Abstract
Maternally transmitted bacteria of the genus Wolbachia are obligate, intracellular symbionts that are frequently found in insects and cause a diverse array of reproductive manipulations, including cytoplasmic incompatibility, male killing, parthenogenesis, and feminization. Despite the existence of a broad range of scientific interest, many aspects of Wolbachia research have been limited to laboratories with insect-rearing facilities. The inability to culture these bacteria outside of the invertebrate host has also led to the existing bias of Wolbachia research toward infections that occur in host insects that are easily reared. Here, we demonstrate that Wolbachia infections can be simply established, stably maintained, and cryogenically stored in vitro using standard tissue culture techniques. We have examined Wolbachia host range by introducing different Wolbachia types into a single tissue culture. The results show that an Aedes albopictus (Diptera: Culicidae) cell line can support five different Wolbachia infection types derived from Drosophila simulans (Diptera: Drosophilidae), Culex pipiens (Culicidae), and Cadra cautella (Lepidoptera: Phycitidae). These bacterial types include infection types that have been assigned to two of the major Wolbachia clades. As an additional examination of Wolbachia host cell range, we demonstrated that a Wolbachia strain from D. simulans could be established in host insect cell lines derived from A. albopictus, Spodoptera frugiperda (Lepidoptera: Noctuidae), and Drosophila melanogaster. These results will facilitate the development of a Wolbachia stock center, permitting novel approaches for the study of Wolbachia infections and encouraging Wolbachia research in additional laboratories.
Wolbachia pipientis refers to a monophyletic clade of intracellular α-proteobacteria that infect a diverse range of invertebrate hosts and display the ability to manipulate the reproduction of their hosts via several distinct phenotypes, including cytoplasmic incompatibility, feminization, parthenogenesis, and male killing (14, 25, 31). Although the mechanisms that mediate these reproductive distortions have yet to be defined, the variety of reproductive manipulations induced by Wolbachia species has made this genus a model for the study of reproductive parasitism (3). Additional research has focused on determining the role of Wolbachia in genetic conflict, host reproductive isolation, and speciation (2, 19, 30). The ability of Wolbachia species to affect host reproduction has also made this genus the focus of applied strategies designed to manipulate field populations of medically and economically important invertebrates (23).
Since the original description of the genus Wolbachia (11), the inability to culture these symbionts outside of the invertebrate host has continued to be a hindrance to research with these bacteria. With the advent of PCR, molecular techniques have been used to partially circumvent traditional bacteriological methods, permitting the phylogenetic characterization of Wolbachia infections (13, 34, 36) and the recognition of additional infections (33). However, additional research with Wolbachia often requires that a colony of infected host invertebrates be maintained. Not surprisingly, studies of Wolbachia infections that occur in host colonies that are difficult to rear have been limited to a relatively small number of laboratories with appropriate facilities and expertise. As a result, a majority of recent Wolbachia research has been biased toward infections that occur within hosts that are easily reared (e.g., Drosophila). Comparative studies with multiple Wolbachia types have also been limited in part because of the difficult logistics of maintaining multiple host colonies.
Recently, a technique permitting maintenance of Wolbachia in vitro has been developed. This prior research established a cell line from Wolbachia-infected Aedes albopictus embryos (i.e., the Aa23 cell line [15]) and demonstrated that Wolbachia could be stably maintained in vitro using standard cell culture techniques. We hypothesized that the shell vial technique could potentially provide a simplified means of establishing in vitro Wolbachia infections. The shell vial technique, which was originally developed as a diagnostic protocol to detect rickettsial infections, permits the establishment of in vitro rickettsial infections via centrifugation of infected host material onto a monolayer of uninfected cells (24).
Here we report the use of the shell vial technique as a simple means to establish different Wolbachia infections and to examine host cell range. Insect cell lines have been stably infected with two Wolbachia infections from Drosophila simulans (Diptera: Drosophilidae), two infections from a superinfected strain of Cadra cautella (Lepidoptera: Phycitidae), and an infection from Culex pipiens (Diptera: Culicidae). These infections represent a phylogenetically diverse range of Wolbachia types that originate from a broad range of invertebrate hosts. The shell vial technique has been used to establish Wolbachia infections in cell lines derived from A. albopictus (Diptera: Culicidae), Spodoptera frugiperda (Lepidoptera: Noctuidae), and Drosophila melanogaster.
MATERIALS AND METHODS
Insect maintenance and egg collection.
D. simulans eggs were collected by standard procedures (7). Cadra cautella eggs were collected by placing adults in a petri dish containing a fitted piece of filter paper for 24 h; females deposited eggs directly onto the filter paper, and eggs were subsequently washed into a microcentrifuge tube for preparation. Culex pipiens eggs were collected using sterile rearing medium as an oviposition site; petri dishes containing this medium were placed in cages with blood-fed females for 24 h.
Cell culture maintenance.
Wolbachia strain wAlbB-infected (Aa23) and uninfected (Aa23T) A. albopictus cells were maintained as previously described (15). Growth medium consisted of equal volumes of Mitsuhashi-Maramorosch and VP12 media (29) augmented with 10% (vol/vol) heat-inactivated fetal bovine serum (HyClone, Logan, Utah). D. melanogaster Schneider's (S2) cells and S. frugiperda (SF9) cells were grown in Shields and Sang M3 insect medium (Sigma, St. Louis, Mo.) and Grace's insect medium (Sigma), respectively. Cells that were cryogenically stored were frozen by a standard cell culture technique in growth medium augmented with 10% (vol/vol) dimethyl sulfoxide (Sigma). The S2, SF9, and C6/36 tissue culture lines were obtained from the American Type Culture Collection (ATCC; Manassas, Va.).
Wolbachia infections were eliminated from cell culture by adding tetracycline to the growth media at a final concentration of 10 μg/ml. Although tetracycline treatment of a single passage is typically sufficient to eliminate the in vitro Wolbachia infection, residual Wolbachia DNA persists transiently in subsequent passages and can be detected by PCR assay (see Fig. 2). Tetracycline treatment was repeated for three sequential passages.
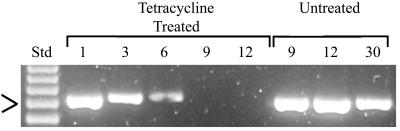
FIG. 2.
Effect of tetracycline treatment on in vitro wRi Wolbachia infection. The numbers indicate the cell culture passages following the initiation of tetracycline treatment. In this experiment, the ability to detect Wolbachia via PCR amplification was lost prior to the ninth passage. The ability to detect Wolbachia via PCR amplification persisted beyond 30 passages with in vitro infections that had not been treated with tetracycline. A molecular size standard (Std) is shown in the first lane (123 Ladder; Gibco). The arrow indicates 492 bp.
Shell vial technique.
Cell monolayers were grown in sterile shell vials (Fisher Scientific; Pittsburgh, Pa.) until they were approximately 80% confluent. Immediately prior to the addition of donor host material (i.e., crushed embryos), the growth medium was removed. Approximately 20 mg of eggs was collected from D. simulans (Riverside and Coffs Harbor strains), Cadra cautella, and Culex pipiens and surface sterilized. Drosophila and Cadra eggs were surface sterilized for 2 min in a 2.5% sodium hypochlorite solution and then treated twice (for 5 min each time) with 70% ethanol. Only the ethanol treatment was used with Culex eggs because of embryo damage resulting from sodium hypochlorite treatment. Following surface sterilization, the eggs were rinsed well with sterile water and suspended in sterile phosphate-buffered saline (PBS). Eggs were crushed and overlaid on the cell culture. The cultures were then centrifuged at 2,500 × g in a Beckman J2-21 centrifuge fitted with a JS-13 swinging-bucket rotor (Beckman, Palo Alto, Calif.) at 15°C for 1 h. After centrifugation, 5 ml of growth medium was added, and the recipient cells were resuspended by striking the vial on a tabletop. The resuspended cells were transferred to a tissue culture flask (Corning, Corning, N.Y.) for maintenance.
PCR amplification and sequencing.
For all PCR amplifications, approximately 106 insect cells were homogenized in 100 μl of STE (0.1 M NaCl, 10 mM Tris-HCl, and 1 mM EDTA [pH 8.0]). Proteinase K (Sigma) was added to a final concentration of 0.4 mg/ml, and this mixture was incubated at 56°C for 1 h. Following heat inactivation at 95°C for 15 min, 1 μl of each of these samples was amplified in a solution containing 50 mM KCl, 20 mM Tris-HCl (pH 8.4), 1.5 mM MgCl2, 0.25 mM deoxynucleoside triphosphates, 0.5 μM primers, and 1 U of Taq DNA polymerase (Life Technologies, Inc., Rockville, Md.) in a total volume of 20 μl. Samples were denatured for 3 min at 94°C and cycled 35 times at 94°C, 55°C, and 72°C (1 min each), using a PTC-200 thermal cycler (MJ Research; Watertown, Mass.); this was followed by a 10-min extension at 72°C. A 10-μl aliquot of each amplification product was electrophoresed on a 1% agarose gel, stained with ethidium bromide, and visualized under UV illumination.
Typically, infection status was monitored using the general wsp primers (primers 81F and 691R [36]). Cultures failing to amplify with the Wolbachia-specific primers were reamplified using 12S mitochondrial primers to verify template quality (13). Diagnostic primers for different Wolbachia types are shown in Table 1. For sequencing, amplified DNA was cleaned using a QiaQuick PCR cleanup kit (Qiagen, Valencia, Calif.) and directly sequenced using an ABI Prism 310 sequencer (Applied Biosystems, Foster City, Calif.).
TABLE 1.
Characteristics of Wolbachia strains established in vitro
| Wolbachia strain designation | Original insect host | Wolbachia clade | Phenotypea | Diagnostic primersb | Reference |
|---|---|---|---|---|---|
| wRic | D. simulans (Diptera) | A | CI; mod+ resc+ | 169F, 691R | 26 |
| wCof | D. simulans (Diptera) | A | CI; mod− resc− | 308F, 691R | 12 |
| wAlbB | A. albopictusd (Diptera) | B | CI; mod+ resc+ | 183F, 691R | 22 |
| wPip | Culex pipiens (Diptera) | B | CI; mod+ resc+ | 183F, 691R | 9 |
| wCauA | Cadra cautellae (Lepidoptera) | A | CI; mod+ resc+ | 178F, 691R | 21 |
| wCauB | Cadra cautella (Lepidoptera) | B | CI; mod+ resc+ | 211F, 691R | 21 |
See reference 4 for a review of CI phenotypes.
Diagnostic primers are discussed in reference 36.
wRi infection was successfully established in four different insect cell lines: Aa23, C6/36, S2, and SF9.
Host superinfected with wAlbA and wAlbB; only the wAlbB infection has been established in vitro (15).
Host superinfected with wCauA and wCauB; both infections have been established in vitro as a superinfected culture.
Immunocytochemistry.
Cells were examined at passage 25 (for S2 cells) or at passage 15 (for SF9 cells). Cells were taken from nearly confluent cultures, extensively rinsed in PBS, and put onto 8-well slides pretreated with poly-l-lysine. The cells were then incubated in a 4% paraformaldehyde solution for 15 min, rinsed in PBST (PBS plus 0.1% Triton X-100), blocked for 30 min with PBST containing 1% (vol/vol) bovine serum albumin, and incubated with anti-WSP antibody (1:500 dilution in PBST) overnight at 4°C. Afterward, the cells were rinsed in PBST, incubated with rhodamine-conjugated anti-rabbit immunoglobulin G antibody (Molecular Probes; 1:200 dilution in PBST) for 1 h at room temperature, rinsed in PBS, and finally mounted with polyvinyl alcohol (Calbiochem). Images were acquired using a Leica TCS confocal microscope and displayed in Photoshop (Adobe, Inc., San Jose, Calif.).
RESULTS AND DISCUSSION
Infections were initially established by centrifugation of infected host material (i.e., early embryos) onto a monolayer of uninfected A. albopictus cells (Aa23T [15]). D. simulans embryos infected with wRi were first used because of the ease of obtaining large quantities of infected embryos. To test the general applicability of the shell vial technique, subsequent attempts focused on establishing additional in vitro infections from a diverse range of invertebrate taxa (Table 1). Although obtaining sufficient quantities of infected host material for establishing in vitro infections was more difficult with some hosts, no significant complications occurred, and in vitro infections were successfully established for each of the infected host taxa.
In addition to examining the robustness of the shell vial technique with a range of invertebrate host taxa, these experiments also demonstrated the ability of the Aa23T cell line to stably maintain a phylogenetically diverse range of Wolbachia infections, including strains from two of the major clades of the genus Wolbachia (i.e., A and B [27]) (Table 1). As an additional test of the shell vial technique, wRi infection was also established in three other uninfected insect cell lines: the dipteran D. melanogaster Schneider's (S2) and A. albopictus C6/36 (ATCC CRL-1660) cell lines and the lepidopteran S. frugiperda (SF9) cell line. All cell lines behaved indistinguishably from the Aa23T cell line in terms of their ability to establish and maintain the wRi Wolbachia infection in vitro.
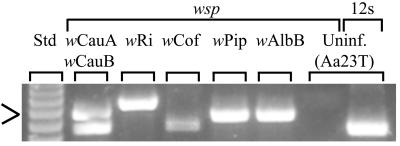
For all in vitro infections, Wolbachia was initially detected by PCR using diagnostic primers specific for the wsp gene (36) (Table 1). In addition to detection, these primers also permitted an initial verification of the Wolbachia type (Fig. 1). Sequencing of the amplified wsp genes was used to provide additional confirmation of the Wolbachia infection types. In each case, the wsp sequences were identical to previously published sequences derived from in vivo infections (36).
FIG. 1.
Typical results of PCR amplifications of the established in vitro Wolbachia-infected cells (Table 1) and an uninfected (Uninf.) cell line (Aa23T [15]) using Wolbachia-specific (wsp) (Table 1) and general mitochondrial (12S) primers. Amplification with 12S primers was used to verify the quality of the DNA template used in the reactions (13). wCauA and wCauB occur together in a superinfected cell culture (Table 1). A molecular size standard (Std) is shown in the first lane (123 Ladder; Gibco). The arrow indicates 492 bp.
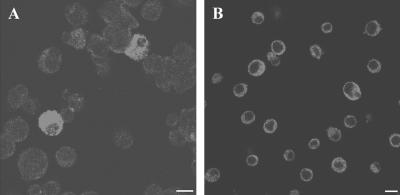
To verify that the Wolbachia DNA that was amplified from in vitro infections was derived from living bacteria and not residual exogenous DNA, the in vitro infection was treated with tetracycline, which has been previously shown to eliminate Wolbachia infection (1, 16, 35). The infected cultures were divided into two portions; one of the aliquots was treated with tetracycline (10 μg/ml), and the other aliquot remained untreated. The ability to amplify Wolbachia PCR products of the expected size was lost in the tetracycline-treated lines (Fig. 2). In contrast, diagnostic primers continued to amplify Wolbachia sequences in the cell culture lines that were not treated with tetracycline. The in vitro Wolbachia infection was also visualized in cell monolayers by using a polyclonal antibody raised against the major surface protein of the bacterium, the WSP protein (8). Similar to previous observations of the Wolbachia infection in the Aa23 cell line (15), the Wolbachia infection was localized to the cell cytoplasm (Fig. 3). As illustrated in Fig. 3, approximately 10% of the S2 cells and >90% of the SF9 cells were infected. Fluorescent staining demonstrated that the cells were not evenly infected in vitro (Fig. 3).
FIG. 3.
Anti-WSP immunofluorescence staining of wRi-infected insect cell lines. (A) D. melanogaster Schneider's (S2) cells; (B) S. frugiperda (SF9) cells. Bars, 10 μm.
The original wRi in vitro infection has been maintained for over 30 passages at high passage rates (>9 months). The remaining in vitro infections have been maintained for at least 10 passages at high passage rates (≥3 months). Cells infected with Wolbachia in vitro can also be frozen and cryogenically stored using standard tissue culture techniques. Stable infections of the wRi infection have been recovered from samples frozen at −70°C for over 6 months. Attempts to maintain Wolbachia in both fresh and spent growth media in the absence of A. albopictus cells were unsuccessful.
wRi infection was used to examine the amount of host material required for the successful establishment of in vitro infections. In one set of experiments, egg tissue in amounts ranging from 150 μg to 78 mg was introduced onto Aa23T monolayers. The monolayers were subsequently categorized by infection status. The mean tissue amounts ± standard errors were as follows: uninfected, 7.9 ± 2.9 mg (n = 16); stably infected, 18.5 ± 4.3 mg (n = 16); and contaminated or lost, 29.8 ± 7.1 mg (n = 12). The last two values were determined to be significantly different from the first (P < 0.05; Student's t test). Thus, a positive correlation was observed between the amount of infected donor material overlaid on the cell monolayer and the successful establishment of a stable in vitro infection. At low levels of donor host material, the ability to detect infections was lost in sequential passages of these cultures (i.e., the infection was not stably established). This was expected since the number of recipient host cells in the current protocol remained relatively constant. Therefore, decreasing amounts of Wolbachia-infected donor material should result in a smaller proportion of the recipient host cells becoming infected and an increased opportunity for loss of the infection in subsequent passages of the culture. However, too much host material is also problematic, as this increases the frequency of undesired microbial contamination (i.e., fungal and other contaminants), which can result in the loss of cell cultures. Therefore, in subsequent attempts to establish additional in vitro infections, a protocol was adopted in which the host material was divided into a dilution series which was then introduced onto identical monolayers. The monolayers receiving the highest concentrations of infected host material that were not lost because of microbial contamination were maintained. Unwanted microbial contamination that results in the loss of the cell culture can also be reduced by surface sterilizing eggs and by the use of early embryos as donor host material. Previous research with in vitro infections determined that both penicillin and streptomycin could be added to the growth medium without harming the Wolbachia-infected cells (15). However, these antibiotics were not found to be generally useful for suppressing unwanted bacterial contamination.
Conclusions.
Prior investigations of Wolbachia host cell range have consisted of interspecific transinfection attempts (5, 6, 17, 18, 20, 28). However, interpretation of these prior experiments has been complicated by technical problems associated with embryonic microinjection. Here, we have used a simple technique to demonstrate that six Wolbachia infection types can be supported by A. albopictus cells. We have also shown that the wRi infection may be stably maintained in Drosophila, Spodoptera, and Aedes host cells. Our success in establishing infections suggests that the Wolbachia host cell range is broader than previously thought (17). As an additional test of Wolbachia host cell range, the presently established in vitro infections could be used as a uniform source of different Wolbachia types for future transinfection attempts. For example, different in vitro infections could be microinjected into Drosophila embryos (separately or simultaneously) by previously developed techniques (5, 6, 20) to determine which infections can be stably maintained in vivo and to compare infection dynamics of the different Wolbachia types.
The shell vial technique described here provides a simple means to establish, maintain, and cryogenically store Wolbachia infections. In addition to simplifying and encouraging Wolbachia research in additional laboratories, the expanded use of this technique will allow the generation of a Wolbachia stock center as a scientific resource, permitting the cataloguing and distribution of infections for future reference. The ability to maintain in vitro infections should also permit novel approaches for the examination of Wolbachia. For example, the ability to maintain multiple infection types within a similar host background should simplify future comparative Wolbachia studies, including ultrastructural investigations and the analysis of gene expression patterns, biochemical pathways, and recombination events (32). The in vitro establishment of Wolbachia infections derived from nematodes will facilitate the identification of antimicrobial agents that are useful for eliminating filaria of medical and veterinary importance (10).
Acknowledgments
We thank Jack Werren and Doug Dahlman for helpful comments and for improving the manuscript.
This research was supported in part by grants from the United States Department of Agriculture (NRICGP grant 9902683 to S.L.D.), the National Institutes of Health (grant AI40620 to S.L.O.), the McKnight Foundation (to S.L.O.), the UNDP/World Bank/World Health Organization Program for Research and Training in Tropical Diseases (to S.L.O.), and the European Commission (QLK3-CT2000-01079 to K.B.).
Footnotes
Publication 01-08-73 of the University of Kentucky Agricultural Experiment Station.
REFERENCES
- 1.Bandi, C., J. W. McCall, C. Genchi, S. Corona, L. Venco, and L. Sacchi. 1999. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 29:357-364. [DOI] [PubMed] [Google Scholar]
- 2.Bordenstein, S. R., F. P. O'Hara, and J. H. Werren. 2001. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409:707-710. [DOI] [PubMed] [Google Scholar]
- 3.Bordenstein, S. R., and J. H. Werren. 2000. Do Wolbachia influence fecundity in Nasonia vitripennis? Heredity 84:54-62. [DOI] [PubMed] [Google Scholar]
- 4.Bourtzis, K., S. L. Dobson, H. R. Braig, and S. L. O'Neill. 1998. Rescuing Wolbachia have been overlooked. Nature 391:852-853. [DOI] [PubMed] [Google Scholar]
- 5.Boyle, L., S. L. O'Neill, H. M. Robertson, and T. L. Karr. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796-1799. [DOI] [PubMed] [Google Scholar]
- 6.Braig, H. R., H. Guzman, R. B. Tesh, and S. L. O'Neill. 1994. Replacement of the natural Wolbachia symbiont of Drosophila simulans with a mosquito counterpart. Nature 367:453-455. [DOI] [PubMed] [Google Scholar]
- 7.Braig, H. R., W. Zhou, S. L. Dobson, and S. L. O'Neill. 1998. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J. Bacteriol. 180:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O'Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153-160. [DOI] [PubMed] [Google Scholar]
- 9.Guillemaud, T., N. Pasteur, and F. Rousset. 1997. Contrasting levels of variability between cytoplasmic genomes and incompatibility types in the mosquito Culex pipiens. Proc. R. Soc. Lond. Ser. B 264:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans, P. G., C. A. Hart, and A. J. Trees. 2001. In vitro activity of antimicrobial agents against the endosymbiont Wolbachia pipientis. J. Antimicrob. Chemother. 47:659-663. [DOI] [PubMed] [Google Scholar]
- 11.Hertig, M., and S. B. Wolbach. 1924. Studies on rickettsia-like micro-organisms in insects. J. Med. Res. 44:329-374. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann, A. A., D. Clancy, and J. Duncan. 1996. Naturally occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76:1-8. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill, S. L., R. Giordano, A. M. Colbert, T. L. Karr, and H. M. Robertson. 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neill, S. L., A. A. Hoffmann, and J. H. Werren. 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom.
- 15.O'Neill, S. L., M. Pettigrew, S. P. Sinkins, H. R. Braig, T. G. Andreadis, and R. B. Tesh. 1997. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol. Biol. 6:33-39. [DOI] [PubMed] [Google Scholar]
- 16.Pijls, J. W. A. M., H. J. van Steenbergen, and J. J. M. van Alphen. 1996. Asexuality cured: the relations and differences between sexual and asexual Apoanagyrus diversicornis. Heredity 76:506-513. [Google Scholar]
- 17.Pintureau, B., S. Grenier, B. Boleat, F. Lassabliere, A. Heddi, and C. Khatchadourian. 2000. Dynamics of Wolbachia populations in transfected lines of Trichogramma. J. Invertebr. Pathol. 76:20-25. [DOI] [PubMed] [Google Scholar]
- 18.Poinsot, D., K. Bourtzis, G. Markakis, C. Savakis, and H. Mercot. 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150:227-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rokas, A. 2000. Wolbachia as a speciation agent. Trends Ecol. Evol. 15:44-45. [DOI] [PubMed] [Google Scholar]
- 20.Rousset, F., H. R. Braig, and S. L. O'Neill. 1999. A stable triple Wolbachia infection in Drosophila with nearly additive incompatibility effects. Heredity 82:620-627. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki, T., and H. Ishikawa. 1999. Wolbachia infections and cytoplasmic incompatibility in the almond moth and the Mediterranean flour moth. Zool. Sci. 16:739-744. [Google Scholar]
- 22.Sinkins, S. P., H. R. Braig, and S. L. O'Neill. 1995. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. R. Soc. Lond. Ser. B 261:325-330. [DOI] [PubMed] [Google Scholar]
- 23.Sinkins, S. P., and S. L. O'Neill. 2000. Wolbachia as a vehicle to modify insect populations, p. 271-287. In A. M. Handler and A. A. James (ed.), Insect transgenesis: methods and applications. CRC Press, Boca Raton, Fla.
- 24.Stenos, J., S. Graves, and B. Dwyer. 1992. Quantification of Rickettsia australis. Am. J. Trop. Med. Hyg. 47:141-146. [DOI] [PubMed] [Google Scholar]
- 25.Stouthamer, R., J. A. J. Breeuwer, and G. D. D. Hurst. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71-102. [DOI] [PubMed] [Google Scholar]
- 26.Turelli, M., and A. A. Hoffmann. 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140:1319-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandekerckhove, T. T. M., S. Watteyne, A. Willems, J. G. Swing, J. Mertens, and M. Gillis. 1999. Phylogenetic analysis of the 16S rDNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda, Collembola) and its implications for wolbachial taxonomy. FEMS Microbiol. Lett. 180:279-286. [DOI] [PubMed] [Google Scholar]
- 28.van Meer, M. M. M., J. Witteveldt, and R. Stouthamer. 1999. Development of a microinjection protocol for the parasitoid Nasonia vitripennis. Entomol. Exp. Appl. 93:325-329. [Google Scholar]
- 29.Varma, M. G. R., and M. Pudney. 1969. The growth and serial passage of cell lines from Aedes aegypti larvae in different media. J. Med. Entomol. 6:432-439. [DOI] [PubMed] [Google Scholar]
- 30.Wade, M. J. 2001. Infectious speciation. Nature 409:675-677. [DOI] [PubMed] [Google Scholar]
- 31.Werren, J. H. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42:587-609. [DOI] [PubMed] [Google Scholar]
- 32.Werren, J. H., and J. D. Bartos. 2001. Recombination in Wolbachia. Curr. Biol. 11:431-435. [DOI] [PubMed] [Google Scholar]
- 33.Werren, J. H., and D. M. Windsor. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. Lond. Ser. B 267:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werren, J. H., W. Zhang, and L. R. Guo. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. Ser. B 261:55-63. [DOI] [PubMed] [Google Scholar]
- 35.Yen, J. H., and A. R. Barr. 1973. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 22:242-250. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, W., F. Rousset, and S. L. O'Neill. 1998. Phylogeny and PCR based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. Ser. B 265:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]