Abstract
The biotransformation and bioconcentration of natural and synthetic steroid estrogens by Chlorella vulgaris were investigated by using batch-shaking experiments with incubation for 48 h in the light or dark. Estradiol and estrone were interconvertible in both light and dark conditions; however, this biotransformation showed a preference for estrone. In the light, 50% estradiol was further metabolized to an unknown product. Apart from biotransformation, estrone, as well as hydroxyestrone, estriol, and ethinylestradiol, was relatively stable in the algal culture, whereas estradiol valerate was hydrolyzed to estradiol and then to estrone within 3 h of incubation. All of the tested estrogens exhibited a degree of partitioning to C. vulgaris; however, the concentrations of estriol, hydroxyestrone, ethinylestradiol, and estradiol valerate were always below the quantification limits. For estradiol and estrone, the partitioning of these estrogens in the algal extracts to the filtrates was <6% of the total amount present. The average concentration factor for estrone was ca. 27; however, the concentration factor for estradiol was not reported since no equilibrium was reached between the aqueous solution and that within the cells due to continuing biotransformation.
Natural phenolic steroid estrogens (estradiol and estrone) and the synthetic estrogen, ethinylestradiol, have been detected in aquatic systems, and their occurrence is associated with vitellogenesis and reproductive abnormalities in wild fish (3, 5, 7, 16, 21). A more complete understanding of their behavior in the aquatic environment is required in order to obtain accurate risk assessments (9). Algae, given their substantial biomass, extensive range of habitat, and diversity play an important role in the fate of organic compounds in the aquatic ecosystem (14, 15). They may degrade or take up contaminants, thereby acting as a medium for bioconcentration and subsequent biomagnification in higher trophic levels (14, 15, 20). Biosorption of heavy metals and organic pollutants by algae has been frequently reported ((1, 12, 14), and some macroalgae have been shown to have the same detoxification enzymes as those found in the mammalian liver, the so-called green liver (15). It has been demonstrated that Ochromonas danica can break down phenolic compounds to pyruvate and carbon dioxide (19).
Ethinylestradiol and estradiol valerate are synthetic estrogens produced by modifying the structure of estradiol. Ethinylestradiol can be de-ethinylated to estradiol in the human liver, although most (up to 80%) ethinylestradiol is excreted from humans unchanged in conjugated forms (4), whereas estradiol valerate is readily hydrolyzed to estradiol in the liver to exhibit its estrogenic functions (18). In the environment, the persistence of ethinylestradiol has been reported in sewage treatment works and rivers (7, 13, 22). With respect to the natural estrogens, estradiol and estrone, they are readily transformed via oxidative and reductive pathways by 17β-hydroxysteroid dehydrogenase in a range of organisms (4, 6). Microorganisms that do not produce estrogens, such as fungi and bacteria, also exhibit the ability to facilitate this biotransformation through such pathways upon exposure to estradiol and estrone (4, 11, 13, 23, 26, 27). These steroid estrogens may also be further metabolized to estriol and hydroxyestrone, which play an important role in a range of organisms (4).
The aim of this study was to investigate the interaction of a common and widely studied freshwater alga, Chlorella vulgaris, with different natural (estradiol, estrone, hydroxyestrone, and estriol) and synthetic (ethinylestradiol and estradiol valerate) estrogens and to establish whether they are biotransformed or bioconcentrated.
MATERIALS AND METHODS
Chemicals.
Individual working solutions of estradiol, estrone, estriol, hydroxyestrone, estradiol valerate, estradiol acetate, and ethinylestradiol (Sigma, Poole, United Kingdom) were prepared in HPLC grade acetone (Rathburn, Walkerburn, United Kingdom) at 10 mg liter−1. C. vulgaris was purchased from the Culture Collection of Algae and Protozoa (CCAP), Windermere, United Kingdom. The alga was grown in Jaworski's medium, as recommended by the CCAP, with continuous aeration and illumination (cool white fluorescent light at 300 lx was provided by Osram L58 W23 tubes) in a temperature-controlled room set at 18 ± 2oC. The algal cultures were allowed to grow into the stationary phase (20 days) achieving ca. 2 g liter−1 (dry weight). Stationary-phase cultures maximized the amount of biomass available to allow for determination of trace levels of steroid estrogens sorbed to the solid phase.
Biotransformation and bioconcentration experiments.
All of the batch experiments were undertaken in triplicate. Individual estrogens (500 ng) (estradiol, estrone, estriol, hydroxyestrone, estradiol valerate, and ethinylestradiol) were spiked separately into 250-ml Teflon bottles (dark) or 500-ml conical flasks (light) and blown to dryness with a gentle nitrogen stream. An algal culture (200 ml) was transferred into the bottles or flasks so that the initial concentration was of 2.5 μg liter−1 and placed on a rotary shaker in the dark or aerated in the light with triplicate samples taken at time intervals of 3, 6, 24, and 48 h. The optical density of the algal culture was measured at 680 nm and converted into the mass of algae (dry weight) for each sampling time. Further kinetic experiments on the biotransformation of estradiol were undertaken by using different algal cell densities (0.8, 1.5, and 2.3 g liter−1, dry weight) with shaking for 1, 18, and 48 h and a range of estradiol concentrations per gram of algae (i.e., 9.7, 97, 388, and 969 μl liter−1 g−1) in the dark for 6 h.
Estrogen determination in filtrate and algal cells.
Algal cells were filtered from the growth culture by using a glass fiber filter (GF6; Scheicher & Schuell, London, United Kingdom). Before extraction, 500 ng of estradiol acetate was spiked into the separated algal cells and filtrate for the determination of the final recovery in each sample. Estrogens in the algal cells were extracted overnight by soxhlet extraction by using 150 ml of dichloromethane (Rathburn, Walkerburn, United Kingdom). Estrogens in the liquid phase were extracted by liquid-liquid extraction with 200 ml of dichloromethane. Extracted estrogens were evaporated to 1 ml by using a rotary evaporator, transferred into a reaction vial, and dried with a gentle nitrogen stream. A 50-μl portion of the derivatization mixture [N-methyl-N-(trimethylsilyl)-tri-fluoroacetamide-trimethylsilylimidazole-dithioerythritol; 1,000:2:2 (vol/vol/wt)] (Sigma, Poole, United Kingdom) was added, and the reaction vial was sealed and placed in a heating block at 60°C for 30 min. The solution was again evaporated to dryness, and 0.2 ml of a 2-mg liter−1 concentration of Mirex (internal standard; Promochem, Welwyn, United Kingdom) in hexane was then added before gas chromatography-mass spectrometry (GC-MS) analysis. The GC-MS conditions and calibration, identification, and quantification procedures have been described previously (8). The GC-MS limit of quantification was 10 μg liter−1 in the final extracts; however, some samples exhibited responses of target ions at the correct retention times below this level, and such results were reported as being less than the quantification level but above the detection limit of 5 μg liter−1.
Quality control.
Before the experiments, quality controls were undertaken to ensure that the estrogens did not sorb to the laboratory equipment. The recovery efficiency of the soxhlet and liquid-liquid extraction for the algae and the filtrate spiked with 500 ng each of estrogen mixture was evaluated with respect to the recovery of estradiol acetate. The recovery for soxhlet extraction was 65%, and for liquid-liquid extraction it was >85% for all of the estrogens, except for estriol, for which the recoveries were 50% in both extraction methods. Moreover, there was no estrogen peak detected from the blanks of the alga and filtrate extracts.
RESULTS
Fate of estrogens in algal culture.
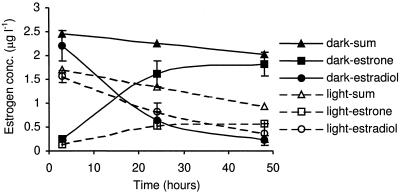
The concentration of estradiol in the culture medium fell rapidly over the first 3 h of incubation, and this decline was more pronounced in the light than the dark (Fig. 1). However, the subsequent change in the estradiol concentration was more rapid in the dark and was accompanied by an increase in the concentration of estrone in the medium solution. The stability of all estrogens was tested by using crude and autoclaved algal filtrate (without algal cells) instead of the algal culture as controls. Experimental conditions were the same for all sample types. The estrogen concentrations in the crude and autoclaved (sterile) filtrates remained constant, which indicated that no biological, physical, or chemical removal occurred in the algal-cell-free or sterile filtrates. The lack of change in the crude filtrates which may have contained bacteria not removed by the GF6 filter would indicate that effects observed were due to the algae rather than to other microorganisms. The most probable explanation for the source of estrone is that it is a result of biotransformation of estradiol by the algae, C. vulgaris. This is also supported by considering the mass balance of estradiol and estrone in the algal culture, which in the dark decreased by <20% overall (Table 1). However, in the light there was an overall decrease in concentration of both compounds, probably as a result of further metabolism (Table 1). These differences in the final concentrations of estradiol and estrone under light and dark conditions suggest that biochemical processes in the algae have a different effect on the estrogens under light and dark conditions.
FIG. 1.
Changes with time (under light and dark conditions) observed after spiking the culture with estradiol.
TABLE 1.
Mass balance and distribution of estrogens in algae and filtrate in samples spiked with estradiol
| Condition and duration (h) | Mass balance (ng) | Amt (ng g−1) in algae
|
Amt (μg liter−1) in filtrate
|
Partitioning to algae (%)a | ||
|---|---|---|---|---|---|---|
| Estradiol | Estrone | Estradiol | Estrone | |||
| Dark | ||||||
| Initial | 500 | 0 | 0 | 2.5 | 0 | 0 |
| 3 | 506 | 98 | 66 | 2.2 | 0.26 | 3 |
| 24 | 485 | 201 | 175 | 0.63 | 1.62 | 8 |
| 48 | 426 | 137 | 72 | 0.22 | 1.81 | 5 |
| Light | ||||||
| Initial | 500 | 0 | 0 | 2.5 | 0 | 0 |
| 3 | 362 | 206 | 17 | 1.57 | 0.13 | 6 |
| 24 | 298 | 59 | 269 | 0.82 | 0.52 | 12 |
| 48 | 252 | 30 | 97 | 0.38 | 0.57 | 9 |
That is, the sum of estrogens in algae versus that in the filtrates.
Although further transformation products, i.e., hydroxyestrone and estriol, were observed in media extracts, the concentrations were below quantification levels, and these are therefore insignificant products or metabolic intermediates with short half-lives. It was also possible that the estrogens were removed from the media solution through bioconcentration within the algae. The amount of estrogens that partitioned to the algae did not exceed >9% of the total estrogens by mass (Table 1). Although the ultimate fate of the estradiol is not clear, it is apparent that in the light C. vulgaris reduces the concentration from 2.50 to 0.37 μg liter−1, although ca. 0.60 μg of the estradiol liter−1 is converted to estrone, which is a potent steroid estrogen.
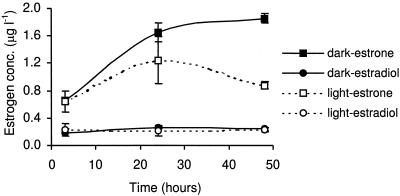
Medium solutions were also spiked with estrone and again incubated under light and dark conditions. In this case, estradiol was observed in the solutions, which indicates that the alga is able to interconvert estrone and estradiol. However, the amount of estrone transformed was <10% compared to the reverse reaction where more than 70% and more than 22% of estradiol was transformed to estrone in the dark and light, respectively (Fig. 2). Both estriol and hydroxyestrone were again observed in solution, yet still at levels below the quantification limit. It was, however, clear that mass balance equations (Table 2) indicate that the overall reduction in the concentration of estrone and estradiol was less than when the solutions were spiked with estradiol. With respect to the partitioning of estrogens to the algae, the maximum percentage partitioning was 7%, with the majority of compounds remaining in solution (Table 2).
FIG. 2.
Changes with time (under light and dark conditions) observed after spiking the culture with estrone.
TABLE 2.
Mass balance, distribution, and concentration factor of estrogens in algae and filtrate in samples spiked with estrone
| Condition and duration (h) | Mass balance (ng) | Amt (ng g−1) in algae
|
Amt (μg liter−1) in filtrate
|
Partitioning to algae (%)a | Concn factorb | ||
|---|---|---|---|---|---|---|---|
| Estradiol | Estrone | Estradiol | Estrone | ||||
| Dark | |||||||
| Initial | 500 | 0 | 0 | 2.5 | 0 | 0 | 0 |
| 3 | 509 | 103 | 47 | 0.31 | 2.16 | 3 | 22 |
| 24 | 445 | 83 | 98 | 0.24 | 1.90 | 4 | 52 |
| 48 | 424 | 109 | 41 | 0.10 | 1.95 | 4 | 21 |
| Light | |||||||
| Initial | 500 | 0 | 0 | 2.5 | 0 | 0 | 0 |
| 3 | 510 | 240 | 70 | 0.11 | 2.29 | 6 | 31 |
| 24 | 481 | 72 | 26 | 0.12 | 2.24 | 2 | 12 |
| 48 | 487 | 157 | 47 | 0.17 | 2.14 | 7 | 22 |
See Table 1, footnote a.
That is, the concentration factor of estrone in algae versus that in the filtrates.
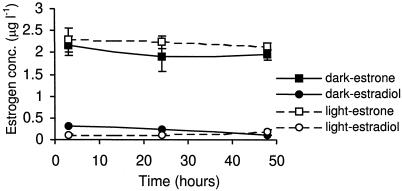
The algae also exhibited an ability to hydrolyze estradiol valerate to estradiol and estrone both in the light and in the dark (Fig. 3). The concentration of estradiol valerate decreased after the first 3 h of incubation to below the quantification level, and this drop was associated with the occurence of both estradiol and estrone. The concentration of estradiol remained constant throughout the experimental period in both dark and light; however, the level of estrone increased continuously in the dark but peaked at 24 h in the light. Traces of both estriol and hydroxyestrone were also detected in the filtrate, but their concentrations were below the quantification limits. These data may suggest that estradiol valerate was rapidly transformed to an intermediate before a slower hydrolysis to estradiol. Some uptake of estradiol valerate by the algae was observed; however, the concentrations were below the limit of quantification.
FIG. 3.
Changes with time (under light and dark conditions) observed after spiking the culture with estradiol valerate.
For the samples spiked with estriol and ethinylestradiol, a slight drop in the concentrations of both compounds were observed; however, the datum points were not significantly different, and thus the evidence for the removal of these compounds was not conclusive. Similarly, it was not possible to quantify any uptake of these estrogens by the algae since the concentrations were generally below the quantification limits and in some cases below detection levels.
The fate of hydroxyestrone in the algal culture was also determined, and the result was compared to that in a culture filtrate (no algae present) as a control. A reduction in the concentration of hydroxyestrone (<10% of initial spiked) was recorded in the culture with a detectable amount of estriol, while the concentration of hydroxyestrone was unchanged in the control. The relative stability of hydroxyestrone would indicate that it is not a rapidly metabolized intermediate in any transformation pathway.
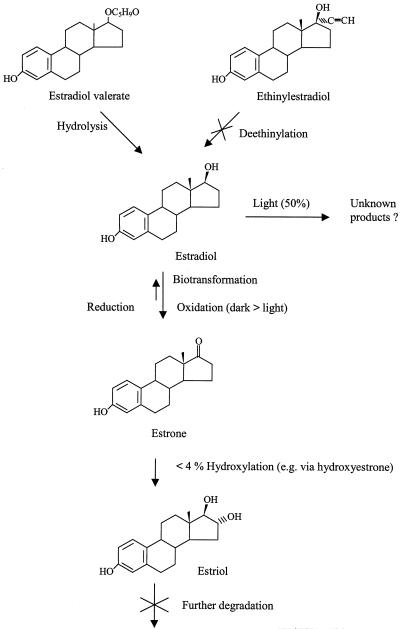
Using the preceding observations, a possible pathway for transformation of the steroid estrogens by C. vulgaris is shown in Fig. 4. Ethinylestradiol was persistent in the algal culture, whereas estradiol valerate was hydrolyzed to estradiol and estrone. Due to the interconversion preference for estrone, it is evident that estradiol valerate was hydrolyzed to estradiol and then transformed to estrone. Under light conditions ca. 50% of estradiol was metabolized into an unknown product and some of the estradiol was transformed to estrone, while under dark conditions biotransformation to estrone was the major pathway. Although estrone, hydroxyestrone, and estriol were detected in the algal culture under light conditions, the low concentration and the stability of these estrogens in the culture indicated that, as a major metabolic pathway, it is insignificant compared to the unknown pathway observed to occur in the light.
FIG. 4.
Proposed biotransformation pathway of steroid estrogens by C. vulgaris.
Bioconcentration of estrogens in the algae.
On exposure of C. vulgaris to estradiol and estrone, a degree of partitioning of both estrogens was observed to occur between the algae and aqueous phases. However, a true “bioconcentration factor” cannot be reported since equilibrium was not reached, and transformation continued. For the samples spiked with estradiol, the concentration of estradiol decreased significantly in the filtrate with time, and the occurence of estrone in the algal extract was most probably a result of intracellular biotransformation (rather than uptake from the filtrate); thus, no bioconcentration factor can be reported (Table 1). For the samples spiked with estrone, although biotransformation did occur, the level of estrone in the filtrate remained relatively constant and the concentration of estradiol was also consistently low. It was therefore likely that estrone extracted from the algae originated from the filtrate solution, and thus a concentration factor for estrone between the algae and filtrate was calculated to give an insight into the probable bioconcentration factor for estrone. The average concentration factors for estrone under both light and dark conditions was ca. 27 (Table 2).
Effect of cell density on transformation of estradiol.
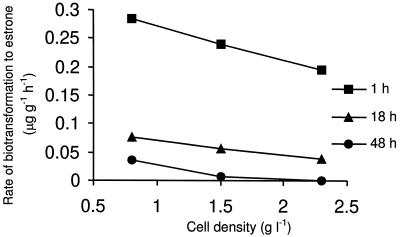
The kinetics of the biotransformation process from estradiol to estrone were determined over a range of cell densities in the dark only since the end products of transformation in light were unknown. The rate of biotransformation of estradiol to estrone declined with an increase in cell density and shaking time (Fig. 5), which indicated that substrate levels were limiting under these experimental conditions.
FIG. 5.
Effect of cell density on the formation of estrone from estradiol.
Effect of substrate level on the rate of transformation.
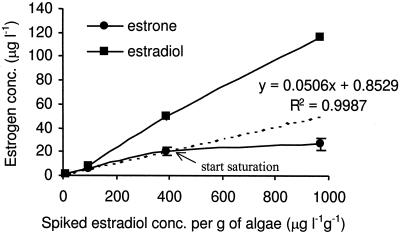
In order to investigate the effect of substrate level on biotransformation, different concentrations of estradiol were spiked into the culture. The final concentration of estrone produced increased with an initial estradiol concentration of up to 400 μg liter−1 g−1 of algae; however, at 969 μg liter−1 g−1 there was no significant increase in the final estrone concentration. The regression line produced by using the first three datum points indicated that, within experimental error, the concentration of estradiol became limiting at <400 μg liter−1 g−1 (Fig. 6), which is equivalent to 2.58 μg of estrone produced per g of algae per h and is likely to indicate the maximum capacity of C. vulgaris to transform estradiol to estrone under experimental conditions.
FIG. 6.
Effect of estradiol concentration on the biotransformation.
DISCUSSION
In the present study, the hydrolysis of estradiol valerate to estradiol by C. vulgaris has been demonstrated; however, under the conditions used in this study, the transformation of ethinylestradiol, through deethinylation or other pathways, was not observed. With respect to estradiol and estrone, C. vulgaris exhibited a preference toward estrone under both light and dark conditions. This biotransformation may be significant to the alga by reducing the estrogenic potency and increasing the polarity of estrogens for excretion or further metabolism (4, 25). The occurrence of estriol and hydroxyestrone is an indicator of the further hydroxylation of estrone to more polar metabolites (4, 25). However, such a pathway accounts for a small proportion, i.e., <4% of the total loss of estrone/estradiol in the light, and thus another, unidentified pathway must be responsible for the 50% loss of the estrogens. It is possible that conjugation of the estradiol may occur in the light. Conjugation increases polarity and is the method by which the steroid estrogens are excreted in urine in a range of animal species (4). However, this process also occurs in plants and partially or completely deactivates plant hormones and phytoestrogens (2). This process has also been observed to occur in plants to detoxify xenobiotics such as DDT and chlorinated phenols (17, 24). It is evident that conjugation is a rapid detoxification mechanism, and an absence of a lag phase for conjugation has been demonstrated for plant hormones (2). Such transformation would explain the rapid decline in estradiol concentration after the first 3 h of shaking. Moreover, in the same review (2) the effect of light on the stimulation and regulation of conjugation was exemplified in different plant hormones.
Estradiol and/or estrone biotransformation by the algae was observed to be dependent on cell density and substrate concentration. In the present study a relatively high concentration of estrogens in comparison to environmental levels of ca. 0.1 to 2 ng liter−1 (9) and algal cells were used to allow for monitoring changes and uptake. The results of the kinetic study demonstrated that the biotransformation process operated under conditions of substrate limitation; however, if the same pattern of substrate limitation occurs in the natural environment, it would be dependent on the ratio of estrogen concentrations present, the amount of algae, and a number of other factors not examined here. However, our results suggest that it is likely that biotransformation would reach a saturation point once the concentration of estradiol in aqueous solution per gram of algae is greater than 400 μg liter−1 g−1. This kinetic study may be used in estimating the probable rate of biotransformation of estrogens by algae in different environmental conditions.
The log Kow values for the steroid estrogens indicate that ethinylestradiol should partition to the organic cellular material to a greater extent than the other estrogens studied here, if we assumed that simple diffusion controls the partitioning process (10, 23). However, this was not the case, and the apparently variable uptake of the compounds suggests that an active transport (or exclusion) or a selective binding mechanism may be operating. Since it is generally agreed that estrogens pass across membranes via a simple diffusion pathway (25), it is likely that the bioconcentration of estrogens in the algae is due to an active binding mechanism such as binding to receptors or enzymes for biotransformation.
It has been suggested that accumulation of persistent organic compounds in phytoplankton is the first step of biomagnification in the food chain (12, 20). However, the concentration factors of estrogens in the algae are significantly lower than those reported for other endocrine-disrupting substances such as DDT (3 × 104) (10). It is likely therefore that the biomagnification of estrone is less significant than that of other endocrine-disrupting substances in these ecosystems.
Acknowledgments
K.M.L. is grateful to the Croucher Foundation for the award of a Ph.D. scholarship.
REFERENCES
- 1.Axelman, J., D. Broman, and C. Naf. 1997. Field measurements of PCB partitioning between water and plankton organisms: influence of growth, particle size, and solute-solvent interactions. Environ. Sci. Technol. 31:665-669. [Google Scholar]
- 2.Bearder, J. R. 1980. Biosynthesis and metabolism of plant hormones. In J. MacMillan (ed.), Hormonal regulation and development: molecular aspects of plants hormones. Springer-Verlag, Berlin, Germany.
- 3.Belfroid, A. C., A. Van der Horst, A. D. Vethaak, A. J. Schafer, G. B. J. Rijs, J. Wegener, and W. P. Cofino. 1999. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci. Total Environ. 225:101-108. [DOI] [PubMed] [Google Scholar]
- 4.Bolt, H. M. 1979. Metabolism of estrogens: natural and synthetic. Pharmacol. Ther. 4:155-181. [DOI] [PubMed] [Google Scholar]
- 5.Desbrow, C., E. J. Routledge, G. C. Brighty, J. P. Sumpter, and M. Waldock. 1998. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 32:1549-1558. [Google Scholar]
- 6.Itagaki, E., and T. Iwaya. 1988. Purification and characterization of 17β-hydroxysteroid dehydrogenase from Cylindrocarpon radicicola. J. Biochem. 103:1039-1044. [DOI] [PubMed] [Google Scholar]
- 7.Jurgens, M. D., A. C. Johnson, and R. J. Williams. 1999. Fate and behavior of steroid oestrogens in rivers: a scoping study. R&D Technical Report P161. Environment Agency, London, United Kingdom.
- 8.Lai, K. M., K. L. Johnson, M. D. Scrimshaw, and J. N. Lester. 2000. Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environ. Sci. Technol. 34:3890-3894. [Google Scholar]
- 9.Lai, K. M., M. D. Scrimshaw, and J. N. Lester. The effects of natural and synthetic steroid estrogens in relation to their environmental occurrence. Crit. Rev. Toxicol., in press. [DOI] [PubMed]
- 10.Lai, K. M., M. D. Scrimshaw, and J. N. Lester. Prediction of the bioaccumulation factors and body burden of natural and synthetic estrogens in aquatic organisms in the river systems. Sci. Total Environ., in press. [DOI] [PubMed]
- 11.Lanisnik, T., M. Zakelj-Mavric, and I. Belic. 1992. Fungal 17β-hydroxysteroid dehydrogenase. FEMS Microbiol. Lett. 99:49-52. [DOI] [PubMed] [Google Scholar]
- 12.Larsson, P., A. Andersson, D. Broman, J. Nordback, and E. Lundberg. 2000. Persistent organic pollutants (POPs) in pelagic systems. Ambio 29:202-209. [Google Scholar]
- 13.Layton, A. C., B. W. Gregory, J. R. Seward, T. W. Schultz, and G. S. Sayler. 2000. Mineralization of steroidal hormones by biosolids in wastewater treatment systems in Tennessee USA. Environ. Sci. Technol. 34:3925-3931. [Google Scholar]
- 14.Mason, R. P., J. R. Reinfelder, and F. M. M. Morel. 1996. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol. 30:1835-1845. [Google Scholar]
- 15.Pflugmacher, S., C. Wiencke, and H. Sandermann. 1999. Activity of phase I and phase II detoxication enzymes in Antarctic and Arctic macroalgae. Marine Environ. Res. 48:23-36. [Google Scholar]
- 16.Routledge, E. J., D. Sheahan, C. Desbrow, G. C. Brighty, M. Waldock, and J. P. Sumpter. 1998. Identification of estrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environ. Sci. Technol. 32:1559-1565. [Google Scholar]
- 17.Sandermann, H., R. Schmitt, H. Eckey, and T. Bauknecht. 1991. Plant biochemistry of xenobiotics: isolation and properties of soybean O- and N-glucosyl and O- and N-malonyltransferases for chlorinated phenols and anilines. Arch. Biochem. Biophys. 287:341-350. [DOI] [PubMed] [Google Scholar]
- 18.Seibert, B. 1996. Data from animal experiments and epidemiological data on turmorigenicity of estradiol valerate and ethinylestradiol. UBA TEXTE 3:63-68. [Google Scholar]
- 19.Semple, K. T., R. B. Cain, and S. Schmidt. 1999. Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 170:291-300. [Google Scholar]
- 20.Sijm, D. T. H. M., K. W. Broersen, D. F. de Roode, and P. Mayer. 1998. Bioconcentration kinetics of hydrophobic chemicals in different densities of Chlorella pyrenodosa. Environ. Toxicol. Chem. 17:1695-1704. [Google Scholar]
- 21.Ternes, T. A., J. Mueller, M. Stumpf, K. Haberer, R. D. Wilken, and M. Servos. 1999. Behaviour and occurrence of estrogens in municipal sewage treatment plants-I. Investigations in Germany, Canada, and Brazil. Sci. Total Environ. 225:81-90. [DOI] [PubMed] [Google Scholar]
- 22.Ternes, T. A., P. Keckel, and J. Mueller. 1999. Behaviour and occurrence of estrogens in municipal sewage treatment plants. II. Aerobic batch experiments with activated sludge. Sci. Total Environ. 225:91-99. [DOI] [PubMed] [Google Scholar]
- 23.Wang, X., Y. Ma, W. Yu, and H. J. Geyer. 1997. Two-compartment thermodynamic model for bioconcentration of hydrophobic organic chemicals by alga: quantitative relationship between bioconcentration factor and surface area of marine algae or octanol/water partition coefficient. Chemosphere 35:1781-1797. [Google Scholar]
- 24.Wetzel, A., and H. Sandermann. 1994. Plant biochemistry of xenobiotics: isolation and characterization of a soybean O-glucosyltransferase of DDT metabolism. Arch. Biochem. Biophys. 314:323-328. [DOI] [PubMed] [Google Scholar]
- 25.Williams, C., and G. M. Stancel. 1996. Estrogens and progestins, p. 1411-1426. In J. G. Hardman, L. E. Limbird, P. B. Molinoff, and R. W. Ruddon (ed.), The pharmacological basis of therapeutics, 9th ed. The McGraw-Hill Book Co., New York, N.Y.
- 26.Zakelj-Mavric, M., T. Kastelic-Suhadolc, A. Plemenitas, T. L. Rizner, and I. Belic. 1995. Steroid hormone signalling system and fungi. Comp. Biochem. Physiol. 112B:637-642. [DOI] [PubMed] [Google Scholar]
- 27.Zeillinger, R., and J. Spona. 1986. Pseudomonas testosteroni: new data about growth and steroid metabolism. FEMS Microbiol. Lett. 37:231-235. [Google Scholar]