Abstract
A rapid and simple two-step procedure suitable for both small- and large-scale purification of pediocin-like bacteriocins and other cationic peptides has been developed. In the first step, the bacterial culture was applied directly on a cation-exchange column (1-ml cation exchanger per 100-ml cell culture). Bacteria and anionic compounds passed through the column, and cationic bacteriocins were subsequently eluted with 1 M NaCl. In the second step, the bacteriocin fraction was applied on a low-pressure, reverse-phase column and the bacteriocins were detected as major optical density peaks upon elution with propanol. More than 80% of the activity that was initially in the culture supernatant was recovered in both purification steps, and the final bacteriocin preparation was more than 90% pure as judged by analytical reverse-phase chromatography and capillary electrophoresis.
Gene-encoded, ribosomally synthesized antimicrobial peptides are widely distributed in nature, being produced by bacteria, plants, and a wide variety of animals, including humans (28, 29, 32, 34). The peptides are often cationic and amphiphilic or hydrophobic, and many of them kill bacteria by permeabilizing the target cell membrane. The peptides may be developed into new and useful antimicrobial additives and drugs. An example of this is the antimicrobial peptide nisin, which is produced by lactic acid bacteria (LAB). This peptide is used as a food preservative (9) and has been considered for use for treatment of gastric Heliobacter infections and/or ulcers (16).
There has especially been considerable interest in antimicrobial peptides (bacteriocins) produced by LAB because of the “food-grade quality” and industrial importance of these bacteria. LAB are used in food production, are part of the natural microbial flora in food that humans have consumed for centuries, and constitute a significant part of the indigenous flora of mammals, including humans. Thus, LAB and the bacteriocins that they produce may be considered safe agents for preventing growth of pathogenic and/or undesirable microorganisms.
Many of the LAB bacteriocins belong to the pediocin-like family; these bacteriocins are of special interest because of their antilisterial activity. The family contains at least 15 different bacteriocins, of which pediocin PA-1 (3, 19, 23, 30), leucocin A UAL-187 (17), mesentericin Y105 (18), sakacin P (35) and curvacin A (identical to sakacin A [20, 35]) were the first to be identified. All pediocin-like bacteriocins are cationic, contain between 35 and 50 amino acid residues, permeabilize target cell membranes, and have very similar primary structures but differ markedly with respect to their target cell specificity (5-7, 10-14, 22, 24, 29, 32, 37).
The use of pediocin-like bacteriocins and other antimicrobial peptides as additives or drugs requires a simple and rapid method by which large quantities may be purified to homogeneity. Present methods for purification of pediocin-like bacteriocins and other cationic bacteriocins generally include a centrifugation step for the removal of cells from the bacterial culture medium, followed by a step in which the peptides may be concentrated. This latter step often involves precipitation of peptides with ammonium sulfate and collecting the precipitate by centrifugation. The peptides are subsequently purified to homogeneity by sequential chromatography on cation exchange, hydrophobic interaction, and/or reverse-phase columns. Although these procedures function reasonably well when one is purifying smaller quantities of peptides from a <1-liter bacterial culture, they tend to become unmanageable when one is working with the large volumes that are needed for large-scale industrial production and for production of peptides for structural and functional studies. The major problem is that the centrifugation and precipitation steps become cumbersome when one is working with large volumes. Moreover, ammonium sulfate precipitation of peptides from culture medium results in variable and often low yields (about 50% but in some instances down to 10%), in part because much of the precipitate floats even after centrifugation and is consequently difficult to collect (8, 15, 26, 27). It is our experience that ammonium sulfate precipitation of the pediocin-like bacteriocin pediocin PA-1 has a yield of 40% ± 20% (average and standard deviation of 22 experiments; unpublished results). In a recently published procedure for purification of pediocin-like bacteriocins, concentration by ammonium sulfate precipitation was replaced with cation-exchange chromatography (15). Although this replacement was reported to increase the yield of purified bacteriocins (overall yields of between 10 and 66% were obtained), purification from a 100-ml culture took 6 to 8 h (15). In this study we present a rapid and simple two-step procedure suitable for both small- and large-scale purification of pediocin-like bacteriocins and other cationic peptides.
Bacterial strains and bacteriocin assay.
Pediocin PA-1 (3, 19, 23, 30) was produced by Pediococcus acidilactici LMG 2351, which was isolated from commercial starter cultures obtained from Christian Hansen Laboratories, Copenhagen, Denmark. Sakacin P (35) and a sakacin P mutant were produced by bacteriocin expression systems developed recently (2, 11, 22). Curvacin A (35), leucocin A (17), lactococcin A (21), lactococcin G (33), and nisin Z (25) were produced by Lactobacillus curvatus LTH1174 (35), Leuconostoc mesenteroides 6 (36), Lactococcus lactis subsp. cremoris LMG 2130 (21), L. lactis LMG 2081 (33), and L. lactis LMG 2077, respectively. Both enterocins A and B were produced by Enterococcus faecium CTC492 (31). The lactococcal strains were grown at 30°C in M17 (Biokar Diagnostica) supplemented with glucose and Tween 80 to final concentrations of 0.4% (wt/vol) and 0.1% (vol/vol), respectively. All the other strains were grown at 30°C in MRS broth (Oxoid).
Bacteriocin activity was measured by using a microtiter plate assay system, essentially as described previously (11, 21). Lactobacillus sake NCDO 2714 (type strain) was used as the indicator strain during the assaying of pediocin PA-1, sakacin P, a sakacin P mutant, curvacin A, leucocin A, nisin Z, and enterocins A and B. L. lactis subsp. lactis IL 1403 was used as the indicator strain during the assaying of lactococcins A and G. One bacteriocin unit (BU) was defined as the amount of bacteriocin that inhibited the growth of the indicator strain by 50%.
Purification of pediocin-like bacteriocins by passage of bacterial cultures through cation exchanger followed by reverse-phase chromatography of peptides.
The rapid two-step purification procedure that was developed avoids centrifugation and precipitation steps and is thus easily scaled up. In the first step, the bacterial culture was applied directly on a SP Sepharose Fast Flow cation-exchange column (Amersham Pharmacia Biotech). This approach is similar to that reported by Callewaert and De Vuyst for purification and concentration of the bacteriocin amylovorin L471 on an expanded bed cation-exchange column to which crude fermentation medium had been applied (4). Bacteria and anionic compounds passed through the SP Sepharose Fast Flow cation-exchange column, whereas cationic bacteriocins were eluted with 1 M NaCl after the column had first been washed with a phosphate buffer containing between 0.1 and 0.2 M (depending on the bacteriocin which is being purified) NaCl. In the second step, the 1 M NaCl fraction from the cation exchanger was applied at a high flow rate on a low-pressure Resource reverse-phase column (Amersham Pharmacia Biotech). Bacteriocins bind to the column and were detected as major peaks on the optical density profile upon elution with propanol. This second step could be repeated if necessary for obtaining peptides with a purity of more than 90%.
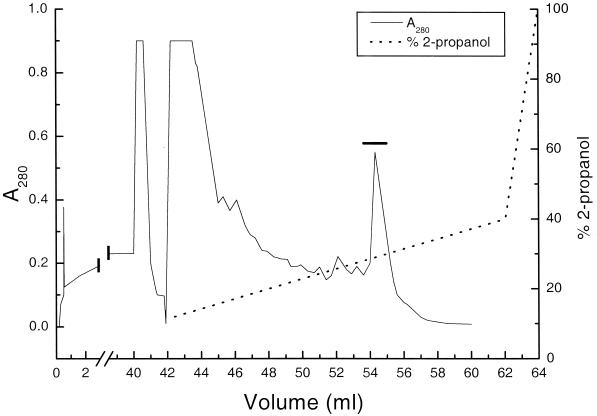
The procedure may be illustrated with the purification of pediocin PA-1. More than 80% of the activity that was initially in the culture supernatant was recovered in both purification steps (Table 1), and the bacteriocin was detected as a major optical density peak upon reverse-phase chromatography in the second step (Fig. 1). The identity of the purified peptide obtained after reverse-phase chromatography was verified by mass spectrometry; it was more than 90% pure as judged by analytical reverse-phase chromatography and capillary electrophoresis (results not shown).
TABLE 1.
Purification of pediocin PA-1 by new procedure
| Fractionation step/fraction | Vol (ml) | Total A280a | Total activity (BU) (106) | Sp actb | Increase in sp actb | Yield (%) |
|---|---|---|---|---|---|---|
| Bacterial culture | 400 | 1.35 × 104 | 8 | 600 | 1 | 100 |
| Step 1c (cation exchange) | 40 | 40 | 7 | 1.7 × 105 | 300 | 85 |
| Step 2d (reverse phase) | ||||||
| First run | 1.4 | 1.25 | 7.5 | 6 × 106 | 1 × 104 | 95 |
| Second run | 1.6 | 1.0e | 9 | 9 × 106 | 1.5 × 104 | 110 |
Total A280 is the A280 multiplied by the volume in milliliters.
Specific activity is total activity (in BU) divided by total A280.
The bacterial culture was applied on a 6-ml SP Sepharose Fast Flow cation-exchange column equilibrated with 20 mM sodium phosphate, pH 5.8. The column was subsequently washed with 15 column volumes of the phosphate buffer and 5 column volumes of the phosphate buffer containing 0.2 M NaCl, and pediocin PA-1 was then eluted with 40 ml of the phosphate buffer containing 1 M NaCl.
Chromatography of the 1 M NaCl fraction from step 1 was performed as described in the Fig. 1 legend.
The amount of pediocin PA-1 purified from a 400-ml bacterial culture was determined to be 300 μg from the UV absorption at 280 nm (1.0) and the molecular extinction coefficients calculated from the contribution of individual amino acid residues.
FIG. 1.
Reverse-phase chromatography of the 1 M NaCl fraction obtained in the cation-exchange chromatography step (step 1, Table 1). After addition of propanol and trifluoroacetic acid to final concentrations of 5 and 0.1% (vol/vol), respectively, the 1 M NaCl fraction from step 1 (Table 1) was applied at a flow rate of 5 ml/min on a 3-ml Resource reverse-phase column. Peptides binding to the column were eluted at a flow rate of 1 ml/min with a linear propanol gradient (shown in the figure) in 0.1% trifluoroacetic acid. Only the fractions under the horizontal bar contained bacteriocin activity, which coincided with the absorbance peak under the bar. The first 42 ml represents the flowthrough fraction that contained substances that did not bind to the column. To prevent fouling of the reverse-phase column, samples applied to the column were cleared of gross contaminants by centrifugation at about 4,000 × g for 10 min.
For comparison, pediocin PA-1 was in parallel also isolated from the same culture using our earlier standard purification procedure (Table 2). Due to the much-higher yields obtained with the new procedure, about 300 μg was purified from 400-ml culture using the new procedure, whereas only about 45 μg (average and standard deviation of 22 earlier experiments were 80 ± 30 μg) was purified using our earlier procedure (Table 1). Moreover, pediocin PA-1 was purified in less than 2 h using the new procedure, whereas our earlier procedure took more than twice that time when starting with a 400-ml culture. This time difference becomes much larger when one starts with larger culture volumes, since the new procedure, in contrast to our earlier procedure, may easily be scaled up with relatively little increase in purification time.
TABLE 2.
Purification of pediocin PA-1 by earlier standard procedure, essentially as described in reference 35
| Fractionation step/fraction | Vol (ml) | Total A280a | Total activity (BU) (106) | Sp actb | Increase in sp actb | Yield (%) |
|---|---|---|---|---|---|---|
| Bacterial culture | 400 | 1.35 × 104 | 8 | 600 | 1 | 100 |
| Step 1c (centrif-ugation) | 400 | 1.35 × 104 | 8 | 600 | 1 | 100 |
| Step 2d (amm sulf ppt) | 75 | 250 | 1 | 4 × 103 | 7 | 15 |
| Step 3e (cation exchange) | 40 | 2.4 | 0.85 | 3.5 × 105 | 600 | 10 |
| Step 4f (HIC) | 10 | 1.0 | 1.5 | 1.5 × 106 | 2.5 × 103 | 20 |
| Step 5g (reverse phase) | ||||||
| First run | 1.3 | 0.20 | 0.9 | 4.5 × 106 | 7.5 × 103 | 10 |
| Second run | 1.3 | 0.15h | 0.7 | 4.7 × 106 | 8 × 103 | 10 |
Total A280 is the A280 multiplied by the volume in milliliters.
Specific activity is total activity (in BU) divided by total A280.
Bacteria were removed from culture by centrifugation at 4,000 × g for 15 min.
Peptides were precipitated (ppt) by adding ammonium sulfate (amm sulf) (400 g per liter) and pelleting the precipitate by centrifugation at 7,000 × g for 20 min.
The fraction from step 2 was applied on a 6-ml SP Sepharose Fast Flow cation-exchange column equilibrated with 20 mM sodium phosphate, pH 5.8. The column was subsequently washed with 40 ml of the phosphate buffer, and pediocin PA-1 was then eluted with 40 ml of 1 M NaCl.
The fraction from step 3 was applied on a 1-ml octyl-Sepharose hydrophobic interaction column (HIC), and pediocin PA-1 was then eluted with 70% (vol/vol) ethanol.
The fraction from step 4 was chromatographed on a reverse-phase column, as described in footnote d to Table 1.
The amount of pediocin PA-1 purified from a 400-ml bacterial culture was determined to be 45 μg, as described in footnote e to Table 1.
Other pediocin-like bacteriocins that have been tested—curvacin A, leucocin A, sakacin P, and a sakacin P mutant—also bound to the cation exchanger column and were recovered with yields of 90% or more after bacterial cultures were passed through the column. However, the optimal concentration of NaCl for washing the column before elution of the bacteriocins with 1 M NaCl (see Table 1, footnote c) differed among these bacteriocins, presumably reflecting their net positive charge. When pediocin PA-1 (net positive charge of about 5 to 6) was being isolated, 0.2 M NaCl could be used without loss of the bacteriocin (less than 1% loss) from the column. When sakacin P (net positive charge of about 3 to 4) was being isolated, the concentration of NaCl had to be reduced to 0.15 M in order to avoid loss of bacteriocin (between 1 and 20% loss at 0.2 M NaCl) when the column was washed. The bacteriocins appeared as a major optical density peak upon reverse-phase chromatography in the second purification step.
Capacity of cation-exchange column: >100-ml cell culture may be applied to 1-ml cation exchanger.
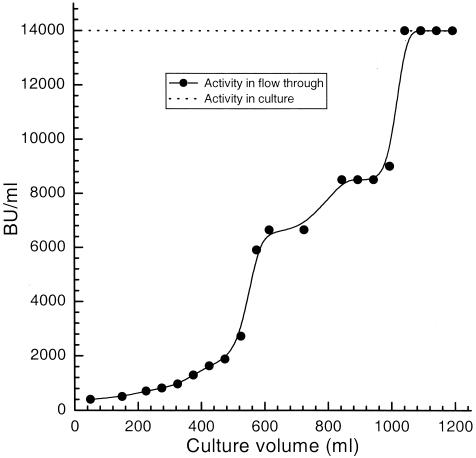
To determine the amount of cell culture that could be applied to the cation exchanger in the first purification step, an overnight stationary bacterial culture producing pediocin PA-1 was applied on a 3-ml SP Sepharose Fast Flow cation-exchange column, and flowthrough fractions were assayed for bacteriocin activity (Fig. 2). Only about 4 and 7% of the bacteriocin activity were lost in the flowthrough fraction when, respectively, 300 and 500 ml of the culture were applied to the column (Fig. 2). Binding of the bacteriocin was, however, significantly reduced when more than 600 ml of culture was applied to the 3-ml column. Of the activity which was applied after 600 ml of culture had passed through the column, less than 50% bound to the column, and essentially no further binding was obtained after 1 liter had passed through the column (Fig. 2). In our bacteriocin purification experiments, no more than 100 ml of culture was applied per 1-ml ion exchanger. After use, the ion exchanger could be regenerated by washing with several column volumes of 2 M NaOH followed by column equilibration.
FIG. 2.
The amount of bacteriocin activity in the flowthrough fraction as a function of the amount of bacterial culture (overnight culture of P. acidilactici LMG 2351; produces pediocin PA-1) that has passed through a 3-ml SP Sepharose Fast Flow cation-exchange column. The horizontal, stippled line indicates the activity in the bacterial culture before it is applied to the column.
Other cationic peptides secreted by bacteria may also be concentrated and purified by simply passing the bacterial cultures through a cation exchanger. After passage of a culture producing the two-peptide bacteriocin lactococcin G (33), 33% of the recovered activity was found in the flowthrough fraction and 67% was found in the 1 M NaCl fraction. Although the percentage of lactococcin G which bound to the column was not as high as that obtained for the pediocin-like bacteriocins, it was higher than that obtained (about 35% binding to the column) in the cation-exchange chromatography step of the earlier standard purification procedure (33). Binding of lactococcin G to cationic exchangers is generally not as efficient as one would expect from the cationic character of the two peptides that constitute lactococcin G, possibly due to interactions between lactococcin G and other components (33). A recovery similar to that obtained with lactococcin G was also obtained when a culture of E. faecium CTC492 was passed through a cation exchanger. This strain produces two bacteriocins: enterocin B and the pediocin-like bacteriocin enterocin A (31). The recovery of the two other bacteriocins that were tested, nisin Z and lactococcin A, was lower, being only about 30 and 15%, respectively. However, there was no effort to optimize chromatography conditions, and optimization, perhaps by reducing the pH of the culture medium before application on the cation exchanger, might possibly improve the recovery.
The influence of the pH on recovery was clearly demonstrated by Callewaert and De Vuyst (4). In their report on the purification of amylovorin L471, crude fermentation medium containing cells was applied directly to an expanded bed cation-exchange column. The highest recovery (30%) was obtained upon applying the bacteriocins to the column at pH 3.6 and eluting it at pH 6.5 (4). The recovery was reduced by 50% or more if both sample application and elution were carried out at the same pH, irrespective of whether this was 3.6 or 6.5 (4). The bacteriocins was lost in the flowthrough fraction when both application and elution were carried out at pH 6.5, whereas the loss appeared to be due to poor release from the column when both application and elution were carried out at pH 3.6 (4). It is our experience that many peptide bacteriocins have affinity to the matrix of commonly used columns. Some bacteriocins, such as plantaracin JK, may interact strongly with the column matrix, and elution requires 3 M guanidine-HCl (1). Other bacteriocins appear to interact weakly with the matrix and may be released under gentler elution conditions. Even for these bacteriocins, however, recovery may in some cases be improved with the use of guanidine-HCl. We consequently recommend that elution with 3 M guanidine-HCl be included as a final elution step upon column chromatography of bacteriocins in order to ascertain that the elution has been complete. The use of guanidine-HCl has one drawback, however, in that guanidine-HCl may interfere with the binding of the bacteriocin to the column in the subsequent chromatography step, thus necessitating dilution or dialysis of the sample.
The new purification procedure has proved to be extremely useful. The short purification time and high yields have enabled us to purify in a few hours milligram quantities of pediocin-like bacteriocins that are needed for our structural studies, whereas this could take up to a week or more using our previous purification protocol.
Acknowledgments
This work was supported by a grant from the Norwegian Research Council.
REFERENCES
- 1.Anderssen, E. L., D. B. Diep, I. F. Nes, V. G. H. Eijsink, and J. Nissen-Meyer. 1998. Antagonistic activity of Lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl. Environ. Microbiol. 64:2269-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson, L., T. Katla, M. Børnslett, V. G. H. Eijsink, and A. Holck. 1998. A system for heterologous expression of bacteriocins in Lactobacillus sake. FEMS Microbiol. Lett. 168:137-143. [DOI] [PubMed] [Google Scholar]
- 3.Biswas, S. R., P. Ray, M. C. Johnson, and B. Ray. 1991. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl. Environ. Microbiol. 57:1265-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callewaert, R., and L. De Vuyst. 1999. Expanded bed adsorption as a unique operation for the isolation of bacteriocins from fermentation media. Bioseparation 8:159-168. [PubMed] [Google Scholar]
- 5.Chen, Y., R. D. Ludescher, and T. J. Montville. 1997. Electrostatic interactions, but not the YGNGV consensus motif, govern the binding of pediocin PA-1 and its fragments to phospholipid vesicles. Appl. Environ. Microbiol. 63:4770-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y., R. Shapira, M. Eisenstein, and T. J. Montville. 1997. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl. Environ. Microbiol. 63:524-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chikindas, M. L., M. J. Garcia-Garcera, A. J. M. Driessen, A. M. Ledeboer, J. Nissen-Meyer, I. F. Nes, T. Abee, W. N. Konings, and G. Venema. 1993. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl. Environ. Microbiol. 59:3577-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras, B. G. L., L. de Vuyst, B. Devreese, K. Busanyova, J. Raymaeckers, F. Bosman, E. Sablon, and E. J. Vandamme. 1997. Isolation, purification, and amino acid sequence of lactobin A, one of the two bacteriocins produced by Lactobacillus amylovorus LMG P-13139. Appl. Environ. Microbiol. 63:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 10.Eijsink, V. G. H., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fimland, G., L. Johnsen, L. Axelsson, M. B. Brurberg, I. F. Nes, V. G. H. Eijsink, and J. Nissen-Meyer. 2000. A C-terminal disulfide bridge in pediocin-like bacteriocins renders bacteriocin activity less temperature dependent and is a major determinant of the antimicrobial spectrum. J. Bacteriol. 182:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fimland, G., O. R. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fimland, G., R. Jack, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1998. The bactericidal activity of pediocin PA-1 is specifically inhibited by a 15-mer fragment that spans the bacteriocin from the center toward the C terminus. Appl. Environ. Microbiol. 64:5057-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fregeau Gallagher, N. L., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 15.Guyonnet, D., C. Fremaux, Y. Cenatiempo, and J. M. Berjeaud. 2000. Method for rapid purification of class IIa bacteriocins and comparison of their activities. Appl. Environ. Microbiol. 66:1744-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, R. E. W. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 17.Hastings, J. W., M. Sailer, K. Johnson, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1991. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 173:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Héchard, Y., B. Dérijard, F. Letellier, and Y. Cenatiempo. 1992. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J. Gen. Microbiol. 138:2725-2731. [DOI] [PubMed] [Google Scholar]
- 19.Henderson, J. T., A. L. Chopko, and D. van Wassenaar. 1992. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch. Biochem. Biophys. 295:5-12. [DOI] [PubMed] [Google Scholar]
- 20.Holck, A., L. Axelsson, S. E. Birkeland, T. Aukrust, and H. Blom. 1992. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb706. J. Gen. Microbiol. 138:2715-2720. [DOI] [PubMed] [Google Scholar]
- 21.Holo, H., Ø. Nilssen, and I. F. Nes. 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173:3879-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen, L., G. Fimland, V. Eijsink, and J. Nissen-Meyer. 2000. Engineering increased stability in the antimicrobial peptide pediocin PA-1. Appl. Environ. Microbiol. 66:4798-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marugg, J. D., C. F. Gonzalez, B. S. Kunka, A. M. Ledeboer, M. J. Pucci, M. Y. Toonen, S. A. Walker, L. C. M. Zoetmulder, and P. A. Vandenbergh. 1992. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0. Appl. Environ. Microbiol. 58:2360-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, K. W., R. Schamber, O. Osmanagaoglu, and B. Ray. 1998. Isolation and characterization of pediocin AcH chimeric protein mutants with altered bactericidal activity. Appl. Environ. Microbiol. 64:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulders, J. W. M., I. J. Boerrigter, H. S. Rollema, R. J. Siezen, and W. M. de Vos. 1991. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur. J. Biochem. 201:581-584. [DOI] [PubMed] [Google Scholar]
- 26.Muriana, P. M., and T. R. Klaenhammer. 1991. Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl. Environ. Microbiol. 57:114-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mørtvedt, C. I., J. Nissen-Meyer, K. Sletten, and I. F. Nes. 1991. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl. Environ. Microbiol. 57:1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nes, I. F., D. B. Diep, L. S. Håvarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 29.Nes, I. F., H. Holo, G. Fimland, H. H. Hauge, and J. Nissen-Meyer. 2001. Unmodified peptide-bacteriocins (class II) produced by lactic acid bacteria, p. 81-115. In C. J. Dutton, M. A. Haxell, H. A. I. McArthur, and R. G. Wax (ed.), Peptide antibiotics: discovery, modes of action and application, section B. Distribution of antimicrobial peptides. Marcel Dekker, Inc., New York, N.Y.
- 30.Nieto Lozano, J. C., J. Nissen-Meyer, K. Sletten, C. Peláz, and I. F. Nes. 1992. Purification and amino acid sequences of a bacteriocin produced by Pediococcus acidilactici. J. Gen. Microbiol. 138:1985-1990. [DOI] [PubMed] [Google Scholar]
- 31.Nilsen, T., I. F. Nes, and H. Holo. 1998. An exported inducer peptide regulates bacteriocin production in Enterococcus faecium CTC492. J. Bacteriol. 180:1848-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nissen-Meyer, J., H. H. Hauge, G. Fimland, V. G. H. Eijsink, and I. F. Nes. 1997. Ribosomally synthesized antimicrobial peptides produced by lactic acid bacteria: their function, structure, biogenesis, and their mechanism of action. Recent Res. Dev. Microbiol. 1:141-154. [PubMed] [Google Scholar]
- 33.Nissen-Meyer, J., H. Holo, L. S. Håvarstein, K. Sletten, and I. F. Nes. 1992. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J. Bacteriol. 174:5686-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nissen-Meyer, J., and I. F. Nes. 1997. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch. Microbiol. 167:67-77. [PubMed] [Google Scholar]
- 35.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH1174 and sakacin P from L. sake LTH673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 36.Vaughan, A., V. G. H. Eijsink, T. F. O' Sullivan, K. O' Hanlon, and D. van Sinderen. 2001. An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J. Appl. Microbiol. 91:131-138. [DOI] [PubMed] [Google Scholar]
- 37.Wang, Y., M. E. Henz, N. L. Fregeau Gallagher, S. Chai, A. C. Gibbs, L. Z. Yan, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 1999. Solution structure of carnobacteriocin B2 and implications for structure-activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry 38:15438-15447. [DOI] [PubMed] [Google Scholar]