AAC CCT TGT TGA CGC TCT GT |
| mcyC | R2 | Elke10 | CTG CCA AGA GTT CAG CAT CT |
| mcyD | R2 | Topaz4 | TTG TTG CCA TTC CTG CAT |
| mcyE | R2 | mcyEpR2 | CGT AAA CCT TCA GGG CAT GA |
| mcyF | R2 | mcyFpR2 | TGC AGC CTT CCT GAA ATA ATT |
| mcyG | R2 | mcyGpR2 | AGC AGT CAT GGA CAG AAT ATT |
| mcyH | R2 | Ebony5 | GAC GCT CAA CAT AAC TGG AA |
| mcyI | R2 | mcyIpR2 | GTT GCA TCT GAT TGC GAA TTA |
| mcyJ | R2 | mcyJpR2 | AAT CCG CTA CAG CCA ACC TT |
| Primers used for RACE-PCR and RT-PCR | |||
| mcyA | R1 | Promi5R | GGT AAT CTA TTG AAA CCA GAT G |
| mcyB | R1 | mcyBpR | CGA GTG ACG CTC TAC AAC TT |
| mcyC | R1 | mcyCpR | CCA GTT CTT GAA AGG CTG CT |
| mcyD | R1a | MpR60R | TTT GTT GAG ACT CGA AAG CTT TTA |
| mcyD | R1b | MpR15R | GTT TTC GGA TAA GTT CTT TTT ATC |
| mcyE | R1 | mcyER | GAC TTC CAC TAT AGC ATC AAG |
| mcyF | R1 | mcyFR | CTA TCA ATA GAA CCT GTG CGA |
| mcyG | R1 | mcyGR | ACA AGC CCA TAG TGC GGG AAT |
| mcyH | R1 | abcpR | TCT CAG AAA CTT AGA GGC ATT |
| mcyI | R1 | Ebony3 | GCC TCA TTA ATT TCC TCC GGT |
| mcyJ | R1 | mcyJpR | TGA TCT CCG GCT TTA AGT TC |
| Primers used for RT-PCR | |||
| mcyB | F | Ruby43 | CGG TTA TTT TGC GAC TCC |
| mcyC | F | Elke12 | GAA AAA TTC ATC GTC AAG CT |
| mcyE | F | mcyDF | GTC TGT CTC TAT AGA TGA ACG |
| mcyF | F | mcyEF | GAT CTG GCC TTG GAC GGA ATT |
| mcyG | F | mcyFF | TTG ACC ATC GCA CCT CTA TCA |
| mcyH | F | abcpF | GAC TTT CCT AAT ATT AGA CGC |
| mcyI | F | mcyIpF | CTC CCT TAG AAC AAT TTC TCT |
| mcyJ | F | mcyJpF | GAG CAA CTT TGC TCA AAG ATT |
| Primers used for RACE ligation and RACE-PCR | |||
| DT89 | CGC CAT TTC CAC CTT CTC TTC | ||
| DT88 | GAA GAG AAG GTG GAA ATG GCG TTT TGG | ||
R, reverse primer (R1 is upstream of R2; R1a and R1b were used interchangeably, leading to the same results); F, forward primer.
RACE.
Transcription start sites were identified by ligation-anchored PCR (33). In this procedure, T4 RNA ligase was used to ligate a 5′-phosphorylated, 3′ end cordycepin-blocked anchor oligonucleotide, DT88 (Table 1), to the single-stranded cDNA. The ligation conditions were as follows: 1× RNA ligase buffer (Fermentas), 0.4 μM DT88, 10 μl of cDNA sample, and 1 μl (3 U) of RNA ligase in a 12-μl (final volume) mixture. The reaction mixture was incubated overnight at room temperature. The PCR was performed directly with the DT88-ligated cDNA (1 μl), 10 pmol of DT89, and R2 primers (Table 1). The PCR was hot started by adding 5 μl of water containing 1 U of Taq DNA polymerase (Biotechnology International) after an initial 2-min denaturation step at 90°C. Then the following touchdown program was used: 95°C for 10 s and 70 to 60°C (1 min each) for 10 cycles, followed by 15 cycles of 95°C for 10 s and 60°C for 1 min. A second, nested PCR was carried out under the same conditions with a reverse primer (R1) internal to the primer originally used for cDNA synthesis (R2). The resulting RACE products were visualized by agarose gel electrophoresis, gel purified, and sequenced to determine the transcription start sites for each mcy gene. All RACE assays were repeated several times with up to six different RNA samples. Analyses of the mcyA and mcyD transcription start sites were repeated with RNA samples from three separate cultures grown under the conditions described above.
RT-PCR.
The presence of transcripts in selected gene regions was checked by RT-PCR. The reverse primer (R1) (Table 1) used to do this was situated upstream of the original reverse primer used for the RT reaction (R2). PCRs were performed with an initial denaturation step of 95°C for 2 min, followed by 30 cycles of 94°C for 10 s, 50°C for 20 s, and 72°C for 1 min, using 1 μl of cDNA prepared as described above, 10 pmol of reverse primer (R1), and 10 pmol of forward primer (Table 1).
Results
Transcription initiation sites for mcyA and mcyD are dependent on light.
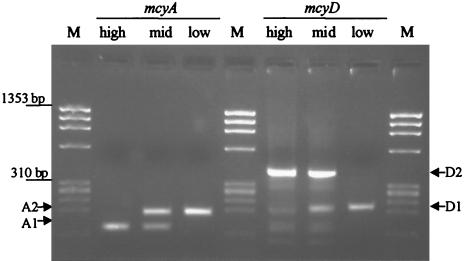
Both mcyA and mcyD had two transcription start points, which appeared to be dependent on the light conditions under which the harvested culture grew. Analysis of RNA from cultures grown with 31 μmol of photons m−2 s−1 (intermediate light conditions) showed that both RACE products, corresponding to the transcription start sites found under high and low light conditions (Fig. 1), were present.
Fig. 1.
RACE products identified for mcyA and mcyD from RNA extracted from cells grown with 31 μmol of photons m−2 s−1 (intermediate light conditions) and exposed prior to sampling to high light conditions (68 μmol of photons m−2 s−1) and low light conditions (16 μmol of photons m−2 s−1). φX174 DNA/HaeIII (Promega) was used as a size marker (lanes M). The bands represent 1,353, 1,078, 872, 603, 310, 281, 271, 234, and 194 bp (from top to bottom).
For mcyA, two transcription start sites were identified, which were 206 bp (AH) and 254 bp (AL) upstream of the translation start site under high and low light conditions, respectively. For mcyD, low and high light conditions resulted in the use of transcription start sites which were 143 bp (DL) and 342 bp (DH) upstream of the initiating methionine (Fig. 2). Promoter motifs at −10 and −35 bp upstream of the transcription initiation site exhibited similarity to the Escherichia coli σ70 consensus promoter sequences (Fig. 2).
Fig. 2.
Nucleotide sequence of the region separating mcyA and mcyD, including portions of each ORF. The site of transcription initiation for each gene is indicated by +1, and the translation start codon is indicated by boldface type. Putative promoter sequences at −10 and −35 are underlined.
mcyABCDEFGHIJ gene cluster is arranged as two polycistronic operons, mcyABC and mcyDEFGHIJ.
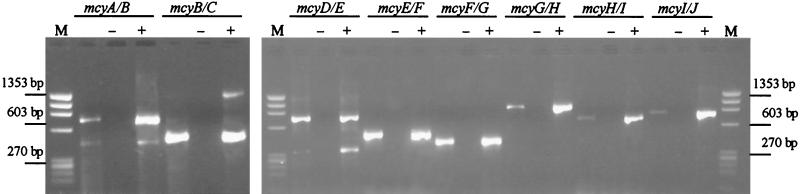
RT-PCR analysis revealed the presence of transcripts in the noncoding regions between mcyA and B, mcyB and C, mcyD and E, mcyE and F, mcyF and G, mcyG and H, mcyH and I, and mcyI and J (Fig. 3). The amplicons obtained from the cDNA were the same sizes as the amplicons obtained from the genomic DNA. Negative control experiments, which revealed a complete absence of DNA in the RNA samples, were performed by subjecting a duplicate RNA sample to the same experimental procedures but omitting the RT enzyme during RT.
Fig. 3.
RT-PCR analysis showing the presence of transcripts between mcyA and mcyB, between mcyB and mcyC, between mcyD and mcyE, between mcyE and mcyF, between mcyF and mcyG, between mcyG and mcyH, between mcyH and mcyI, and between mcyI and mcyJ. For each gene, negative controls in which RNA was used for the PCR (lanes −) and positive controls in which DNA was used for the PCR (lanes +) were included. φX174 DNA/HaeIII (Promega) was used as a size marker (lanes M). The bands represent 1,353, 1,078, 872, 603, 310, 281, 271, 234, and 194 bp (from top to bottom).
Detection of putative intercistronic promoters.
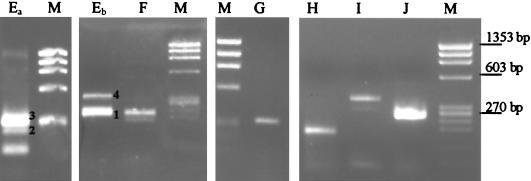
The RACE assay was also employed to check for potential individual ORF promoters internal to the polycistronic transcripts. RACE products were identified for most of the microcystin synthetase genes. Some of these products were repeatedly amplified to obtain a quantity useful for sequencing and visualization by gel electrophoresis (Fig. 4). The single amplicons observed for mcyF, mcyG, mcyH, mcyI, and mcyJ indicated that there were additional intercistronic transcription start sites for these genes (Fig. 4 and 5). Putative transcription start sites and promoters for each of the genes are shown in Table 2. Interestingly, four different RACE products were found for mcyE. These products were identified repeatedly with the same and successive RNA samples. All four start sites exhibited low levels of similarity to the E. coli σ70 promoter motifs (Table 2). A fifth mcyE band below band 2 (Fig. 4) was too small to extend upstream of the translation start site of mcyE. This band was also checked by sequencing and may have indicated that incomplete cDNA synthesis, RNase processing, or transcript degradation occurred. There were no RACE products identified for mcyB and mcyC.
Fig. 4.
RACE products detected for mcy genes in the polycistronic mcyDEFGHIJ transcript (mcyEa, mcyEb, mcyF, mcyG, mcyH, mcyI, and mcyJ). Lanes Ea and Eb show results obtained with two different RNA samples. mcyE RACE products 1, 2, 3, and 4 were purified and sequenced (Table 2). φX174 DNA/HaeIII (Promega) was used as a size marker (lanes M). The bands represent 1,353, 1,078, 872, 603, 310, 281, 271, 234, and 194 bp (from top to bottom).
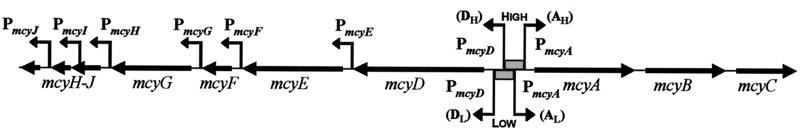
Fig. 5.
Organization of the microcystin synthetase gene cluster, mcyABCDEFGHIJ, showing putative promoters for mcyEFGHIJ (PmcyE to PmcyJ) and the alternate promoters identified for mcyA and mcyD (PmcyA and PmcyD) under high light conditions (AH and DH) and low light conditions (AL and DL).
Table 2.
Putative intercistronic transcription start points, +1 nucleotides, promoter sequences at −10 and −35, and locations of the transcription start points and the translation start sites (ORF) in the mcyABCDEFGHIJ cluster (1 to 55,000 bp)
| Gene | Location of:
|
Sequence
|
|
|---|---|---|---|
| Transcription start point (bp)a | ORF (bp)a | −35−10+1 | |
| mcyE1 | 24175 | 24173 | ATTTATGTTTAACGGCGTATAAATTTAGAAATTATAGAGAATAGGG |
| mcyE2 | 24187 | 24173 | CACAGCATCCTAATTTATGTTTAACGGCGTATAAATTTAGAAATTA |
| mcyE3 | 24232 | 24173 | GCTTAACTCTCTGGGGTTAAATTTGGGTTTATATTTACTGATAGCA |
| mcyE4 | 24288 | 24173 | AAAATTATTTTTCAAAAGCCATGAGATGTGGTAGGATAGGTAGATG |
| mcyF | 13703 | 13678 | GAGGGGCAGGATATGTTCACAGAAGATTTTAATTTTTAGTCAACAG |
| mcyG | 12883 | 12791 | CCTTGACCATCGCACCTCTATCAGGTACAATTCAAACTTGAGTTGA |
| mcyH | 4692 | 4672 | CTCCTTACTGGTATCCAACTGATCCTAAAGGAAGGACATTTTCAGA |
| mcyI | 3167 | 3017 | CTTATATCAGCAGTTACAAAAAGCACAGACAACTTTTATTAGCGTA |
| mcyJ | 1909 | 1828 | CTGCAAAATCCTTAGGAGGATAATAATTTGGAAAACATCGGCAAAA |
| E. coli σ70 | TTGACA TATAAT | ||
Location starting from the 3′ mcyJ end (accession no. AF183408).
Discussion
Findings presented here showed that the microcystin synthetase gene cluster, mcyABCDEFGHIJ, is transcribed as two polycistronic transcripts, mcyABC and mcyDEFGHIJ, from a bidirectional central promoter situated between mcyA and mcyD. For both mcyA and mcyD, two transcription start sites were identified, and the use of these start sites appeared to depend on the light intensity. Furthermore, most of the polyketide and tailoring mcy genes (mcyE, mcyF, mcyG, mcyH, mcyI, and mcyJ) may also possess individual promoters (Fig. 5).
Light-dependent transcription start sites were identified for mcyA and mcyD when cells were exposed to 68 μmol of photons m−2 s−1 (high light conditions) and 16 μmol of photons m−2 s−1 (low light conditions) prior to harvesting and RNA extraction. When cells were grown with constant light of 31 μmol of photons m−2 s−1, both transcription start sites were detected (Fig. 1). The use of multiple promoters for expression of the same cyanobacterial gene has previously been reported for glnA (glutamine synthetase) and argD (N-acetylornithine aminotransferase) in Anabaena sp. strain PCC7120 (15, 35), for zwf (glucose-6-phosphate dehydrogenase) in Nostoc punctiforme (32), and for the petH gene encoding ferredoxin:NADP+ reductase in a heterocyst-forming Anabaena sp. (36). In the case of petH, one of the promoters directs constitutive transcription under different nitrogen nutrition conditions, while the other is induced in cells subjected to nitrogen stepdown and in nitrogen-fixing filaments (36). Similarly, Microcystis cells may use different promoters depending on exposure of the cells to different light conditions. Light-dependent transcription has previously been demonstrated for mcyB and mcyD and may indicate that microcystin has a function that is related to irradiance (18).
One of the initial steps in which transcription initiation is regulated is the interaction of the RNA polymerase with conserved promoter sequences situated −10 and −35 bp upstream of the transcription initiation site. Most cyanobacterial −10 promoter sequences are highly conserved with the consensus E. coli σ70 promoter motif (TANNNT) (11). The −35 motif, however, shows only weak conservation with the E. coli σ70 consensus sequence (TTGACA) in approximately one-half of the promoters mapped for different cyanobacterial genera (11). The transcription initiation sites identified under high light conditions for mcyA and mcyD indicated that there were conserved −10 motifs consisting of TACAAT and TCTATT, respectively (Fig. 2). Conservation was less prevalent in the −10 basal promoter sequences of mcyA (AAAAAT) and mcyD (CTGAAT) transcripts generated under low light conditions. Less conserved promoter motifs generally indicate weaker transcription. However, the −35 regions of the low-light-conditions transcripts of mcyA (TTGTCA) and mcyD (GTGACA) exhibited higher levels of similarity to the E. coli σ70 consensus than those identified for the mcyA (TGTGCA) and mcyD (TTCACG) transcripts under high light conditions (Fig. 2).
The less conserved −35 motif in the mcyA and mcyD high-light-conditions transcripts could indicate involvement of cis factors superimposed on the promoter region, as found in regulated E. coli σ70 promoters (10). In these types of promoters, the binding sites of activators are close to the −35 region, leading to less conservation of this motif. Thus, it may be speculated that mcyABC and mcyDEFGHIJ are constitutively transcribed from the weak promoters exhibited under low light conditions and that an activator is involved in transcription initiation under high light conditions. Initiation from the alternate promoters under high light conditions may lead to increased transcription under these conditions, as previously determined for mcyB and mcyD (18).
RACE products identified for mcyEFGHIJ indicated that intercistronic promoters were present. Promoter elements at −10 and −35, however, exhibited only weak similarity to the consensus E. coli σ70 (Table 2). The absence of typical promoter elements could indicate false predictions of transcription start sites due to incomplete processing of a larger transcript during RT or due to RNA cleavage (6, 14). This may be true especially for the four transcription start sites detected for mcyE. However, weak conservation of the −10 and −35 motifs or the complete absence of −35 regions has previously been documented in genes from various cyanobacterial genera, including the glnA(IV) and psbB(I)(II) genes from Anabaena sp. strain PCC7120, in which the transcription initiation site was verified by in vitro transcription assays (19, 35).
Internal promoters have been identified in the polycistronic operons of several other bacterial gene clusters (9, 22, 38). The his operon in E. coli and Salmonella enterica serovar Typhimurium, for example, consisting of eight polycistronic genes, contains two internal promoters within hisC and hisF, as well as the primary promoter at the 5′ end (9). The distal cistrons of the operon are expressed from the two internal promoters, in addition to the primary element. Expression from these alternate promoters appears to depend on various physiological states (17). Similarly, it could be speculated that the intercistronic mcy promoters are functional or more active only under particular growth conditions.
It has also been proposed that internal promoters may have a function, especially in long and generally unstable transcripts, in ensuring adequate expression of the distal genes of the operon or cluster (26). This has been suggested for the 14-kb cap1 gene cluster of Staphylococcus aureus, which is transcribed from one strong primary promoter and five weak internal promoters which exhibit significant activity when the primary promoter is removed (26). The internal promoter of a particular gene, such as ilvEp in E. coli, can contribute as much as 90% of the total expression (21). However, as transcription through a promoter inhibits its activity (promoter occlusion), the internal promoters probably function only when the primary transcript is interrupted, degraded, or otherwise repressed (1). This may also be true for the large 55-kb mcyABCDEFGHIJ gene cluster. RACE products of the mcyEFGHIJ genes were identified in only approximately one-half of the RNA samples tested. Under conditions which favor transcription from the primary promoters at mcyA and mcyD and high transcript stability, the internal promoters may be subject to promoter occlusion. Thus, without knowing the precise conditions which lead to activity of the internal promoters rather than transcription from the primary promoter, we could not consistently reisolate the RACE products which identify the mcy internal promoters.
Alternatively, our results may be explained by the presence of processing (cleavage) sites in the large transcripts. Processing could produce monocistronic mRNAs for translation, as observed in chloroplasts (4, 29). The internal promoters identified for the mcyEFGHIJ genes are somewhat analogous to the chloroplast gene petD. This gene possesses its own promoter for production of a monocistronic transcript. However, it may also utilize the promoter of the upstream gene petA and be transcribed as part of a polycistronic message (29). Here, promoter usage depends on RNA processing events and RNA secondary structures at the 5′ end of the gene (4).
Another structural feature of the mcy mRNA was the long untranslated leader regions identified for both mcyA and mcyD (Fig. 2). Transcription initiation sites under high and low light conditions for mcyA were located 206 and 254 bp upstream of the ORF start codon, respectively. This provided a longer mcyABC transcript when cells were cultured under low light conditions. For mcyD, the longer mcyDEFGHJ transcript was identified under high light conditions, under which the transcriptional start site was situated 342 bp upstream of the ORF, compared to 143 bp upstream of the ORF when the organism was grown under low light conditions. Light-stimulated differences in the lengths of transcripts have previously been documented in the pea chloroplast (37). Long untranslated leader regions between the basal promoter motifs and the ORF start codon are also characteristic of light-responsive expression of the psbA and psbD genes in cyanobacteria (7). In these systems, the consensus basal promoter elements are not affected by changes in light intensity, and light-responsive expression is driven by specific cis elements in the untranslated leader regions of the mRNA (8). Light-responsive elements have been identified 11 to 84 bp downstream of the transcription start site for psbDII and 1 to 41 and 39 downstream of the transcription start site for psbAII and psbAIII, respectively (7, 20). Such regions are being investigated further for the mcy cluster by using a reporter vector system. For the psb genes, these regions alone were able to confer a reproducible level of light-responsive expression on a heterologous promoter (8, 20).
In conclusion, we found that the microcystin synthetase gene cluster (mcyABCDEFGHIJ) consists of two polycistronic transcripts, mcyABC and mcyDEFGHIJ, which are transcribed from a central promoter between mcyA and mcyD. Most interestingly, both polycistronic transcripts have alternate transcription start sites which appear to be light dependent. Furthermore, identification of putative intercistronic transcription start sites for individual mcy genes indicated that there is complex gene regulation involving multiple and alternate messages. These findings may prove to be highly significant for our understanding of regulation of the microcystin synthetase gene cluster under particular light conditions and may increase our knowledge concerning a function of microcystin under these growth conditions.
Acknowledgments
This work was supported by grants from the Australian Research Council and the CRC for Water Quality and Treatment to B.A.N. and M.K., by a grant from the DFG to T.B., and by a grant from the Alexander von Humboldt foundation to E.D.
REFERENCES
- 1.Adhya, S., and M. Gottesman. 1982. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell 29:939–944. [DOI] [PubMed] [Google Scholar]
- 2.Arment, A. R., and W. W. Carmichael. 1996. Evidence that microcystin is a thiotemplate product. J. Phycol. 32:591–597. [Google Scholar]
- 3.Axelsson, R., F. Oxelfelt, and P. Lindblad. 1999. Transcriptional regulation of Nostoc uptake hydrogenase. FEMS Microbiol. Lett. 170:77–81. [DOI] [PubMed] [Google Scholar]
- 4.Barkan, A., M. Walker, M. Nolasco, and D. Johnson. 1994. A nuclear mutation in maize blocks the processing and translation of several chloroplast mRNAs and provides evidence for the differential translation of alternative mRNA forms. EMBO J. 13:3170–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boison, G., H. Bothe, and O. Schmitz. 2000. Transcriptional analysis of hydrogenase genes in the cyanobacteria Anacystis nidulans and Anabaena variabilis monitored by RT-PCR. Curr. Microbiol. 40:315–321. [DOI] [PubMed] [Google Scholar]
- 6.Boudreau, E., Y. Takahashi, C. Lemieux, M. Turmel, and J.-D. Rochaix. 1997. The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J. 16:6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustos, S. A., and S. S. Golden. 1991. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J. Bacteriol. 173:7525–7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustos, S. A., and S. S. Golden. 1992. Light-regulated expression of the psbD gene family in Synechococcus sp. strain PCC7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol. Gen. Genet. 232:221–230. [DOI] [PubMed] [Google Scholar]
- 9.Carlomagno, M. S., L. Chiariotti, P. Alifano, A. G. Nappo, and C. B. Bruni. 1988. Structure and function of the Salmonella typhimurium and Escherichia coli K-12 histidine operons. J. Mol. Biol. 203:585–606. [DOI] [PubMed] [Google Scholar]
- 10.Collado-Vides, J., B. Magasanik, and J. D. Gralla. 1991. Control site location and transcriptional regulation in Escherichia coli. Microbiol. Rev. 55:371–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis, S. E., and J. A. Martin. 1994. The transcription apparatus and the regulation of transcription initiation, p. 613–639. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Press, Dordrecht, The Netherlands.
- 12.Dittmann, E., B. A. Neilan, M. Erhard, H. von Doehren, and T. Boerner. 1997. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 26:779–787. [DOI] [PubMed] [Google Scholar]
- 13.Domanski, T. L., B. L. M. de Jonge, and K. W. Bayles. 1997. Transcriptional analysis of the Staphylococcus aureus gene encoding penicillin-binding protein 4. J. Bacteriol. 179:2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominic, B., Y.-B. Chen, and J. P. Zehr. 1998. Cloning and transcriptional analysis of the nifUHDKgenes of Trichodesmium sp. IMS101 reveals stable nifD, nifDK and nifK transcripts. Microbiology 144:3359–3368. [DOI] [PubMed] [Google Scholar]
- 15.Floriano, B., A. Herrero, and E. Flores. 1994. Analysis of expression of the argC and argD genes in the cyanobacterium Anabaena sp. strain PCC7120. J. Bacteriol. 176:6397–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisler, M., B. Jakobs, J. Richter, and J. Schumann. 1996. Cotranscription of a GTPase gene from the cyanobacterium Synechocystis PCC6803 and a P-type Ca(2+)ATPase gene. Biochim. Biophys. Acta 1309:189–193. [DOI] [PubMed] [Google Scholar]
- 17.Grisolia, V., A. Riccio, and C. B. Bruni. 1983. Structure and function of the internal promoter (hisBp)of the Escherichia coli K-12 histidine operon. J. Bacteriol. 155:1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaebernick, M., B. A. Neilan, T. Börner, and E. Dittmann. 2000. Light and the transcriptional response of the microcystin biosynthetic gene cluster. Appl. Environ. Microbiol. 66:3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang, J. D., and R. Haselkorn. 1989. Isolation, sequence and transcription of the gene encoding the photosystem II chlorophyll-binding protein, CP-47, in the cyanobacterium Anabaena PCC7120. Plant Mol. Biol. 13:441–457. [DOI] [PubMed] [Google Scholar]
- 20.Li, R., and S. S. Golden. 1993. Enhancer activity of light-responsive regulatory elements in the untranslated leader regions of cyanobacterial psbA genes. Proc. Natl. Acad. Sci. USA 90:11678–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes, J. M., N. Soliman, P. K. Smith, and R. P. Lawther. 1989. Transcriptional polarity enhances the contribution of the internal promoter, ilvEp, in the expression of the ilvGMEDA operon in wild-type Escherichia coli K12. Mol. Microbiol. 3:1039–1051. [DOI] [PubMed] [Google Scholar]
- 22.Lortie, L.-A., M. Pelletier, C. Vadeboncoeur, and M. Frenette. 2000. The gene encoding IIABLMan in Streptococcus salivarius is part of a tetracistronic operon encoding a phosphoenolpyruvate: mannose/glucose phosphotransferase system. Microbiology 146:677–685. [DOI] [PubMed] [Google Scholar]
- 23.Moore, R. E., J. L. Chen, B. S. Moore, G. M. L. Patterson, and W. W. Carmichael. 1991. Biosynthesis of microcystin-LR. Origin of the carbons in the Adda and Masp units. J. Am. Chem. Soc. 113:5083–5084. [Google Scholar]
- 24.Nishizawa, T., M. Asayama, K. Fujii, K. Harada, and M. Shirai. 1999. Genetic analysis of the peptide synthetase genes for a cyclic heptapeptide microcystin in Microcystis spp. J. Biochem. (Tokyo) 126:520–526. [DOI] [PubMed] [Google Scholar]
- 25.Nishizawa, T., A. Ueda, M. Asayama, K. Fujii, K. Harada, K. Ochi, and M. Shirai. 2000. Polyketide synthase gene coupled to the peptide synthetase module involved in the biosynthesis of the cyclic heptapeptide microcystin. J. Biochem. (Tokyo) 127:779–789. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang, S., and C. Y. Lee. 1997. Transcriptional analysis of type 1 capsule genes in Staphylococcus aureus. Mol. Microbiol. 23:473–482. [DOI] [PubMed] [Google Scholar]
- 27.Rinehart, K. L., M. Namikoshi, and B. W. Choi. 1994. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria). J. Appl. Phycol. 6:159–176. [Google Scholar]
- 28.Rippka, R., and M. Herdman. 1992. Pasteur culture collection (PCC) of cyanobacterial strains in axenic culture, vol. 1. Institut Pasteur, Paris, France.
- 29.Sakamoto, W., N. R. Sturm, K. L. Kindle, and D. B. Stern. 1994. petD mRNA maturation in Chlamydomonas reinhardtii chloroplast: role of 5′ endonucleolytic processing. Mol. Cell. Biol. 14:6180–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer, B. C. 1995. Revolutions in rapid amplification of cDNA ends: new strategies for polymerase chain reaction cloning of full-length cDNA ends. Anal. Biochem. 20:255–273. [DOI] [PubMed] [Google Scholar]
- 31.Sivonen, K. 1996. Cyanobacterial toxins and toxin production. Phycologia 35:12–24. [Google Scholar]
- 32.Summers, M. L., and J. C. Meeks. 1996. Transcriptional regulation of zwf, encoding glucose-6-phosphate dehydrogenase, from the cyanobacterium Nostoc. Mol. Microbiol. 22:473–480. [DOI] [PubMed] [Google Scholar]
- 33.Tillett, D., B. P. Burns, and B. A. Neilan. 2000. Optimized rapid amplification of cDNA ends (RACE) for mapping bacterial mRNA transcripts. BioTechniques 28:448, 450, 452–453, 456. [DOI] [PubMed] [Google Scholar]
- 34.Tillett, D., E. Dittmann, M. Erhard, H. von Doehren, T. Boerner, and B. A. Neilan. 2000. Structural organisation of microcystin biosynthesis in M. aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem. Biol. 7:753–764. [DOI] [PubMed] [Google Scholar]
- 35.Tumer, N. E., S. J. Robinson, and R. Haselkorn. 1983. Different promoters for the Anabaena glutamine synthetase gene during growth using molecular or fixed nitrogen. Nature 306:337–342. [Google Scholar]
- 36.Valladares, A., A. M. Muro-Pastor, M. F. Fillat, A. Herrero, and E. Flores. 1999. Constitutive and nitrogen-regulated promoters of the petH gene encoding ferredoxin:NADP+ reductase in the heterocyst-forming cyanobacterium Anabaena sp. FEBS Lett. 449:159–164. [DOI] [PubMed] [Google Scholar]
- 37.Woodbury, N. W., L. L. Roberts, J. D. Palmer, and W. F. Thompson. 1988. A transcription map of the pea chloroplast genome. Curr. Genet. 14:75–89. [Google Scholar]
- 38.Yanofsky, C., T. Platt, I. P. Crawford, B. P. Nichols, G. E. Christie, H. Horowitz, M. VanCleemput, and A. M. Wu. 1981. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 9:6647–6668. [DOI] [PMC free article] [PubMed] [Google Scholar]