Abstract
The acetogens Sporomusa silvacetica, Moorella thermoacetica, Clostridium magnum, Acetobacterium woodii, and Thermoanaerobacter kivui (i) grew in both semisolid and liquid cultivation media containing O2 and (ii) consumed small amounts of O2. Low concentrations of O2 caused a lag phase in growth but did not alter the ability of these acetogens to synthesize acetate via the acetyl coenzyme A pathway. Cell extracts of S. silvacetica, M. thermoacetica, and C. magnum contained peroxidase and NADH oxidase activities; catalase and superoxide dismutase activities were not detected.
Acetogens have been termed obligate or strict anaerobes. They do not grow aerobically, are isolated mostly from anoxic habitats, and utilize a pathway (the acetyl coenzyme A pathway) that contains enzymes that are extremely sensitive to O2 (6, 7, 22). However, acetogens (i) can be readily isolated from leaf litter and the mineral soil of well-drained, oxic soils (8, 9), (ii) can tolerate periods of oxygenation in soils (21), (iii) are active in termite guts that have steep oxygen gradients (20), and (iv) occur in high numbers in transiently oxygenated rhizosphere sediments colonized by sea grass (12). These observations suggest that certain acetogens must cope with O2 under in situ conditions. In preliminary studies, the classic acetogen Moorella thermoacetica was found to reduce resazurin in O2-supplemented medium (A. Gößner and H. L. Drake, unpublished data), and the objective of the present study was to determine the tolerance and metabolic response of model acetogens toward O2 (for a preliminary report of this study, see A. Karnholz, K. Küsel, and H. L. Drake, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. I-91, p. 401, 2000).
Organisms, media, and growth conditions.
The acetogens used in this study were selected because each is a well-described model acetogen that has been isolated from a different habitat. The temperatures of incubation for Sporomusa silvacetica (DSM 10669; isolated from soil), M. thermoacetica (DSM 1974; isolated from horse manure), Clostridium magnum (DSM 2767; isolated from fresh water sediment), Acetobacterium woodii (DSM 1030; isolated from a marine estuary), and Thermoanaerobacter kivui (DSM 2030; isolated from lake sediment) were 30, 55, 30, 30, and 55°C, respectively. The acetogens were cultivated in a carbonate-buffered, undefined (U) medium containing yeast extract, vitamins, and trace metals but no reducing agents (4). Medium was dispensed under CO2 into 27-ml crimp seal culture tubes (7 ml of medium per tube) or 1-liter infusion bottles (500 ml of medium per bottle; used for preparation of cell extracts), which were then sealed and autoclaved; the pH was approximately 6.7. Anoxic aqueous stock solutions of glucose or fructose (prepared under argon) were filter sterilized and added to the medium by syringe injection by using O2-free techniques. Culture tubes and bottles containing liquid medium were incubated in a horizontal, static position. Culture tubes were shaken vigorously before optical densities were measured. Escherichia coli K-12 (DSM 423) was cultivated aerobically in nutrient broth (8 g per liter; Difco Laboratories, Detroit, Mich.) at pH 6.8 and 37°C.
Measurement of O2 in semisolid medium with microsensors.
Semisolid U medium was U medium supplemented with 5 g of Gelrite (Carl Roth GmbH, Karlsruhe, Germany) per liter. The 27-ml crimp seal culture tubes containing 10 ml of semisolid U medium were autoclaved, cooled to approximately 50°C, and then inoculated with 0.5 ml of freshly grown culture. As soon as the inoculated medium had solidified at room temperature, sterile O2 was injected by syringe until a final concentration of 21% (vol/vol) was reached. The tubes were incubated vertically, and O2 in the semisolid medium was measured with a microelectrode immediately after the tubes were opened. The microelectrode setup consisted of a micromanipulator-controlled O×10 sensor (5- to 10-μm tip diameter; Unisense, Aarhus, Denmark) and a PA 2000 picoammeter (Unisense) that was attached to a strip chart recorder. The microelectrode was calibrated with air-saturated (for 100% control) and N2-saturated (for 0% control) water that had the same temperature as the culture tubes (i.e., either 30 or 55°C), and O2 concentrations were calculated according to standard tables (http://www.unisense.com/support/pdf/gas-tables.pdf). The detection limit was 1 μM O2. Since tubes were only assayed once, a series of replicate tubes were inoculated for each organism so that O2 concentrations could be assessed periodically.
Enzyme assays and analytic methods.
Cell extracts were prepared under anoxic conditions (10). Cell extracts used for enzyme assays were obtained from cells grown in U medium supplemented with 10 mM fructose (for S. silvacetica) or 10 mM glucose (for M. thermoacetica and C. magnum) and with 0.5% (vol/vol) O2 in the gas phase. Catalase, peroxidase, NADH oxidase, and superoxide dismutase activities were assayed according to standard protocols (1, 2, 18, 19) at room temperature (22°C). The assays with extracts from M. thermoacetica, however, were conducted at 50°C. The catalase, peroxidase, NADH oxidase, and superoxide dismutase activities are expressed, respectively, in the following units: micromoles of H2O2 consumed per minute, milligrams of pyrogallol oxidized per minute, micromoles of NADH oxidized per minute, and micromoles of nitrotetrazolium blue chloride not reduced per minute. Growth was measured as the optical density at 660 nm (OD660); the optical path width (i.e., the inner diameter of the culture tubes) was 1.6 cm. Uninoculated medium served as a reference. Protein in cell extracts was determined colorimetrically (3). The amounts of substrates and products present in culture fluids and headspaces were determined by high-performance liquid chromatography and gas chromatography (4, 11, 17). The results are representative of replicate experiments.
Effect of O2 on the growth of acetogens.
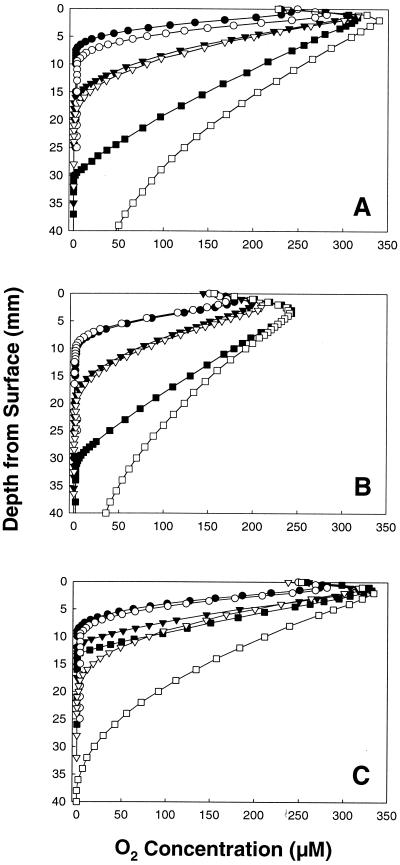
When nonreduced, semisolid medium was inoculated with S. silvacetica, M. thermoacetica, C. magnum, A. woodii, or T. kivui and then incubated with a gas phase that contained 21% (vol/vol) O2 (equivalent to the concentration of O2 in air), the extent of the oxidized surface of the medium was less in inoculated tubes than it was in uninoculated controls (Fig. 1 and data not shown). Growth, determined visually as an increase in opaqueness or in the formation of colonies within the semisolid medium was apparent only in the portions of the tubes with negligible O2. For example, as shown in Fig. 1C, the growth of C. magnum at 12 h was apparent at and below a depth of 13 mm, the portion of the tube where O2 was not detectable, but growth was not apparent between depths of 0 and 12 mm, where O2 was detectable. Culture tubes were under positive pressure during incubation, and the slight increase (i.e., peak) in the concentration of O2 in the first few millimeters of the semisolid medium was due to the initial release of O2 as the medium equilibrated to atmospheric pressure when the tubes were opened.
FIG. 1.
O2 gradients in cultures of S. silvacetica (A), M. thermoacetica (B), and C. magnum (C) in semisolid U medium with headspaces containing 21% (vol/vol) O2. Tubes were incubated vertically. Open and solid symbols are O2 in uninoculated and inoculated culture tubes, respectively. Gradients were determined at the following times subsequent to incubation: 1 (circles), 6 (triangles), and 48 (squares) h (A); 1 (circles), 6 (triangles), and 72 (squares) h (B); 1 (circles), 6 (triangles), and 12 (squares) h (C). Growth had reached a maximum height at the last time interval.
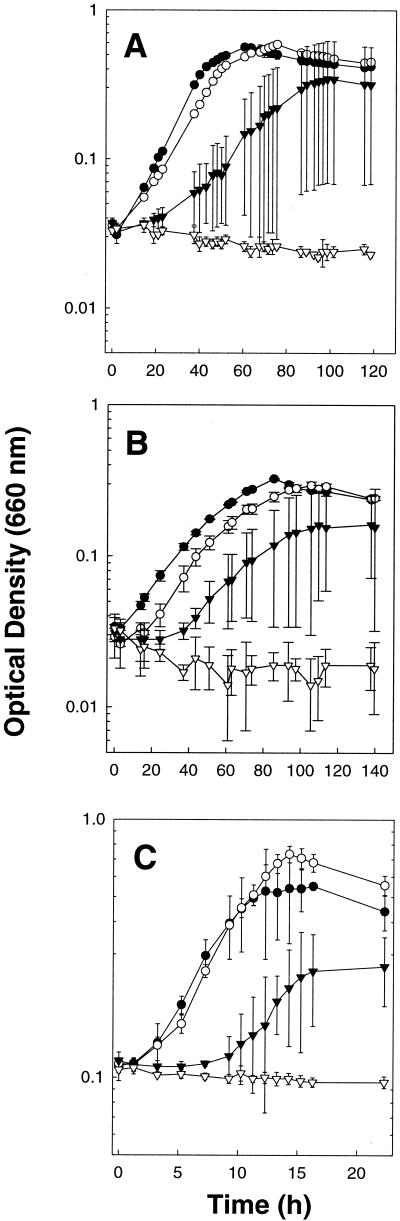
S. silvacetica, M. thermoacetica, C. magnum, A. woodii, and T. kivui also grew in nonreduced liquid culture medium containing small amounts of O2 in the gas phase (Fig. 2 and data not shown). Increasing amounts of O2 caused an increase in the lag phase of growth and a decrease in the final optical densities achieved. As illustrated by the large error bars in some of the growth curves in Fig. 2, growth became less reproducible once the concentration of O2 became at least partially inhibitory. Of the five acetogens tested, A. woodii and T. kivui were the most sensitive to O2 and did not grow in nonreduced U medium when O2 in the gas phase exceeded 0.3 and 0.5% (vol/vol), respectively.
FIG. 2.
Effect of O2 on the growth of S. silvacetica (A), M. thermoacetica (B), and C. magnum (C) cultivated in nonreduced U medium. Culture tubes were incubated horizontally and shaken prior to each measurement. The initial concentrations (percentages by volume) of O2 in the headspaces of culture tubes were as follows: 0 (•), 1.1 (○), 1.9 (▾), and 2.8 (▿) (A); 0 (•), 0.5 (○), 1.0 (▾), and 2.0 (▿) (B); 0 (•), 1.0 (○), 1.9 (▾), and 2.9 (▿) (C). Data points are the mean values of three replicate cultures.
Metabolic response of acetogens to O2.
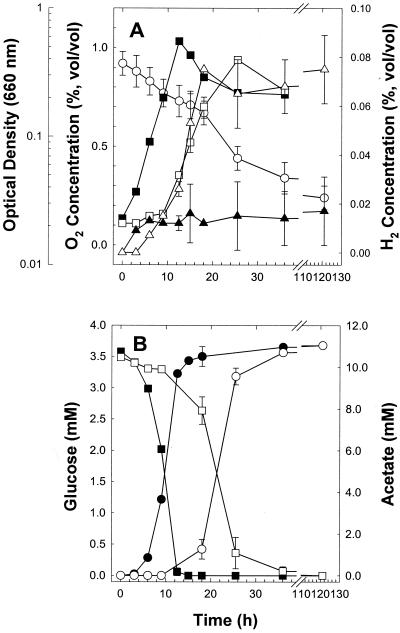
C. magnum displayed the shortest doubling times of the acetogens examined (Fig. 2), so it was chosen for a detailed evaluation of the potential effects of O2 on the formation of products during growth (Fig. 3). When the gas phase of cultures was supplemented with approximately 1% O2 (vol/vol), O2 was consumed throughout lag, log, and stationary phases of growth and trace levels of H2 were produced (Fig. 3A). O2 was not consumed in uninoculated controls. In contrast to the case with anoxic cultures, the initial presence of O2 in the gas phase of cultures caused a delay in the conversion of glucose to acetate; however, the final ratio of acetate produced to glucose consumed for both anoxic controls and O2-supplemented cultures approximated 3 (Fig. 3B), a value indicative of homoacetogenesis, C6H12O6 → 3 CH3COOH.
FIG. 3.
Growth and substrate-product profiles of C. magnum cultivated in nonreduced U medium with (open symbols) and without (solid symbols) O2 in the headspace. (A) Circles, O2; squares, optical density; triangles, H2. (B) squares, glucose; circles, acetate. Data points are the average values of duplicate cultures.
S. silvacetica, M. thermoacetica, A. woodii, and T. kivui also consumed low amounts of O2 (Table 1 and data not shown). As with C. magnum, the presence of low amounts of O2 did not alter the ratios of acetate produced to glucose consumed for S. silvacetica and M. thermoacetica. Acetate-to-biomass ratios can be used to evaluate the bioenergetics and growth efficiencies of acetogens (4). In general, the amount of acetate production that was required for growth increased when the headspaces of cultures was supplemented with O2 (Table 1), indicating that maintenance energy requirements increased in response to oxidative stress.
TABLE 1.
Effect of O2 on growth and product profiles of acetogens cultivated in U mediuma
| Acetogen | O2 (% [vol/vol] in headspace)
|
Max ΔOD660b | Substrate consumed (mM) | Acetate produced (mM)c | Acetate/substrate ratiod | Acetate/biomass ratio e | |
|---|---|---|---|---|---|---|---|
| Initial | Final | ||||||
| S. silvacetica | 0.0 | 0.0 | 0.47 (60) | 4.5f | 11.4 | 2.5 | 24 |
| 0.6 | 0.0 | 0.46 (64) | 4.6f | 11.7 | 2.5 | 25 | |
| 1.0 | 0.2 | 0.43 (78) | 4.5f | 11.9 | 2.6 | 28 | |
| M. thermoacetica | 0.0 | 0.0 | 0.33 (86) | 5.2g | 13.3 | 2.6 | 40 |
| 0.2 | 0.0 | 0.29 (104) | 5.0g | 12.5 | 2.5 | 43 | |
| 0.7 | 0.3 | 0.15 (138) | 5.4g | 12.9 | 2.4 | 86 | |
| C. magnum | 0.0 | 0.0 | 0.55 (12) | 3.6g | 11.0 | 3.1 | 20 |
| 0.5 | 0.1 | 0.46 (15) | 3.5g | 11.1 | 3.2 | 24 | |
| 0.9 | 0.2 | 0.40 (26) | 3.5g | 11.0 | 3.1 | 28 | |
Values are the means of data for three replicate experiments.
Values in parentheses are the times (in hours) required to reach the maximum OD.
Values are corrected with data for control cultures lacking substrates.
The theoretical ratio of acetate produced to substrate consumed for homoacetogenesis is 3.0.
The concentration of acetate produced (millimolar) divided by the OD660 of the culture.
The substrate was fructose.
The substrate was glucose.
Enzyme activities possibly associated with aerotolerance.
Cell extracts of S. silvacetica, M. thermoacetica, and C. magnum contained various levels of peroxidase (which consumes H2O2) and NADH oxidase (which consumes O2) activities; catalase (which consumes H2O2) and superoxide dismutase (which consumes O2·−) activities were not detected (Table 2).
TABLE 2.
Oxidative stress enzymes in cell extracts
| Organism | Enzyme level (mU/mg of protein)a
|
|||
|---|---|---|---|---|
| Catalase | Superoxide dismutase | Peroxidase | NADH oxidase | |
| S. silvacetica | 0 | 0 | 0.34 ± 0.01 | 8.0 ± 0.6 |
| M. thermoacetica | 0b | 0b | 1.13 ± 0.15 | 32.0 ± 1.2 |
| C. magnum | 0 | 0 | 0.06 ± 0.02 | 141 ± 6 |
| E. coli K-12 | 22,527 ± 657 | 0.51 ± 0.16 | 66 ± 4 | 220 ± 28 |
Values are the means ± standard deviations of data from three replicate experiments.
No activity was observed at room temperature as well.
Concluding remarks.
The capacity of so-called strict anaerobes to tolerate and consume trace levels of O2 was first demonstrated for sulfate-reducing bacteria (16). Since acetogens occur in, and can be easily isolated from, habitats that are subject to transient fluxes of O2, it is not surprising that acetogens have the ability to consume low amounts of O2 and to grow in medium supplemented with low concentrations of O2. In addition to the acetogens evaluated in the present study, Clostridium glycolicum RD-1, an abundant culturable acetogen that inhabits the sea grass root, also displays tolerance to O2 (13). C. glycolicum RD-1 can tolerate up to 6% (vol/vol) O2 in the headspace of cultures and undergoes a metabolic shift towards classic ethanol and lactate fermentations when challenged with oxic conditions (13). It has recently been reported that methanogens and acetogens that inhabit the termite gut can likewise tolerate and consume traces of O2 (14; H. Boga and A. Brune, Abstr. Annu. Meet. Verein. Allgem. Angewand. Mikrobiol., Biospectrum, Abstr. 15. P. 11. 33, p. 143, 2000).
The occurrence of NADH oxidase activity in cell extracts indicates that the acetogens tested can catalytically consume O2. O2 can yield H2O2 and O2·− within cells, and several enzymes are known to rid cells of these toxic products. Peroxidase activity was low but detectable. The absence of catalase and superoxide dismutase in acetogens was not entirely unexpected, since these enzymes produce O2. Rubrerythrin (which consumes H2O2) and rubredoxin oxidoreductase (which consumes O2·−) are alternative oxidative-stress defense proteins in the sulfate reducer Desulfovibrio vulgaris and do not produce O2 (15). Genes for similar proteins have been identified in M. thermoacetica (5). A full understanding of the biochemical processes that are responsible for the aerotolerance of acetogens will require additional investigations.
Acknowledgments
We thank Carola Matthies for helpful discussions and evaluation of the manuscript.
Support for this study was provided by the German Ministry of Education, Science, Research, and Technology (PT BEO 51-0339476C).
REFERENCES
- 1.Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. [DOI] [PubMed] [Google Scholar]
- 2.Beers, R. F., Jr., and I. W. Sizers. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195:133.. [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Daniel, S. L., T. Hsu, S. I. Dean, and H. L. Drake. 1990. Characterization of the H2- and CO-dependent chemolithotrophic potentials of the acetogens Clostridium thermoaceticum and Acetogenium kivui. J. Bacteriol. 172:4464-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das, A., E. D. Coulter, D. M. Kurtz, Jr., and L. G. Ljungdahl. 2001. Five-gene cluster in Clostridium thermoaceticum consisting of two divergent operons encoding rubredoxin oxidoreductase-rubredoxin and rubrerythrin—type A flavoprotein—high-molecular-weight rubredoxin. J. Bacteriol. 183:1560-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake, H. L. 1994. Acetogenesis, acetogenic bacteria, and the acetyl-CoA Wood/Ljungdahl pathway: past and current perspectives, p. 3-60. In H. L. Drake (ed.), Acetogenesis. Chapman and Hall, Inc., New York, N.Y.
- 7.Drake, H. L., K. Küsel, and C. Matthies. Ecological consequences of the phylogenetic and physiological diversities of acetogens. Antonie Leeuwenhoek, in press. [DOI] [PubMed]
- 8.Gößner, A. S., R. Devereux, N. Ohnemüller, G. Acker, E. Stackebrandt, and H. L. Drake. 1999. Thermicanus aegyptius gen. nov., sp. nov., isolated from oxic soil, a fermentative microaerophile that grows commensally with the thermophilic acetogen Moorella thermoacetica. Appl. Environ. Microbiol. 65:5124-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhner, C. H., C. Frank, A. Grießhammer, M. Schmittroth, G. Acker, A. Gößner, and H. L. Drake. 1997. Sporomusa silvacetica sp. nov., an acetogenic bacterium isolated from aggregated forest soil. Int. J. Syst. Bacteriol. 47:352-358. [DOI] [PubMed] [Google Scholar]
- 10.Kuhner, C. H., C. Matthies, G. Acker, M. Schmittroth, A. S. Gößner, and H. L. Drake. 2000. Clostridium akagii sp. nov. and Clostridium acidisoli sp. nov.: acid-tolerant, N2-fixing clostridia isolated from acidic forest soil and litter. Int. J. Syst. E vol. Microbiol. 50:873-881. [DOI] [PubMed] [Google Scholar]
- 11.Küsel, K., and H. L. Drake. 1995. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl. Environ. Microbiol. 61:3667-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Küsel, K., H. C. Pinkart, H. L. Drake, and R. Devereux. 1999. Acetogenic and sulfate-reducing bacteria inhabiting the rhizoplane and deep cortex cells of the sea grass Halodule wrightii. Appl. Environ. Microbiol. 65:5117-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Küsel, K., A. Karnholz, T. Trinkwalter, R. Devereux, G. Acker, and H. L. Drake. 2001. Physiological ecology of Clostridium glycolicum RD-1, an aerotolerant acetogen isolated from sea grass roots. Appl. Environ. Microbiol. 67:4734-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leadbetter, J. R., and J. A. Breznak. 1996. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl. Environ. Microbiol. 62:3620-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumppio, H. L., N. V. Shenvi, A. O. Summers, G. Voordouw, and D. M. Kurtz, Jr. 2001. Rubrerythrin and rubredoxin oxidoreductase in Desulfovibrio vulgaris: a novel oxidative stress protection system. J. Bacteriol. 183:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marschall, C., P. Frenzel, and H. Cypionka. 1993. Influence of oxygen on sulfate reduction and growth of sulfate-reducing bacteria. Arch. Microbiol. 159:168-173. [Google Scholar]
- 17.Matthies, C., A. Freiberger, and H. L. Drake. 1993. Fumarate dissimilation and differential reductant flow by Clostridium formicoaceticum and Clostridium aceticum. Arch. Microbiol. 160:273-278. [Google Scholar]
- 18.Stanton, T. B., and N. S. Jensen. 1993. Purification and characterization of NADH oxidase from Serpulina (Treponema) hyodysenteriae. J. Bacteriol. 175:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stellmach, B., W. Gottschick, F. Battermann, and K. Zabel. 1988. Bestimmungsmethoden enzyme, p. 222-223. Steinkopff Verlag, Darmstadt, Germany.
- 20.Tholen, A., and A. Brune. 1999. Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl. Environ. Microbiol. 65:4497-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner, C., A. Grießhammer, and H. L. Drake. 1996. Acetogenic capacities and the anaerobic turnover of carbon in a Kansas prairie soil. Appl. Environ. Microbiol. 62:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood, H. G., and L. G. Ljungdahl. 1991. Autotrophic character of acetogenic bacteria, p. 201-250. In J. M. Shively and L. L. Barton (ed.), Variations in autotrophic life. Academic Press, San Diego, Calif.