Abstract
The Colilert-18 system for enumeration of total coliforms and Escherichia coli is approved by the U.S. Environmental Protection Agency for use in drinking water analysis and is also used by various agencies and research studies for enumeration of indicator organisms in fresh and saline waters. During monitoring of Pinellas County, Fla., marine waters, estimates of E. coli numbers (by Colilert-18) frequently exceeded fecal coliform counts (by membrane filtration) by 1 to 3 orders of magnitude. Samples from freshwater sites did not display similar discrepancies. Fecal coliforms, including E. coli, could be cultured from 100% of yellow fluorescent wells (denoting E. coli-positive results) inoculated with freshwater samples but could be cultured from only 17.1% of the “positive” wells inoculated with marine samples. Ortho-nitrophenyl-β-d-galactopyranoside (ONPG)-positive or 4-methylumbelliferyl-β-d-glucuronide (MUG)-positive noncoliform bacteria were readily cultured from Colilert-18 test wells inoculated with marine samples. Filtered cell-free seawater did not cause false positives. Coculture preparations of as few as 5 CFU of Vibrio cholerae (ONPG positive) and Providencia sp. (MUG positive) ml−1 inoculated into Colilert-18 caused false-positive E. coli results. Salinity conditions influenced coculture results, as the concentration of coculture inoculum required to cause false positives in most wells increased from about 5 CFU ml−1 in seawater diluted 1:10 with freshwater to ≈5,000 CFU ml−1 in seawater diluted 1:20 with freshwater. Estimated E. coli numbers in various marine water samples processed at the 1:10 dilution ranged from 10 to 7,270 CFU·100 ml−1, while E. coli numbers in the same samples processed at the 1:20 dilution did not exceed 40 CFU·100 ml−1. The lower estimates of E. coli numbers corresponded well with fecal coliform counts by membrane filtration. This study indicates that assessment of E. coli in subtropical marine waters by Colilert-18 is not accurate when the recommended 1:10 sample dilution is used. The results suggest that greater dilution may diminish the false-positive problem, but further study of this possibility is recommended.
Coliform bacteria are widely used as indicators of fecal contamination of both fresh and marine waters. Certain members of the coliform group live outside of the gastrointestinal tract in the environment and may create a false indication of fecal contamination. A more specific indicator of fecal contamination is Escherichia coli, a thermotolerant fecal coliform bacterium distinguished by its ability to grow at 44.5°C and by its expression of the enzyme β-d-glucuronidase. This enzyme hydrolyzes 4-methylumbelliferyl-β-d-glucuronide (MUG) to form a fluorescent (F) product that is visible with UV light (11, 23) and that is used to differentiate E. coli from other fecal coliforms.
The Colilert-18 defined-substrate technology system (Idexx Laboratories, Inc., Westbrook, Maine) is intended to provide rapid (18-h), standardized quantitation of total coliforms and E. coli. Various versions of the Colilert system were shown to yield results that are statistically consistent with the standard methods of membrane filtration and multiple-tube fermentation testing used for the detection of coliforms and E. coli in freshwater (2, 3, 6, 7). The U.S. Environmental Protection Agency has approved Colilert for use in drinking water monitoring (9, 10), and analyses of E. coli in fresh (6, 12, 21) and saline (18, 22) natural waters have been published.
The Colilert-18 assay system is based on a defined substrate medium containing MUG and ortho-nitrophenyl-β-d-galactopyranoside (ONPG) and is used for the one-step detection of both total coliforms and E. coli in water samples. ONPG is a colorless lactose analog that is hydrolyzed by β-d-galactosidase, an enzyme common to all coliforms, to form a yellow (Y) product. β-d-Glucuronidase is present in relatively few bacterial species but is found in most E. coli strains. In the Colilert system, a Y color change following incubation is indicative of total coliforms, while a Y reaction in conjunction with fluorescence under UV light is considered indicative of E. coli. A most probable number (MPN) estimate of total coliform and E. coli numbers is achieved though use of a plastic Quantitray partitioned into 48 small (120-μl) and 49 large (1.6-ml) wells.
Discrepancies, including E. coli false positives and false negatives, have been published (6, 8, 16, 17) for various Colilert formulations. It has been reported that up to 34% of E. coli strains in human feces (1) and 10 to 20% of E. coli strains isolated from environmental sources (21) are not MUG positive. These isolates would be false negatives in the Colilert system. Organisms, including Aeromonas spp. (16), pseudomonads (14), some Salmonella and Shigella spp. (15, 20), and Flavobacterium spp. (19), can produce MUG-positive reactions, which may lead to false-positive results if the organisms are also lactose positive or are growing in a mixed culture with lactose-positive bacteria. It has been noted that the efficacy of the test should first be evaluated for source waters, such as storm runoff, wastewater effluent, or marine waters (7), yet the major work on the quantitative accuracy of Colilert assay systems for E. coli in U.S. marine waters was carried out exclusively in California waters (18). Marine waters in California are much colder than subtropical waters, and the dominant autochthonous bacteria would therefore be expected to differ from those of warmer waters. No studies, to our knowledge, have compared the accuracy of Colilert to that of standard enumeration techniques for indicator bacteria in tropical or subtropical waters. In spite of this paucity of information, the Colilert-18 system is frequently used by local and state agencies and by other entities for water quality monitoring in fresh and saline waters.
This study was prompted by the observation of high E. coli counts (103 to 104 CFU·100 ml−1) by Colilert-18 in subtropical marine and estuarine waters monitored by the Pinellas County Health Department, Florida Department of Health. The high E. coli counts contrasted sharply with fecal coliform counts obtained by standard membrane filtration analysis of the same samples. It was hypothesized that autochthonous MUG-positive marine bacteria acting in tandem with ONPG-positive, MUG-negative isolates can cause false-positive E. coli results in the Colilert-18 test. Because previous studies indicated that various plant and algal extracts can significantly interfere with the system's detection of both coliforms and E. coli (4), filtered, cell-free marine and estuarine water samples were also examined as a source of MUG and ONPG activity in the Colilert-18 system.
MATERIALS AND METHODS
Enumeration of E. coli cells by Colilert-18.
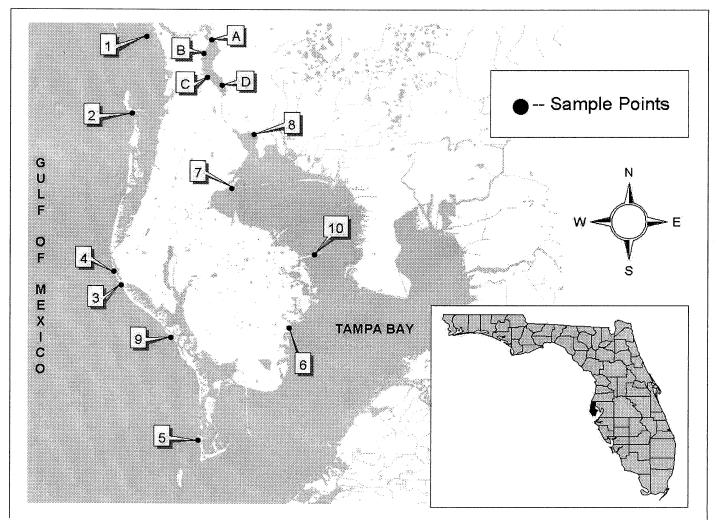
Marine, estuarine, and freshwater samples were collected in sterile 100-ml glass bottles from sites located around Tampa Bay, Fla. (Fig. 1). Eight marine, two estuarine, and four freshwater sites were sampled. The samples were transported on ice to the laboratory within 5 h and were each inoculated into one Colilert-18 Quantitray. Undiluted freshwater samples were assayed directly, whereas marine and estuarine samples were diluted 1:10 with sterile deionized water in accordance with the manufacturer's instructions. The inoculated Quantitrays were subsequently sealed and incubated at 35.0°C for 18 to 20 h. Following incubation, the Quantitray wells were read for Y color, indicating ONPG hydrolysis, and fluorescence, indicating MUG cleavage. A handheld UV light (366 nm) was used to identify F wells. The number and types of well reactions in each Quantitray were translated into MPN estimates for E. coli according to the manufacturer's instructions. One 100-ml sample from each of the eight marine and two estuarine sites was collected on 11 July, 25 July, and 29 August 2000 (see Table 2). On 11 October 2000, three 100-ml samples were collected at each marine site and processed as follows: one by Colilert-18 at a 1:10 (sample-deionized water) dilution, one by Colilert-18 at a 1:20 dilution, and one by the membrane filtration test for fecal coliforms. One 100-ml sample from each of the four freshwater sites was collected on 29 August and 11 October 2000. Freshwater samples were not collected on 11 July 2000, and only one freshwater site was sampled on 25 July 2000.
FIG. 1.
Map of sample sites for the study. The numbered sites are marine (1 to 6, 9, and 10) or estuarine (7 and 8) water. The lettered sites (A to D) are freshwater.
TABLE 2.
Percentage of YF Colilert-18 wells from which fecal coliforms were cultured
| Sitea | Percentage of wells from which fecal coliforms were culturedb
|
|||
|---|---|---|---|---|
| 7/11/00 | 7/25/00 | 8/29/00 | 10/11/00 | |
| Marine and estuarine | ||||
| 1 | 0 | 100 | 0 | 0 |
| 2 | 0 | 50 | 0 | 0 |
| 3 | 0 | 100 | 50 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 50 | 0 | 0 |
| 6 | 0 | 100 | 100 | 0 |
| 7 | 0 | 0 | 0 | 0 |
| 8 | ND | 50 | 0 | 0 |
| 9 | 0 | 0 | 50 | 0 |
| 10 | 0 | 0 | 0 | 0 |
| Total (avg) | 0 | 45 | 20 | 0 |
| n | 18 | 20 | 20 | 18 |
| Freshwater | ||||
| A | ND | 100 | 100 | 100 |
| B | ND | ND | 100 | 100 |
| C | ND | ND | 100 | 100 |
| D | ND | ND | 100 | 100 |
| Total (avg) | 100 | 100 | 100 | |
| n | 2 | 8 | 8 | |
The total number of wells sampled on each date (usually two wells per site) is denoted by n.
ND, not determined. Dates are given as month/day/year.
Recovery of fecal coliforms.
Following incubation, the backing material of each Quantitray was disinfected by application of 70% ethanol with a sterile swab. After the residual ethanol evaporated, sterile pipette tips were used to pierce the backing material of two MUG-positive, ONPG-positive wells; two MUG-positive, ONPG-negative wells; and one MUG-negative, ONPG-positive well per tray. One tray was processed per water sample.
One hundred microliters of fluid was withdrawn from each well and added to a separate tube containing 5 ml of EC broth (Difco) and to a Durham tube. The samples from the Colilert wells, an E. coli-positive control, and an uninoculated control were incubated at 44.5°C in a water bath. After 24 h, all of the tubes were examined for turbidity and the Durham tubes were examined for gas.
At the same time the EC tubes were inoculated, fluid from each well was used to inoculate selective-differential media. One drop (approximately 20 μl) of well content was streaked for isolation on MacConkey agar and Trypticase soy agar (TSA) amended with 10 mg of MUG/liter (TSA plus MUG). Following incubation at 35.0°C for 24 h, colonies were examined for lactose utilization on MacConkey agar and for MUG activity on TSA plus MUG. Selected colonies isolated from each Colilert well on TSA plus MUG were cross-checked on MacConkey agar in order to assess their phenotypes with respect to lactose fermentation and on thiocitrate bile salts (TCBS) agar to determine if they belonged to the family Vibrionaceae. The same colonies were also inoculated into Colilert medium in order to confirm their phenotypes with respect to ONPG and MUG cleavage in the Colilert system.
Identification of environmental isolates from Colilert wells.
Selected colonies isolated from Colilert wells were identified to the genus and/or species level using the API 20E biochemical test system (BioMerieux, Inc., Hazelwood, Mo.). Twenty-four-hour-old colonies were transferred from TSA-plus-MUG plates to separate 5-ml tubes containing a sterile solution of 0.85% NaCl and were resuspended and transferred to API 20E strips. The API 20E strips were prepared, incubated, treated, and interpreted according to the manufacturer's instructions. The numerical profile was compared with corresponding Analytab Products (API) codes in order to determine genus and species.
Examination of cell-free MUG activity.
Colilert wells inoculated from six different marine sites were tested for supernatant-mediated MUG activity. Fluid (200 μl) from MUG-positive, ONPG-negative and MUG-positive, ONPG-positive wells was aseptically recovered from strongly F, 24-h-old Colilert-18 wells. The fluid was filtered through a syringe filter (0.45-μm pore size) to remove cells. Twenty microliters of the filtrate from each sample was transferred to a well of a microtiter plate containing 180 μl of Luria-Bertani broth with 10 mg of MUG/liter. Twenty microliters of the resulting 10−1 dilution was carried through a dilution series to yield 10−1 to 10−6 dilutions of each sample in Luria-Bertani broth plus MUG. The microtiter plates were examined immediately under a UV transilluminator for fluorescence and were then incubated at 35.0°C for 72 h. The microtiter wells were examined for fluorescence and turbidity daily.
Marine coculture.
Marine isolates with specific phenotypes (one MUG positive, ONPG negative and one MUG negative, ONPG positive) were used to test the hypothesis that two species growing in a coculture could produce a reaction in the Colilert system that would mimic E. coli. A MUG-positive, ONPG-negative marine isolate was recovered from an F Colilert-18 well on TSA plus MUG. The isolate showed no growth on MacConkey agar or TCBS agar, indicating that it was neither a coliform nor a member of the family Vibrionaceae. The API 20E identification indicated closest resemblance to a member of the genus Providencia. Vibrio cholerae O/1 was used as the ONPG-positive, MUG-negative isolate. Streaking on TSA plus MUG showed V. cholerae O/1 to be negative for MUG activity, while API 20E analysis verified ONPG activity.
Demonstration of false-positive results from cocultures of marine bacteria.
The two marine isolates described above were inoculated on separate TSA plates and incubated at 35.0°C for 48 h. Sterile swabs were used to transfer cultures from TSA into correspondingly labeled tubes of filtered, autoclaved seawater. Cell concentrations were normalized to 0.50 absorbance unit using a spectrophotometer set at 660 nm. A 1:1 (MUG-positive, ONPG-positive) coculture was prepared by adding 1 ml of the suspension containing the MUG-positive isolate and 1 ml of the suspension containing the ONPG-positive isolate to 8 ml of sterile seawater (diluted coculture). A 10-fold dilution series of the diluted coculture (10−2 to 10−7) was prepared in sterile seawater. Finally, 1-ml aliquots from each of the 10−3 through 10−7 coculture dilutions were distributed to bottles containing 99 ml of undiluted seawater or a 1:5, 1:10, or 1:20 seawater-deionized water dilution. This was done in order to evaluate the relationship between salinity levels and Colilert-18 assay results while maintaining uniform initial inoculum concentrations and MUG-positive-ONPG-positive isolate ratios. Thus, a final coculture dilution series of 10−5, 10−6, 10−7, 10−8, and 10−9 was prepared for each salinity level.
Culturable counts for the coculture dilution series were obtained immediately by spread plating 100 μl from each dilution on TSA and incubating it for 24 h at 35.0°C. The contents of one Colilert-18 defined-nutrient packet was then added to each sample bottle, and the samples were transferred to Quantitrays, which were incubated for 18 h at 35.0°C. The reactions were then read as specified above.
As a positive control for the coculture experiment, E. coli was cultured in a parallel manner, i.e., four 1-ml aliquots from the 10−6 dilution prepared in sterile seawater were inoculated into undiluted seawater and the three seawater dilutions specified above. E. coli cells were immediately enumerated on TSA plates, and Quantitrays were prepared and incubated as for the experimental samples. A Quantitray containing autoclaved seawater was incubated in order to verify the lack of cell-free ONPG and MUG activity. A plate count on TSA was also carried out to verify sterility.
Fecal coliform counts on water samples were obtained by filtering 100 ml of sample through a membrane filter (0.45-μm pore size). The filters were placed on mFC agar (Difco) and incubated for 20 to 24 h in a water bath at 44.5°C.
RESULTS
Recovery of bacteria.
Ten marine and estuarine sites located in Tampa Bay and the Gulf of Mexico (Fig. 1) and four freshwater sites were sampled for this study. One Colilert-18 Quantitray was prepared for each water sample, and several wells per Quantitray with various reactions were assessed for the presence of fecal coliforms (Table 1).
TABLE 1.
Characterization of bacteria in individual Colilert wells by thermotolerance, lactose fermentation, and β-d-glucuronidase activity
| Site | Well reaction | na | No. of isolates
|
|||||
|---|---|---|---|---|---|---|---|---|
| Growth plus gas (EC at 44.5°C) | No growth on TSA plus MUG | Lactose+ MUG+ | Lactose+ MUG− | Lactose− MUG+ | Lactose− MUG− | |||
| Marine water | YF | 60 | 15 | 0 | 17 | 0 | 38 | 5 |
| Marine water | F | 38 | 0 | 3 | 0 | 0 | 26 | 9 |
| Marine water | Y | 28 | 1 | 0 | 0 | 1 | 11 | 16 |
| Freshwater | YF | 18 | 18 | 0 | 18 | 0 | 0 | 0 |
| Freshwater | F | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Freshwater | Y | 8 | 1 | 0 | 0 | 2 | 1 | 5 |
Number of Colilert wells sampled. The number of isolates in columns 5 to 9 add up to n (column 3).
Attempts to culture fecal coliforms from Colilert-18 wells inoculated with marine water samples were generally unsuccessful, even when those wells showed the yellow fluorescent (YF) reaction characteristic of E. coli. Only 25% (15 of 60) of EC broth tubes inoculated with fluid from YF (ONPG-positive, MUG-positive) Colilert wells showed turbidity and gas (Table 1). MUG-positve, lactose-positive bacteria were isolated from 17 of the YF wells, including all of the EC broth-positive wells. MUG-positive, lactose-negative colonies, but not lactose-positive, MUG-negative colonies, were readily isolated from YF wells that were negative by EC broth. No EC broth-positive tubes were observed for F (MUG-positive only) wells, and only one EC broth tube inoculated from a Y well was positive. Isolates from this culture were, as expected, lactose positive and MUG negative. Over 75% of all isolates from marine sites grew on TCBS agar, indicating the presence of Vibrio spp. and other members of the family Vibrionaceae.
On no date were more than 50% of YF Colilert wells from marine sites positive for fecal coliforms by EC broth assay, and on two sample dates all wells were negative by EC broth assay (Table 2).
Results from the four freshwater sites contrasted sharply with those from the marine and estuarine sites (Tables 1 and 2). One hundred percent (n = 18) of YF Quantitray wells sampled from freshwater sites yielded turbidity and gas in EC broth. Lactose-positive, MUG-positive bacteria were cultured from all of the YF Colilert-18 wells sampled (Table 1).
Biochemical analysis of recovered isolates.
In order to identify organisms that could be responsible for MUG-positive (F) reactions in Colilert wells, biochemical analysis of isolates recovered on TSA plus MUG from wells showing fluorescence (YF and F) was carried out. When MUG-positive isolates from four different marine sites that were positive for fecal coliforms (growth and gas in EC broth at 44.5°C) were further characterized, three were identified as E. coli and one isolate did not correspond to a known profile. Vibrio alginolyticus and Photobacterium damselae were recovered from YF wells and from F wells. All V. alginolyticus and P. damselae isolates were ONPG negative, indicating that an unrecovered organism was responsible for ONPG activity in YF wells.
Some lactose-positive isolates originating from Colilert wells inoculated with marine water were also characterized. Lactose-positive, MUG-negative colonies isolated from Y wells on TSA plus MUG were identified as Burkholderia cepacia, although the certainty of identification was weak. V. cholerae was identified by biochemical characterization (unconfirmed by serology or molecular techniques) in three Y wells.
From freshwater sites, biochemical characterization indicated that 72.7% of MUG-positive, lactose-positive isolates tested (n = 11) from YF wells were E. coli. One isolate was identified as Salmonella enterica serovar Arizonae, and two isolates did not correspond to any listed API profile.
Examination of cell-free MUG activity.
In order to assess whether extracellular agents, such as enzymes or chemicals, in seawater samples had caused development of MUG-positive reactions, analysis of cell-free filtrate from YF and F Colilert wells was carried out. Uninoculated controls showed no fluorescence or turbidity over the course of incubation. Cell-free filtrate of F wells from marine samples showed no development of turbidity or fluorescence at any time or at any dilution level. Filtrate from one YF well developed slight fluorescence but no yellowing in the 10−1 and 10−2 dilutions after 24 h. The fluorescence did not appreciably increase or extend across the series with time. Turbidity, which would have indicated bacterial contamination, did not develop in any well.
False-positive results caused by marine isolates.
Colilert-18 Quantitrays inoculated with a marine coculture (V. cholerae [ONPG positive] and Providencia sp. [MUG positive]) demonstrated the YF reactions characteristic of E. coli (Table 3). Colilert tests for each salinity level were inoculated with identical coculture preparations, but inoculum plate counts differed over approximately a twofold range, depending upon the salinity of the medium into which the organisms were incubated (Table 3). Positive controls inoculated with ≈50 CFU of E. coli ml−1 developed the expected YF reaction in all wells and at each salinity level. No fluorescence or yellowing was seen in the uninoculated sterile-seawater control.
TABLE 3.
Inoculum plate counts and numbers of positive (YF) reactions for a marine coculture inoculated in the Colilert-18 system at various inoculum concentrations and in several seawater dilutions
| Inoculum dilution | Result for seawater at indicated dilution
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Undiluted
|
1:5
|
1:10
|
1:20
|
||||||||||
| Inoculum plate count (CFU ml−1)a | Positive (YF) wellsb
|
Inoculum plate count (CFU ml−1) | Positive (YF) wells
|
Inoculum plate count (CFU ml−1) | Positive (YF) wells
|
Inoculum plate count (CFU ml−1) | Positive (YF) wells
|
||||||
| L | S | L | S | L | S | L | S | ||||||
| 10−5 | TNTC | 49 | 48 | TNTC | 49 | 48 | TNTC | 49 | 48 | TNTC | 49 | 48 | |
| 10−6 | 312 | 49 | 48 | 349 | 49 | 48 | 297 | 49 | 48 | 171 | 0 | 0 | |
| 10−7 | 29 | 49 | 48 | 45 | 49 | 46 | 33 | 49 | 18 | 24 | 0 | 0 | |
| 10−8 | 4 | 49 | 48 | 7 | 48 | 9 | 5 | 48 | 43 | 2 | 0 | 1 | |
| 10−9 | 2 | 46 | 0 | 0 | 38 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
TNTC, too numerous to count.
Number of YF Colilert wells per test. L, large wells (1.6 ml); S, small wells (120 μl).
The intensity of coculture-mediated fluorescence was brightest, and yellowing was weakest, in Quantitrays containing undiluted seawater. At the 1:10 salinity level recommended by the manufacturer, false-positive E. coli results (YF) were noted in all wells except those receiving the most dilute inoculum (0 to 2 CFU ml−1, corresponding to 0 to 3 CFU in each 1.6-ml well) (Table 3). High false-positive E. coli counts were obtained from Colilert tests inoculated with less than 50 CFU of coculture cells ml−1. For example, wells containing 1:10-diluted seawater that were inoculated with 33 CFU of coculture cells ml−1 (10−7 dilution) were scored YF in 49 large and 18 small wells, corresponding to an E. coli MPN of 307.6 CFU·100 ml−1. Wells containing 1:10-diluted seawater inoculated with 297 CFU of coculture cells ml−1 (10−6 dilution) were scored YF in 49 large and 48 small YF wells, representing an E. coli MPN of >2,419 CFU·100 ml−1.
Coculture-mediated false positives dropped dramatically for the 1:20 seawater dilution (Table 3). An inoculum concentration of several thousand CFU of coculture cells per milliliter (10−5 dilution) gave false positives in all wells, but several hundred CFU of coculture cells per milliliter (10−6 dilution) caused no false-positive results.
Unamended water samples from the marine and estuarine sites were examined concurrently by membrane filtration for fecal coliforms and by Colilert-18 tests prepared at the recommended 1:10 dilution for E. coli. Colilert-18 estimates of E. coli numbers were much higher at 8 of the 10 sites than corresponding membrane filtration estimates of fecal coliform numbers (Table 4). However, the same samples examined at a 1:20 dilution yielded Colilert-18 results that were much more consistent with membrane filtration results.
TABLE 4.
Colilert-18 results for E. coli at marine sites in 1:10- and 1:20-diluted samples compared with membrane filtration results for fecal coliforms
| Testa | Estimated E. coli (Colilert) or fecal coliform (MF) no. (CFU·100 ml−1)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1:10 Colilert | 143 | 132 | 7,270 | 373 | 437 | 130 | 20 | 10 | 1,467 | 107 |
| 1:20 Colilert | <20 | <20 | <20 | 20 | <20 | 20 | 20 | <20 | 40 | <20 |
| MF | <20 | <20 | 120 | <20 | <20 | <20 | <20 | 20 | <20 | <20 |
MF, membrane filtration.
Site number.
DISCUSSION
As coastal communities continue to expand, rapid, accurate quantitation of fecal contaminants in nearshore marine waters will continue to be a pressing public health and environmental concern. While many regulatory agencies employ membrane filtration or multiple-tube fermentation assays to detect and quantify indicator bacteria, these methods are time- and labor-intensive. Consequently, assays based on disposable rapid-development kits, such as the Colilert-18 test, have been increasingly employed in recent years as monitoring and research tools. Because false-positive results for E. coli can have economically harmful consequences, such as unnecessary beach closures, it is essential that test systems yield low percentages of false-positive and false-negative results.
This study investigated the cause of overestimates of E. coli numbers in subtropical marine waters in the United States. A disparity in numbers estimated by Colilert-18 (E. coli) versus membrane filtration (fecal coliforms) could not be readily explained, as Colilert-18 estimates were routinely 1 to 2 orders of magnitude higher than estimates by membrane filtration. The discrepancy occurred only for marine samples and was not apparent for freshwater samples. This study showed that fecal coliforms could not, in most cases, be cultured from the YF wells that indicate E. coli when Colilert-18 wells were inoculated with marine samples. Fecal coliforms, most of which were identified as E. coli, were recovered from all YF wells that had been inoculated with freshwater samples. Control Colilert-18 wells inoculated with low numbers of E. coli in seawater consistently yielded E. coli cultures after identical incubation times, indicating that the organism remained culturable under the assay conditions.
Cell-free filtrate from YF and F marine wells did not produce appreciable fluorescence or yellowing in microwells containing MUG, indicating that extracellular enzymes, plant extracts, or free chemical agents were not the cause of the reactions. MUG-positive, ONPG-negative marine bacteria that could have caused the development of fluorescence, including V. alginolyticus and P. damselae, were readily recovered from YF and F wells.
Only one lactose-positive, MUG-negative isolate was cultured from the marine samples, although more were expected given the high rate of false-positive YF wells. This phenomenon may be due to the difference between the substrate used in the Colilert test (ONPG) and the substrate in MacConkey agar (lactose), from which lactose-positive colonies were recovered. Vibrio vulnificus has previously been implicated as a source of β-galactosidase activity in marine waters (5), as have other Vibrio spp. and Aeromonas spp. (13).
A coculture of two marine isolates originally isolated from Colilert-18 wells demonstrated that false-positive YF (MUG-positive, ONPG-positive) reactions can occur when the overlapping biochemical activities of two organisms gave rise to the Y color and fluorescence produced by E. coli. Cocultures inoculated into a 1:20 seawater dilution produced false positives only at the highest inoculum level (approximately 3 × 103 CFU ml−1), and the fluorescence was extremely faded. Unamended marine water samples diluted 1:10 and processed by Colilert greatly overestimated E. coli numbers, while results for the 1:20 dilution were comparable to those obtained for fecal coliforms by membrane filtration. Although it could be argued that the broth medium of Colilert is more favorable for recovery of E. coli than membrane filtration, thus consistently yielding higher estimates of E. coli numbers than the membrane filtration estimates of fecal coliform numbers, the results from the unamended marine water samples do not support this hypothesis. When E. coli concentrations estimated by Colilert-18 from the 1:10- and 1:20-diluted samples were compared, a greater-than-10-fold difference was generally observed (Table 4) rather than the expected 2-fold difference. The results of this work support the hypothesis that bacteria other than fecal coliforms caused the YF reactions at the 1:10 dilution. These bacteria may have been incapable of growth at the low salinity of the 1:10 dilution, or they may have been diluted out if they were originally present in low numbers.
While this work suggests that a 1:20 dilution of marine samples may increase the accuracy of the Colilert-18 system for estimating E. coli numbers in tropical waters, it is important to note that the diminution of false positives at 1:20 dilution was demonstrated for one pair of experimental organisms and in one set of unamended marine water samples. As there are doubtless other species capable of causing false-positive results in the Colilert-18 system, the effect of sample dilution should be further explored before this type of defined-substrate test is used for water quality testing in marine and estuarine waters. Furthermore, if this system is to be used in marine waters, confirmation of positive E. coli results in a medium such as EC-MUG incubated at 44.5°C would prevent most false positives caused by marine bacteria.
Acknowledgments
Funding for this study was provided in part by the Pinellas County Health Department, Florida Department of Health.
We thank Joseph Sowers of Pinellas County Health Department, Florida Department of Health, for the map.
REFERENCES
- 1.Chang, G. W., J. Brill, and R. Lum. 1989. Proportion of β-d-glucuronidase-negative Escherichia coli in human fecal samples. Appl. Environ. Microbiol. 56:335-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covert, T. C., L. C. Shadix, E. W. Rice, J. R. Haines, and R. W. Freyberg. 1989. Evaluation of the autoanalysis Colilert test for detection and enumeration of total coliforms. Appl. Environ. Microbiol. 55:2443-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covert, T. C., E. W. Rice, S. A. Johnson, D. Berman, C. H. Johnson, and P. J. Mason. 1992. Comparing defined-substrate coliform tests for the detection of Escherichia coli in water. J. Am. Water Works Assoc. 84:98-104. [Google Scholar]
- 4.Davies, C. M., S. C. Apte, S. M. Peterson, and J. L. Stauber. 1994. Plant and algal interference in bacterial β-d-galactosidase and β-d-glucuronidase assays. Appl. Environ. Microbiol. 60:3959-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, C. M., S. C. Apte, S. M. Peterson, and J. L. Stauber. 1995. Possible interference of lactose-fermenting marine vibrios in coliform β-d-galatosidase assays. J. Appl. Bacteriol. 78:287-393. [DOI] [PubMed] [Google Scholar]
- 6.Eckner, K. F. 1998. Comparison of membrane filtration and multiple-tube fermentation by the Colilert and Enterolert methods for detection of waterborne coliform bacteria, Escherichia coli, and enterococci used in drinking and bathing water quality monitoring in southern Sweden. Appl. Environ. Microbiol. 64:3079-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edberg, S. C., M. J. Allen, D. B. Smith, and the National Collaborative Study. 1988. National Field Evaluation of a defined substrate method for the simultaneous enumeration of total coliforms and Escherichia coli from drinking water: comparison with the standard multiple tube fermentation method. Appl. Environ. Microbiol. 54:595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edberg, S. C., M. J. Allen, D. B. Smith, and the National Collaborative Study. 1989. National Field Evaluation of a defined substrate method for the simultaneous enumeration of total coliforms and Escherichia coli from drinking water: comparison with presence-absence techniques. Appl. Environ. Microbiol. 55:1003-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federal Register. 1989. National primary drinking water regulations: total coliforms (including fecal coliforms and E. coli). Fed. Regist. 54:27554-27567. [Google Scholar]
- 10.Federal Register. 1992. National primary drinking water regulations: analytical techniques, coliform bacteria, final rule. Fed. Regist. 57:24774. [Google Scholar]
- 11.Feng, P., R. Lum, and G. W. Chang. 1991. Identification of uidA gene sequence in β-d-glucuronidase-negative Escherichia coli. Appl. Environ. Microbiol. 57:320-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fricker, E. J., and C. R. Fricker. 1996. Use of two presence/absence systems for the detection of E. coli and coliforms from water. Water Res. 30:2226-2228. [Google Scholar]
- 13.Geissler, K., M. Manafi, I. Amoros, and J. L. Alonso. 2000. Quantitative determination of coliforms and Escherichia coli in marine waters with chromogenic and fluorogenic media. J. Appl. Microbiol. 88:280-285. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo, C., J. Reyes, and R. Goldschmidt. 1977. Induction and properties of β-galactosidase and β-galactoside permease in Pseudomonas BAL-31. J. Bacteriol. 129:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilian, M., and P. Bulow. 1979. Rapid identification of Enterobacteriaceae. II. Use of β-glucuronidase detecting agar medium (PGUA) for the identification of E. coli in primary cultures of urine samples. Acta Pathol. Microbiol. Scand. B 87:271-276. [PubMed] [Google Scholar]
- 16.Landre, J. P., A. A. Gavriel, and A. J. Lamb. 1998. False-positive coliform reaction mediated by Aeromonas in the Colilert defined substrate technology system. Lett. Appl. Microbiol. 26:352-354. [DOI] [PubMed] [Google Scholar]
- 17.Lewis, C. M., and J. L. Mak. 1989. Comparison of membrane filtration and autoanalysis Colilert presence-absence techniques for analysis of total coliforms and Escherichia coli in drinking water samples. Appl. Environ. Microbiol. 55:3091-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer, C. J., Y. L. Tsai, A. L. Lang, and L. R. Sangermano. 1993. Evaluation of Colilert-Marine Water for detection of total coliforms and Escherichia coli in the marine environment. Appl. Environ. Microbiol. 59:786-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petzel, J. P., and P. A. Hartman. 1986. A note on starch hydrolysis and β-glucuronidase activity among flavobacteria. J. Appl. Bacteriol. 61:421-426. [DOI] [PubMed] [Google Scholar]
- 20.Ralovich, B., G. A. M. Ibrahim, A. Fabian, and M. Herpay. 1991. What is the diagnostic value of β-d-glucuronidase (BDG) activity of bacteria using fluorocult ECD agar for their cultivation. Acta Microbiol. Hung. 38:147-154. [PubMed] [Google Scholar]
- 21.Shadix, L. C., and E. W. Rice. 1991. Evaluation of β-glucuronidase assay for the detection of Escherichia coli from environmental waters. Can. J. Microbiol. 37:908-911. [DOI] [PubMed] [Google Scholar]
- 22.Solo-Gabriele, H. M., M. A. Wolfert, T. R. Desmarais, and C. J. Palmer. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 66:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trepeta, R. W., and S. C. Edberg. 1984. Methylumbelliferyl-β-d-glucuronide-based medium for rapid isolation and identification of Escherichia coli. J. Clin. Microbiol. 19:172-174. [DOI] [PMC free article] [PubMed] [Google Scholar]