Abstract
Anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin (1,2,3,4-tetrahydronaphthalene) was investigated with a sulfate-reducing enrichment culture obtained from a contaminated aquifer. Degradation studies with tetralin revealed 5,6,7,8-tetrahydro-2-naphthoic acid as a major metabolite indicating activation by addition of a C1 unit to tetralin, comparable to the formation of 2-naphthoic acid in anaerobic naphthalene degradation. The activation reaction was specific for the aromatic ring of tetralin; 1,2,3,4-tetrahydro-2-naphthoic acid was not detected. The reduced 2-naphthoic acid derivatives tetrahydro-, octahydro-, and decahydro-2-naphthoic acid were identified consistently in supernatants of cultures grown with either naphthalene, 2-methylnaphthalene, or tetralin. In addition, two common ring cleavage products were identified. Gas chromatography-mass spectrometry (GC-MS) and high-resolution GC-MS analyses revealed a compound with a cyclohexane ring and two carboxylic acid side chains as one of the first ring cleavage products. The elemental composition was C11H16O4 (C11H16O4-diacid), indicating that all carbon atoms of the precursor 2-naphthoic acid structure were preserved in this ring cleavage product. According to the mass spectrum, the side chains could be either an acetic acid and a propenic acid, or a carboxy group and a butenic acid side chain. A further ring cleavage product was identified as 2-carboxycyclohexylacetic acid and was assumed to be formed by β-oxidation of one of the side chains of the C11H16O4-diacid. Stable isotope-labeling growth experiments with either 13C-labeled naphthalene, per-deuterated naphthalene-d8, or a 13C-bicarbonate-buffered medium showed that the ring cleavage products derived from the introduced carbon source naphthalene. The series of identified metabolites suggests that anaerobic degradation of naphthalenes proceeds via reduction of the aromatic ring system of 2-naphthoic acid to initiate ring cleavage in analogy to the benzoyl-coenzyme A pathway for monoaromatic hydrocarbons. Our findings provide strong indications that further degradation goes through saturated compounds with a cyclohexane ring structure and not through monoaromatic compounds. A metabolic pathway for anaerobic degradation of bicyclic aromatic hydrocarbons with 2-naphthoic acid as the central intermediate is proposed.
In recent years, substantial progress has been made in the understanding of anaerobic degradation of monoaromatic hydrocarbons. Similar to aerobic degradation pathways, the substrates are channeled through peripheral pathways to central intermediates where ring cleavage takes place.
Anaerobic degradation of monoaromatic hydrocarbons proceeds in every case investigated through the benzoyl-coenzyme A (benzoyl-CoA) pathway and differs only with respect to the peripheral degradation pathways (11). The aromatic ring of benzoyl-CoA is reduced in an ATP-dependent mechanism by benzoyl-CoA reductase to initiate ring cleavage (10, 13, 14). In different organisms, this reaction may comprise a two- or four-electron reduction step (10). Water is added to a double bond in the β-position to the carboxy group to introduce a hydroxy group that allows subsequent oxidation and thiolytic ring cleavage.
In contrast to the degradation of monoaromatic compounds, only a little information exists on the anaerobic degradation of polycyclic aromatic hydrocarbons (PAH). This is obviously due to the extremely difficult cultivation of the respective bacteria and the limited number of available cultures. Besides several microcosm studies that showed 14CO2 evolution from radiolabeled PAHs with nitrate or sulfate as electron acceptors (7, 8, 15, 18, 21), transferable naphthalene-degrading cultures have been described only recently (2, 9, 19, 20, 24). Naphthalene degradation by sulfate-reducing bacterial cultures was studied with a marine enrichment culture from harbor sediment (24, 25) and a freshwater culture enriched from a tar oil-contaminated aquifer (1, 17, 19). In these studies, 2-naphthoic acid was formed from naphthalene by incorporation of bicarbonate into the carboxy group. Further degradation products were reduced 2-naphthoic acid derivatives, suggesting a stepwise reduction of the aromatic ring system in analogy to the benzoyl-CoA degradation pathway of monoaromatic compounds. The same metabolites were reported for degradation of 2-methylnaphthalene by a sulfate-reducing freshwater culture (1). It was demonstrated that 2-methylnaphthalene was activated by the addition of fumarate to the methyl group, analogous to anaerobic toluene degradation, and subsequently degraded to 2-naphthoic acid and reduced derivatives of 2-naphthoic acid (1, 3-5, 16).
Here we provide indications that anaerobic degradation of naphthalene and 2-methylnaphthalene proceeds via reduction of the bicyclic aromatic ring system of 2-naphthoic acid, analogous to the benzoyl-CoA pathway. The formation of identical ring fission products produced during the degradation of naphthalene, 2-methylnaphthalene, and tetralin (1,2,3,4-tetrahydronaphthalene) indicates that ring cleavage is initiated with a reduced bicyclic compound and further degradation proceeds through cyclohexane structures and not through monoaromatic compounds.
MATERIALS AND METHODS
Cultivation of bacteria.
A naphthalene-degrading, sulfate-reducing bacterial culture was enriched from a contaminated aquifer as described previously (17, 19). Subcultures were inoculated with a 10% volume of the liquid phase in 100-ml serum bottles half-filled with carbonate-buffered, sulfide-reduced freshwater mineral medium (pH 7.4), with trace element solution SL10 and 10 mM sulfate as an electron acceptor (23). Naphthalene and 2-methylnaphthalene were added as solid crystals, and tetralin (1,2,3,4-tetrahydronaphthalene) was added as a liquid (2 to 4 mg/50 ml). Bottles were flushed with N2-CO2 (80:20), closed with Viton rubber stoppers (Maag Technik, Dübendorf, Switzerland), and incubated at 30°C in the dark without shaking. Sulfide was measured continuously in the cultures (6), and increasing concentrations indicated anoxic conditions.
Synthesis of GC-MS reference compounds.
[1-13C]naphthalene and decahydro-2-naphthoic acid were synthesized as described elsewhere (17, 22). 2-Carboxycyclohexylacetic acid was synthesized by hydrogenation of a methanolic solution of 2-carboxyphenylacetic acid (homophthalic acid) with molecular hydrogen (250 kPa) and rhodium on activated aluminum (Fluka, Deisenhofen, Germany) as a catalyst (20°C, 24 h) (12). 1H and 13C nuclear magnetic resonance (NMR) spectra indicated complete hydrogenation. The 13C-NMR spectrum and gas chromatography-mass spectrometry (GC-MS) analyses showed the presence of two isomers in the ratio 90 (cis) to 10 (trans).
Reference compounds and educts for chemical synthesis were obtained from Aldrich (Steinheim, Germany). Organic solvents (analytical quality) were purchased from Merck (Darmstadt, Germany) and distilled before use. All other chemicals were obtained from Merck.
Analysis of metabolites.
Culture samples were preserved with 100 mM sodium hydroxide and stored at −20°C until extraction. Ten milliliters of the alkaline samples was extracted once with 2 ml of hexane to remove the nondegraded aromatic hydrocarbons. The water phase was acidified to pH 2 (HCl) and extracted three times with 1 ml of diethyl ether. The extracts were combined, and the solvent was removed by vacuum evaporation. The residue was derivatized with 90 μl of a mixture of trimethylchlorosilane (Supelco, Deisenhofen, Germany) and methanol (1/8 [vol/vol]) to methylate carboxy groups. The samples were heated in closed glass vessels at 75°C for 1 h. After cooling to room temperature, 1 ml of acidified water (pH 2 [HCl]) was added, and the water phase was extracted two times with 1 ml of hexane and once with 1 ml of diethyl ether. The combined derivatized extracts were dried over anhydrous sodium sulfate and subjected to GC-MS analysis. As a control in case the alkaline conditions in sample preservation influenced the metabolites, culture samples were also preserved in 100 mM HCl before storage, which generated the same results in the subsequent GC-MS analysis.
GC-MS measurements were performed with a Hewlett-Packard 6890 gas chromatograph coupled with a Quattro II mass spectrometer (Micromass, Altrincham, United Kingdom). One microliter of the samples was injected on a 30-m capillary column (DB-5; J & W Scientific [0.32-mm internal diameter, 0.25-μm film thickness]), and helium was used as the carrier gas. The temperature program was 80°C (3 min, isothermal), 80 to 220°C (4°C/min), 220 to 310°C (10°C/min), and 310°C (10 min, isothermal).
The following MS conditions were applied: ionization mode, EI+; ionization energy, 70 eV; source temperature, 180°C; mass range, m/z 50 to 400.
For identification of metabolites, coelution analyses and comparison of the mass spectra with those of commercially available and chemically synthesized reference compounds were applied. The total concentration of extracted metabolites was always below 1% of the supplied substrates.
High-resolution GC-MS.
High-resolution GC-MS analyses were performed with a Hewlett-Packard 5890 gas chromatograph equipped with a BPX5 fused silica column (30-m length, 0.25-mm internal diameter, 0.25-μm film thickness [SGE, Darmstadt, Germany]). The injection mode was splitless, and the injector was heated at 270°C. The temperature program was 80°C (3 min, isothermal), 80 to 220°C (4°C/min), and 220°C to 300°C (10°C/min). The gas chromatograph was coupled with a VG 70-250 SE mass spectrometer (magnet scan, m/z 500 to 35; scan rate, 1 s per mass decade; resolution, 8,000; source temperature, 200°C). Perfluorokerosene was used as a reference compound.
RESULTS
Supernatants of the sulfate-reducing bacterial enrichment culture N47 grown with either naphthalene, 2-methylnaphthalene, or tetralin (1,2,3,4-tetrahydronaphthalene) were screened for metabolites to obtain information on the ring cleavage mechanism during anaerobic PAH degradation.
Growth with tetralin.
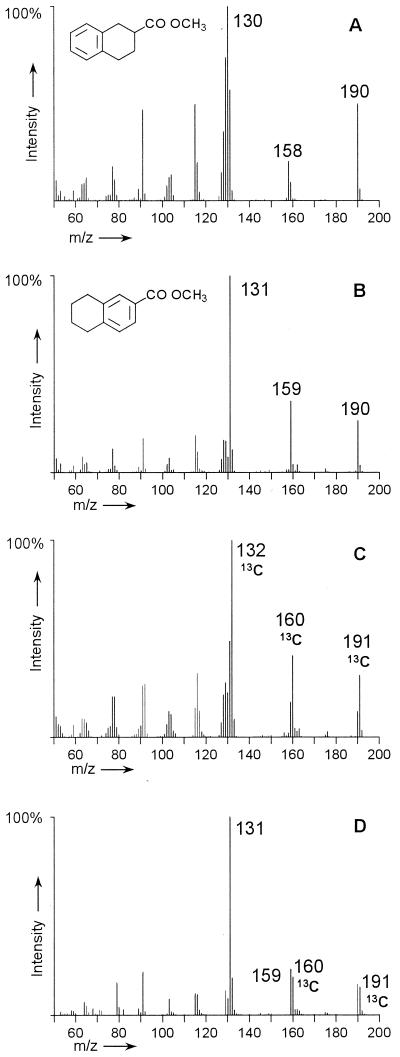
The sulfate-reducing enrichment culture N47 was able to grow with tetralin as the sole source of carbon and electrons and was transferred two times on tetralin. A tetrahydro-2-naphthoic acid was identified as major metabolite by GC-MS analyses, but the mass spectrum and GC retention time differed from those of a commercially available 1,2,3,4-tetrahydro-2-naphthoic acid, which was not detected in the culture supernatants. The mass spectrum of 1,2,3,4-tetrahydro-2-naphthoic acid methyl ester is characterized by the peaks at m/z 130, 158, and 190 (Fig. 1A). The fragment at m/z 190 represents the molecular peak [M]. The loss of CH3-OH [M − 32] is typical for methyl esters of alicyclic acids and results in the fragment at m/z 158. The further cleavage α to the carbonyl function [M − 60] leads to the peak at m/z 130.
FIG. 1.
Mass spectra of tetrahydro-2-naphthoic acids (as methyl esters) identified in supernatants of the sulfate-reducing enrichment culture N47 grown on different substrates. (A) Mass spectrum of commercially available 1,2,3,4-tetrahydro-2-naphthoic acid. (B to D) Mass spectra of the tentatively identified 5,6,7,8-tetrahydro-2-naphthoic acid produced in anaerobic degradation experiments with nonlabeled tetralin (B), [1-13C]naphthalene (C), and nonlabeled naphthalene in a [13C]bicarbonate-buffered medium (D).
In contrast, the mass spectrum of the tetrahydro-2-naphthoic acid metabolite identified in the culture supernatants showed intensive peaks at m/z 159 [M − 31] due to the expulsion of a methoxy group (O-CH3) and at m/z 131 [M − 59] and due to the cleavage of an OC-O-CH3 group (Fig. 1B). This fragmentation pattern is typical for methyl esters of aromatic acids. The metabolite was therefore tentatively identified as 5,6,7,8-tetrahydro-2-naphthoic acid (1, 17).
Other metabolites of tetralin degradation were isomers of octahydro- and decahydro-2-naphthoic acids (spectra not shown). These degradation products were also identified in growth experiments with naphthalene or 2-methylnaphthalene with the same culture (1, 17).
Confirmation of the precursor-product relationship between naphthalene and 5,6,7,8-tetrahydro-2-naphthoic acid.
In degradation experiments with [1-13C]naphthalene, all identified metabolites carried the 13C label (17). Their mass spectra showed a shift of 1 atomic mass unit (amu) compared to the degradation products of nonlabeled naphthalene, as shown for the tentatively identified 5,6,7,8-tetrahydro-2-naphthoic acid (131→132, 159→160, 190→191) (Fig. 1C).
The use of a [13C]bicarbonate-buffered medium with nonlabeled naphthalene as a growth substrate confirmed that the 13C label was preserved in the carboxy group of 5,6,7,8-tetrahydro-2-naphthoic acid (Fig. 1D) as well as in the carboxy group of the octahydro- and decahydro-2-naphthoic acid isomers (data not shown). The mass spectrum of the methyl ester of the tentatively assigned 5,6,7,8-tetrahydro-2-naphthoic acid revealed double peaks at m/z 190/191 and m/z 159/160, which are due to a partially labeled carboxy group. Due to the carryover of nonlabeled [12C]bicarbonate with the inoculum, the mass spectrum represented a mixture of nonlabeled and 13C-labeled compounds. The remaining tetralin fragment (m/z 131) after the mass spectrometric elimination of the methylated carboxy group was not labeled.
Identification of ring cleavage products.
Further studies on the degradation of naphthalene, 2-methylnaphthalene, and tetralin revealed two metabolites as common ring cleavage products for all three substrates.
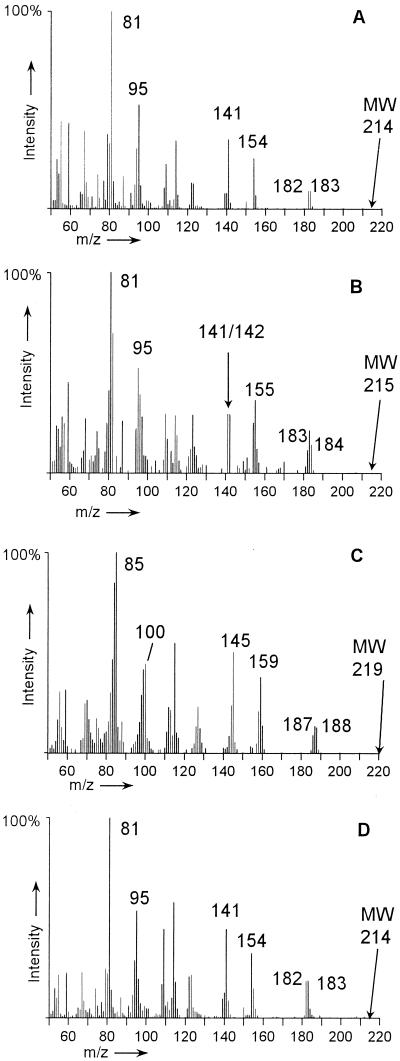
The first metabolite showed the GC-MS spectrum of a saturated cyclohexane ring structure with two carboxylic acid side chains (Fig. 2A). Through application of high-resolution GC-MS, an elemental composition of C11H16O4 was determined, indicating that all carbon atoms of the precursor 2-naphthoic acid were preserved in this ring cleavage product (C11H16O4-diacid). Because it is characteristic of dimethyl esters of dicarboxylic acids under the conditions applied, the molecular ion of the metabolite with m/z 240 was detected only very weakly due to the instability of the molecular ion. The spectrum is determined by the fragmentation of the two methyl ester groups. Two times CH3-OH [M − 32 and M − 64] was cleaved off, producing fragments at m/z 208 and 166, respectively. In both cases, the expulsion of CH3-OH was followed by the cleavage of CO, resulting in fragments with m/z 180 [M − 32 − 28], m/z 148 [M − 32 − 28 − 32], and m/z 120 [M − 32 − 28 − 32 − 28]. Thus, the fragment with m/z 120 represents the hydrocarbon body of the diacid. The peak at m/z 74 is typical for methyl esters of aliphatic carboxylic acids (H2C-COH-OCH3) and results from the fragmentation of the carboxylic side chains. According to the mass spectrum, the side chains could either be an acetic acid and a propenic acid [3-(2-aceticcyclohexylpropenic acid] or a carboxy group and a butenic acid side chain [4-(2-carboxycyclohexyl)-butenic acid] (structures labeled VII in Fig. 5). The C11H16O4-diacid was a transient metabolite that was not detected in considerable amounts in degradation experiments with [1-13C]naphthalene as a substrate, but in multiple studies with nonlabeled and per-deuterated naphthalene as well as with 2-methylnaphthalene.
FIG. 2.
Mass spectra of the dimethyl ester of the C11H16O4-diacid metabolite produced during anaerobic degradation of nonlabeled naphthalene (A), per-deuterated naphthalene-d8 (B), and per-deuterated naphthalene-d8 in a [13C]bicarbonate-buffered medium (C). For chemical structure, see Fig. 5 (compound VII). MW, molecular weight.
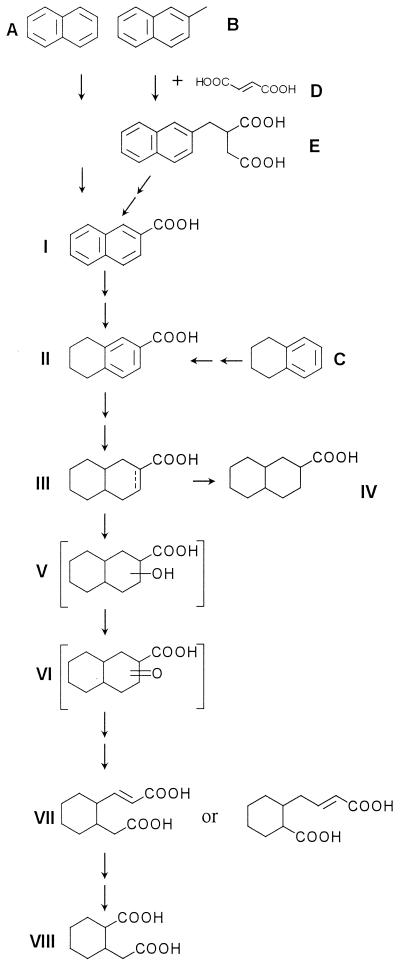
FIG. 5.
Proposed reductive 2-naphthoic acid pathway of anaerobic PAH degradation. Compounds A to E depict substances of the upper degradation pathways leading to either 2-naphthoic acid or 5,6,7,8-tetrahydro-2-naphthoic acid (for tetralin degradation). Compounds labeled with I to VIII depict metabolites of the reductive 2-naphthoic acid degradation pathway. Compounds: A, naphthalene; B, 2-methylnaphthalene; C, tetralin; D, fumaric acid; E, naphthyl-2-methyl-succinic acid; I, 2-naphthoic acid; II, 5,6,7,8-tetrahydro-2-naphthoic acid; III, octahydro-2-naphthoic acid (position of the double bond is unknown); IV, decahydro-2-naphthoic acid; V, hydroxydecahydro-2-naphthoic acid; IV, β-oxo-decahydro-2-naphthoic acid; VII, C11H16O4-diacid (cis/trans configuration not determined); VIII, cis-2-carboxycyclohexylacetic acid.
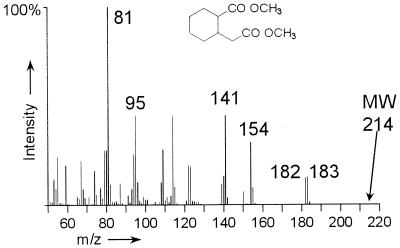
The second identified metabolite after ring cleavage was 2-carboxycyclohexylacetic acid (hexahydrohomophthalic acid) (Fig. 3A). The identity of 2-carboxycyclohexylacetic acid was confirmed by comparison with the mass spectrum and GC retention time of the chemically synthesized reference compound (Fig. 4). Chemical synthesis of 2-carboxycyclohexylacetic acid by catalytic reduction of 2-carboxyphenylacetic acid (homophthalic acid) with molecular hydrogen produced two isomers in the ratio 90 (cis) to 10 (trans) (12). Only the cis isomer was present in extracts of the culture supernatants of all three PAH substrates as determined by GC coelution analyses. The molecular peak of the 2-carboxycyclohexylacetic acid dimethyl ester (m/z 214) was not detected under the applied conditions due to the instability of the molecular ion. Characteristic peaks at m/z 182/183 are due to the fragmentation of a methyl ester group by the cleavage of (CH3-O) and (CH3-OH). α-Cleavage of the protonated methyl ester group (OHC-OCH3) results in m/z 154. The peak at m/z 141 is produced by the expulsion of the acetic acid methyl ester side chain. The ion of m/z 81 is formed by the further cleavage of the carboxy methyl ester group [M − 73 − 60].
FIG. 3.
Mass spectra of the dimethyl esters of 2-carboxycyclohexylacetic acid produced in anaerobic degradation experiments with nonlabeled naphthalene (A), [1-13C]naphthalene (B), per-deuterated naphthalene-d8 (C), and nonlabeled naphthalene in a [13C]bicarbonate-buffered medium (D) used as substrates. MW, molecular weight.
FIG. 4.
Mass spectrum of the dimethyl ester of the chemically synthesized reference compound cis-2-carboxycyclohexylacetic acid (11). MW, molecular weight.
Verification of the precursor-product relationship between naphthalene, the C11H16O4-diacid metabolite, and 2-carboxycyclohexylacetic acid.
Growth experiments with 13C-labeled or deuterated naphthalene-d8 were performed to verify that the ring fission products (the C11H16O4-diacid and 2-carboxycyclohexylacetic) derived from the precursor naphthalene.
When [1-13C]naphthalene was used as a growth substrate, the C11H16O4-diacid could not be detected in high enough concentrations to obtain GC-MS spectra. In contrast, the ring fission product 2-carboxycyclohexylacetic acid carried a 13C label, proving that it derived from the introduced [1-13C]naphthalene (Fig. 3B). The fragments exhibited a shift of 1 amu compared to the mass spectrum of the nonlabeled compound. For example, 13C-labeled fragments are represented by peaks at m/z 184, 183, 155, and 142. Because only 1 of the 10 carbon atoms of the parent naphthalene is a 13C, the label is eliminated in specific mass fragments, and the mass spectrum represents a mixture of labeled and nonlabeled fragments. The applied substrate is 13C labeled in position 1 of the naphtha lene structure ([1-13C]naphthalene). Thus, the expulsion of a former C1 carbon atom (e.g., the carboxy group positioned at the cyclohexane ring or the CH2 group of the acetic acid side chain) may lead to elimination of the 13C-label-producing fragments at m/z 154 and 141.
By application of per-deuterated naphthalene-d8 as a growth substrate, the 2-naphthoic acid derivatives and the ring cleavage metabolites were deuterium labeled. The C11H16O4-diacid and 2-carboxycyclohexylacetic acid both showed a mass shift of 5 amu (Fig. 2B and 3C).
When nonlabeled naphthalene was used as substrate in a [13C]bicarbonate-buffered growth medium, the concentration of the C11H16O4-diacid was again too low to obtain mass spectra. 2-Carboxycyclohexylacetic acid was found in high enough concentrations in the degradation experiments with [13C]bicarbonate, but it was not 13C labeled, demonstrating that the degradation reactions leading to 2-carboxycyclohexylacetic acid eliminated the 13C-carboxy group of the precursor 2-naphthoic acid (Fig. 3D). The mass spectrum was identical to that of a 2-carboxycyclohexylacetic acid produced during the degradation of naphthalene in a nonlabeled bicarbonate buffer (Fig. 3A).
With a [13C]bicarbonate-buffered medium supplemented with per-deuterated naphthalene-d8 as a growth substrate, 2-naphthoic acid and its reduced analogues carried the deuterium label, and the 13C label was incorporated in the carboxy group. The two ring cleavage products carried five deuterium labels. The C11H16O4-diacid was deuterium labeled and carried the 13C-labeled carboxy group (Fig. 2C), whereas the 13C-labeled carboxy group was not preserved in 2-carboxycyclohexylacetic acid (described above).
DISCUSSION
In the present study, we investigated the anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin (1,2,3,4-tetrahydronaphthalene) by a sulfate-reducing enrichment culture. It is demonstrated that, in analogy to the benzoyl-CoA pathway, the central intermediate 2-naphthoic acid is reduced to 5,6,7,8-tetrahydro-2-naphthoic acid and probably octahydro-2-naphthoic acid prior to ring cleavage (1, 17). The further degradation pathway after cleavage of the first ring proceeds through saturated intermediates with a cyclohexane ring structure and two carboxylic acid side chains and not through monoaromatic compounds.
Previous experiments revealed that pathways for anaerobic naphthalene and 2-methylnaphthalene degradation converge at the common intermediate 2-naphthoic acid (1, 17). In analogy to the benzoyl-CoA pathway for degradation of monoaromatic compounds, the aromatic ring system is activated by a stepwise reduction of 2-naphthoic acid producing the metabolites tetrahydro-, octahydro-, and decahydro-2-naphthoic acid (1, 10, 13, 14, 17, 25). The cooccurrence of traces of 1,2,3,4-tetrahydro-2-naphthoic acid together with 5,6,7,8-tetrahydro-2-naphthoic acid in cultures with naphthalene as a carbon source was ambiguous, because it was not clear if the ring cleavage reaction was initiated from a monoaromatic compound or a further reduced intermediate. If 1,2,3,4-tetrahydro-2-naphthoic acid were the ring cleavage substrate, the further degradation of the generated monoaromatic compounds would probably proceed via the benzoyl-CoA pathway, and the further reduced metabolites identified here would be dead-end metabolites.
In the present study, degradation experiments with tetralin as a carbon source revealed 5,6,7,8-tetrahydro-2-naphthoic acid as a major metabolite together with octahydro- and decahydro-2-naphthoic acid. No 1,2,3,4-tetrahydro-2-naphthoic acid was detected, indicating that the addition of the C1 unit occurred exclusively at the aromatic ring of tetralin. Apart from the finding that the addition of the C1 unit needs an aromatic ring as a substrate, this is an important clue to the later ring cleavage mechanism. Due to the electron-withdrawing effect of carboxy groups, the ring cleavage is expected to proceed in the β-position to the carboxy group. As in 5,6,7,8-tetrahydro-2-naphthoic acid, because the carboxy group is positioned at the aromatic ring, ring cleavage is assumed to generate products with a cyclohexane structure. Therefore, the further degradation pathway after ring cleavage goes via cyclohexanoic and not via monoaromatic compounds.
This is in accordance with the identification of the more reduced metabolite octahydro-2-naphthoic acid in tetralin- and naphthalene-grown cultures (17). The further degradation of 5,6,7,8-tetrahydro-2-naphthoic acid goes most likely through an octahydro-2-napthoic acid, which is hydrated to introduce a hydroxy group in β-position to the carboxy group to yield either 1- or 3-hydroxydecahydro-2-naphthoic acid. A further oxidation to 1- or 3-oxo-decahydro-2-naphthoic acid would allow a thiolytic ring cleavage. Nevertheless, such intermediates have not been identified so far. Decahydro-2-naphthoic acid is probably a dead-end metabolite.
Substrate utilization tests in a previous study with the same sulfate-reducing enrichment culture confirm the results of the metabolite analyses presented here. The culture could grow with 2-naphthoic acid as the sole carbon and energy source, whereas decaline, 1,2,3,4-tetrahydro-2-naphthoic acid, and decahydro-2-naphthoic acid did not support growth (17). The results suggest that decaline could not be activated because the addition of a C1 unit is specific to an aromatic ring. 1,2,3,4-Tetrahydro-2-naphthoic acid is not supposed to be an intermediate of the 2-naphthoic acid degradation pathway and cannot be converted to 5,6,7,8-tetrahydro-2-naphthoic acid. Decahydro-2-naphthoic acid is probably a dead-end metabolite of an accidental reduction of octahydro-2-naphthoic acid.
The conclusions drawn from the tetralin growth experiments are also in accordance with the identification of the alicyclic metabolites C11H16O4-diacid and 2-carboxycyclohexylacetic acid as common ring cleavage products of the 2-naphthoic acid degradation pathway. No aromatic analogues of these metabolites occurred in the batch cultures investigated, giving evidence that the second ring of the naphthalene system is completely reduced before opening. The stable isotope-labeling experiments showed unequivocally that the identified compounds are ring cleavage products of anaerobic naphthalene degradation. In addition, they give further indications about the nature of the putative precursor for the ring cleavage reaction, although such a compound has not been identified so far. In labeling experiments with [13C]bicarbonate-buffered medium, the tentatively identified C11H16O4-diacid still carried the label from the carboxy group of the [13C]2-naphthoic acid precursor. The fact that the C11H16O4-diacid contains the same number of carbon atoms as 2-naphthoic acid indicates that it is presumably the first or second metabolite after the cleavage of the first ring. However, our GC-MS analyses could not differentiate between the two possible ring cleavage products, 3-(2-aceticcyclohexyl)-propenic acid and 4-(2-carboxycyclohexyl)-butenic acid.
The ring cleavage product 2-carboxycyclohexylacetic acid is likely to be produced from the C11H16O4-diacid by a degradation reaction of one of the carboxylic acid side chains analogous to the β-oxidation of fatty acids. Stable isotope experiments revealed that the carboxy group of the precursory 2-napthoic acid was eliminated during this degradation reaction.
At present, it remains unclear how the further degradation of 2-carboxycyclohexylacetic acid proceeds. Nevertheless, the culture investigated here was able to grow with cyclohexanecarboxylic acid and cyclohex-1-ene-carboxylic acid (17), indicating that the organisms are able to cleave cyclohexanecarboxylic acid ring structures.
Based on the results presented here and in earlier studies of anaerobic naphthalene and 2-methylnaphthalene degradation (1, 17, 19, 24, 25), we propose a reductive 2-naphthoic acid degradation pathway for naphthalene-related compounds by sulfate-reducing bacteria (Fig. 5). Although it is very likely that most of the metabolites described react as CoA esters, we depict the compounds as free acids because the corresponding CoA esters have not been identified so far. The alkaline conditions in the sample preparation would have led to the hydrolysis of all potential CoA esters present. However, in control experiments without alkaline treatment, the same metabolite patterns were observed.
As designated in Fig. 5, naphthalene (compound A) or 2-methylnaphthalene (B) is activated in peripheral, upper degradation pathways to generate 2-naphthoic acid (I). Analogous to the anaerobic benzoyl-CoA pathway, 2-naphthoic acid is then reduced to 5,6,7,8-tetrahydro-2-naphthoic acid (II), which is also the entry of anaerobic tetralin (C) degradation into the pathway. A further hydrogenation may lead to octahydro-2-napthoic acid (III), which could be hydrated to generate hydroxydecahydro-2-naphthoic acid (V) and subsequently oxidized to oxodecahydro-2-naphthoic acid (VI). Decahydro-2-naphthoic acid (IV) is probably a dead-end metabolite. A thiolytic ring cleavage and a subsequent β-oxidation can generate the tentatively identified C11H16O4-diacid (VII). Cleaving off an acetic acid from a side chain of the tentatively identified C11H16O4-diacid (VII) by β-oxidation generates 2-carboxycyclohexylacetic acid (VIII).
Acknowledgments
We are grateful to Bernhard Schink, Konstanz, Germany, for continuous support. We thank Stephan Franke, Hamburg, Germany, for high-resolution GC-MS analyses and Christian Garms, Sven Possner, and Wittko Francke, Hamburg, Germany, for chemical synthesis of [1-13C]naphthalene, decahydro-2-naphthoic acid, and 2-carboxycyclohexylacetic acid.
Financial support for part of this work was provided by the Deutsche Forschungsgemeinschaft through grants Mi 157/11-3 and Schi 180/7-3 and is gratefully acknowledged.
Footnotes
Publication 174 of the Deutsche Forschungsgemeinschaft priority program 546, Geochemical Processes with Long-Term Effects in Anthropogenically Affected Seepage and Groundwater.
REFERENCES
- 1.Annweiler, E., A. Materna, M. Safinowski, A. Kappler, H. H. Richnow, W. Michaelis, and R. U. Meckenstock. 2000. Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Appl. Environ. Microbiol. 66:5329-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedessem, M. E., N. G. Swoboda-Colberg, and P. J. S. Colberg. 1997. Naphthalene mineralization coupled to sulfate-reduction in aquifer-derived enrichments. FEMS Microbiol. Lett. 152:213-218. [Google Scholar]
- 3.Beller, H. R., and A. M. Spormann. 1997. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J. Bacteriol. 179:670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beller, H. R., and A. M. Spormann. 1997. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl. Environ. Microbiol. 63:3729-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biegert, T., G. Fuchs, and J. Heider. 1996. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 238:661-668. [DOI] [PubMed] [Google Scholar]
- 6.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 7.Coates, J. D., R. T. Anderson, and D. R. Lovley. 1996. Oxidation of polycyclic aromatic hydrocarbons under sulfate-reducing conditions. Appl. Environ. Microbiol. 62:1099-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates, J. D., J. Woodward, J. Allen, P. Philp, and D. R. Lovley. 1997. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl. Environ. Microbiol. 63:3589-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galushko, A., D. Minz, B. Schink, and F. Widdel. 1999. Anaerobic degradation of naphthalene by a pure culture of a novel type of marine sulphate-reducing bacterium. Environ. Microbiol. 1:415-420. [DOI] [PubMed] [Google Scholar]
- 10.Harwood, C. S., G. Burchardt, H. Herrmann, and G. Fuchs. 1999. Anaerobic metabolism of aromatic compounds via the benzoyl-CoA pathway. FEMS Microbiol. Rev. 22:439-458. [Google Scholar]
- 11.Heider, J., A. M. Spormann, H. R. Beller, and F. Widdel. 1999. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol. Rev. 22:459-473. [Google Scholar]
- 12.Jackson, W. R., M. R. Moffat, P. Perlmutter, and E. E. Tasdelen. 1992. The stereochemistry of organometallic compounds. XXXVIII. Regio- and stereo-control in the rhodium catalysed hydroformylation of some alkenyl phosphites. Aust. J. Chem. 45:823-834. [Google Scholar]
- 13.Koch, J., W. Eisenreich, A. Bacher, and G. Fuchs. 1993. Products of enzymatic reduction of benzoyl-CoA, a key reaction in anaerobic aromatic metabolism. Eur. J. Biochem. 211:649-661. [DOI] [PubMed] [Google Scholar]
- 14.Koch, J., and G. Fuchs. 1992. Enzymatic reduction of benzoyl-CoA to alicyclic compounds, a key reaction in anaerobic aromatic metabolism. Eur. J. Biochem. 205:195-202. [DOI] [PubMed] [Google Scholar]
- 15.Langenhoff, A. A. M., A. J. B. Zehnder, and G. Schraa. 1996. Behaviour of toluene, benzene, and naphthalene under anaerobic conditions in sediment columns. Biodegradation 7:267-274. [Google Scholar]
- 16.Leuthner, B., C. Leutwein, H. Schultz, P. Hörth, W. Haehnel, E. Schlitz, H. Schägger, and J. Heider. 1998. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol. Microbiol. 28:615-628. [DOI] [PubMed] [Google Scholar]
- 17.Meckenstock, R. U., E. Annweiler, W. Michaelis, H. H. Richnow, and B. Schink. 2000. Anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Appl. Environ. Microbiol. 66:2743-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milhelcic, J. R., and R. G. Luthy. 1988. Degradation of polycyclic aromatic hydrocarbon compounds under various redox conditions in soil-water systems. Appl. Environ. Microbiol. 54:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morasch, B., E. Annweiler, R. J. Warthmann, and R. U. Meckenstock. 2001. The use of a solid adsorber resin for enrichment of bacteria with toxic substrates and to identify metabolites: degradation of naphthalene, o-, and m-xylene by sulfate-reducing bacteria. J. Microbiol. Methods 44:183-191. [DOI] [PubMed] [Google Scholar]
- 20.Rockne, K. J., J. C. Chee-Sanford, R. A. Sanford, B. P. Hedlund, J. T. Staley, and S. E. Strand. 2000. Anaerobic naphthalene degradation by microbial pure cultures under nitrate-reducing conditions. Appl. Environ. Microbiol. 66:1595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockne, K. J., and S. E. Strand. 1998. Biodegradation of bicyclic and polycyclic aromatic hydrocarbons in anaerobic enrichments. Environ. Sci. Technol. 32:3962-3967. [Google Scholar]
- 22.Staab, H. A., and M. Haenel. 1970. [1-13C]-Naphthalin: Synthese, NMR-Spektrum, ESR-Spektrum des Radikalanions und Automerisierungsversuche. Chem. Ber. 103:1095-1100. [Google Scholar]
- 23.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer Verlag, New York, N.Y. [Google Scholar]
- 24.Zhang, X., and L. Y. Young. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulfidogenic consortia. Appl. Environ. Microbiol. 63:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, X. M., E. R. Sullivan, and L. Y. Young. 2000. Evidence for aromatic ring reduction in the biodegradation pathway of carboxylated naphthalene by a sulfate-reducing consortium. Biodegradation 11:117-124. [DOI] [PubMed] [Google Scholar]