Abstract
A first application of a multiplexed, bead-based method is described for determining the abundances of target sequences in an environmental PCR product. Target sequences as little as 0.3% of the total amount of DNA can be quantified. Tests were conducted on 16S ribosomal DNA sequences from microorganisms collected from contaminated groundwater.
We are developing a multiplexed, bead-based method involving polystyrene microspheres, nucleic acid hybridizations, and flow cytometry instrumentation with an aim towards environmental field testing. High-throughput, quantitative methods which can simultaneously measure the abundances of many microorganisms would help us gain a broader view of microbial activity involved with degrading, eliminating, or immobilizing contaminants in the environment (4, 9). When PCR is used, a critical step is the accurate measurement of the amount of individual sequences in the PCR product derived from community DNA. In this paper, we developed a procedure for multiplexed quantitation of PCR products using the bead method in a manner which can lead to high-throughput testing. Measurements were made on 16S ribosomal DNA sequences obtained from groundwater contaminated with TCE and TKEBS at the Lawrence Livermore National Laboratory, Well-834-D3, Site 300.
Microorganisms were collected by passing groundwater through filters. A phenol chloroform-isoamyl alcohol method, adapted from reference 1, was used to extract DNA. The 16S rRNA gene was amplified from community DNA or plasmid DNA obtained from clones using bacterial primers 338Fbs2 (5"[Biotin]*T*C*C*T*A CGG GAG GCA GC) and 907R (5"CCG TCA ATT CMT TTR AGT TT; Oligos Etc.) (3), where 338Fbs2 was synthesized with a 5" biotin modification, 12-carbon linker, five phosphorothioate bonds (*), and reverse-phase high-performance liquid chromatography purification (Synthegen, Inc.). The terms “PCR mix,” “environmental PCR product,” and “pure PCR product” refer to PCR products prepared from no-template DNA, community DNA, and plasmid DNA, respectively. Typical concentrations were 50 to 60 fmol/μl for a pure PCR product and 12 or 13 fmol/μl for the environmental PCR product.
Capture probes for four target sequences labeled d006, d011, d023, and Geothrix (Gx) and a universal probe 533FA (3) were designed, synthesized, and attached to beads, as described in reference 7. The bead probes were designated plain/d006, 154/d023, 138/d011, 134/Gx, and 133/533FA, where 154/d023 means that the d023 capture probe was attached to bead type 154.
A “bead mix” and single-stranded amplicons were prepared according to reference 7. In each tube, 17 μl of single-stranded amplicons was combined with 34 μl of bead mix containing five types of bead probes (10,000 beads of each type) and were hybridized at 46°C for 2 h. The beads were then washed in 46°C 1× TMAC buffer (7). Twelve microliters of a 20-μg/ml streptavidin-R-phycoerythrin (Molecular Probes) solution, prepared in 1× TMAC buffer at room temperature, was added to the hybridization mix, vortexed, and incubated at 46°C for 10 min. The beads were then washed two times in 46°C 1× TMAC buffer and resuspended in 100 μl of 1× TMAC buffer at room temperature.
The beads were detected with a Luminex 100 flow cytometer. The intensity values of the reporter signals were converted into units known as molecules of equivalent soluble fluorochrome (MESF) using Quantum 27 (R-PE) Reference Standards (Bangs Laboratories, Inc.) according to standard procedures. Cytometry data were analyzed with FCS Express version 1.065 (De Novo Software). The mean intensity (Is) of the reporter signal and intersample standard deviation (SD) were determined by running ≤7 replicate tubes. A similar procedure was used for the background signal (Ib). The uncertainty in the fluorescence response F = Is − Ib was calculated using the standard error SD in the difference of means (5).
The quality of the bead probes was checked as follows. The background signal for each bead probe was determined using PCR mix as the analyte. A typical value for plain/d006 was 203 ± 25 MESF with higher values for the fluorescent beads. Sequence discrimination was evaluated by exposing bead probes to noncomplementary pure or environmental PCR products diluted with PCR mix. No cross-hybridization signal was found.
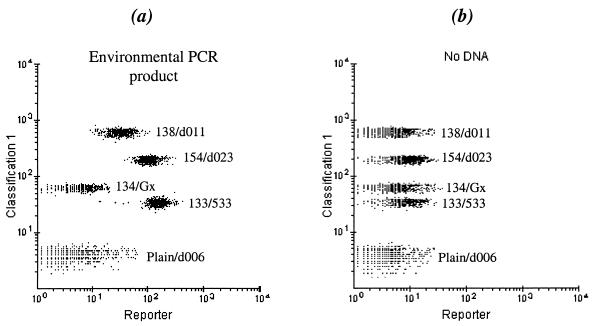
An example of the flow cytometer output is shown in Fig. 1, demonstrating the fluorescence response of the bead probes after hybridization to 115 fmol of environmental PCR product. A visual comparison between Fig. 1a and b shows that the reporter signals for 138/d011, 154/d023, and 133/533FA in the environmental sample are higher than the background, indicating the presence of d011 and d023. For a given bead population, the standard error of the mean intensity was ≤ 3% of the mean intensity; however, intersample variability was larger. For a measurement of abundance, the fluorescence response must be related to the actual amount of a sequence in the PCR product.
FIG. 1.
Fluorescence detection of the bead-probes with the Luminex 100 flow cytometer. The dot plots show the orange reporter signal (abscissa) and the red bead classification color (ordinate). (a) Analyte containing 16S ribosomal DNA from the contaminated well. (b) Negative control (PCR mix).
Since the hybridization efficiency between probes and targets can vary considerably, standard concentration curves were developed for each probe. We have found that the concentration response in the environmental PCR product is different from that in the pure PCR product. Therefore, quantitation was accomplished by adding known amounts (Ci) of a target (measured in, for example, fmoles) to a fixed amount of environmental PCR product, containing an unknown amount (Ce) of that target. The total amount of target in the analyte was therefore C = Ce + Ci.
Model.
To determine Ce, a model equation was developed. Estimates in reference 7 showed that not more than a few tenths of a percent of the total amount of target DNA in the analyte actually hybridize to capture probes on the bead surface. In this case, the fluorescence response of a specific target sequence may be described by a Scatchard-type equation for a unireactant system (probe-target) (6): Fi = [kaImax(Ce + Ci)]/[1 + ka(Ce + Ci)]) (equation 1), where Fi is the fluorescence response of a particular target in the sample (with background subtracted); ka = Ka/V; Ka is the association constant; V is the reaction volume, and Imax is the maximum response corresponding to complete saturation occurring at ka(Ce + Ci) ≫ 1. In practice, ka, Imax, and Ce must be determined experimentally. Equation 1 represents a system of equations where i = 0, 1, 2, 3… This model assumes (i) that there is no cross-hybridization, consistent with our observations, and (ii) that parameters ka and Imax are constant for all Ci.
At minimum, three spiking values are required to determine Ce, ka, and Imax. When Co = 0 (no spike), equation 1 yields a simple, exact solution: Ce = [αC1C2]/[C2(1 − α) − C1] (equation 2), where α = F0(F2 − F1)/[F1(F2 − F0 )]. Parameters Imax, ka, and Ce can also be determined by directly fitting equation 1 to the experimental data {Ci, Fi}.
Verification of model.
The model was tested by spiking equimolar mixtures of pure PCR products for d006, d011, and d023 and Gx into 106 fmol of environmental PCR product, using PCR mix as diluent. The Ce value for each sequence was calculated from equation 2 using Ci = 0, 8, and 16 fmol. The uncertainties in Ce were determined from SD and conventional error propagation formulas (2). Ce was found to be 10.0 ± 4.7 fmol (9.4%) for d023, 4.3 ± 1.2 fmol (4.1%) for d011, and 0.31 ± 0.17 fmol (0.3%) for d006, where the percentage refers to the relative abundance out of 106 fmol of environmental PCR product. In the case of 138/Gx beads, F0 was −16 ± 24 MESF, indicating that this sequence was not detected (0%) in the environmental PCR product within the error. Except for d023, the relative abundances of d011, d006, and Gx were in agreement with cloning and sequencing results based on 63 clones. The cloning results were 28.6% (17.9 to 41.4) for d023; 4.8% (0.99 to 13.3) for d011; 1.6% (0.04 to 8.53) for d006; and 0% (0.00 to 4.64), where the bracketed numbers represent the 95% confidence interval determined by using Minitab software (J. H. Klotz, L. M. Leemis, and K. S. Trivedi, Letter, Am. Statistician 50:388-389, 1996).
Figure 2 illustrates the experimental data {Ci, Fi} for d023 and a weighted, nonlinear fit of equation 1 using the calculated Ce to obtain ka and Imax (Table 1). For this and other sequences, the fits using the model equation are excellent.
FIG. 2.
Application of model to determine the amount (Ce) of d023 in the environmental PCR product (106 fmol). Fi, indicated by ○, is shown for Ci at 0, 2, 8, and 16 fmol. Ce was determined from equation 2 by using errors given by SD. The solid line is a weighted, nonlinear fit to equation 1. The “+” symbols and the dotted line are the Fi and fit for pure d023 PCR product diluted in PCR mix.
TABLE 1.
Fitting parameters associated with the standard curve for each target sequence in an analyte consisting of either pure or environmental PCR producta
| Target sequence | Results for:
|
||||
|---|---|---|---|---|---|
| Pure PCR
|
Environmental PCR
|
||||
| ka | Imax | ka | Imax | ||
| d023 | (5.23 ± 0.98) × 10−3 | 64,100 ± 12,000 | (31.80 ± 0.04) × 10−3 | 17,700 ± 200 | |
| d011 | (7.20 ± 1.10) × 10−3 | 41,400 ± 6,300 | (29.8 ± 7.5) × 10−3 | 11,800 ± 3,000 | |
| d006 | (7.84 ± 0.92) × 10−3 | 31,200 ± 3,700 | (8.86 ± 0.98) × 10−3 | 20,900 ± 2,300 | |
| Gx | (2.25 ± 0.43) × 10−3 | 26,000 ± 5,100 | (27.1 ± 0.2) × 10−3 | 2,200 ± 200 | |
Units used are fmoles−1 and MESF for ka and Imax, respectively.
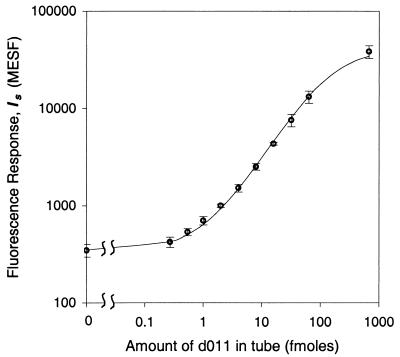
The values of ka and Imax are different in the pure and environmental PCR products, indicating different hybridization conditions (see dotted and solid curves in Fig. 2). Concentration curves for pure PCR products were obtained with equimolar mixtures of d023, d011, and d006 and Gx diluted in PCR mix. Parameters ka and Imax were calculated by a weighted, nonlinear fit of equation 1 with Ce = 0 (Table 1). In general Imax is greater in the pure PCR product than in the environmental PCR product. As shown in Fig. 3 for d006, the excellent fit to the experimental concentration curve spanning 3 orders of magnitude in the amount of target DNA in the tube again supports the model equation for this system.
FIG. 3.
Concentration curve of pure d011 PCR product diluted in PCR mix. The reporter signal Is is displayed on the ordinate axis indicating that Ib = 348 ± 51 MESF for 138/d011 beads. The solid line is a weighted, nonlinear fit to the model. The error bars represent intersample SD.
We sought further validation of the model by comparing our values for the dissociation constant Kd (= 1/Ka) to those in the literature. In our experiments the Kd values for a 570-nucleotide target DNA hybridized to a 20-mer capture probe ranged from 2.5 × 10−9 M for d006 to 8.7 × 10−9 M for Gx. Detailed studies (8) of hybridizations on microparticle surfaces between a short target (22-mer) and a particle-bound 22-mer capture probe obtained Kd values of ∼10−9 M at 50°C. Our values are similar but somewhat higher; we speculate that this may be due to a decrease in hybridization efficiency for long-target DNA. We note, however, that the design of the capture probe can affect the hybridization response more than the target sequence length.
As expected, there is substantial variation of the coefficients ka and Imax for different target sequences, most likely due to secondary structure. In practice, for assays targeting a broad array of microorganisms, it will be impossible to design probes with the desired specificity and identical coefficients. Good quantitation will therefore require separate determination of ka and Imax for each control strain. Correspondingly, fine quantitation with universal probes is probably not achievable, as demonstrated by the data for 133/533FA beads in which the total amount of DNA was found to be 17.6 ± 10.5 fmol, compared to 106 fmol determined by gel-based quantitation.
The coefficients ka and Imax are different between an environmental sample and a pure PCR product. Cross-hybridization is unlikely to be the cause since none was observed in our experiments. (For example, the Gx capture probe subjected to noncomplementary pure or environmental PCR products produced no signal.) At this time, we suggest two possible causes: (i) chemicals in the groundwater or extraction process which reduce the efficiency of hybridization or labeling and (ii) many similar sequences in the PCR product which interfere with each other. Tests on more types of environmental samples will be required to elucidate this point.
In summary, we have developed and verified a quantitative method for multiplexed determination of abundances in environmental PCR products.
Acknowledgments
We acknowledge B. Methe and D. Lovley for providing Geobacter and Geothrix clones. This work was supported by DOE NABIR grant DE-FG02-99ER62868.
REFERENCES
- 1.Barns, S. M., R. E. Fundyga, M. W. Jeffries, and N. R. Pace. 1994. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc. Natl. Acad. Sci. USA 91:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevington, P. R. 1969. Data reduction and error analysis for the physical sciences. McGraw-Hill Book Co., New York, N.Y.
- 3.Dojka, M. A., P. Hugenholtz, S. K. Haack, and N. R. Pace. 1998. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 64:3869-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pieper, D. H., and W. Reineke. 2000. Engineering bacteria for bioremediation. Curr. Opin. Biotechnol. 11:2626.. [DOI] [PubMed] [Google Scholar]
- 5.Press, W. H., S. A. Teukolsky, W. T. Vetterling, and B. P. Flannery. 1992. Numerical recipes in C: the art of scientific computing, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 6.Segel, I. H. 1976. Biochemical calculations, 2nd ed. John Wiley & Sons, New York, N.Y.
- 7.Spiro, A., M. Lowe, and D. Brown. 2000. A bead-based method for multiplexed identification and quantitation of DNA sequences using flow cytometry. Appl. Environ. Microbiol. 66:4258-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens, P. W., M. R. Henry, and D. M. Kelso. 1999. DNA hybridization on microparticles: determining capture-probe density and equilibrium dissociation constants. Nucleic Acids Res. 27:1719-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wackett, L. P., and D. C. Hershberger. 2001. Biocatalysis and biodegradation. ASM Press, Washington, D.C.