Abstract
Xylene monooxygenase of Pseudomonas putida mt-2 catalyzes multistep oxidations of one methyl group of toluene and xylenes. Recombinant Escherichia coli expressing the monooxygenase genes xylM and xylA catalyzes the oxygenation of toluene, pseudocumene, the corresponding alcohols, and the corresponding aldehydes, all by a monooxygenation type of reaction (B. Bühler, A. Schmid, B. Hauer, and B. Witholt, J. Biol. Chem. 275:10085-10092, 2000). Using E. coli expressing xylMA, we investigated the kinetics of this one-enzyme three-step biotransformation. We found that unoxidized substrates like toluene and pseudocumene inhibit the second and third oxygenation steps and that the corresponding alcohols inhibit the third oxygenation step. These inhibitions might promote the energetically more favorable alcohol and aldehyde dehydrogenations in the wild type. Growth of E. coli was strongly affected by low concentrations of pseudocumene and its products. Toxicity and solubility problems were overcome by the use of a two-liquid-phase system with bis(2-ethylhexyl)phthalate as the carrier solvent, allowing high overall substrate and product concentrations. In a fed-batch-based two-liquid-phase process with pseudocumene as the substrate, we observed the consecutive accumulation of aldehyde, acid, and alcohol. Our results indicate that, depending on the reaction conditions, product formation could be directed to one specific product.
During the last few decades, the microbial degradation pathways of aromatic and aliphatic hydrocarbons have received a lot of scientific interest because of the high potential of the enzyme systems involved for environmental (43) and preparative applications (38, 59). These pathways are usually initiated by an oxygenase-catalyzed chemo-, regio-, and stereoselective hydroxylation of the hydrocarbons, a reaction for which often no organic chemical counterpart is known (9, 13).
The xylene degradation pathway of Pseudomonas putida mt-2 and its initiating oxygenase, the xylene monooxygenase (XMO), are among the best-studied examples of aromatic hydrocarbon degradation (5, 36, 57, 61). The enzymes for xylene degradation are encoded on a catabolic plasmid, the TOL plasmid pWW0. XMO is the first enzyme in the upper degradation pathway for toluene and xylenes, in which a carboxylic acid is formed (1, 16, 58). The upper pathway also involves benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase, which catalyze the oxidation of benzyl alcohols via benzaldehydes to benzoic acids (46-48). The carboxylic acid is then transformed to substrates of the Krebs cycle through the meta cleavage pathway (10, 14, 36, 55).
XMO consists of two polypeptide subunits, encoded by xylM and xylA (16, 52). XylA, the NADH:acceptor reductase component, is an electron transport protein transferring reducing equivalents from NADH to XylM (45). XylM, the hydroxylase component, is located in the membrane, and its activity depends on phospholipids and ferrous ion, with a pH optimum of 7 (44, 62).
The substrate spectrum of XMO was investigated, with focus on preparative applications. XMO expressed in Escherichia coli oxidizes toluene and xylenes but also m- and p-ethyl-, methoxy-, nitro-, and chloro-substituted toluenes, as well as m-bromo-substituted toluene, to the corresponding benzyl alcohol derivatives (21, 65). Furthermore, styrene is transformed into S-styrene oxide with an enantiomeric excess (ee) of 95% (64, 65). The one-step oxygenation of styrene catalyzed by recombinant XMO in growing cells of E. coli was applied to produce S-styrene oxide on a 2-liter scale with hexadecane as the second organic phase (30).
The wild-type strain P. putida mt-2 was used to oxidize methyl groups on aromatic heterocyclics to the corresponding carboxylic acids (20). In large-scale fermentations, a 5-methyl-2-pyrazinecarboxylic acid titer up to 20 g liter−1 was reached. This system exploits the inability of the wild-type strain to further degrade heteroaromatic carboxylic acids. In P. putida mt-2, all three enzyme activities of the upper xylene degradation pathway are responsible for the three-step oxidation. Early reports suggested that XMO also catalyzes alcohol and aldehyde oxidations (15, 16). Later, such activities were attributed to dehydrogenases present in the E. coli host (16, 44). Recently, we verified by in vivo experiments that XMO indeed catalyzes the oxidation of benzyl alcohols and benzaldehydes, both via a monooxygenation type of reaction (4). E. coli cells expressing XMO genes under the control of the alk regulatory system (12, 51, 62, 67) were used for these experiments.
Potential preparative in vivo applications of XMO are hampered by low water solubilities and high toxicities of possible substrates and products, limiting the performance of aqueous systems. Nonconventional reaction media such as an aqueous-organic two-liquid-phase system are promising alternatives (8, 40). A second immiscible phase can act as a reservoir for substrate and products, regulating the concentration of such compounds in the biocatalyst microenvironment, minimizing toxicity and simplifying product recovery (24, 60, 63).
In the present study, we characterized the multistep oxidation of substrates such as pseudocumene and toluene by whole cells of E. coli containing XMO with the aims of clarifying the natural role of such a multistep catalysis and identifying possible applications. The biotechnological conversion of pseudocumene is of special interest because a controlled regio- and chemospecific multistep oxidation of only one methyl group is difficult to achieve by purely chemical methods. We determined the kinetics of the one-enzyme multistep reaction and analyzed the whole-cell biocatalyst in a two-liquid-phase biotransformation on a 2-liter scale. Our results indicate that, depending on the reaction conditions, product formation may be directed to one specific product, either benzylic alcohols, aldehydes, or acids.
MATERIALS AND METHODS
Bacterial strain and plasmid.
E. coli JM101 [supE thi Δ(lac-proAB) F" (traD36 proAB+ lacIq lacZΔM15)] (28), an E. coli K-12 derivative, was used as the recombinant host strain. As the expression vector, we used the pBR322-derived plasmid pSPZ3 containing the XMO genes xylM and xylA under the control of the alk regulatory system (30). The Qiaprep spin miniprep kit (Qiagen, Basel, Switzerland) was used to prepare plasmid DNA following the supplier's protocol.
Media and growth conditions.
Bacteria were grown either in Luria-Bertani (LB) broth (Difco, Detroit, Mich.) or in M9* minimal medium, which is identical to M9 mineral medium (37) except that it contained a threefold-higher concentration of phosphate salts to increase the buffer capacity and did not contain calcium chloride. Glucose was added to the M9* mineral medium at a concentration of 0.5% (wt/vol) as the single carbon source or to complex media at a concentration of 1% (wt/vol) for catabolite repression of tryptophanase synthesis in E. coli. Tryptophanase is involved in the formation of indole on complex media; indole can subsequently be converted to indigo by XMO (26, 27). When necessary, cultures were supplemented with kanamycin (final concentration, 50 mg/liter), thiamine (10 mg/liter) and 1 ml/liter of trace element solution US*, which contained 1 M hydrochloric acid and (per liter) 4.87 g of FeSO4·7H2O, 4.12 g of CaCl2·2H2O, 1.50 g of MnCl2·4H2O, 1.05 g of ZnSO4, 0.30 g of H3BO3, 0.25 g of Na2MoO4·2H2O, 0.15 g of CuCl2·2H2O, and 0.84 g of disodium EDTA·2H2O. Solid media contained 1.5% (wt/vol) agar. Liquid cultures were routinely incubated in baffled Erlenmeyer flasks shaken at 200 rpm and 30°C.
Chemicals.
Chemicals were obtained from Fluka (Buchs, Switzerland) [toluene, >99.5% pure; benzyl alcohol, >99%; benzaldehyde, >99%; benzoic acid, >99.5%; pseudocumene, ≈99%; 3,4-dimethylbenzoic acid, ≈97%; and bis(2-ethylhexyl)phthalate (BEHP), 97%], Aldrich (Buchs, Switzerland) (3,4-dimethylbenzyl alcohol, 99%), Lancaster (Muehlheim, Germany) (3,4-dimethylbenzaldehyde, 97%), and Acros Organics (Geel, Belgium) (n-octane, >98.5%).
Analysis of metabolites.
For the separation of n-octane, toluene/pseudocumene, and the respective alcohols, aldehydes, and acids we used gas chromatography (GC) as described elsewhere (4). Alternatively, benzyl alcohol, benzaldehyde, and benzoic acid were separated via high-performance liquid chromatography (HPLC), using a Nucleosil C18 HD column (100-Å pore size, 5-μm particle size, 25 cm by 4 mm inner diameter; Macherey-Nagel, Oensingen, Switzerland) with a mobile phase of 64.9% H2O-35% acetonitrile-0.1% H3PO4 at a flow rate of 0.7 ml/min. For the separation of 3,4-dimethylbenzyl alcohol, 3,4-dimethylbenzaldehyde, and 3,4-dimethylbenzoic acid, we used the same column at the same flow rate with a mobile phase of 59.9% H2O-40% acetonitrile-0.1% H3PO4. The UV detector was set at a wavelength range of 210 to 265 nm.
Whole-cell assays.
Whole-cell assays to study the kinetics and inhibitions of the three monooxygenation steps catalyzed by XMO included cell growth, induction, resting-cell biotransformations at a 1-ml scale, and sample preparation for GC and HPLC analysis and were performed as described before (4) with the following modifications: xylMA expression was induced by n-octane (0.1%, vol/vol) exclusively, and cell concentrations in the resting-cell biotransformations varied between 0.1 and 1.1 g of cell, dry weight (CDW) per liter.
In experiments to follow product formation over time, samples of cells were incubated with the same substrates for different time periods (0 to 80 min). The assays were carried out twice independently. Initial specific activities were calculated as average activities based on the sum of all products formed in 5 min of reaction. One unit (U) is defined as the activity that forms 1 μmol of total products in 1 min. Specific activity was expressed as activity per gram of CDW.
Vmax and Ks for pseudocumene, toluene, and their derivatives were determined in reactions carried out for 5 min except for the aldehydes as substrate (10 min of reaction) with various substrate concentrations (0.04 to 30 mM). Specific activities were calculated based on the amount of products formed. Vmax and Ks values were calculated using the program Leonora, designed to analyze enzyme kinetic data and described by Cornish-Bowden (7). Experiments were repeated at least three times independently.
Determination of toxicities of pseudocumene and its metabolites for E.coli JM101.
The toxicities of pseudocumene, 3,4-dimethylbenzyl alcohol, 3,4-dimethylbenzaldehyde, and 3,4-dimethylbenzoic acid were determined with E. coli JM101 incubated in complete M9* medium. After reaching the exponential growth phase, the culture was subdivided into sterile baffled Erlenmeyer flasks with screw-on caps containing a Teflon seal to avoid evaporation, and the different compounds were added to concentrations between 0 and 40 mM. Subsequently, incubation was continued and growth was determined by monitoring the optical density at 450 nm. An optical density of 1 corresponded to 0.29 g of CDW per liter.
The effect of BEHP containing 1% (vol/vol) n-octane and different amounts of pseudocumene on growth of E. coli JM101 was determined by a similar procedure. After subdividing an exponentially growing culture, an equal volume of organic phase was added and incubation was continued. One culture was further incubated without a second phase as a control.
Two-liquid-phase culture.
A stirred-tank reactor with a total volume of 3 liters (33, 63) was used in the reactor experiments. Precultivation and batch cultivation of freshly transformed E. coli JM101(pSPZ3) were performed as described earlier (30, 31). The reactor was aerated at a rate of 0.5 liters min−1 throughout the entire experiment. After batch cultivation, when the cell concentration amounted to 3 g of CDW liter−1, the culture of 1 liter in volume was supplemented with 4 ml of US* trace element solution and 4 ml of a 1% (wt/vol) thiamine solution. Subsequently, the culture was fed at a rate of 10 g h−1 with an aqueous solution containing (per liter) 450 g of glucose, 50 g of yeast extract (Difco, Detroit, Mich.), and 9 g of MgSO4·7H2O, adjusted to pH 3 with hydrochloric acid. One hour after feed initiation, 1 liter of the organic phase was added, resulting in a phase ratio of 0.5. The organic phase consisted of BEHP as the carrier solvent, which contained 1% (vol/vol) n-octane as an inducer of the alk regulatory system and 2% (vol/vol) pseudocumene as the substrate. Concomitantly, the stirrer speed was increased to 2,000 rpm. Organic phase addition served as the time point of induction. Foam formation was limited by the addition of Antifoam 289 (Sigma, Buchs, Switzerland).
Process analytics.
The dissolved oxygen tension was determined with an autoclavable amperometric probe (Mettler Toledo, Greifensee, Switzerland). CDW and octane, pseudocumene, 3,4-dimethylbenzyl alcohol, 3,4-dimethylbenzaldehyde, and 3,4-dimethylbenzoic acid concentrations in the organic phase as well as in the aqueous phase of the reactor were monitored over time. To do this, 2-ml samples were withdrawn from the reactor at regular intervals and added to 2-ml Eppendorf tubes. The reaction was stopped by placing the samples on ice and immediately acidifying them to pH 2 by the addition of 40 μl of perchloric acid stock solution (10%, vol/vol). The samples were centrifuged to separate the phases, and the position of the interphase was marked. The organic phase was removed, diluted 50-fold with diethyl ether supplemented with dodecane as an internal standard, dried over sodium sulfate, and analyzed by GC to determine substrate, product, and octane concentrations. Then 0.5 ml of the aqueous supernatant of each centrifuged sample was removed with a syringe into and extracted with an equal volume of diethyl ether as described earlier (4). Subsequently, the ether phase was analyzed by GC to determine the concentrations in the aqueous phase. The remaining aqueous supernatant was completely removed and discarded. The cell pellet was resuspended in an amount of M9* medium corresponding to the original amount of aqueous supernatant, and the optical density at 450 nm was determined.
The apparent volumetric productivities and specific product formation rates were calculated as averages for intervals between two data points. The term apparent refers to the fact that activities may have been limited by substrate availability. Furthermore, since aldehyde and acid accumulation from pseudocumene demands multistep catalysis, apparent specific aldehyde and acid formation rates require doubled and threefold specific biocatalyst activities, respectively.
RESULTS
Product formation patterns in the presence of two substrates.
Recently, we showed that whole cells of E. coli JM101 containing XMO catalyze the multistep oxidation of toluene and pseudocumene via corresponding alcohols and aldehydes to benzoic acids (4). When benzaldehydes were added as substrates, the cells transformed benzaldehyde and 3,4-dimethylbenzaldehyde to the corresponding acids at initial rates of 10 and 55 U (g of CDW)−1, respectively. In contrast, when toluene and pseudocumene were added as substrates, we observed that benzoic acids did not form until concentrations of toluene or pseudocumene reached low levels (4).
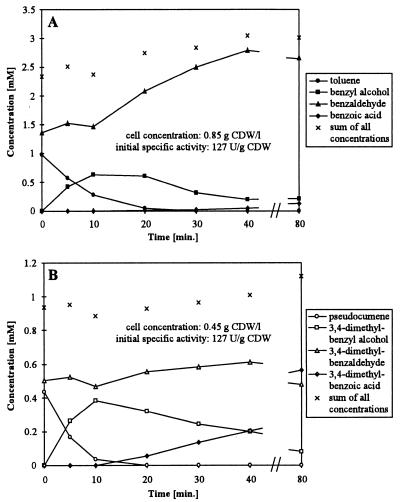
In order to investigate this phenomenon in more detail, cells were incubated with the same amounts of toluene and benzaldehyde for different time periods (Fig. 1A). Benzoic acid was not formed until the toluene concentration reached a value as low as 0.05 mM, although benzaldehyde was present from the start of the reaction. Analogous biotransformations were carried out with pseudocumene and 3,4-dimethylbenzaldehyde as substrates (Fig. 1B). The corresponding acid started to be formed at a pseudocumene concentration below 0.036 mM. These results demonstrate that acid formation is completely prevented in the presence of toluene or pseudocumene.
FIG. 1.
Product formation after simultaneous addition of toluene and benzaldehyde (A) and of pseudocumene and 3,4-dimethylbenzaldehyde (B) to resting E. coli JM101(pSPZ3) in 50 mM potassium phosphate buffer, pH 7.4, containing 1% (wt/vol) glucose. The assay was performed as described in Materials and Methods.
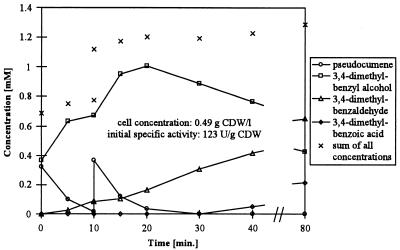
We also tested the influence of pseudocumene and toluene on alcohol oxidation. Cells were incubated with equal amounts of pseudocumene and 3,4-dimethylbenzyl alcohol (Fig. 2). After the reaction started, the alcohol accumulated at a high rate of 109 U (g of CDW)−1, whereas the aldehyde accumulated at a rate as low as 9 U (g of CDW)−1. Then, at reduced pseudocumene concentrations, the mean aldehyde formation rate increased to 25 U (g of CDW)−1. After 10 min, a pseudocumene pulse established pseudocumene concentrations similar to those observed at the beginning of the reaction. The product formation pattern between 10 and 20 min of reaction looked very similar to the product formation pattern in the first 10 min. The aldehyde formation rate was reduced to 8.5 U (g of CDW)−1, whereas the alcohol accumulated at a high rate of 115 U (g of CDW)−1. At reduced pseudocumene concentrations, the aldehyde formation rate recovered to 25 U (g of CDW)−1. In identical experiments without a pseudocumene pulse, low initial aldehyde formation rates increased up to 30 U (g of CDW)−1 as pseudocumene concentrations decreased and remained roughly constant throughout the rest of the experiment (results not shown). The acid slowly accumulated when pseudocumene had completely disappeared from the reaction mixtures. Toluene also reduced the aldehyde formation rate, but only at concentrations above 0.5 mM and to a lesser extent than pseudocumene (results not shown). The presence of pseudocumene or toluene obviously not only prevents aldehyde oxidation but also reduces the alcohol oxidation rate. For the impairment of alcohol oxidation, higher pseudocumene or toluene concentrations are required than for the prevention of aldehyde oxidation.
FIG. 2.
Product formation after simultaneous addition of pseudocumene and 3,4-dimethylbenzyl alcohol to resting E. coli JM101(pSPZ3) in 50 mM potassium phosphate buffer, pH 7.4, containing 1% (wt/vol) glucose. Assays were performed as described in Materials and Methods. After 10 min, pseudocumene was pulsed to the reaction mixtures.
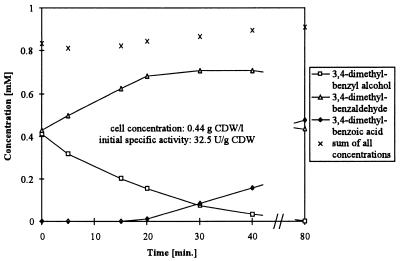
In order to investigate the product formation pattern in the presence of 3,4-dimethylbenzyl alcohol and 3,4-dimethylbenzaldehyde, similar amounts of the two substrates were incubated with induced E. coli JM101(pSPZ3) (Fig. 3). Initially only alcohol was oxidized. Aldehyde oxidation started after the concentrations of alcohol and aldehyde had decreased and increased, respectively. For benzyl alcohol and benzaldehyde as substrates, a similar product formation pattern with an initial aldehyde formation rate of 123 U (g of CDW)−1 was obtained. Obviously not only pseudocumene and toluene but also the corresponding alcohols prevent acid formation. The inhibition of acid formation by the alcohols seems to be weaker than the inhibition by pseudocumene and toluene, since alcohol concentrations must be higher than toluene and pseudocumene concentrations to prevent acid formation.
FIG. 3.
Product formation after simultaneous addition of 3,4-dimethylbenzyl alcohol and 3,4-dimethylbenzaldehyde to resting E. coli JM101(pSPZ3) in 50 mM potassium phosphate buffer, pH 7.4, containing 1% (wt/vol) glucose. Assays were performed as described in Materials and Methods.
Vmax and Ks values for E.coli JM101(pSPZ3) with different substrates.
In our previous study, we speculated that the biocatalyst might take up toluene and pseudocumene more efficiently than the corresponding aldehydes, which would explain the lack of acid formation in the presence of unoxidized substrates such as toluene and pseudocumene (4). In order to evaluate this presumption and to learn more about the catalytic features of XMO present in E. coli, we investigated the kinetics of the consecutive in vivo oxygenation of xylenes to benzoic acids. Apparent maximal reaction velocities (Vmax) and substrate uptake constants (Ks, corresponding to the substrate concentrations at which whole cells show half-maximal transformation rates) were determined by whole-cell assays (Table 1). The term apparent is used because the kinetic values were determined with whole cells.
TABLE 1.
Apparenta Vmax and Ks values of E. coli JM101(pSPZ3) for different substrates
| Substrate | Apparent Vmax [U (g of CDW)−1] | Apparent Ks (μM) | Vmax/Ks [U μM−1 (g of CDW)−1] |
|---|---|---|---|
| Pseudocumene | 351 ± 8 | 202 ± 8 | 1.7 |
| 3, 4-Dimethylbenzyl alcohol | 93 ± 3 | 24 ± 4 | 3.9 |
| 3, 4-Dimethylbenz-aldehydeb | ≥560 | ≥ 8,000 | NDc |
| Toluene | 134 ± 9 | 87 ± 17 | 1.5 |
| Benzyl alcohol | 225 ± 9 | 85 ± 4 | 2.6 |
| Benzaldehydeb | ≥18 | ≥ 20,000 | ND |
The term apparent refers to the fact that these kinetic values were determined for whole cells. Values are means ± standard errors.
The kinetic values for benzaldehydes could not be determined accurately because relevant substrate concentrations were in a range at which measurements were hampered by toxicity and limited solubility of the substrates.
ND, not determined.
The cells showed Michaelis-Menten-like kinetics for the formation of benzyl alcohols from pseudocumene or toluene and the formation of benzaldehydes from benzyl alcohols. For the natural substrate pseudocumene, we found a higher uptake constant than for the corresponding alcohol, whereas the maximal reaction velocity of pseudocumene oxidation was higher than of 3,4-dimethylbenzyl alcohol oxidation. The kinetic values for benzaldehydes were difficult to determine because the relevant substrate concentrations were in a range at which measurements were hampered by toxicity and limited solubility of the substrates. Surprisingly, at concentrations above the solubility of 3,4-dimethylbenzaldehyde in aqueous solution (>4 mM), increasing aldehyde amounts still caused increasing reaction rates, suggesting aldehyde uptake from organic-phase droplets. Nevertheless, we were able to estimate lower limits for the Ks values of the aldehydes (Table 1). The minimal Vmax values presented in Table 1 correspond to the maximal reaction velocities measured experimentally. The uptake of benzaldehydes by the biocatalyst is in fact significantly less efficient than the uptake of toluene and pseudocumene.
Toxicities of pseudocumene and its derivatives for E.coli in an aqueous medium.
Xylenes as well as their oxidized derivatives are expected to be toxic to microorganisms such as E. coli. Metabolically active cells are essential to maintain biocatalytic activity of E. coli(pSPZ3) because NADH, a cofactor of the XMO-catalyzed oxidations, must be regenerated by the E. coli host. Therefore, the toxicities of substrates and products are important parameters for a possible preparative application of XMO in recombinant E. coli.
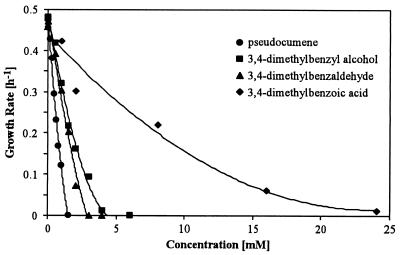
We tested the toxicities of pseudocumene and its products in cultures of E. coli JM101 by monitoring growth at different substrate and product concentrations. Figure 4 shows the influence of various inhibitor concentrations on the growth rate. Pseudocumene and the corresponding alcohol and aldehyde were found to inhibit growth even at low concentrations, whereas 3,4-dimethylbenzoic acid was considerably less toxic. Thus, the concentrations of pseudocumene and the corresponding alcohol and aldehyde in the microenvironment of the cells have to be kept low for practical application of E. coli JM101(pSPZ3) as a biocatalyst. A two-liquid-phase system with a second phase consisting of an organic solvent offers a possible solution to accomplish this requirement.
FIG. 4.
Growth rates of E. coli JM101 after addition of different amounts of pseudocumene, 3,4-dimethylbenzyl alcohol, 3,4-dimethylbenzaldehyde, or 3,4-dimethylbenzoic acid. After entering exponential growth, a 400-ml culture was split into 40-ml subcultures, to which different amounts of the substance of interest were added. Experimental details are described in Materials and Methods.
Fed-batch-based biotransformation in a two-liquid-phase system.
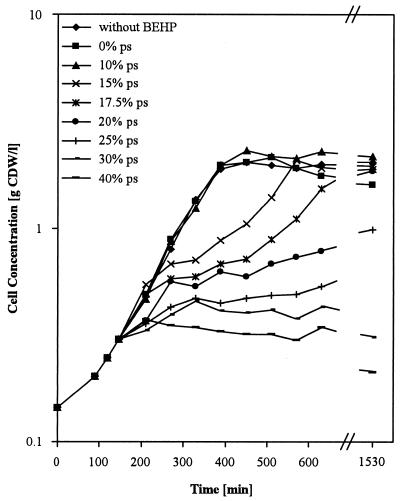
We used BEHP for the investigation of the multistep biotransformation of pseudocumene by xylMA-expressing recombinant E. coli in a two-liquid-phase system. In order to ensure the suitability of BEHP for our system, we examined the effect of BEHP, present at a phase ratio of 0.5, and different concentrations of pseudocumene in BEHP on growth ofE. coli JM101 (Fig. 5). BEHP containing up to 10% (vol/vol) pseudocumene had no effect on cell growth. Higher pseudocumene concentrations increasingly reduced the growth rate. Thus, the use of BEHP allows the addition of large amounts of pseudocumene to the biotransformation reaction mixture and thereby meets the main demand for a second liquid organic phase.
FIG. 5.
Growth of E. coli JM101 in the absence and in the presence of BEHP containing different volume fractions of pseudocumene. After reaching exponential growth, a 200-ml culture was split into 20-ml subcultures, to which no or 20 ml of BEHP containing different volume fractions of pseudocumene (ps) was added. Experimental details are described in Materials and Methods.
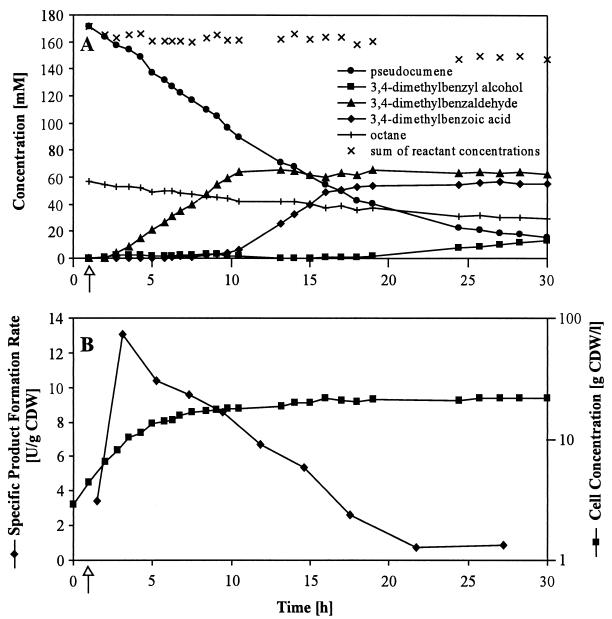
To analyze the product formation pattern in the BEHP/M9* two-liquid-phase system, we performed biotransformations in fed-batch mode. This approach was based on our previous experiments designed to produce S-styrene oxide from styrene (30, 31, 63). The biotransformation was started 1 h after feed initiation by the addition of the organic phase containing 2% (vol/vol) pseudocumene and 1% (vol/vol) n-octane as the inducer for xylMA expression. Results are shown in Fig. 6 and were confirmed by repeating the experiment. Substrate and product concentrations (Fig. 6A) were calculated as the sum of the concentrations in the organic and the aqueous phase for each compound and correspond to the doubled overall concentrations in the total volume.
FIG. 6.
Fed-batch-based two-liquid-phase biotransformation with E. coli JM101(pSPZ3) at a phase ratio of 0.5. The second organic phase consisted of BEHP as the organic carrier solvent, 2% (vol/vol) pseudocumene, and 1% (vol/vol) n-octane (as the inducer). Addition of the organic phase occurred 1 h after feed initiation (arrow). Experimental details of the fed batch are described in Materials and Methods. (A) Reactant and octane concentrations during the fed-batch experiment. All concentrations represent the sum of the respective concentrations in the organic and aqueous phases. (B) Formation of E. coli JM101(pSPZ3) biomass and development of the specific product formation rate, which was calculated by determining the rate of total product formation per gram of CDW as the average for an interval between two data points.
Aldehyde and alcohol formation began about 1 h after addition of the second phase, confirming the short induction period observed in shaking flask experiments (4). The subsequent biotransformation can be divided into three stages. In the first stage, pseudocumene was directly channeled through two oxygenation steps, and 3,4-dimethylbenzaldehyde accumulated as the sole product to a concentration of 65 mM. The second stage was characterized by a complete cessation of aldehyde accumulation and exclusive formation of 3,4-dimethylbenzoic acid at pseudocumene concentrations below 90 mM. In the third stage, 3,4-dimethylbenzyl alcohol accumulated at a very low rate to a concentration of 13.5 mM, the aldehyde concentration remained constant, and the acid concentration increased slightly. The final concentrations of pseudocumene and 3,4-dimethylbenzaldehyde amounted to 16 and 63 mM, respectively. The aqueous pseudocumene, alcohol, and aldehyde concentrations remained very low during the whole experiment, whereas the acid accumulated to concentrations of 48.5 and 6.5 mM in the aqueous and organic phases, respectively. Such aqueous acid concentrations are expected to be toxic for E. coli JM101 (Fig. 4). The octane concentration decreased continuously during the biotransformation, which is due to its volatility and gives an indirect measure of substrate stripping by aeration, which caused a slight decrease in the total reactant concentration.
As expected, growth continued after organic phase addition without any interruption (Fig. 6B). After 4 h of exponential growth, the culture was limited by oxygen over 5 h. Linear growth to a CDW of 17 g liter−1 after 7.5 h of fed-batch cultivation was followed by slow growth to a maximal cell density of 22 g liter−1. The specific activity concerning the formation of all three products, referred to as the specific product formation rate, was maximal 2 h after induction in growing cells (13 U [g of CDW]−1), then decreased continuously, and was low during the third stage of the biotransformation (Fig. 6B). The maximal volumetric activity concerning the formation of one specific product, in this case 3,4-dimethylbenzaldehyde, amounted to 133 U literaq−1, corresponding to a maximal volumetric productivity of 1.1 g literaq−1 h−1. This productivity was reached 3.5 h after induction and remained constant for 7 h.
The biocatalyst E. coli JM101(pSPZ3), cultivated in a fed-batch mode, supported the accumulation of 3,4-dimethylbenzaldehyde, 3,4-dimethylbenzoic acid, and 3,4-dimethylbenzyl alcohol in three consecutive stages of the biotransformation.
DISCUSSION
Kinetic properties of E.coli JM101(pSPZ3).
The high specific product formation rates observed in this study for resting cells of E. coli JM101(pSPZ3) containing XMO together with results obtained earlier with this system indicate that pSPZ3 is highly suited for xylMA expression under the control of the alk promoter (4, 30). The efficient inhibition of the XMO-catalyzed aldehyde oxygenation by toluene and pseudocumene might be explained by the high uptake constants for aldehydes (Table 1) and competitive inhibition. Besides substrate affinity, kinetics of biocatalysts based on whole cells are determined by various factors such as membrane permeability and cofactor availability. Pseudocumene and toluene also reduce aldehyde formation rates, which cannot be explained by competitive inhibition, since the biocatalyst has lower apparent specificity constants (Vmax/Ks) for pseudocumene and toluene than for the corresponding alcohols (Table 1). Moreover, higher concentrations of 3,4-dimethylbenzyl alcohol than of pseudocumene are needed to inhibit aldehyde oxidation, which is not expected from the Vmax/Ks values. Therefore, the lack of acid formation in the presence of pseudocumene also cannot be ascribed only to competitive inhibition. A comparison with earlier experiments using the same whole cells and unoxidized substrates (e.g., toluene and pseudocumene) (4) shows that pseudocumene and toluene oxidation rates are not reduced in the presence of alcohols or aldehydes, another argument against substrate competition. Transient accumulation and degradation of substrate, product, and inhibitor impeded kinetic analysis of the mutual inhibitions.
Nevertheless, the results presented in this study indicate that the inhibitions by pseudocumene and toluene are not purely competitive, whereas the inhibition of aldehyde oxygenation by the corresponding alcohols may be. The presence of unoxidized substrates might induce an enzyme conformation in which their own oxidation is favored over alcohol and aldehyde oxidation. P450 monooxygenases, for instance, were proposed to undergo conformational changes caused by salt (66), inhibitor (34), or substrate (32, 53) or during the reaction (3), e.g., by transition from the ferric to the ferrous state (35). A hypothesis assuming a single active site and not necessarily requiring a conformational change implies that unoxidized substrates bind to a putative substrate access channel, to which the membrane might also contribute, and thereby inhibit the access of more polar substrates to the active site.
Considering that the natural host P. putida mt-2 expresses three enzymes for the three oxidation steps of the upper pathway, the ability of XMO to catalyze all reactions from the unoxidized substrates to the carboxylic acids is surprising. An analogous example is the bile acid synthesis pathway, in which sterol side chain oxidations from alcohol to carboxylic acid can proceed by sterol 27-hydroxylase, also responsible for alcohol formation, but also by the action of alcohol and aldehyde dehydrogenases (6, 17). This dual ability was suggested to be an example of evolution producing functional similarity from disparate structures and mechanisms.
In our case, a recombinant strain of P. putida KT2440 expressing only the XMO genes from its chromosome (29) grew on benzoic acid by the action of enzymes of the ortho cleavage pathway encoded on the chromosome but not on toluene provided via the gas phase (results not shown). The lack of growth on toluene could be caused by an inhibition of alcohol and aldehyde oxygenation by toluene. Nevertheless, early in evolution XMO might have been the only enzyme in the upper pathway catalyzing the three-step oxidation at low rates with a high demand for reducing equivalents. Later, dehydrogenases catalyzing energetically more favorable reactions, in which reducing equivalents are produced and not consumed, might have evolved. Such dehydrogenations might have become favored through the inhibition of alcohol and aldehyde oxygenation by xylenes.
The high specificity constants of XMO-containing E. coli for benzyl alcohols are surprising, but in the wild type a high catalytic efficiency in alcohol oxygenation might be necessary, because the equilibrium catalyzed by XylB, the benzyl alcohol dehydrogenase, lies on the side of the alcohols (4). With purified XylB, Shaw et al. found Km values of 155 and 65 μM and Vmax values of 320 and 4,800 μmol min−1 mg−1 for benzyl alcohol (forward reaction) and benzaldehyde (reverse reaction), respectively (47). As suggested earlier, XylB may prevent the formation of high intracellular concentrations of the particularly reactive benzaldehydes (4). In the case of high fluxes through the degradation pathways and low aldehyde concentrations, XylB contributes to aldehyde formation. Thus, at high concentrations of unoxidized substrates, the inhibition of alcohol oxygenation could promote alcohol dehydrogenation. Furthermore, the inhibition of the second and the third oxygenation steps of the XMO-catalyzed reaction cascade by unoxidized substrates may explain why the alcohol and aldehyde oxidation activities of XMO have not been more widely reported.
One-enzyme multistep catalysis—mechanism and examples.
At least 11 nonheme integral membrane enzymes, including XylM, contain a highly conserved 8-histidine motif (41, 42) which is essential for catalytic activity. Based on studies on the alkane hydroxylase of P. putida GPo1, such integral membrane enzymes were proposed to contain diiron clusters as O2-activating sites, for which the 8-histidine motifs provide ligands (41). Furthermore, alkane hydroxylase of P. putida catalyzes the oxygenative formation of medium-chain-length alkanals from terminal alkanols (25), also part of a multistep oxygenation. The histidine cluster containing C-4 sterol methyl oxidase of Saccharomyces cerevisiae catalyzes, like XMO, the three-step oxidation of a methyl group to the carboxylic acid (2). Like C-4 sterol methyl oxidase, several P450 enzymes also catalyze multistep oxidations of steroids and beyond of other substrates such as ethanol, which is oxidized via acetaldehyde to acetic acid (3). Purified naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4 and ammonia-grown cells of Nitrosomonas europaea catalyze, among other reactions, the oxidation of toluene via benzyl alcohol to benzaldehyde but not to benzoic acid (19, 23). Obviously, multistep oxidations catalyzed by a single enzyme, of which many more could be cited, are widespread among oxygenases.
As in the XMO-catalyzed oxidation of benzyl alcohols (4), the formation of gem-diol intermediates, which spontaneously dehydrate to the more stable carbonyl compounds, was also proposed in the P450-catalyzed oxidations of ethanol (3), 8-hydroxylinalool (54), hydroxy fatty acids (39, 49), and benzyl alcohols (56). The P450-catalyzed multistep oxidation of ethanol and of some steroids is considered to involve little exchange of the reaction intermediates with the medium. However, in the XMO-catalyzed multistep oxygenation, substantial exchange of intermediates with the medium must be assumed, since transient accumulation and degradation of alcohol and aldehyde intermediates occur (4) (Fig. 1 to 3).
Product formation patterns in the BEHP/M9* two-liquid-phase fed-batch system.
Cyclic hydrocarbons can be toxic to bacteria, inhibiting a potential whole-cell-based biocatalytic process; Sikkema et al. reviewed mechanisms of membrane toxicity of hydrocarbons (50). In our study, we also found pseudocumene as well as 3,4-dimethylbenzyl alcohol and 3,4-dimethylbenzaldehyde to be toxic at low concentrations. We therefore chose the two-liquid-phase concept to overcome toxicity and solubility problems (11, 24, 63). As expected for very hydrophobic solvents (22), BEHP (log Poct, 9.6) did not affect bacterial growth when used as second organic phase and proved to be highly suited to prevent substrate toxicity.
In fed-batch-based two-liquid-phase biotransformations of pseudocumene, the products 3,4-dimethylbenzyl alcohol, 3,4-dimethylbenzaldehyde, and 3,4-dimethylbenzoic acid accumulated in three diverse stages. The exclusive accumulation of the aldehyde during the first stage suggests that pseudocumene concentrations in the system were high enough to inhibit aldehyde oxidation but not to inhibit alcohol oxidation. The switch from aldehyde to acid accumulation in the second stage may be explained by pseudocumene concentrations falling below an inhibitory limit at around 90 mM in the organic phase. At this point of the biotransformation, the aqueous pseudocumene concentration was below the detection limit of 20 μM. An inhibitory limit in the aqueous phase below 20 μM is in accordance with the results shown in Fig. 1, which suggest that the inhibitory limit is below 36 μM. The slow alcohol accumulation accompanied by a slight increase in the acid concentration during the third stage of the biotransformation might be ascribed to low XMO activities, which might be exceeded by the transformation of aldehyde to alcohol by the nonspecific alcohol dehydrogenase activity of E. coli (4). The accumulation of mainly one product in each of the three stages of the biotransformation indicates that the use of the two-liquid-phase concept may allow the accumulation of a single product in a kinetically controlled one-enzyme multistep process.
Factors influencing biocatalyst activity.
The parallel decreases in substrate concentration and specific product formation rate in two-liquid-phase biotransformations point to substrate limitation. The maximal product formation rate of 13 U (g of CDW)−1 was much lower than 127 U (g of CDW)−1, the maximal specific activity measured for resting cells. The results of resting-cell experiments are not expected to coincide perfectly with the results of fed-batch experiments. However, the huge difference between the specific product formation rates nevertheless strongly suggests substrate limitation during the fed-batch experiment. Moreover, the exclusive formation of 3,4-dimethylbenzaldehyde in the beginning of the biotransformation also indicates substrate limitation. The exclusive accumulation of the aldehyde is only possible when the alcohol oxidation rate equals or exceeds the pseudocumene oxidation rate. According to the kinetic values for the first two oxidation steps (Table 1), exclusive aldehyde accumulation from pseudocumene requires clearly limiting pseudocumene concentrations, at which the lower uptake constant of the biocatalyst for 3,4-dimethylbenzyl alcohol than for pseudocumene dominates the higher maximal reaction velocity of pseudocumene oxidation.
High aqueous substrate and product concentrations can also impair biocatalyst activity in two-liquid-phase systems (18). The avoidance of toxic aqueous substrate and product concentrations is crucial for the maintenance of high biocatalyst activities (24). Aqueous concentrations of pseudocumene, 3,4-dimethylbenzyl alcohol, and 3,4-dimethylbenzaldehyde did not reach toxic levels and had no obvious effect on biocatalyst activity in the fed-batch experiments, but we observed toxic aqueous acid concentrations, which may have affected cell metabolism. Furthermore, the metabolic activity of the cells, which is necessary for NADH regeneration, is reduced in the late stationary phase anyway.
In general, at the beginning of the biotransformation, the specific product formation rate was dependent on the amount of pseudocumene present in the two-liquid-phase system. With progression of the biotransformation, most probably biocatalyst activities were increasingly influenced by toxic acid concentrations and other factors, such as the metabolic state of the cells in the late stationary phase.
Possible strategies towards the accumulation of a single product.
The exclusive accumulation of 3,4-dimethylbenzyl alcohol might be achieved by high pseudocumene concentrations in the BEHP/M9* two-liquid-phase system. At high substrate concentrations, we expect higher biocatalyst activities and, besides aldehyde formation, alcohol accumulation when the high Vmax of the pseudocumene oxidation dominates the low Ks for the alcohol (Table 1). Furthermore, high pseudocumene concentrations are expected to inhibit aldehyde formation, which could be further minimized by coexpression of benzyl alcohol dehydrogenase (4). Exclusive 3,4-dimethylbenzaldehyde formation can be reached via substrate concentrations at which the alcohol oxidation rate equals or exceeds the pseudocumene oxidation rate. Coexpression of dehydrogenases is expected to impair aldehyde accumulation. Finally, exclusive 3,4-dimethylbenzoic acid formation may be reached by maintaining the organic pseudocumene concentration below 90 mM or by coexpression of benzaldehyde dehydrogenase and possibly also benzyl alcohol dehydrogenase.
The two dehydrogenases may also have contributed to the production of carboxylic acids from aromatic heterocyclics in an industrial process developed by Lonza AG using the wild-type strain P. putida mt-2 (20). Acid production is limited in the BEHP/M9* two-liquid-phase system because the deprotonated form of the acid is poorly extracted by BEHP and therefore exerts toxic effects on the cells.
By controlling the pseudocumene concentration in a two-liquid-phase system and selective coexpression of dehydrogenases, the process presented may be driven to the exclusive accumulation of the alcohol, aldehyde, or acid derivative of pseudocumene. This principle might also apply to other substrates of XMO. The experimental application of these strategies and the further characterization of the presented kinetically controlled multistep biotransformation system are topics of ongoing research.
Acknowledgments
This work was supported by the BASF Corporation (Ludwigshafen, Germany).
We thank Jim Spain for critical reading of the manuscript.
REFERENCES
- 1.Abril, M.-A., C. Michan, K. N. Timmis, and J. L. Ramos. 1989. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J. Bacteriol. 171:6782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bard, M., D. A. Bruner, C. A. Pierson, N. D. Lees, B. Biermann, L. Frye, C. Koegel, and R. Barbuch. 1996. Cloning and characterization of ERG25, the Saccharomyces cerevisiae gene encoding C-4 sterol methyl oxidase. Proc. Natl. Acad. Sci. USA 93:186-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell-Parikh, L. C., and F. P. Guengerich. 1999. Kinetics of cytochrome P450 2E1-catalyzed oxidation of ethanol to acetic acid via acetaldehyde. J. Biol. Chem. 274:23833-23840. [DOI] [PubMed] [Google Scholar]
- 4.Bühler, B., A. Schmid, B. Hauer, and B. Witholt. 2000. Xylene monooxygenase catalyzes the multistep oxygenation of toluene and pseudocumene to corresponding alcohols, aldehydes, and acids in Escherichia coli JM101. J. Biol. Chem. 275:10085-10092. [DOI] [PubMed] [Google Scholar]
- 5.Burlage, R. S., S. W. Hooper, and G. S. Sayler. 1989. The TOL (pWW0) catabolic plasmid. Appl. Environ. Microbiol. 55:1323-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cali, J. J., and D. W. Russell. 1991. Characterization of human sterol 27-hydroxylase, a mitochondrial cytochrome P-450 that catalyzes multiple oxidation reactions in bile acid biosynthesis. J. Biol. Chem. 266:7774-7778. [PubMed] [Google Scholar]
- 7.Cornish-Bowden, A. 1995. Analysis of enzyme kinetic data. Oxford University Press, New York, N.Y.
- 8.de Smet, M.-J., H. Wynberg, and B. Witholt. 1981. Synthesis of 1,2-epoxyoctane by Pseudomonas oleovorans during growth in a two-phase system containing high concentrations of 1-octene. Appl. Environ. Microbiol. 42:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faber, K. 1997. Biotransformations in organic chemistry, 3rd ed. Springer, Berlin, Germany.
- 10.Franklin, F. C. H., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta-cleavage pathway. Proc. Natl. Acad. Sci. USA 78:7458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman, A., J. M. Woodley, and M. D. Lilly. 1993. In situ product removal as a tool for bioprocessing. Bio/Technology 11:1007-1012. [DOI] [PubMed] [Google Scholar]
- 12.Grund, A., J. Shapiro, M. Fennewald, P. Bacha, J. Leahy, K. Markbreiter, M. Nieder, and M. Toepfer. 1975. Regulation of alkane oxidation in Pseudomonas putida. J. Bacteriol. 123:546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harayama, S., M. Kok, and E. L. Neidle. 1992. Functional and evolutionary relationships among diverse oxygenases. Annu. Rev. Microbiol. 46:565-601. [DOI] [PubMed] [Google Scholar]
- 14.Harayama, S., P. R. Lehrbach, and K. N. Timmis. 1984. Transposon mutagenesis analysis of meta-cleavage pathway operon genes of the TOL plasmid of Pseudomonas putida mt-2. J. Bacteriol. 160:251-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harayama, S., R. A. Leppik, M. Rekik, N. Mermod, P. R. Lehrbach, W. Reineke, and K. N. Timmis. 1986. Gene order of the TOL catabolic plasmid upper pathway operon and oxidation of both toluene and benzyl alcohol by the xylA product. J. Bacteriol. 167:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harayama, S., M. Rekik, M. Wubbolts, K. Rose, R. A. Leppik, and K. N. Timmis. 1989. Characterization of five genes in the upper-pathway operon of TOL plasmid pWW0 from Pseudomonas putida and identification of the gene products. J. Bacteriol. 171:5048-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmberg-Betsholtz, I., E. Lund, I. Björkhem, and K. Wikvall. 1993. Sterol 27-hydroxylase in bile acid biosynthesis. Mechanism of oxidation of 5b-cholestane-3a,7a,12a,27-tetrol into 3a,7a,12a-trihydroxy-5b-cholestanoic acid. J. Biol. Chem. 268:11079-11085. [PubMed] [Google Scholar]
- 18.Kawakami, K., and T. Nakahara. 1994. Importance of solute partitioning in biphasic oxidation of benzyl alcohol by free and immobilized whole cells of Pichia pastoris. Biotechnol. Bioeng. 43:918-924. [DOI] [PubMed] [Google Scholar]
- 19.Keener, W. K., and D. J. Arp. 1994. Transformation of aromatic compounds by Nitrosomonas europaea. Appl. Environ. Microbiol. 60:1914-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiener, A. 1992. Enzymatic oxidation of methyl groups on aromatic heterocycles: a versatile method for the preparation of heteroaromatic carboxylic acids. Angew. Chem. Int. Ed. Engl. 31:774-775. [Google Scholar]
- 21.Kunz, D. A., and P. J. Chapman. 1981. Catabolism of pseudocumene and 3-ethyltoluene by Pseudomonas putida (avarilla) mt-2: evidence for new functions of the TOL (pWWO) plasmid. J. Bacteriol. 146:179-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laane, C., S. Boeren, K. Vos, and C. Veeger. 1987. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 30:81-87. [DOI] [PubMed] [Google Scholar]
- 23.Lee, K., and D. T. Gibson. 1996. Toluene and ethylbenzene oxidation by purified naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl. Environ. Microbiol. 62:3101-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leon, R., P. Fernandes, H. M. Pinheiro, and J. M. S. Cabral. 1998. Whole-cell biocatalysis in organic media. Enzyme Microb. Technol. 23:483-500. [Google Scholar]
- 25.May, S. W., and A. G. Katopodis. 1986. Oxygenation of alcohol and sulphide substrates by a prototypical non-haem iron monooxygenase: catalysis and biotechnological potential. Enzyme Microb. Technol. 8:17-21. [Google Scholar]
- 26.McFall, E., and E. B. Newman. 1996. Amino acids as carbon sources, p. 358-379. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: molecular and cellular biology, vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 27.Mermod, N., S. Harayama, and K. N. Timmis. 1986. New route to bacterial production of indigo. Bio/Technology 4:321-324. [Google Scholar]
- 28.Messing, J. 1979. A multipurpose cloning system based on single-stranded DNA bacteriophage M13. Recomb. DNA Tech. Bull. 2:43-49. [Google Scholar]
- 29.Panke, S., V. de Lorenzo, A. Kaiser, B. Witholt, and M. G. Wubbolts. 1999. Engineering of a stable whole-cell biocatalyst capable of (S)-styrene oxide formation for continuous two-liquid-phase applications. Appl. Environ. Microbiol. 65:5619-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panke, S., A. Meyer, C. M. Huber, B. Witholt, and M. G. Wubbolts. 1999. An alkane-responsive expression system for the production of fine chemicals. Appl. Environ. Microbiol. 65:2324-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panke, S., M. G. Wubbolts, A. Schmid, and B. Witholt. 2000. Production of enantiopure styrene oxide by recombinant Escherichia coli synthesizing a two-component styrene monooxygenase. Biotechnol. Bioeng. 69:91-100. [DOI] [PubMed] [Google Scholar]
- 32.Poulos, T. L., J. Cupp-Vickery, and H. Li. 1995. Structural studies on prokaryotic cytochromes P450, p. 125-150. In P. R. O. de Montellano (ed.), Cytochrome 450: structure, mechanism and biochemistry, 2nd ed. Plenum Press, New York, N.Y.
- 33.Preusting, H., R. v. Houten, A. Hoefs, E. K. v. Langenberghe, O. Favre-Bulle, and B. Witholt. 1993. High cell density cultivation of Pseudomonas oleovorans: growth and production of poly (3-hydroxyalkanoates) in two-liquid-phase batch and fed-batch systems. Biotechnol. Bioeng. 41:550-556. [DOI] [PubMed] [Google Scholar]
- 34.Raag, R., H. Li, B. C. Jones, and T. L. Poulos. 1993. Inhibitor-induced conformational change in cytochrome P-450cam. Biochemistry 32:4571-4578. [DOI] [PubMed] [Google Scholar]
- 35.Raag, R., and T. L. Poulos. 1989. Crystal structure of the carbon monoxide-substrate-cytochrome P-450cam ternary complex. Biochemistry 28:7586-7592. [DOI] [PubMed] [Google Scholar]
- 36.Ramos, J. L., S. Marqués, and K. N. Timmis. 1997. Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid-encoded regulators. Annu. Rev. Microbiol. 51:341-373. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, S. 1998. Ph.D. thesis. Swiss Federal Institute of Technology, Zurich, Switzerland.
- 40.Schwartz, R. D., and J. McCoy. 1977. Epoxidation of 1,7-octadiene by Pseudomonas oleovorans: fermentation in the presence of cyclohexane. Appl. Environ. Microbiol. 34:47-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanklin, J., C. Achim, H. Schmidt, B. G. Fox, and E. Münck. 1997. Mössbauer studies of alkane ω-hydroxylase: evidence for a diiron cluster in an integral-membrane enzyme. Proc. Natl. Acad. Sci. USA 94:2981-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shanklin, J., E. Whittle, and B. G. Fox. 1994. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787-12794. [DOI] [PubMed] [Google Scholar]
- 43.Shannon, M. J. R., and R. Unterman. 1993. Evaluating bioremediation: distinguishing fact from fiction. Annu. Rev. Microbiol. 47:715-738. [DOI] [PubMed] [Google Scholar]
- 44.Shaw, J. P., and S. Harayama. 1995. Characterization in vitro of the hydroxylase component of xylene monooxygenase, the first enzyme of the TOL-plasmid-encoded pathway for the mineralization of toluenes and xylenes. J. Ferment. Bioeng. 79:195-199. [Google Scholar]
- 45.Shaw, J. P., and S. Harayama. 1992. Purification and characterisation of the NADH:acceptor reductase component of xylene monooxygenase encoded by the TOL plasmid pWW0 of Pseudomonas putida mt-2. Eur. J. Biochem. 209:51-61. [DOI] [PubMed] [Google Scholar]
- 46.Shaw, J. P., and S. Harayama. 1990. Purification and characterisation of TOL plasmid-encoded benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase of Pseudomonas putida. Eur. J. Biochem. 191:705-714. [DOI] [PubMed] [Google Scholar]
- 47.Shaw, J. P., M. Rekik, F. Schwager, and S. Harayama. 1993. Kinetic studies on benzyl alcohol dehydrogenase encoded by TOL plasmid pWW0. J. Biol. Chem. 268:10842-10850. [PubMed] [Google Scholar]
- 48.Shaw, J. P., F. Schwager, and S. Harayama. 1992. Substrate-specificity of benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase encoded by TOL plasmid pWW0, metabolic and mechanistic implications. Biochem. J. 283:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shet, M. S., C. W. Fisher, P. L. Holmans, and R. W. Estabrook. 1996. The omega-hydroxylation of lauric acid: oxidation of 12-hydroxylauric acid to dodecanedioic acid by a purified recombinant fusion protein containing P450 4A1 and NADPH-P450 reductase. Arch. Biochem. Biophys. 330:199-208. [DOI] [PubMed] [Google Scholar]
- 50.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staijen, I. E., R. Marcionelli, and B. Witholt. 1999. The PalkBFGHJKL promoter is under carbon catabolite repression control in Pseudomonas oleovorans but not in Escherichia coli alk+ recombinants. J. Bacteriol. 181:1610-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki, M., T. Hayakawa, J. P. Shaw, M. Rekik, and S. Harayama. 1991. Primary structure of xylene monooxygenase: similarities to and differences from the alkane hydroxylation system. J. Bacteriol. 173:1690-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueng, Y.-F., T. Kuwabara, Y.-J. Chun, and F. P. Guengerich. 1997. Cooperativity in oxidations catalyzed by cytochrome P450 3A4. Biochemistry 36:370-381. [DOI] [PubMed] [Google Scholar]
- 54.Ullah, A. J. H., R. I. Murray, P. K. Bhattacharyya, G. C. Wagner, and I. C. Gunsalus. 1990. Protein components of a cytochrome P-450 linalool 8-methyl hydroxylase. J. Biol. Chem. 265:1345-1351. [PubMed] [Google Scholar]
- 55.van der Meer, J. R., W. M. de Vos, S. Harayama, and A. J. B. Zehnder. 1992. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol. Rev. 56:677-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaz, A. D. N., and M. J. Coon. 1994. On the mechanism of the action of cytochrome P450: evaluation of hydrogen abstraction in oxygen-dependent alcohol oxidation. Biochemistry 33:6442-6449. [DOI] [PubMed] [Google Scholar]
- 57.Williams, P. A., and K. Murray. 1974. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (avarilla) mt-2: evidence for the existence of a TOL plasmid. J. Bacteriol. 120:416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, P. A., L. M. Shaw, C. W. Pitt, and M. Vrecl. 1997. xylUW, two genes at the start of the upper pathway operon of TOL plasmid pWW0, appear to play no essential part in determining its catabolic potential. Microbiology 143:101-107. [DOI] [PubMed] [Google Scholar]
- 59.Witholt, B., M.-J. de Smet, J. Kingma, J. B. von Beilen, R. G. Lageveen, and G. Eggink. 1990. Bioconversions of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. Trends Biotechnol. 8:46-52. [DOI] [PubMed] [Google Scholar]
- 60.Witholt, B., O. Favre-Bulle, R. Lageveen, J. Kingma, J. B. van Beilen, H. Marvin, and H. Preusting. 1992. Synthesis of apolar organic compounds by Pseudomonas spp. and Escherichia coli in two-liquid-phase fermentations, p. 301-314. In E. Galli, S. Silver, and B. Witholt (ed.), Pseudomonas: molecular biology and biotechnology. ASM Press, Washington, D.C.
- 61.Worsey, M. J., and P. A. Williams. 1975. Metabolism of toluene and xylenes by Pseudomonas putida (avarilla) mt-2: evidence for a new function of the TOL plasmid. J. Bacteriol. 124:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wubbolts, M. 1994. Ph.D. thesis. Rijksuniversiteit Groningen, Groningen, The Netherlands.
- 63.Wubbolts, M. G., O. Favre-Bulle, and B. Witholt. 1996. Biosynthesis of synthons in two-liquid-phase media. Biotechnol. Bioeng. 52:301-308. [DOI] [PubMed] [Google Scholar]
- 64.Wubbolts, M. G., J. Hoven, B. Melgert, and B. Witholt. 1994. Efficient production of optically active styrene epoxides in two-liquid-phase cultures. Enzyme Microb. Technol. 16:887-893. [Google Scholar]
- 65.Wubbolts, M. G., P. Reuvekamp, and B. Witholt. 1994. TOL plasmid-specified xylene oxygenase is a wide substrate range monooxygenase capable of olefin epoxidation. Enzyme Microb. Technol. 16:608-615. [DOI] [PubMed] [Google Scholar]
- 66.Yun, C.-H., M. Song, T. Ahn, and H. Kim. 1996. Conformational change of cytochrome P450 1A2 induced by sodium chloride. J. Biol. Chem. 271:31312-31316. [DOI] [PubMed] [Google Scholar]
- 67.Yuste, L., I. Canosa, and F. Rojo. 1998. Carbon source-dependent expression of the PalkB promoter from the Pseudomonas oleovorans alkane degradation pathway. J. Bacteriol. 180:5218-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]