Abstract
Through the analysis of spontaneous insertion mutants of Lactobacillus sakei L45, a second operon involved in lactocin S production was identified and characterized. The new, bicistronic unit, termed lasXY, is situated immediately upstream of the previously characterized nine-open reading frame (ORF) lactocin S operon (lasA-W) and is transcribed in the opposite direction. The proximal of the two newly identified genes, lasX, specifies a 285-residue protein that is similar to a group of proteins with reported gene regulation functions in gram-positive bacteria. It was demonstrated that the lasX mutants have a strongly reduced level of lasA and lasA-W mRNA, thus indicating the likely cause of the Bac− phenotype of these mutants. The second ORF in the operon, lasY, specifies a 300-residue ABC transporter homolog, the function of which is currently obscure. Transcription initiation mapping of the lasXY operon demonstrates that the two lactocin S promoters overlap such that both transcripts initiate within the −35 region of the oppositely oriented promoter. This organization of promoters is unique among this group of regulons and may constitute a modulatory site in the proposed LasX-dependent expression of lasA and downstream genes.
Lactocin S (22, 23), which is produced by some Lactobacillus sakei strains, belongs to the group of antimicrobial peptides known as lantibiotics (35). Common to all lantibiotics (for a recent review on biosynthesis and biological activities, see reference 31) is the presence of one or more of the ring-forming residues lanthionine and/or methyl-lanthionine, which are generated through a sequence of posttranslational modifications of precursor peptides. The lantibiotics are divided into subcategories, type A and B, based on molecular shape and biological activities. Type A peptides, which constitute the majority of known lantibiotics (including lactocin S), are elongated, flexible molecules and probably have in common a membrane-disrupting (pore-forming) mode of action. Structural heterogeneity is, however, observed within both subclasses, comprising differences in the number of thioether rings and their location relative to each other, as well as the presence of additional amino acid modifications. Biosynthetic functions, such as prepeptide processing, may also vary between different lantibiotic systems, as may functions such as producer self-protection (immunity) and genetic regulation of production.
The structural and biosynthetic heterogeneity among the lantibiotics is reflected in the complexity of the genetic loci, which may contain as many as 11 (e.g., nisin producers [6]) or as few as 6 (e.g., lacticin 481 producers [29, 30]) genes dedicated to the production of and protection against a particular lantibiotic. The specific genes may be organized in one or more transcriptional units, but they form a single, compact gene cluster in all known cases.
We have previously reported the sequence and expression of a nine-open reading frame (9 ORF) lactocin S (lasA-W) operon, containing the pre-lactocin S encoding gene, lasA, and candidate genes for functions such as peptide export (lasT), dehydration-thioether bridge formation (lasM), and prepeptide processing (lasP) (38). For two of these genes, lasM and lasT, the tentative functional assignments are supported by the Bac− phenotype of spontaneous mutations caused by the insertion of the insertion (IS) element IS1163 (39). The translation of lasJ, which is the penultimate ORF in the operon, is similar to the lantibiotic immunity factors PepI and EciI (11), suggesting that lasJ may be involved in producer self-protection in the lactocin S system. The four remaining ORFs in the lasA-W operon are either unique (i.e., no significantly similar sequences were found in publicly available nucleic acid or protein databases) or they encode proteins that are novel in a lantibiotic context. We have, so far, not identified mutations in any of the latter genes, and thus their possible significance in determining the lactocin S phenotype, if any, remains to be established.
Recently, a second transpositionally active IS element, IS1520, was identified in the lactocin S-producing strain (40). As is the case with the related IS1163 element, IS1520 causes the cessation of bacteriocin production by transposition to the pCIM1 las locus. In one of the isolated mutants, the element interrupted the third ORF (lasN, previously known as orf239) in the lasA-W operon, thereby suggesting a role for this gene in maintaining the lactocin S phenotype. The remaining IS1520 insertions, however, mapped outside the characterized las operon, indicating the presence of an additional gene(s) involved in lactocin S production. Below, we present an analysis of these mutants, demonstrating that the affected gene, lasX, is necessary for normal expression of the lasA-W operon. lasX is the proximal gene in the bicistronic operon lasXY, which is located upstream of lasA-W and transcribed in the opposite direction. The absence of relevant genetic information in the regions flanking the two operons, suggests that lasXY and lasA-W constitute a complete lactocin S locus.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains, plasmids, and phage lambda derivatives used here are listed in Table 1. MRS liquid or solid (1.5% agar) media were used for propagation of the Pediococcus acidilactici PAC1.0 (lactocin S sensitive indicator strain) and L. sakei strains at 30°C. Bacteriocin assays (deferred plate assay or microtiter plate assay) were performed as described previously (22). Escherichia coli strains were cultivated in Luria-Bertani medium (supplied with 100 μg of ampicillin/ml when appropriate for selection) at 37°C or as specified by the supplier of the lambda DASHII cloning vector (Stratagene Cloning Systems, La Jolla, Calif.). Plasmid DNA was purified in CsCl gradients subsequent to a standard alkaline lysis protocol (32) by using elevated lysozyme concentrations (10 mg/ml) when plasmid was isolated from L. sakei. Phage lambda DNA was isolated as suggested by the vector supplier.
TABLE 1.
Bacterial strains, bacteriophages, and plasmids used in this study
| Strain, plasmid, or phage | Genotype and/or description | Comment and/or other featuresa | Source and/or reference |
|---|---|---|---|
| Strains | |||
| E. coli | |||
| DH5α | F−φ80d lacZΔM15Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rk− mk+) deoR thi-1 supE44 λ-gyrA96 relA1 | Cloning host (blue-white selection) | Gibco-BRL |
| LE392 | F−e14−(McrA−) hsdR514 (rk− mk+) supE44 supF58 lacY1 or Δ(lacIZY)6 galK2 galT22 metB1 trpR55 | Propagation of bacteriophage λ and derivatives | 21 |
| P2392 | LE392(P2) | As for LE392; P2 lysogeny allows spi selection of λ recombinants | 37 |
| L. sakei | |||
| L45-2.1 | L45 derivative cured of pCIM2 | Bac+ Imm+ (lactocin S wild type) | 22 |
| L45-3.1 | L45 derivative cured of pCIM1 | Bac− Imm− | 22 |
| L45-5.1 | lasM::IS1163 | Bac− Imm+ | 38 |
| L45-5.2 | lasT::IS1163 | Bac− Imm+ | 38 |
| L45-5.3 | lasX1::IS1520 | Bac− Imm+ | This study |
| L45.5.6 | lasX2::IS1163 | Bac− Imm+ | This study |
| L45-5.7 | lasX3::IS1520 | Bac− Imm+ | This study |
| L45-5.8 | lasN::IS1520 | Bac− Imm+ | 40; this study |
| Plasmids | |||
| pUC18 | High-copy-number E. coli cloning vectors | None | 48 |
| pGEM7-Zf(+) | MCS flanked by T7 and SP6 promoters | Promega | |
| pCIM1 | lactocin S-encoding plasmid | 22 | |
| plas7.5-1 | pUC derivative carrying a 7.45-kb pCIM1 HindIII fragment | Sequencing template; nt 5445 to 12887 | 38 |
| plas3.5-1 | pGEM-derivative carrying a 3.5-kb EcoRI-HindIII fragment subcloned from λ-las43 | Sequencing template; nt 1 to 3576 | This study |
| λ derivatives | |||
| λ-DASHII | Replacement vector | MCS flanked by T7 and T3 promoters | Stratagene |
| λ-las43 | λ-DASHII derivativeb | Source of plas3.5-1 insert | 38; this study |
| λ-las44 | λ-DASHII derivativeb | PCR-sequencing template; nt 13431 to 15175 | 38; this study |
| λ-las46 | λ-DASHII derivativeb | PCR-sequencing template; nt 5048 to 5495 | 38; this study |
| λ-las62 | λ-DASHII derivativeb | PCR-sequencing template; nt 4173 to 5140 | 38; this study |
| λ-las63 | λ-DASHII derivativeb | PCR-sequencing template; nt 3518 to 4220 and nt 14992-15996 | 38; this study |
| λ-las103 | λ-DASHII derivativeb | PCR-sequencing template; nt 12694 to 13707 | 38; this study |
nt, nucleotides.
That is, a DASHII derivative carrying overlapping sections of the pCIM1 las region
DNA manipulations, amplification, and sequencing.
Enzymes used for restriction digestion, cloning, amplification, and sequencing of DNA were purchased from Promega (Madison, Wis.), New England Biolabs (Beverly, Mass.), or Applied Biosystems (Foster City, Calif.) and used according to the manufacturers' instructions. Oligonucleotide primers for sequencing and amplification of DNA were purchased from Life Technologies (Rockville, Md.). For amplification of DNA, 1 to 10 ng of template and 0.5 μM concentrations of each primer were used. All reactions included a 3-min hot start at 97°C, after which Taq polymerase was added and the reaction allowed to proceed for 30 to 35 cycles of denaturing (94°C for 1 min), annealing (50 to 55°C for 1 min), and primer extension (72°C for 1 min/kb of product). PCR products were purified, when necessary, by using Qiaquick cartridges (Qiagen, Hilden, Germany) according to the protocol provided by the manufacturer.
The nucleotide sequence presented below was determined either by amplifying and sequencing overlapping regions of lambda clones from a pCIM1 library or by sequencing nested deletion derivatives of subcloned DNA. The primers used were derived from the vector (T3 and T7 promoters of λ-DASHII for the amplified ends of inserts [Table 2]; M13 and pUC universal primers for the deletion derivatives) and a previously established pCIM1 sequence. Nested deletion derivatives of cloned DNA were generated by using the Promega Erase-a-Base kit according to the manufacturer's instructions, and plasmids intended for sequencing were purified as described previously (17). See Table 1 for details about the different clones used for completing the sequencing of the pCIM1 las region.
TABLE 2.
Oligonucleotide primers used in this studya
| Primer | Sequence (5"-3") | las region coordinates | Orientation |
|---|---|---|---|
| 1297 | GGTGGAACAGTATGAAAACAG | 6575-6595 | Forward |
| 1298 | GCAATCTATCTAACAATGATG | 6781-6801 | Reverse |
| 1295TC | CGCAGGTATATTTTTAGATTCTC | 6350-6370 | Forward |
| lasX-XF | GGGATTCCATATGAGAGTAGGAGAACTAGTAC | 6382-6403 | Reverse |
| lasX-XR | CGGGATCCACACTAGATTAGCACAGAAG | 5489-5511 | Forward |
| lasY-CF | TACCCCGGGAACAGATACTTCTTTAAAG | 5648-5668 | Reverse |
| lasY-CR | GCGCTCTAGACATATCTTTCAATGCC | 4516-4523 | Forward |
| 4614 | TTTCTTCCAAAGTAGAGCGGTC | 4614-4635 | Forward |
| 5566 | AATGTTTAAAAGCTCTTGATGGTCT | 5567-5591 | Forward |
| 5751 | GGAAACAATACATCTTTCTGAAATAGCTG | 5724-5751 | Reverse |
| 6371 | GAGAGTAGGAGAACTAGTACATCTTATTAG | 6372-6401 | Reverse |
| 2476 | AGAACGGGCTGTGCCATAAC | 4258-4277 | Reverse |
| 2648 | TTGGATGGACAGATTCGACG | 5121-5140 | Reverse |
| 2374 | GTGTGGAGGAAGGTGAAATCTTG | 5473-5495 | Reverse |
| lasW-term | CTCAGTAGAAGTGGAGTTC | 14955-14973 | Forward |
| T3-DASHII | CGCGAATTAACCCTCACTAAAGG | NA | NA |
| T7-DASHII | CCGCGTAATACGACTCACTATAG | NA | NA |
Primers used only for sequencing were excluded. NA, not applicable.
Both plasmids and amplified DNA were sequenced by using the Thermo Sequenase II (Amersham Pharmacia Biotech, Little Chalfont, England) or BigDye (Perkin-Elmer/Applied Biosystems) sequencing kits and the ABI Prism 377 sequencer.
Transcriptional analysis.
Total RNA was isolated from L. sakei as described previously (38) and further purified by using RNeasy purification cartridges (Qiagen). Residual DNA was removed from the RNA preparations by DNase I (RQI; Promega) digestion, followed by RNeasy purification or phenol extraction and ethanol precipitation.
Separation and transfer of RNA for Northern analysis was carried out as described previously (38). lasA- and lasX-specific probes were generated by amplification from pCIM1 by using the primer pairs 1297-1298 and lasX-XF-lasX-XR, respectively (Table 2). After purification (Qiaquick cartridges; Qiagen) the probes were labeled by using a random priming kit (Roche Molecular Biochemicals, Basel, Switzerland) and [α-32P]ATP (3,000 Ci/mmol; Amersham).
For the reverse transcriptase PCR (RT-PCR) analyses, first-strand cDNA synthesis was carried out according to the supplier of the RT (AMV-RT; Promega) by using 0.5 to 1.5 μg of total RNA per reaction. Amplification of the extension products was carried out essentially as described above with a 1/10 mixture (5 μl) of the extension reaction mix as a template and increasing the number of cycles to 40. In all cases, the forward amplification primer was identical to the one used in the corresponding RT reactions. Control reactions where RT had been omitted from otherwise identical reaction mixtures were processed simultaneously.
The primer used for identification of the lasXY transcript 5" end (primer 1295; see Table 2) was labeled with [γ-32P]ATP (5,000 Ci/mmol) and polynucleotide kinase (Promega) according to the standard protocol provided by the enzyme supplier. Then 2 pmol of labeled primer was mixed with 2.5 to 5 μg of RNA, and the volume was adjusted to 11 μl. After a denaturing-primer-annealing step (10 min at 70°C, followed by slow [20- to 30-min] cooling to room temperature), deoxynucleoside triphosphate (50 μM final concentration), AMV-RT buffer (1×), and enzyme (10 U) were added, and the volume was adjusted to 20 μl. The reaction was allowed to proceed at 45°C for 60 min and was terminated by the addition of 10 μl of stop solution-loading buffer (95% formamide, 20 mM EDTA, 0.05% each of xylene cyanol FF and bromophenol blue). After heat denaturation (70°C for 5 min), 2.5 to 5 μl from each reaction was loaded onto a 6% polyacrylamide sequencing gel. The α-35S-labeled sequencing ladders included as molecular size markers were generated with the same primer and pUC75-1 as a template in a standard Sequenase (Amersham) sequencing reaction.
Database searching, sequence retrieval, and sequence comparisons.
All database searches were performed by using the basic local alignment search tool (BLAST) programs (1, 2) from the NCBI BLAST website (http://www.ncbi.nlm.nih.gov/BLAST/). Preliminary sequence data was obtained from The Institute of Genome Research website (http://www.tigr.org). Multiple alignments of proteins were generated by using CLUSTAL X (45) v.1.64b for the Macintosh.
Nucleotide accession number.
The nucleotide accession number for the sequence reported in this study is Z54312.
RESULTS
Sequence of the regions flanking the lasA-W operon.
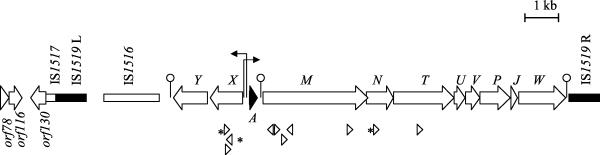
The sequence of pCIM1 upstream of lasA and further downstream of lasW was compiled after sequencing overlapping clones from a pCIM1 lambda library and the plasmids pLas7.5-1 and pLas3.5-1 (Table 1). A graphic representation of the complete, 15,996-bp region is shown in Fig. 1.
FIG. 1.
The genetic organization of the las cluster and flanking regions. The boxed arrows represent ORFs; IS elements are shown as rectangles. The positions of transcription signals are indicated by arrows (promoters) and ball-and-stick symbols (terminators), and the positions and orientation of IS1520 (triangles and asterisks) and IS1163 insertions (triangles) are indicated.
Analysis of the sequence immediately upstream of lasA revealed two consecutive major ORFs, termed lasX and lasY, with an orientation opposite to the lasA-W operon. lasX encodes a 285-residue putative protein with 20 to 25% sequence identity to the members of a group of bacterial regulatory proteins. These proteins, which are usually described as Rgg-like, have been reported to regulate target gene expression by stimulating transcription initiation and have so far been found exclusively in gram-positive organisms. The target genes described include the glucosyl transferase genes in Streptococcus gordonii (42, 43) and Streptococcus oralis (8), the gene encoding a secreted cysteine protease involved in Streptococcus pyogenes virulence (4, 19), and genes involved in glutamate-dependent acid stress resistance in Lactococcus lactis (33). Recently, the involvement of an Rgg homologue in the regulation of gene expression was demonstrated in the mutacin II system of Streptococcus mutans (25), and a related protein is probably also involved in the expression of at least one other Streptococcus mutans lantibiotic, mutacin III (26). An alignment of LasX with the closest related sequences found in the databases is shown in Fig. 2.
FIG. 2.
Alignment (CLUSTAL X [see Materials and Methods]) of LasX with related sequences from Streptococcus gordonii (Rgg-Sg [42, 43]), Streptococcus oralis (Rgg-So [8]), Streptococcus pyogenes (RopB [4, 19]), Streptococcus mutans (MutR-II and MutR-III [25, 26]), and Lactococcus lactis (GadR [33]). Positions where at least four of the sequences have identical residues are highlighted (black boxes). Positions with nonidentical but similar residues are indicated with shaded boxes, and invariant positions are marked by asterisks.
The 300-residue putative LasY protein contains the highly conserved nucleotide binding motifs associated with the ABC transporter family of proteins (7, 12, 13). However, unlike the related product of lasT, which is expressed as part of the lasA-W operon and which is essential for the lactocin S phenotype (38), LasY lacks the integral domain of membrane-spanning helices. The most similar sequences (36 to 38% identity in ungapped 288- to 289-amino-acid overlaps) identified in database searches were NatA from Bacillus pseudofirmus (44, 47) and Bacillus halodurans and YhaQ from Bacillus subtilis (18), but significant scores were also observed for NatA from Mycobacterium smegmatis and for the hypothetical proteins MJ1023 and MTH1370 from the archaea Methanococcus jannaschii (3) and Methanobacterium thermoautotrophicum (41), respectively. A search of the incomplete microbial genome database (at NCBI [see Materials and Methods]) resulted in the identification of additional, related sequences in Enterococcus faecalis, Staphylococcus aureus, Bacillus anthracis, and Streptococcus pneumoniae. Except for NatA from Bacillus pseudofirmus, which is involved in Na+ extrusion (47), no function has yet been assigned to any of the putative LasY homologues. Interestingly, all of the genes specifying the LasY-like proteins are closely linked to, and presumably cotranscribed with, genes encoding a homologue of the putative transmembrane protein LasW (previously Orf414 [38]).
As depicted in Fig. 1, the las cluster is flanked by an array of one truncated and three full-length insertion sequences belonging to three different IS families (20). Two of these elements, IS1519L and IS1519R, are nearly identical (98%), and their position and orientation (direct repeats) suggest that they may constitute the termini of a composite transposon harboring the las gene cluster. IS1519L and IS1519R belong to the IS6 family of IS elements, which typically generate 8-bp direct repeats of the target sequence upon transposition (20). In the case of pCIM1, such repeats were not found, neither flanking each element nor associated with the IS1519L-las cluster-IS1519R unit. The absence of direct repeats was also noted for IS1516, which is an ISL3-type element situated ca. 350 and 450 bp from lasY and IS1519L, respectively. IS1516 is probably nonfunctional, since the putative transposase gene has a frameshift after codon 151. The 267 bp immediately distal (with respect to the las-region, see Fig. 1) to IS1519L shows significant similarity to the right end of elements belonging to the IS30 family of insertion sequences, and this truncated IS element has been designated IS1517.
The leftmost 1,200 bp (according to the orientation shown in Fig. 1) of the sequenced region contain three ORFs that could be involved in the replication or maintenance of pCIM1: the translation of orf78 is 40% identical to the C terminus of RepB, which is a replication-associated protein of the Enterococcus faecalis plasmid pAD1 (46). orf116, which overlaps the 3" end of orf78 by 5 bp, encodes a protein similar (25% identical) to the 123-residue protein RepB implicated in stable maintenance of the Lactobacillus reuteri plasmid pTE15 (C. F. Lin and T. C. Chung, unpublished data; GenBank accession no. AF036766). The third ORF in this region, orf130, overlaps the proposed left inverted repeat of the truncated insertion sequence IS1517 by 6 bp. The translation of orf130 is 32% identical (over a 100-residue region containing one gap) to the C terminus of FipA, which is a pKM101-encoded inhibitor of IncP plasmid transfer functions (34).
Transcription analysis.
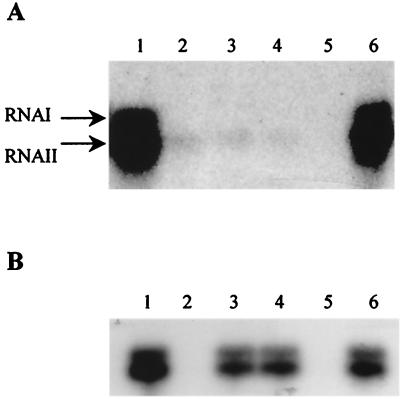
The similarity to Rgg and related proteins suggested that LasX might be a transcriptional regulator of lactocin S expression. In order to assay the effect of lasX inactivaton on the transcription of the lasA-W operon, RNA from L45 and the lasX insertion mutants was analyzed by Northern hybridization by using a lasA-specific probe. As shown in Fig. 3A, the three mutants tested had a strongly reduced level of lasA-specific RNA compared to the wild type. The level of lasA-specific mRNA was not significantly affected in any of the lasT, lasM, or lasN mutants tested (Fig. 3B). All of the mutations represented in Fig. 3B are nonpolar, as determined by Northern hybridization (38) or RT-PCR of downstream sequences (40).
FIG. 3.
Northern blots of RNA isolated from L. sakei L45 wt and mutants hybridized with a lasA-specific probe (see Materials and Methods and Table 1). (A) Lanes 1 and 6, L45-2.1 (wild type); lanes 2 to 4, lasX mutants L45-5.3, L45-5.6, and L45-5.7, respectively; lane 5, L45-3.1 (Bac−Imm−). (B) Lane 1, L45-2.1; lane 2, L45-3.1; lane 3, L45-5.1(lasM::IS1163); lane 4, L45-5.2(lasT::IS1163); lane 5, L45-5.7 (lasX::IS1520); lane 6, L45-5.8 (lasN::IS1520).
The close spacing (70 bp) between lasX and lasY and the absence of likely promoter sequences upstream of lasY suggests that that the two genes may be cotranscribed, an assumption which was initially tested by carrying out hybridization experiments like the one described above. However, this approach failed to detect lasX and lasXY transcripts in any of the RNA preparations, indicating a low level of transcription and/or rapid degradation of the transcript(s). Therefore, a series of RT-PCR experiments were carried out with combinations of primers derived from the ends and interior of both ORFs. The results of these experiments (Fig. 4) demonstrate the presence of mRNA representing both genes, thereby supporting the notion that lasX and lasY are expressed as an operon.
FIG. 4.
RT-PCR detection of lasX and lasY transcripts. The approximate positions of the primers used (see Table 2) are indicated at the top. The reactions were performed with L45-2.1 RNA and primers 5566 (first-strand cDNA synthesis and PCR) and 6371 (lanes 1 and 2) or primers 4614 (first-strand cDNA and PCR) and 5751 (lanes 3 and 4). As a control of RNA-dependent amplification, RT was omitted from the samples run in lanes 2 and 4. The 5566-6371 and 4614-5751 PCR products with pCIM1 DNA as a template were run as molecular size controls in lanes 5 and 6, respectively.
Identification of the 5" end of the lasXY mRNA.
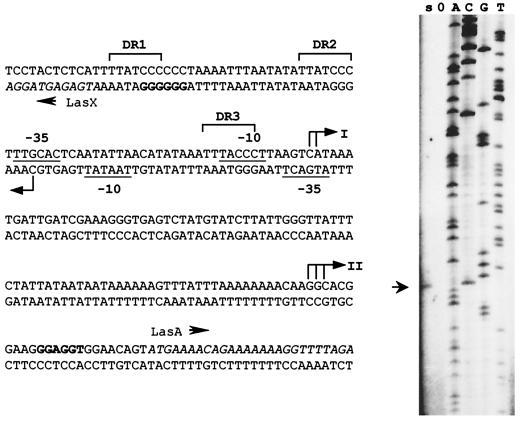
The initiation codons of the divergently oriented lasA and lasX ORFs are situated only 182 bp apart, and transcription of the lasA-W unit initiates ca. 110 bp upstream of lasA. This condensed organization suggested some degree of overlap between the promoter of the lasXY unit, PXY, and PA-W. The transcription start of the lasX or lasXY mRNA was identified (see Materials and Methods) by using RNA isolated from late-growth-phase cultures and a primer complementary to nucleotides 32 to 54 of the lasX coding sequence (las region coordinates 6350 to 6372 [see Table 2]). The results of the primer extension experiment (Fig. 5) indicate the C, located 36 nucleotides upstream of the coding sequence, as the 5"-terminal residue of the lasXY transcript. As shown in Fig. 5, the distance between the 5" ends of the divergent lasXY and lasA-W transcripts is only 34 bp, and both of the suggested −35 regions mask the initiation point of the oppositely directed transcript. This organization of regulator and target promoters is unique among the Rgg-type regulons.
FIG. 5.
On the left-hand side is shown a representation of the las cluster regulatory region. The features indicated are the experimentally determined transcription initiation points (bent arrows), the deduced promoter elements (underlined), direct repeats (marked DR1 to DR3 [with brackets]), and the proposed ribosome-binding sites (boldface letters). The roman numerals refer to the 5" ends of the two RNA species representing lasA (38). To the right is shown the result of the primer extension experiment performed to determine the 5" end of lasX (and/or lasXY) mRNA. The arrow indicates the extension product of the primer 1295TC (Table 2), which was run alongside a sequence reaction with 1295TC and plas7.5-1 as a template.
DISCUSSION
We report here the results of the continued sequencing of the lactocin S-encoding region of pCIM1, an effort spurred by the identification of las insertion mutations mapping outside of the previously reported lactocin S gene cluster (40). Two consecutive ORFs, termed lasX and lasY, were identified immediately upstream of the PA-W promoter. Transcription analysis indicates that lasX and lasY are cotranscribed, thereby constituting a second lactocin S operon. The translation of lasX, which is the affected gene in all of the new mutants, is similar to the streptococcal Rgg (42, 43) and other proteins shown to be involved in the regulation of gene expression in gram-positive bacteria. The observed correlation between the Bac− phenotype and the level of lasA-specific mRNA in lasX mutants suggests that the product of lasX regulates lactocin S gene expression at the transcription level. These findings agree well with what has been observed for Rgg and its homologs in other systems, where it has been concluded that they function as transcriptional activators (4, 19, 25, 42, 43).
Given the similarity of the sequence, as well as of apparent function, it seems reasonable to assume that the Rgg-type regulators may also have a common mechanism of target gene activation. Positive regulation of prokaryotic gene expression often involves the binding of transcription factor molecules to short, specific sequence motifs in the vicinity of the target promoter (14). In the lactocin S promoter region, the most conspicuous candidates for cis-acting regulatory elements are two directly repeated heptanucleotide motifs (TTATCCC) located in the region from −40 to −70 (relative to the A/A-W transcript 5" end), with a third, imperfect unit (TTtaCCC) overlapping the −10 promoter element. The position and spacing of the direct repeats is similar to the corresponding features in the otherwise unrelated phosphorelay systems regulating bacteriocin production in several lactic acid bacteria (for a recent review, see reference 24). It is tempting to speculate that the proposed stimulation of transcription from the PlasA-W promoter is triggered by the binding of LasX to the upstream repeats. The overlapping, divergently oriented organization of regulator and target promoters suggests that interdependent expression of the two las operons may occur by steric interaction alone. In addition, the condensed organization of the lasX-lasA intergenic region (Fig. 5) points to the interesting possibility that the putative protein-DNA interaction promoting transcription from PA-W could, simultaneously, specifically downregulate transcription from PXY.
Putative cis-acting elements have been identified in other Rgg-regulons also, but there is a notable lack of structural similaritities between the different target promoter regions. In the absence of experimental data, therefore, one has to consider the possibility that the transcription-promoting interaction between Rgg-type proteins and RNA polymerase takes place without prior binding of the transcription factor to the promoter region (14).
We are currently investigating in more detail, by techniques such as quantitative RT-PCR and band-shift analysis, the interaction between LasX and its suspected recognition sites in the lasX-lasA intergenic region. Hopefully, the results of these efforts will contribute to a better understanding of how the lactocin S and, possibly, related regulons work.
The lasA-W and lasXY operons comprise a tightly clustered, 11 ORF unit, which is punctuated by an insertion sequence(s) on both sides. At face value, the organization of the nearly identical elements IS1519L and IS1519R indicates that they may constitute the termini of a compound transposon carrying a second IS element, as well as the genes specifying the lactocin S phenotype. Similar arrangements have been observed in several cases for the nisin (10, 15, 16, 27, 28, 36) and, more recently, for the lacticin 481 (5) gene clusters. The absence of direct repeats flanking the IS1519 elements weakens the hypothesis of a lactocin S transposon. It should be noted, however, that transposition by the IS6 type insertion sequences generally lead to replicon fusions or cointegrates (9, 20), in which the fused replicons are separated by directly repeated copies of the IS element. It is possible that the lactocin S region initially was generated by an IS1519-mediated cointegrate formation and that the present locus is the result of a subsequent homologous recombination reaction(s) involving one or both of the flanking repeats. Since there are no additional copies of IS1519 in the L. sakei L45 genome, such (hypothetical) events must have occurred prior to the establishment of the lactocin S gene cluster in L45.
Although the organization of the two las operons suggests that they constitute a complete locus, we cannot at this point exclude the existence of additional, specific genes involved in the production of lactocin S. It is also still an open question whether all, or just a subset of the genes within the cluster are necessary for production, since many of the ORFs have no obvious function in determining the lactocin S phenotype. These and related issues are currently being investigated in our laboratory by heterologous expression experiments in conjunction with systematic mutational analysis of the complete region.
.
Acknowledgments
Preliminary sequence data were obtained from The Institute of Genome Research website (http://www.tigr.org).
The work presented was financed through grants 110729/130 (E.L.A.) and 114322/112 (M.S.) from the Norwegian Research Council.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bult, C. J., O. White, G. J. Olsen, L. Zhou, R. D. Fleischmann, G. G. Sutton, J. A. Blake, L. M. FitzGerald, R. A. Clayton, J. D. Gocayne, A. R. Kerlavage, B. A. Dougherty, J. F. Tomb, M. D. Adams, C. I. Reich, R. Overbeek, E. F. Kirkness, K. G. Weinstock, J. M. Merrick, A. Glodek, J. L. Scott, N. S. M. Geoghagen, and J. C. Venter. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058-1073. [DOI] [PubMed] [Google Scholar]
- 4.Chaussee, M. S., D. Ajdic, and J. J. Ferretti. 1999. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect. Immun. 67:1715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dufour, A., A. Rince, P. Uguen, and J. P. Le Pennec. 2000. IS1675, a novel lactococcal insertion element, forms a transposon-like structure including the lacticin 481 lantibiotic operon. J. Bacteriol. 182:5600-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Entian, K. D., and W. M. de Vos. 1996. Genetics of subtilin and nisin biosyntheses: biosynthesis of lantibiotics. Antonie Leeuwenhoek 69:109-117. [DOI] [PubMed] [Google Scholar]
- 7.Fath, M. J., and R. Kolter. 1993. ABC transporters: bacterial exporters. Microbiol. Rev. 57:995-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara, T., T. Hoshino, T. Ooshima, S. Sobue, and S. Hamada. 2000. Purification, characterization, and molecular analysis of the gene encoding glucosyltransferase from Streptococcus oralis. Infect. Immun. 68:2475-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galas, D. J., and M. Chandler. 1989. Bacterial insertion sequences, p. 109-162. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. ASM Press, Washington, D.C.
- 10.Gireesh, T., B. E. Davidson, and A. J. Hillier. 1992. Conjugal transfer in Lactococcus lactis of a 68-kilobase-pair chromosomal fragment containing the structural gene for the peptide bacteriocin nisin. Appl. Environ. Microbiol. 58:1670-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidrich, C., U. Pag, M. Josten, J. Metzger, R. W. Jack, G. Bierbaum, G. Jung, and H. G. Sahl. 1998. Isolation, characterization, and heterologous expression of the novel lantibiotic epicidin 280 and analysis of its biosynthetic gene cluster. Appl. Environ. Microbiol. 64:3140-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 13.Higgins, C. F., I. D. Hiles, G. P. Salmond, D. R. Gill, J. A. Downie, I. J. Evans, I. B. Holland, L. Gray, S. D. Buckel, A. W. Bell, et al. 1986. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature 323:448-450. [DOI] [PubMed] [Google Scholar]
- 14.Hochschild, A., and S. L. Dove. 1998. Protein-protein contacts that activate and repress prokaryotic transcription. Cell 92:597-600. [DOI] [PubMed] [Google Scholar]
- 15.Horn, N., S. Swindell, H. Dodd, and M. Gasson. 1991. Nisin biosynthesis genes are encoded by a novel conjugative transposon. Mol. Gen. Genet. 228:129-135. [DOI] [PubMed] [Google Scholar]
- 16.Immonen, T., G. Wahlstrom, T. Takala, and P. E. Saris. 1998. Evidence for a mosaic structure of the Tn5481 in Lactococcus lactis N8. DNA Sequence 9:245-261. [DOI] [PubMed] [Google Scholar]
- 17.Kraft, R., J. Tardiff, K. S. Krauter, and L. A. Leinwand. 1988. Using mini-prep plasmid DNA for sequencing double-stranded templates with Sequenase™. BioTechniques 6:544-547. [PubMed] [Google Scholar]
- 18.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 19.Lyon, W. R., C. M. Gibson, and M. G. Caparon. 1998. A role for trigger factor and an Rgg-like regualtor in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 17:6263-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Mørtvedt, C. I., and I. F. Nes. 1990. Plasmid-associated bacteriocin production by a Lactobacillus sakei strain. J. Gen. Microbiol. 136:1601-1607. [Google Scholar]
- 23.Mørtvedt, C. I., J. Nissen-Meyer, K. Sletten, and I. F. Nes. 1991. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sakei L45. Appl. Environ. Microbiol. 57:1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nes, I. F., and V. G. H. Eijsink. 1999. Regulation of group II peptide bacteriocin synthesis by quorum-sensing mechanisms, p. 175-192. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 25.Qi, F., P. Chen, and P. W. Caufield. 1999. Functional analyses of the promoters in the lantibiotic mutacin II biosynthetic locus in Streptococcus mutans. Appl. Environ. Microbiol. 65:652-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi, F., P. Chen, and P. W. Caufield. 1999. Purification of mutacin III from group III Streptococcus mutans UA787 and genetic analyses of mutacin III biosynthesis genes. Appl. Environ. Microbiol. 65:3880-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauch, P. J., M. M. Beerthuyzen, and W. M. de Vos. 1994. Distribution and evolution of nisin-sucrose elements in Lactococcus lactis. Appl. Environ. Microbiol. 60:1798-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauch, P. J. G., and W. M. de Vos. 1992. Characterization of the novel nisin-sucrose conjugative transposon Tn 5276 and its insertion in Lactococcus lactis. J. Bacteriol. 174:1280-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rince, A., A. Dufour, S. Le Pogam, D. Thuault, C. M. Bourgeois, and J. P. Le Pennec. 1994. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 60:1652-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rince, A., A. Dufour, P. Uguen, J. P. Le Pennec, and D. Haras. 1997. Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl. Environ. Microbiol. 63:4252-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahl, H. G., and G. Bierbaum. 1998. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Sanders, J. W., K. Leenhouts, J. Burghoorn, J. R. Brands, G. Venema, and J. Kok. 1998. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol. 27:299-310. [DOI] [PubMed] [Google Scholar]
- 34.Santini, J. M., and V. A. Stanisich. 1998. Both the fipA gene of pKM101 and the pifC gene of F inhibit conjugal transfer of RP1 by an effect on traG. J. Bacteriol. 180:4093-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnell, N., K.-D. Entian, U. Schneider, F. Götz, H. Zähner, R. Kellner, and G. Jung. 1988. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333:276-278. [DOI] [PubMed] [Google Scholar]
- 36.Siegers, K., and K. D. Entian. 1995. Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Appl. Environ. Microbiol. 61:1082-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silhavy, T., M. Berman, and L. Enquist. 1982. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Skaugen, M., C. I. M. Abildgaard, and I. F. Nes. 1997. Organization and expression of a gene cluster involved in the biosynthesis of the lantibiotic lactocin S. Mol. Gen. Genet. 253:674-686. [DOI] [PubMed] [Google Scholar]
- 39.Skaugen, M., and I. F. Nes. 1994. Transposition in Lactobacillus sakei and its abolition of lactocin S production by insertion of IS 1163, a new member of the IS 3 family. Appl. Environ. Microbiol. 60:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skaugen, M., and I. F. Nes. 2000. Transposition in Lactobacillus sakei: inactivation of a second lactocin S operon by the insertion of IS1520, a new member of the IS3 family of insertion sequences. Microbiology 146:1151-1156. [DOI] [PubMed] [Google Scholar]
- 41.Smith, D. R., L. A. Doucette-Stamm, C. Deloughery, H. Lee, J. Dubois, T. Aldredge, R. Bashirzadeh, D. Blakely, R. Cook, K. Gilbert, D. Harrison, L. Hoang, P. Keagle, W. Lumm, B. Pothier, D. Qiu, R. Spadafora, R. Vicaire, Y. Wang, J. Wierzbowski, R. Gibson, N. Jiwani, A. Caruso, D. Bush, J. N. Reeve, et al. 1997. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J. Bacteriol. 179:7135-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sulavik, M. C., G. Tardif, and D. B. Clewell. 1992. Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the Spp phenotype of Streptococcus gordonii challis. J. Bacteriol. 174:3577-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sulavik, M. S., and D. B. Clewell. 1996. Rgg is a positive transcriptional regulator of the Streptococcus gordonii gtfG gene. J. Bacteriol. 178:5826-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takami, H., and T. A. Krulwich. 2000. Reidentification of facultatively alkaliphilic Bacillus firmus OF4 as Bacillus pseudofirmus OF4. Extremophiles 4:19-22. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver, K. E., and D. J. Tritle. 1994. Identification and characterization of an Enterococcus faecalis plasmid pAD1-encoded stability determinant which produces two small RNA molecules necessary for its function. Plasmid 32:168-181. [DOI] [PubMed] [Google Scholar]
- 47.Wei, Y., A. A. Guffanti, and T. A. Krulwich. 1999. Sequence analysis and functional studies of a chromosomal region of alkaliphilic Bacillus firmus OF4 encoding an ABC-type transporter with similarity of sequence and Na+ exclusion capacity to the Bacillus subtilis NatAB transporter. Extremophiles 3:113-120. [DOI] [PubMed] [Google Scholar]
- 48.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]