Abstract
A novel ProteinChip-interfaced tandem mass spectrometer was employed to identify collagen binding proteins from biosurfactant produced by Lactobacillus fermentum RC-14. On-chip tryptic digestion of the captured collagen binding proteins resulted in rapid sequence identification of five novel tryptic peptide sequences via collision-induced dissociation tandem mass spectrometry.
Advances within the field of mass spectrometry (MS) have greatly accelerated proteomic research. Improvements in the ability of MS to rapidly identify and characterize proteins from complex biological materials have made it one of the most powerful tools for studying the function of genes (3, 5). One recent and exciting advance in MS instrumentation is the development of a tandem MS (MS/MS) system with a matrix-assisted laser desorption ionization source (2) that is capable of surface-enhanced laser desorption ionization (SELDI) ProteinChip analysis (4). Recently, the SELDI ProteinChip time-of-flight MS technique (SELDI-TOF-MS) was used to analyze the collagen binding (Cnb) protein content of biosurfactants (BSF) produced by probiotic strains of lactobacilli (1). As an extension to this methodology, we now report the use of a SELDI ProteinChip-interfaced tandem quadrupole TOF instrument (MALDI QSTAR, MDS Sciex, Concord, Ontario, Canada), termed ProteinChip LDI-Qq-TOF-MS (4), to perform MS/MS tryptic peptide sequencing of Lactobacillus fermentum RC-14 BSF Cnb proteins. The BSF produced by probiotic strains Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 have been shown to inhibit the adhesion of several bacterial pathogens in vitro (6). However, only the RC-14-produced BSF has been shown to inhibit Staphylococcus aureus adhesion to surgical implants in vivo (7). In light of these findings, we have attempted in this study to identify potential RC-14 BSF factors that may be responsible for the observed antistaphylococcal activity.
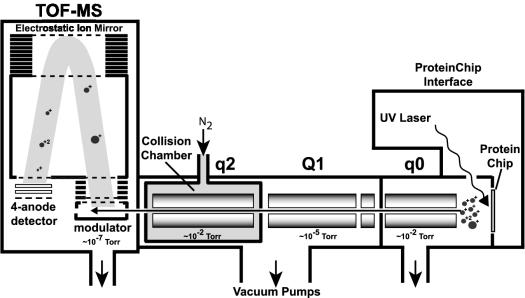
As shown in Fig. 1, the ProteinChip LDI-Qq-TOF-MS machine consists of three quadrupole chambers and a TOF analyzer. Charged ions produced by the SELDI process at the ProteinChip interface enter the initial quadrupole (q0), where they are cooled by collisions with inert gas and ultimately guided into the quadrupole mass filter compartment (Q1). When the device is operated in single-MS mode, quadrupoles Q1 and q2 are run in radio frequency-only mode to allow direct transmission of formed ions into the TOF analyzer. For MS/MS analysis, individual precursor species (present mass-to-charge [m/z] limit, ∼3,000 Da) are selected by quadrupole mass filter Q1 and are transmitted into quadrupole assembly q2, which in this case functions as a collision cell. Transmitted ions collide with inert gas to undergo collision-induced dissociation (CID), which produces three primary characteristic fragment series: b ions, for which the charge is retained on the amino terminus; y ions, for which the charge is retained on the carboxy terminus; and internal product ions. CID fragments of the precursor ion are then analyzed by TOF-MS, which records the m/z distribution of the resulting mixture of product ions. Computer-assisted analysis of the resulting spectra helps to identify the peptide by using the obtained fragmentation spectrum to mine protein, cDNA, or expressed sequence tag databases. In the event of artifactual or nascent modifications of amino acids, these results may prove to be unfruitful. In such instances, the fragmentation spectrum is scrutinized for de novo sequence information among manifesting ion series by using either manual or computer-assisted algorithms.
FIG. 1.
ProteinChip LDI-Qq-TOF-MS system. ProteinChip SELDI is supported by a UV laser source. Ions created in q0 are injected into the quadrupole mass filter (Q1), where precursor ions are selected for subsequent CID fragmentation within the gas collision chamber (q2). Parent and product ions are then injected orthogonally into a modulator region of the TOF-MS compartment, where they are accelerated into an ion mirror (reflectron) and detected via a four-anode detector.
To unambiguously identify the Cnb proteins by on-chip MS/MS tryptic peptide sequencing, we first captured the proteins by using preactivated surface arrays (PS1; Ciphergen Biosystems, Fremont, Calif.) containing either immobilized collagen type I (Cn-I) or Cn-III. Identical preparation and analysis procedures were performed for both types of collagen cross-linked PS1 arrays. Briefly, Cn-I or -III (Sigma, St. Louis, Mo.) was covalently cross-linked using the iminodiacetate chemistry of the PS1 ProteinChip by incubating each spot on the array with 3 μl of an aqueous solution containing 1 μg of collagen for 1 h at room temperature (RT) in a humidified chamber (HC). Any residual non-cross-linked areas on the arrays were inactivated by incubating each of the spots with 3 μl of an aqueous solution containing 1 M Tris (pH 7.5) for 30 min (RT, HC). After the arrays were washed in phosphate-buffered saline and high-pressure liquid chromatography-grade H2O (Sigma), each spot was incubated with 3 μl of a phosphate-buffered saline solution containing 1 μg of BSF (L. fermentum RC-14 or L. rhamnosus GR-1) (6) for 1 h (RT, HC). After the arrays were briefly washed with high-pressure liquid chromatography-grade H2O, the captured Cnb proteins were subjected to on-chip digestion with 25 ng of sequencing-grade modified trypsin (Promega, Madison, Wis.) for 2 h (RT, HC). Limited trypsin digestion of the Cnb proteins was sufficient for protein identification via MS/MS sequencing. After digestion, the arrays were air dried, spotted with 1 μl of a saturated solution of matrix (either α-cyano-4-hydroxycinnamic acid or sinapinic acid [obtained from Ciphergen Biosystems] prepared in an aqueous solution containing 50% acetonitrile and 0.5% trifluoroacetic acid), and allowed to air dry.
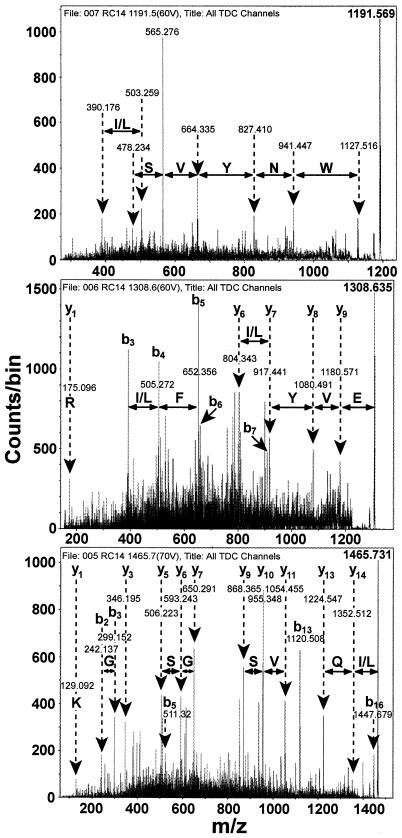
Initially, tryptic peptide maps of the Cnb proteins were generated with the ProteinChip LDI-Qq-TOF-MS in single-MS mode. This allowed identification of unique tryptic peptides for subsequent CID-MS/MS sequencing. As shown in Fig. 2, using the Cn-I PS1 array, three tryptic fragments were chosen for CID-MS/MS sequencing. MS/MS spectra of the product ions for each of the three tryptic fragments (m/z = 1,191.569, 1,308.635, and 1,465.731 Da) were analyzed to assign the b and y ions with the aid of the Predict Fragment routine from the SCIEX program BioMultiView (Fig. 3).
FIG. 2.
Mass analysis of trypsin-digested Cnb proteins from Lactobacillus GR-1 (upper panel) and RC-14 BSF (lower panel). BSF Cnb proteins isolated from L. fermentum RC-14 and L. rhamnosus GR-1, using Cn-I cross-linked PS1 arrays, were on-chip digested with sequencing-grade modified trypsin. The resulting tryptic fragments were analyzed by the ProteinChip LDI-Qq-TOF instrument in single-MS mode to identify peptides that are unique to RC-14. These precursor fragments (*) were then chosen for CID-MS/MS analysis.
FIG. 3.
CID-MS/MS analysis of tryptic fragments of Cnb proteins from L. fermentum RC-14 BSF. Tryptic peptides at m/z 1,191.569, 1,308.635, and 1,465.731 Da were subjected to CID fragmentation. The resulting MS/MS spectra were analyzed with the aid of the Predict Fragment routine from the SCIEX program BioMultiView in order to assign the b and y ion peaks. The amino acid sequence of each fragmented tryptic peptide is determined by calculating the differences in the masses of consecutive b or y ions (e.g., y14 − y13).
In total, five tryptic peptide fragments analyzed by CID-MS/MS generated amino acid sequence information that was used to search available protein databases (Table 1). For three of the sequenced peptides, no significant homologies were found. Homology matches found for the remaining two sequences were from distinct bacterial or nonbacterial species with predicted Blastp E values of ≥25.
TABLE 1.
ProteinChip LDI-Qq-TOF-MS tryptic peptide sequence analysis
| PS1 ProteinChip | Peptide mass (Da) | Amino acid sequencea | Database homology |
|---|---|---|---|
| Cn-I | 1,191.569 | LQGLVS[FA]GSCTCAQK | Yo1026cp (S. cervevisiae), probable membrane protein (E = 31b) |
| 1,308.635 | EVYLFQE[QP]R | NSHc | |
| 1,465.731 | WNYVSd | NSH | |
| Cn-III | 1,263.696 | RAFALLSNWPVK | Cyclic β-1,2-glucan synthase (Mesorhizobium loti) (E = 25) |
| 1,329.685 | GLQGGDL[SH][SH]R | NSH |
Underlining indicates, leucine-isoleucine uncertainty (identical mass), double underlining indicates potential GG = N (identical mass), and brackets indicate tentative order of sequence.
Blastp search E value.
NSH, no significant homologies.
W oxidation prevented further sequence analysis.
In conclusion, the ProteinChip MS/MS tryptic peptide sequencing technique is a very powerful method of rapidly identifying proteins. In particular, the use of biologically modified probe surfaces (i.e., PS1 or PS2 arrays) in combination with LDI-Qq-TOF-MS will permit rapid analysis and identification of physiologically important biomolecules (e.g., proteins, DNA, and RNA, etc.) that bind to proteins. The finding of five novel peptides from L. fermentum RC-14 may prove to be significant with respect to its probiotic activity.
Acknowledgments
We thank Bradley J. Thatcher for his expert technical assistance.
This work was supported by grants from the St. Joseph's Health Care Imperial Oil Fund for Geriatric Medicine, the Lawson Research Institute's Pooled Research Trust Fund, and the Plastic Surgery Educational Foundation to B.S.G.; the Natural Sciences and Engineering Research Council of Canada to G.R.; and the Canadian Institutes of Health Research to J.C.H.
REFERENCES
- 1.Howard, J. C., C. Heinemann, B. J. Thatcher, B. Martin, B. S. Gan, and G. Reid. 2000. Identification of collagen-binding proteins in Lactobacillus spp. with surface-enhanced laser desorption ionization-time-of-flight ProteinChip technology. Appl. Environ. Microbiol. 66:4396-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loboda, A. V., A. N. Krutchinsky, M. Bromirski, W. Ens, and K. G. Standing. 2000. A tandem quadrupole/time-of-flight mass spectrometer with a matrix-assisted laser desorption/ionization source: design and performance. Rapid Commun. Mass. Spectrom. 14:1047-1057. [DOI] [PubMed] [Google Scholar]
- 3.Mann, M., R. C. Hendrickson, and A. Pandey. 2001. Analysis of proteins and proteomes by mass spectrometry. Annu. Rev. Biochem. 70:437-473. [DOI] [PubMed] [Google Scholar]
- 4.Merchant, M., and S. R. Weinberger. 2000. Recent advancements in surface-enhanced laser desorption/ionization-time of flight-mass spectrometry. Electrophoresis 21:1164-1177. [DOI] [PubMed] [Google Scholar]
- 5.Pandey, A., and M. Mann. 2000. Proteomics to study genes and genomes. Nature 405:837-846. [DOI] [PubMed] [Google Scholar]
- 6.Reid, G., C. Heinemann, M. Velraeds, H. C. van der Mei, and H. J. Busscher. 1999. Biosurfactants produced by Lactobacillus. Methods Enzymol. 310:426-433. [DOI] [PubMed] [Google Scholar]
- 7.Reid, G., J. Howard, and B. S. Gan. 2001. Can bacterial interference prevent infection? Trends Microbiol. 9:424-428. [DOI] [PubMed] [Google Scholar]