Abstract
Intracellular rickettsia-like structures were found in the tissues of a glossiphoniid leech, Torix tagoi, by transmission electron microscopy. Diagnostic PCR analysis using specific primers suggested that of the nine glossiphoniid species examined, two species, T. tagoi and Hemicrepsis marginata, harbored bacteria of the genus Rickettsia. A 1.5-kb eubacterial 16S rRNA gene segment obtained from each of these species was amplified by PCR, cloned, and sequenced. Phylogenetic analysis of the 16S rRNA gene demonstrated that the Rickettsia species found in the leeches constituted a novel clade that is distinct from the clade of arthropod-associated Rickettsia species. In natural populations, 97.7% (43 of 44) of T. tagoi leeches and 100% (9 of 9) of H. marginata leeches carried Rickettsia, suggesting that infection with Rickettsia is prevalent in these leeches. This is the first report of Rickettsia found in annelids.
The genus Rickettsia is a phylogenetically well-defined bacterial group that belongs to the order Rickettsiales in the α subdivision of the class Proteobacteria (15, 17, 19). The members of the genus Rickettsia are gram-negative bacteria that have an obligate association with eukaryotic cells (20). This group of bacteria is known to include human and animal pathogens. For example, Rickettsia rickettsii and some other Rickettsia species in the spotted fever group are causative agents of spotted fever. Rickettsia typhi and Rickettsia prowazekii belong to the typhus group and cause murine and epidemic typhus, respectively (3). Although some Rickettsia species facultatively infect humans and other vertebrates, their primary hosts are bloodsucking arthropods that can act as disease vectors. Ticks are vectors of most members of the spotted fever group, mites are vectors of Rickettsia akari, lice are vectors of R. prowazekii, and fleas are vectors of R. typhi (3). In addition to the medically important Rickettsia species, a number of nonpathogenic Rickettsia spp. whose primary hosts are various terrestrial arthropods, such as ladybird beetles, aphids, planthoppers, bruchid beetles, and ticks, have been described (2, 4, 8, 13, 21). Thus, all the primary hosts of the Rickettsia spp. known to date belong to the phylum Arthropoda.
Here, we present the first report of Rickettsia spp. from leeches (class Hirudinea, phylum Annelida).
Biology of glossiphoniid leeches.
Here, we briefly describe the basic biology of the leeches that we investigated. Glossiphoniid leeches (family Glossiphoniidae, order Rhynchobdellida) live mostly in freshwater environments, where they feed on the blood of vertebrates, including fish, amphibians, reptiles, and mammals. Leeches are generally hermaphroditic. In maturity, they copulate and deposit a number of eggs on their own ventral surfaces. The hatchlings are, in general, carried on the ventral surface of the parent for a considerable period, probably to protect the newborns from predators. The hatchlings have plenty of yolk in their bodies and attach themselves to their parent's skin using their posterior suckers. It is believed, though not confirmed, that the hatchlings get no food materials from the parent (16).
Electron microscopy.
In an electron microscopic examination of tissues from leeches, we found intracellular rickettsia-like structures in a glossiphoniid species, Torix tagoi. Four young T. tagoi leeches were collected from a creek at the Ogawa Natural Forest Reserve, northern Ibaraki, Japan, on 12 September 1999 (Table 1). The leeches were dissected to obtain tissues of the epidermis, esophagus, and intestine. Some of the tissues were subjected to electron microscopy, whereas others were preserved in acetone for DNA analysis (6). For those subjected to electron microscopy, the tissues were prefixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 90 min at 4°C and postfixed in 2% osmium tetroxide for 60 min at 4°C. After dehydration through an ethanol series, the materials were embedded in LR white acrylic resin (Sigma) and cut into 80-nm-thick sections. The ultrathin sections were double stained with uranyl acetate and lead citrate and observed under a transmission electron microscope.
TABLE 1.
Grossiphoniid leeches used in this study
| Species | Original location in Japan | Collection date(s) |
|---|---|---|
| Alboglossiphonia lata | Inugami River, Shiga | 13 August 2000 |
| Ancyrobdella smaragdina | Lake Biwa, Shiga | 13 August 2000 |
| Glossiphonia complanata | Lake Yuno, Tochigi | 1 August 2000 |
| Glossiphonia sp. | Suga Pond, Aomori | 7 September 2000 |
| Helobdella stagnalis | Suga Pond, Aomori | 7 September 2000 |
| Hemicrepsis marginata | Tenryu River, Nagano | 1 August 2000 |
| Placobdelloides sp. | Akamatsu Pond, Tottori | 12 August 2000 |
| Torix tagoi | Ogawa Natural Forest, Ibaraki | 12 September 1999a, 10 November 2000b |
| Torix tukubana | Bekanbeushi River, Hokkaido | July 2000 |
Subjected to electron microscopy and initial DNA analysis.
Subjected to diagnostic PCR analysis to survey the infection rate in a natural population.
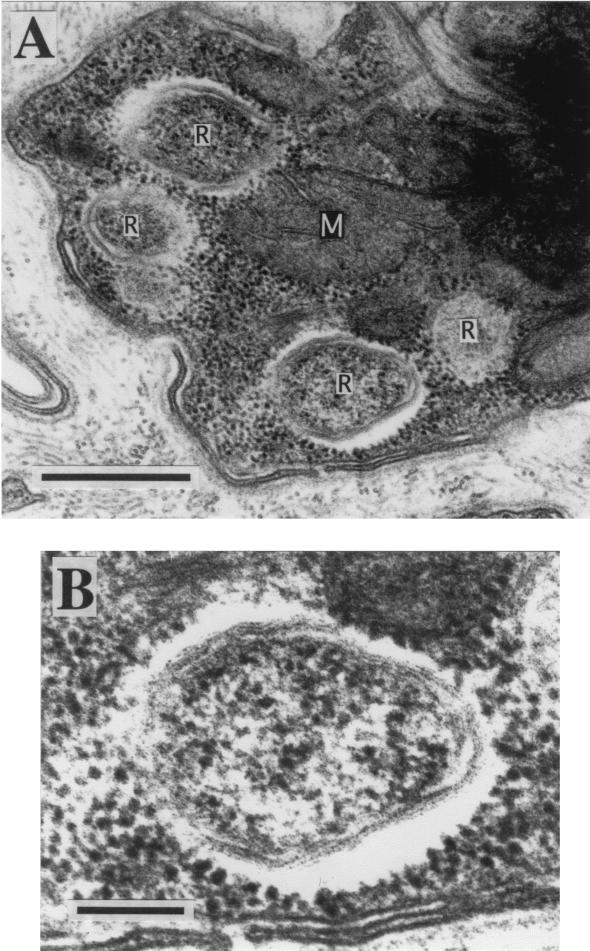
Figure 1 shows an electron microscopic image of an epidermal cell from a T. tagoi leech. A number of bacterial cells of various lengths and widths whose characteristics were consistent with those of members of the order Rickettsiales, such as Rickettsia spp. and Wolbachia spp., were found intracellularly. The same structures were also observed in cells from the esophagus and intestine (data not shown).
FIG. 1.
Transmission electron microscopic images of the epidermal tissue of T. tagoi. (A) Intracellular rickettsia-like structures. Bar, 500 nm. (B) Enlarged image of the intracellular rickettsia-like structure. Bar, 100 nm. Abbreviations: R, rickettsia-like structure; M, mitochondrion.
PCR detection of Rickettsia spp.
To characterize the rickettsia-like organisms in the leech tissues, we conducted diagnostic PCR analyses with the universal and specific primers listed in Table 2. The epidermal tissues of leeches preserved in acetone were subjected to DNA extraction by the use of standard Tris-sodium dodecyl sulfate-proteinase K digestion and phenol-chloroform extraction procedures. PCR was conducted with the primer sets listed in Table 2 under the following conditions: incubation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at the temperature described for each primer set listed in Table 2 for 1 min, and extension at 72°C for 2 min. The PCR products were subjected to electrophoresis in agarose gels, stained with ethidium bromide, and observed on a UV transilluminator.
TABLE 2.
Primer sets used for diagnostic PCR
| Target organism | Target gene | Primer names | Nucleotide sequence (5"-3") | Approx product size (kb) | Annealing temp (°C) | Reference |
|---|---|---|---|---|---|---|
| Leech and insect (universal) | Mitochondrial cytochrome oxidase I | LCO1490 | GGTCAACAAATCATAAAGATATTGG | 0.7 | 48 | 5 |
| HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA | |||||
| Eubacteria (universal) | 16S rRNA | 16SA1 | AGAGTTTGATCMTGGCTCAG | 1.5 | 55 | 7 |
| 16SB1 | TACGGYTACCTTGTTACGACTT | |||||
| Rickettsia sp. (specific 1) | Citrate synthase | CS1 | GGGGGCCTGCTCACGGCGG | 0.4 | 50 | 14 |
| CS2 | ATTGCAAAAAGTACAGTGAACA | |||||
| Rickettsia sp. (specific 2) | 16S rRNA | 16SA1 | AGAGTTTGATCMTGGCTCAG | 0.2 | 55 | 9 |
| Rick16SR | CATCCATCAGCGATAAATCTTTC | |||||
| Wolbachia sp. (specific) | ftsZ homolog | ftsF | GTATGCCGATTGCAGAGCTTG | 0.8 | 50 | 10 |
| ftsR | GCCATGAGTATTCACTTGGCT |
All four T. tagoi leeches examined were Rickettsia positive and Wolbachia negative: PCRs with two sets of Rickettsia-specific primers for 16S rRNA gene and citrate synthase gave amplified products of the expected molecular sizes, whereas PCR with Wolbachia-specific primers for ftsZ exhibited no specific amplified products (data not shown).
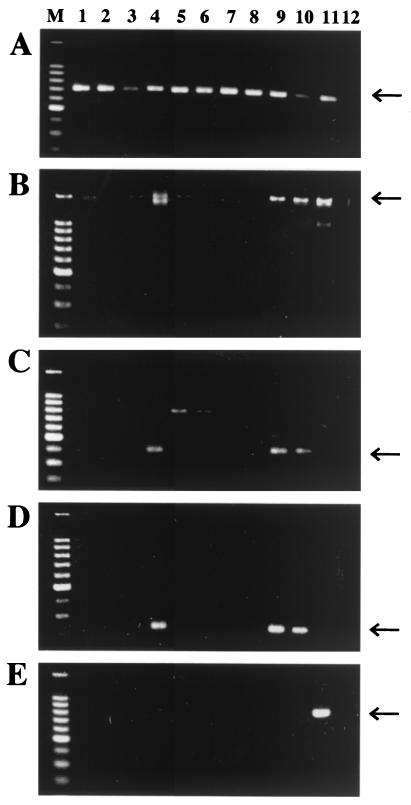
In addition to T. tagoi, eight other species of glossiphoniid leeches (listed in Table 1) were subjected to PCR analysis. The leech samples preserved in acetone were subjected to DNA extraction and PCR as described above. Figure 2 shows the results of diagnostic PCR analysis. Of the nine glossiphoniid leeches examined, only two species, T. tagoi and Hemicrepsis marginata, were Rickettsia positive, whereas all species were Wolbachia negative.
FIG. 2.
PCR detection of Rickettsia spp. in glossiphoniid leeches. (A) Detection of mitochondrial cytochrome oxidase I gene of leeches with primers LCO1490 and HCO2198; (B) detection of eubacterial 16S rRNA gene with primers 16SA1 and 16SB1; (C) specific detection of citrate synthase gene of Rickettsia spp. with primers CS1 and CS2; (D) specific detection of 16S rRNA gene of Rickettsia spp. with primers 16SA1 and Rick16SR; (E) specific detection of the ftsZ gene of Wolbachia spp. with primers ftsF and ftsR. Lane M, DNA size markers (1,500, 1,000, 900, 800, 700, 600, 500, 400, 300, and 200 bp from top to bottom); lane 1, Helobdella stagnalis; lane 2, Placobdelloides sp.; lane 3, Ancyrobdella smaragdina; lane 4, H. marginata; lane 5, Glossiphonia complanata; lane 6, Glossiphonia sp.; lane 7, Alboglossiphonia lata; lane 8, T. tukubana; lane 9, T. tagoi; lane 10, a bruchid beetle, Kytorhinus sharpianus, harboring a Rickettsia sp.; lane 11, a bruchid beetle, Callosobruchus chinensis, harboring a Wolbachia sp.; lane 12, no template control. Arrows indicate specific PCR products. Other faint bands are due to nonspecific amplifications.
Eubacterial 16S rRNA gene sequences from T. tagoi and H. marginata.
To unequivocally identify the bacteria harbored by T. tagoi and H. marginata, a 1.5-kb segment of the eubacterial 16S rRNA gene was amplified with primers 16SA1 and 16SB1 (Fig. 2B) and subjected to cloning, restriction fragment length polymorphism typing, and DNA sequencing as previously described (8).
Restriction fragment length polymorphism profiles of the 16S rRNA gene clones, 10 from T. tagoi and 10 from H. marginata, exhibited the same patterns, respectively (data not shown), indicating that a single bacterial species dominates the endosymbiotic microbiota in these leeches. For each T. tagoi and H. marginata leech, two 16S rRNA gene clones from each of the two leeches were subjected to DNA sequencing. The four sequences from T. tagoi, 1,429 bp in size, were completely identical to each other, and the four sequences from H. marginata, 1,424 bp in size, were identical to each other but not to those of T. tagoi. These sequences exhibited very high levels of similarity (around 95%) to the 16S rRNA gene sequences of Rickettsia spp. in the DDBJ, EMBL, and GenBank DNA databases, confirming that the bacteria harbored by T. tagoi and H. marginata are members of the genus Rickettsia.
Phylogenetic analysis.
To determine the phylogenetic placement of the Rickettsia spp. from the leeches, their 16S rRNA gene sequences were subjected to molecular phylogenetic analysis together with 16S rRNA gene sequences from representatives of the genus Rickettsia and other members of the α subdivision of the Proteobacteria deposited in the DNA databases. A neighbor-joining (NJ) phylogeny was constructed using the program Clustal W (18). A maximum parsimony (MP) phylogeny was constructed using sequences aligned by the Clustal W program and then manually realigned using the program Winclada (version 0.9.99m 24, 2000) to minimize both the maximum and minimum number of steps in the character statistics. The resulting matrix was subjected to parsimony analysis with the program NONA (version 1.6, 1997), in which all insertion and deletion events, transitions, and transversions were equally weighted.
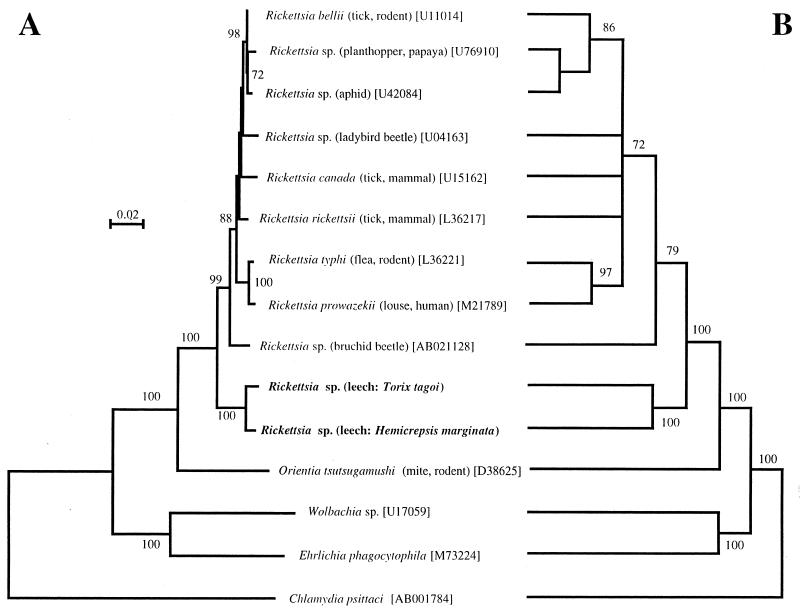
Figure 3 shows the results of molecular phylogenetic analysis. The NJ tree (Fig. 3A) and the MP tree (Fig. 3B) exhibited essentially the same topologies. The 16S rRNA gene sequences from the leeches were definitely placed in the clade of the genus Rickettsia, which was supported by bootstrap values of 100% in both the NJ and MP trees. Interestingly, the genus Rickettsia was divided into two sister monophyletic groups, the clade of leech-associated Rickettsia spp. (100% support in both NJ and MP trees) and the clade of arthropod-associated Rickettsia spp. (99% support in NJ tree and 79% in MP tree). From these results, we concluded that the endosymbionts harbored by T. tagoi and H. marginata belong to the genus Rickettsia and constitute a distinct basally branched clade in the genus. It should be noted, however, that an alternative taxonomic treatment is conceivable. In the event that future biological studies of the leech-associated Rickettsia spp. reveal characteristics significantly distinct from those of arthropod-associated Rickettsia, the creation of a new genus for them would be considered.
FIG. 3.
Phylogenetic positions of the Rickettsia spp. from T. tagoi and H. marginata based on the 16S rRNA gene sequences in the α subdivision of the Proteobacteria. (A) An NJ tree; (B) an MP tree. The bootstrap values that were higher than 70%, obtained by 1,000 resamplings for NJ analysis and 100 resamplings for MP analysis, are shown at the nodes. For Rickettsia and Orientia species, the host organisms are given in parentheses. The numbers in brackets are accession numbers.
Infection rate in natural populations.
We examined infection rates of the Rickettsia spp. in natural populations of T. tagoi and H. marginata by PCR using Rickettsia-specific primers. In addition to the four young T. tagoi leeches collected in 1999, 40 specimens were collected at the same locality on 10 November 2000. Nine H. marginata leeches were collected at the Tenryu River, Nagano, Japan, on 1 August 2000 and subjected to the same analysis (Table 1).
Of the 44 individual T. tagoi leeches, 43 (97.7%) were Rickettsia positive. Of the nine H. marginata leeches, all (100%) were infected with Rickettsia spp. Therefore, at least in these populations of T. tagoi and H. marginata leeches, infection with Rickettsia is prevalent; however, a more extensive survey of other individuals and populations is needed to reach any general conclusions.
Vertical transmission.
In glossiphoniid leeches, in general, mature adults carry eggs and hatchlings on their ventral surfaces for a considerable period, probably for the purpose of protection (16). Of the 40 T. tagoi leeches collected in 2000, we found one Rickettsia positive individual which carried 160 hatchlings. The 50 examined hatchlings were all Rickettsia positive, suggesting that the Rickettsia is vertically transmitted from the parent to the offspring, although the mechanism of infection still needs to be investigated. At this stage, however, the possibility that the adult and the offspring simultaneously acquired the Rickettsia through horizontal transmission cannot be ruled out.
Evolutionary process of the host shift.
In this study, we demonstrated that the genus Rickettsia is divided into two sister monophyletic groups, the clade of leech-associated Rickettsia spp. and the clade of arthropod-associated Rickettsia spp. (Fig. 3). This finding implies that in the common ancestor of extant Rickettsia species, a host shift must have occurred between an arthropod and a leech. What was the evolutionary process of the host shift? Did the switch occur from an arthropod to a leech or in the opposite direction? Previous molecular phylogenetic studies have indicated that the nearest outgroup taxon of the genus Rickettsia is Orientia tsutsugamushi, the causative agent of scrub typhus (3). Because the primary host of O. tsutsugamushi is the trombiculid mite (3), the ancestral primary host of the genus Rickettsia is expected to be an arthropod, probably a bloodsucking mite or tick. If so, it is more parsimonious to conjecture that the evolutionary direction of the host shift was from an arthropod to a leech. However, it is unclear how this sort of host switch could occur. The arthropod hosts of Rickettsia spp., such as ticks, mites, fleas, and lice, are all terrestrial and live on the blood of vertebrates (3), whereas T. tagoi and H. marginata are aquatic and live on the blood of amphibians and fish (11, 16). It is plausible that, due to the isolation of terrestrial from aquatic habitats, the host shift from bloodsucking arthropods to leeches is a rare evolutionary event that occurred only once in an early evolutionary stage of the genus Rickettsia.
Association between the host leeches and the Rickettsia spp.
As discussed above, it appears likely that a lineage of the Rickettsia spp. anciently diverged from the main lineage of arthropod-borne Rickettsia spp. and has been associated with leeches for a long time. It is likely that the phylogenetic pattern of association between the host leeches and their Rickettsia endosymbionts is an evolutionary product of two components: maintenance of the already established association through vertical transmission (association by descent) and occasional host shifts through horizontal transmission (association by colonization). At least for T. tagoi, Rickettsia spp. are suggested to be vertically transmitted, as are the Rickettsia spp. found in many terrestrial arthropods (20). On the other hand, the phylogenetic relationship between T. tagoi and H. marginata strongly suggests possible horizontal transfers and/or losses of the Rickettsia spp. in an evolutionary time course because the leech genera Torix and Hemicrepsis are phylogenetically not sister groups (12) and the Torix tukubana leech examined in this study was not infected with Rickettsia spp. (Fig. 2). Both T. tagoi and H. marginata feed on the blood of amphibians such as frogs, newts, and salamanders (11, 16). Therefore, one possible, though speculative, route of horizontal transmission might be via the blood of amphibian hosts.
Ecological and epidemiological implications.
A majority of the members of the genus Rickettsia so far described are harbored by bloodsucking terrestrial arthropods such as ticks, mites, fleas, and lice and facultatively infect and cause disease in vertebrate hosts such as mammals and birds (3). In this context, it appears meaningful that leeches, bloodsucking aquatic invertebrates, harbor Rickettsia spp. Most glossiphoniid leeches are hematophagous on aquatic vertebrates such as fish, amphibians, and reptiles (16). The combination of bloodsucking arthropods and vertebrates in the terrestrial ecosystem is reminiscent of the combination of bloodsucking leeches and amphibians and fish in the aquatic ecosystem. Therefore, we suggest the possibilities that, in the aquatic ecosystem, leeches are the primary hosts of Rickettsia spp., amphibians and fish are the vertebrate hosts, and Rickettsia spp. may occasionally cause diseases in these vertebrates just as they cause spotted fever and typhus in terrestrial vertebrates. Although speculative at present, this idea will be supported if Rickettsia spp. are identified in amphibians and fish attacked by the leeches.
Perspective.
In this study, we identified a Rickettsia endosymbiont for two out of nine species of glossiphoniid leeches examined. However, the number of species and taxa sampled was quite limited. To assess the prevalence and importance of the Rickettsia infection in leeches, more extensive research is needed. To understand the life cycle, ecology, and epidemiology of rickettsiae, host organisms of leeches should also be examined. Other than the Rickettsia spp., various morphotypes of endosymbiotic bacteria from leeches have been histologically recorded, although their microbiological aspects are poorly understood (1, 16).
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of the Rickettsia spp. from T. tagoi and H. marginata were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession numbers AB066351 and AB066352, respectively.
Acknowledgments
We thank T. Itoh, H. Miyata, and S. Izawa for leech samples; A. Sugimura, S. Kumagai, and K. Sato for technical and secretarial assistance; H. Noda and N. Shinzato for their advice; and T. E. Leonardo for reading of the manuscript.
This research was supported by the Industrial Science and Technology Frontier Program “Technological Development of Biological Resources in Bioconsortia” of the Ministry of International Trade and Industry of Japan.
REFERENCES
- 1.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, N.Y.
- 2.Chen, D. Q., B. C. Campbell, and A. H. Purcell. 1996. A new Rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris). Curr. Microbiol. 33:123-128. [DOI] [PubMed] [Google Scholar]
- 3.Dasch, G. A., and E. Weiss. 1992. The genera Rickettsia, Rochalimaea, Ehrlichia, Cowdria, and Neorickettsia, p. 2407-2470. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, vol. 3. Springer-Verlag, New York, N.Y. [Google Scholar]
- 4.Davis, M. J., Z. Ying, B. R. Brunner, A. Pantoja, and F. H. Ferwerda. 1998. Rickettsial relative associated with papaya bunchy top disease. Curr. Microbiol. 36:80-84. [DOI] [PubMed] [Google Scholar]
- 5.Folmer, O., M. Black, W. Hoeh, R. Lutz, and R. Vrijenhoek. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3:294-299. [PubMed] [Google Scholar]
- 6.Fukatsu, T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol. Ecol. 8:1935-1945. [DOI] [PubMed] [Google Scholar]
- 7.Fukatsu, T., and N. Nikoh. 1998. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl. Environ. Microbiol. 64:3599-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukatsu, T., and M. Shimada. 1999. Molecular characterization of Rickettsia sp. in a bruchid beetle Kytorhinus sharpianus (Coleoptera: Bruchidae). Appl. Entomol. Zool. 34:391-397. [Google Scholar]
- 9.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holden, P. R., J. F. Y. Brookfield, and P. Jones. 1993. Cloning and characterization of an ftsZ homologue from a bacterial symbiont of Drosophila melanogaster. Mol. Gen. Genet. 40:213-220. [DOI] [PubMed] [Google Scholar]
- 11.Jung, T. 1955. Zur Kenntnis der Ernährungsbiologie der in dem Raum zwischen Harz und Heide vorkommenden Hirudineen. Zool. Jb. (Allg. Zool.) 66:79-123. [Google Scholar]
- 12.Light, J. E., and M. E. Siddall. 1999. Phylogeny of the leech family Glossiphoniidae based on mitochondrial gene sequences and morphological data. J. Parasitol. 85:815-823. [PubMed] [Google Scholar]
- 13.Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regnery, R. L., C. L. Spruill, and B. D. Plikaytis. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roux, V., and D. Raoult. 1995. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146:385-396. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer, R. T. 1986. Leech biology and behavior. Oxford University Press, Oxford, England.
- 17.Stothard, D. R., and P. A. Fuerst. 1995. Evolutionary analysis of the spotted fever and typhus groups of Rickettsia using 16S rRNA gene sequences. Syst. Appl. Microbiol. 18:52-61. [Google Scholar]
- 18.Thompson, J. D., D. G. Higgins, and J. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisburg, W. G., M. E. Dobson, J. E. Samuel, G. A. Dasch, L. P. Mallavia, O. Baca, L. Mandelco, J. E. Sechrest, E. Weiss, and C. R. Woese. 1989. Phylogenetic diversity of the rickettsiae. J. Bacteriol. 171:4202-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss, E., and J. W. Moulder. 1984. Order I. Rickettsiales Gieszczkiewicz 1939, 25AL, p. 687-704. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 21.Werren, J. H., G. D. D. Hurst, W. Zheng, J. A. J. Breeuwer, R. Stouthamer, and M. E. N. Majerus. 1994. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J. Bacteriol. 176:388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]