Abstract
Mitochondrial cytochrome b was isolated from the dinoflagellate Pfiesteria piscicida, and the utility of the gene for species identification was examined. One of the primer sets designed was shown to be highly specific for P. piscicida. A time step PCR protocol was used to demonstrate the potential of this primer set for quantification of this species.
Pfiesteria piscicida is a heterotrophic dinoflagellate that has been implicated in fish kill events in some estuaries on the east coast of the United States (4, 5). Accurate identification and quantification of P. piscicida and differentiation of this species from morphologically similar dinoflagellates are essential for verifying the association of P. piscicida with fish kills and for studying the population dynamics of this species. Molecular techniques that can be used for rapid and accurate identification and quantification of this species (e.g., quantitative PCR) are highly desirable (13), because the traditional method that relies on thecal plate tabulation and electron microscopy is not feasible for processing a large number of samples.
The mitochondrial cytochrome b (cob) gene has been found to be useful for phylogenetic analysis and identification of many organisms (1, 6, 7, 8, 14, 19) because of its relatively high mutation rate (for reviews see references 2 and 16). In this study, we isolated this gene from dinoflagellates and examined its utility for identifying and quantifying P. piscicida.
Algal cultures and sample collection.
The Pfiesteria spp. and other algae used in this study are listed in Table 1. Pfiesteria spp., Cryptoperidiniopsis sp., and Gyrodinium galatheanum were grown in 15-practical salinity unit (PSU) seawater that was filtered (pore size, 0.45 μm) and autoclaved; the cryptophyte Rhodomonas sp. strain CCMP768 was provided as food for these organisms. The Rhodomonas strain was grown in 15-PSU seawater amended with f/2 nutrients (9). Other algae were grown in f/2 medium that was prepared with 28-PSU seawater. Illumination was provided at a photon flux of approximately 100 microeinsteins m−2 s−1.
TABLE 1.
Algal species that were tested with P. piscicida-specific mitochondrial cytochrome b (Ppcob) and 18S rRNA (Pp18S) primers
| Taxon | Strain (source) | Results obtained with:
|
|
|---|---|---|---|
| Ppcob primers | Pp18S primers | ||
| Pfiesteria piscicida | CCMP1831a | + | + |
| P. piscicida | CCMP1834a | + | + |
| P. piscicida | CCMP1921a | + | + |
| P. piscicida | CCMP1928a | + | + |
| Pfiesteria-like | CCMP1929a | + | + |
| P. piscicida | NCSU113-3 (J. M. Burkholder) | + | + |
| P. shumwayae | P. A. Tester | − | −b |
| Pfiesteria-like | CCMP1829a | − | −b |
| Pfiesteria-like | CCMP1835a | − | −b |
| Cryptoperidiniopsis sp. | CCMP1827a | − | − |
| Cryptoperidiniopsis sp. | CCMP1828a | − | −b |
| Gyrodinium galatheanum | D. K. Stoecker | − | − |
| Prorocentrum minimum | CCMP696a | − | − |
| P. minimum | Exuv (G. Wikfors) | − | − |
| P. minimum | JA9801 (P. M. Glibert) | − | − |
| P. minimum | JA2001 (P. M. Glibert) | − | − |
| P. minimum | P. A. Tester | − | − |
| Alexandrium tamarense | CB307 (D. M. Anderson) | − | − |
| Oxyrrhis marina | Z1 (H. Zhang) | − | − |
| Rhodomonas sp. | CCMP768a | − | − |
Culture obtained from Provasoli-Guillard National Center for Culture of Marine Phytoplankton.
A nonspecific band(s) was generated, and sequencing results indicated that the band(s) was not an 18S rRNA product(s).
When Pfiesteria cultures were in the exponential growth phase, feeding was discontinued for 2 to 3 days and samples were harvested by centrifugation at 3,000 × g for 20 min at 4°C. Samples of all other algae were collected in a similar manner from actively growing cultures.
DNA extraction, cloning, and sequencing.
DNA was extracted by using a previously described protocol (11), with a slight modification of the DNA extraction buffer (0.1 M EDTA, 1% sodium dodecyl sulfate, 200 μg of proteinase K per ml). Partial cob (Ppcob) and cox3 (subunit 3 of cytochrome oxidase, Ppcox3) fragments were obtained by screening a P. piscicida cDNA library (Lin and Zhang, unpublished data). Based on the sequences of these fragments, Ppcob- and Ppcox3-specific primers were designed (PPCOB and PPCOX3, respectively) (Table 2), and these primers were used in PCR performed with genomic DNA as templates to amplify the 5"-terminal and upstream regions of Ppcob. Amplification was carried out by using the following program: 1 min at 95°C, followed by 35 cycles of 20 s at 94°C, 30 s at 58°C, and 1 min at 72°C. PCR products were subcloned into the pCR vector (Invitrogen) and were sequenced by using a BigDye terminator cycle sequencing kit (Applied Biosystems).
TABLE 2.
Primers used in this study
| Primer | Sequence (5"-3") | Source |
|---|---|---|
| PPCOB | GATGTGATAAAGAAGAGACCCCGAAATGAGTTTC | This study |
| PPCOX3 | CCTCCAGAAGGTTTCTATCTTCCAGATCCTTG | This study |
| PPCOB1F | TTCATTATCAATTCCCATTTCTTG | This study |
| PPCOB1R | AAAACCATTATTATTTAAGAGACAC | This study |
| PPCOB2F | AACTACCAACGGTCGAGAAATGC | This study |
| PPCOB2R | TGAAGATAACGTAAACACCATCC | This study |
| PPCOB3F | CTTAATTGGTAACGACAAACAACAG | This study |
| PPCOB3R | AAGAAGAGATCCCGAAATGAGTTTC | This study |
| PP18SF1 | GCCTAAGCTTGTTAAACGGCAATGC | Rublee et al.a |
| PP18SR1 | GGTAATTTGCGCGCCTGCTGCC | This study |
| 18SCOMF | TGCATGGCCGTTCTTAGTTGGTGG | This study |
| 18SCOMR | CACCTACGGAAACCTTGTTACGAC | This study |
See reference 15. This primer is equivalent to primer 65 For.
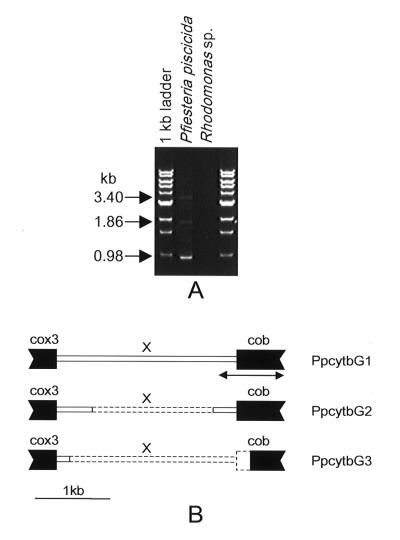
Three structurally different DNA fragments were isolated, and each of these fragments contained cob, cox3, and an unidentified sequence (X region) flanking the 5" end of Ppcob (Fig. 1). The first clone, PpcytbG1 (3,404 bp; GenBank accession number AF357520), contained the majority of the coding region of Ppcob and the 3"-terminal region of cox3, interrupted by a 2,332-bp X region. The second clone, PpcytbG2 (1,861 bp; GenBank accession number AF357521), contained the same Ppcob and cox3 regions as PpcytbG1 but had a deletion (1,516 bp) in the X region. In the third fragment (PpcytbG3; 977 bp; GenBank accession number AF357522), almost the whole X region and 282 bp of the Ppcob 5"-terminal coding region were deleted, suggesting that this may be a pseudogene. The Ppcob sequences in the three fragments were identical.
FIG. 1.
PCR amplification of P. piscicida mitochondrial cytochrome b and its 5" flanking region and organization of the gene fragments. (A) PCR amplification with primers PPCOX3 and PPCOB (Table 2). (B) Organization of the three PCR-amplified gene fragments. cox3, cytochrome oxidase subunit 3; cob, Ppcob; X, sequence that does not exhibit similarity to known genes in DNA databases. Coding regions are indicated by solid boxes. The dashed lines in the X region in PpcytbG2 and PpcytbG3 indicate deleted regions. The arrow indicates the region used to design P. piscicida-specific primers.
Phylogenetic analysis.
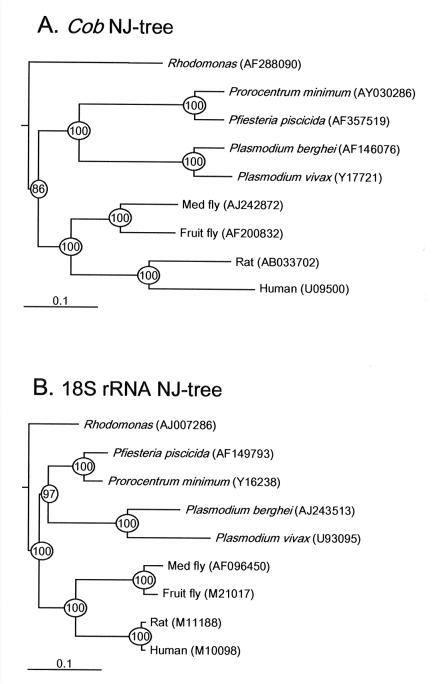
A comparison of the Ppcob genomic sequence with the corresponding sequences of other organisms (Fig. 2) indicated that the Ppcob sequence exhibited about 94% identity to the partial cob sequence of the dinoflagellate Prorocentrum minimum CCMP696, 60% identity to protozoan sequences, and <55% identity to the sequences of other eukaryotes. In contrast, the 18S rRNA sequence of P. piscicida exhibited 95% identity to the P. minimum sequence and >70% identity to the sequences of other eukaryotes.
FIG. 2.
Rooted neighbor-joining (NJ) trees based on nucleotide sequences. (A) Tree constructed by using genomic sequences encoding cytochrome b (cob) of dinoflagellates and other organisms. (B) Tree constructed by using 18S rRNA of dinoflagellates and other organisms. Scale bars = 0.1 nucleotide substitution per site. The circled numbers are bootstrap confidence values based on 1,000 replicates. The numbers in parentheses are accession numbers.
Phylogenetic trees were constructed by using the nucleotide sequences of both cob and 18S rRNA and the neighbor-joining method (17) and were corrected by the Kimura method (10); the trees were rooted with the green alga Scenedesmus obliquus (cob accession number, X17375; 18S rRNA accession number, AB037088). Sequences were aligned by using the CLUSTAL W (version 1.8) server at the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp/Welcome-e.html) and the default values, and the alignments were adjusted by using the Sea View program to maintain codon integrity prior to analysis with the Phylo_win package. The reliability of the tree topology was evaluated by bootstrap analysis with 1,000 replicates. The tree constructed by using the cob genes of dinoflagellates and other organisms showed clear clustering of P. piscicida with P. minimum (the bootstrap values for this cluster were 100%) (Fig. 2A). This tree also showed that there is a close relationship to the apicomplexan Plasmodium. The 18S rRNA tree and the cob gene tree indicated similar phylogenetic relationships for all of the organisms (Fig. 2B). However, the branch lengths in the cob tree were more even than those in the 18S rRNA tree, in agreement with the recent finding that mitochondrial genes behave more like a molecular clock and can be ideal genes for phylogenetic analysis (16).
Design and test of P. piscidida-specific primers.
All of the primers used in this study are listed in Table 2. Based on a multiple-sequence alignment of cob nucleotide sequences of P. piscicida and other organisms, the following species-specific primers were designed for P. piscicida: PPCOBF1 (forward primer) and PPCOBR1 (reverse primer). The other primers (forward primers PPCOBF2 and PPCOBF3 and reverse primers PPCOBR2 and PPCOBR3) were designed based on the sequences of the 5"-terminal and upstream regions of Ppcob. For comparison, the following P. piscicida-specific primers for 18S rRNA (Pp 185) were also designed based on the reported sequence (13, 15): PP18SF1 and PP18SR1. The forward primer (PP18SF1) was Pfiesteria specific and identical to primer 65 For designed by Rublee et al. (15), while the reverse primer (PP18SR1) was universal for eukaryotes. As a control for PCR, a set of universal primers for eukaryotic 18S rRNA (18SCOMF and 18SCOMR) was also designed (Table 2).
PCR were performed by using the specific primers and approximately 50 ng of genomic DNA from each species or strain (Table 1). Amplification was carried out by using the following program: 1 min at 95°C, followed by 35 cycles of 20 s at 94°C, 30 s at 60°C, and 40 s at 72°C. PCR products obtained with both cob and 18S rRNA were sequenced directly.
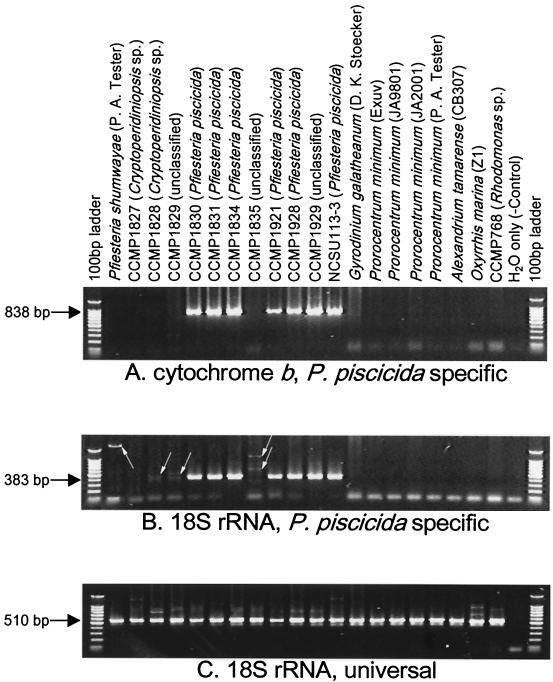
The specificities and sensitivities of the primer sets tested varied slightly. Primers PPCOB3F and PPCOB3R appeared to be the most specific (Fig. 3), while primers PPCOB2F and PPCOB2R appeared to be the most sensitive (data not shown). Since specificity is more important, the PPCOB3F-PPCOB3R primer pair was used for further tests. The Ppcob PCR results were consistently positive when DNA templates were derived from cultures previously identified as P. piscicida (CCMP1830, CCMP1831, CCMP1834, CCMP1921, CCMP1928, and NCSU113-3) (Fig. 3A). The nucleotide sequences of all of the PCR fragments were identical, in complete agreement with the results of P. piscicida-specific 18S rRNA PCR (Fig. 3B).
FIG. 3.
PCR amplification with P. piscicida-specific and universal primer sets. (A) P. piscicida-specific mitochondrial cytochrome b primer set (primers PPCOB3F and PPCOB3R). (B) P. piscicida-specific 18S rRNA primer set (primers PP18SF1 and PP18SR1). (C) Eukaryote universal 18S rRNA primer set (primers 18SCOMF and 18SCOMR).
The P. piscicida-specific cob primers never produced positive results in cases in which the Pp18S primers gave negative results. The Ppcob and Pp18S primers produced no DNA product with a blank control (H2O) and with samples of Rhodomonas, the Pfiesteria-like dinoflagellates Cryptoperidiniopsis sp. strains CCMP1827 and CCMP1828, and other dinoflagellates. The absence of a PCR product in these cases was not due to poor quality of the DNA, as the same DNA templates yielded abundant PCR products when a universal 18S rRNA primer set was used (Fig. 3C). On the contrary, the Pp18S primers sometimes generated weak DNA bands (e.g., with Pfiesteria shumwayae, CCMP1828, CCMP1829, and CCMP1835) (Fig. 3B), while the Ppcob primers did not produce positive results (Fig. 3A) and sequencing results showed that the weak bands were nonspecific products.
The clear absence of PCR products when the Ppcob primers were used and the sequencing results obtained with the faint Pp18S PCR bands suggested that CCMP1829 and CCMP1835, two unclassified Pfiesteria-like dinoflagellates, were unlikely to be P. piscicida. In contrast, when CCMP1929, another unclassified Pfiesteria-like dinoflagellate, was examined, PCR with both Ppcob and Pp18S primers generated P. piscicida type bands (Fig. 3A and B), and the nucleotide sequences of the DNA fragments were identical to the nucleotide sequences of the DNA fragments of P. piscicida strains (GenBank accession numbers AF357519 [cob] and AF149793 [18S rRNA]). These results suggest that CCMP1929 is a P. piscicida strain.
Time step PCR to determine sensitivity and quantitation capability.
To test the ability of the Ppcob primers to quantify P. piscicida, genomic DNA was isolated from 2 × 107 P. piscicida cells, dissolved in 500 μl of TE buffer, and used as a stock preparation. Serial dilutions of this DNA stock preparation equivalent to 0.2, 1, 5, 25, 125, 625, 3,125, and 15,625 P. piscicida cells were PCR amplified by using primers PPCOB3F and PPCOB3R. Each reaction mixture was overlaid with 1 drop of mineral oil. A 5-μl aliquot was removed from each reaction mixture at cycles 25 and 30 during the 35-cycle PCR (called time step PCR in this study).
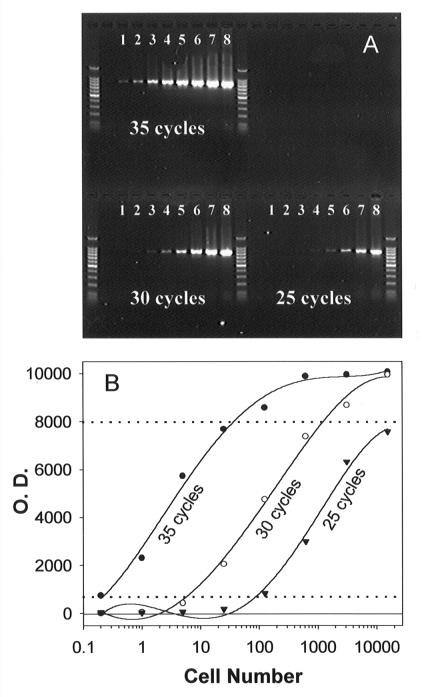
The results of the time step PCR when the DNA serial dilutions were used showed that there was a good linear correlation between cell number and the quantity of the PCR product (Fig. 4). After 35 cycles, as little as 0.2 cell was detected, and the PCR product reached a plateau when DNA from more than 125 cells was used. A linear increase in the amount of the PCR product was obtained for 5 to 625 cells with 30 cycles. When fewer cycles (25 cycles) were used, no saturation problem was encountered even for the highest number of cells (15,625 cells), although the detection limit increased to 125 cells. A wide range of cell numbers (0.2 to 15,625 cells) can be quantified by this method.
FIG. 4.
Time step PCR of Ppcob and standard curves. (A) PCR performed with DNA templates equivalent to 0.2 (lanes 1), 1 (lanes 2), 5 (lanes 3), 25 (lanes 4), 125 (lanes 5), 625 (lanes 6), 3,125 (lanes 7), and 15,625 (lanes 8) P. piscicida cells and the PPCOB3F-PPCOB3R primer set. A 5-μl aliquot removed from a reaction tube after 25, 30, or 35 cycles was loaded into each lane. (B) Plot of DNA band intensity (optical density [O.D.]) versus log cell number used in PCR. The dashed lines indicate the range of cell numbers that can be quantified by the time step PCR protocol.
Tests with utermohl’s solution-preserved and field-collected samples.
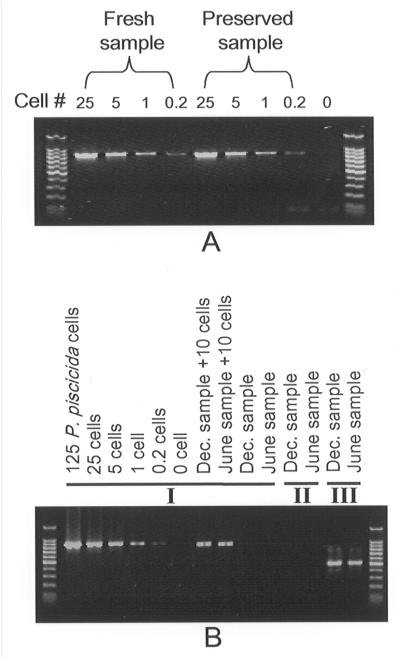
We examined the potential effects on PCR of Utermohl's solution (18), a fixative commonly used to preserve plankton samples collected in the field. Two P. piscicida culture samples were collected; one was fixed in Utermohl's solution for 2 months at room temperature before DNA extraction, and the other was processed immediately. The Ppcob PCR results obtained with the fresh samples and the samples preserved with Utermohl's solution were similar (Fig. 5A).
FIG. 5.
PCR of Utermohl's solution-preserved P. piscicida cells and field-collected samples. (A) PCR of fresh and Utermohl's solution-preserved P. piscicida cells. (B) PCR of preserved field samples collected from Avery Point in November 2000 and June 2001. I, P. piscicida-specific mitochondrial cytochrome b primer set (primers PPCOB3F and PPCOB3R); II, P. piscicida-specific 18S rRNA primer set (primers PP18SF1 and PP18SR1); III, eukaryote universal 18S rRNA primer set (primers 18SCOMF and 18SCOMR).
Field samples were collected in December 2000 and June 2001 from the dock at the Avery Point Campus of the University of Connecticut, which is located at the eastern end of Long Island Sound, where no P. piscicida has been found. Examination of the samples by light microscopy revealed that diatoms were dominant and that few dinoflagellates were present. Samples were fixed with Utermohl's solution, and 50-ml subsamples were centrifuged to obtain cell pellets. DNA was extracted and dissolved in 50 μl of TE buffer, and 1 μl of each DNA solution (equivalent to 1 ml of a field sample) was used in a PCR performed as described above. In parallel, the same amounts of the field DNA samples were spiked with P. piscicida stock DNA equivalent to the DNA from 10 cells and analyzed. While PCR with both Ppcob and Pp18S primers yielded negative results for the field samples (Fig. 5B), samples spiked with P. piscicida cells yielded positive results, and the sensitivity was as expected.
Utility of Ppcob AND Pp18S in ecological studies.
High levels of accuracy and sensitivity are required to detect P. piscicida in the field, especially because P. piscicida normally is only a minor component of the plankton community. Results of this study showed that the Ppcob-specific primer set consisting of primers PPCOB3F and PPCOB3R meets these requirements. The accuracy of this primer set was manifested by the PCR results obtained with several P. piscicida strains and the unclassified P. piscicida-like dinoflagellates. The high level of sensitivity was shown by the low detection limit, 0.2 cell ml−1 based on our time step PCR protocol, which is far lower than the minimum P. piscicida concentration that causes ichthyotoxicity (more than 250 cells ml−1) (3, 4) and concentrations often found in affected estuaries (>40 cells ml−1) (3, 4, 12).
In the natural environment, there are numerous unknown plankton species that may sometimes produce spurious positive results in a survey based on a single gene sequence. As mentioned above, occasional nonspecific positive results were obtained in our Pp18S rRNA experiments. Although no nonspecific positive results have been found for Ppcob so far, the possibility that some nonspecific positive results may occur cannot be eliminated. Use of both Ppcob and 18S rRNA simultaneously, as demonstrated in our tests, can provide more reliable identification of P. piscicida.
In addition, often field-collected samples may need to be preserved and stored for some period of time before they can be processed. The results of this study showed that storage of samples fixed with Utermohl's solution for up to 2 months did not have noticeable effects on the PCR results compared with the results obtained with fresh unfixed samples. Finally, unknown organic materials present in field samples often coprecipitate with DNA and inhibit PCR, and variations may occur between PCR and between gels. Thus, we propose that for each field sample two subsamples should be analyzed and that to one of the subsamples a known amount of P. piscicida DNA should be added. A standard PCR in which serial dilutions of a known amount of DNA are used should also be performed simultaneously with the PCR performed with samples, and standard curves, such as those shown in Fig. 4B, should be constructed. With these precautions, the use of Ppcob and Pp18S to identify P. piscicida and the use of Ppcob to quantify P. piscicida are feasible with the simple time step PCR.
Acknowledgments
We thank Robert Andersen of Bigelow Laboratory and JoAnne Burkholder of North Carolina State University for P. piscicida and Pfiesteria-like cultures, Donald M. Anderson of WHOI for the Alexandrium tamarense culture, Patricia Tester of National Ocean Services for the P. shumwayae culture, and Gary Wikfors of National Marine Fisheries Service, and Patricia M. Glibert and Diane K. Stoecker of Horn Point Environmental Laboratory for Prorocentrum and Gyrodinium cultures. We also thank Timothy Feinstein and Keri Costa for assistance with growing the cultures and collecting field samples.
This research was supported by NOAA-ECOHAB grant NA86OP0491.
Footnotes
ECOHAB publication number 34.
REFERENCES
- 1.Akihito, I. A., T. Kobayashi, K. Ikeo, T. Imanishi, H. Ono, Y. Umehara, C. Hamamatsu, K. Sugiyama, Y. Ikeda, Y. Sakemoto, A. Fumihito, S. Ohno, and T. Gojobori. 2000. Evolutionary aspects of gobioid fishes based upon a phylogenetic analysis of mitochondrial cytochrome b genes. Gene 259:5-15. [DOI] [PubMed] [Google Scholar]
- 2.Avise, J. C. 1994. Molecular markers, natural history and evolution. Chapman & Hall, New York, N.Y.
- 3.Burkholder, J. M., and H. B. Glasgow, Jr. 1997. Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: behavior, impacts, and environmental controls. Limnol. Oceanogr. 42:1052-1055. [Google Scholar]
- 4.Burkholder, J. M., H. B. Glasgow, and N. Deamer-Melia. 2001. Overview and present status of the toxic Pfiesteria piscicida (Dinophyceae). Phycologia 40:186-214. [Google Scholar]
- 5.Burkholder, J. M., E. J. Noga, C. H. Hobbs, and H. B. Glasgow, Jr. 1992. New “phantom” dinoflagellate is the causative agent of major estuarine fish kills. Nature 358:407-410. [DOI] [PubMed] [Google Scholar]
- 6.Callejas, C., and M. D. Ochando. 2000. Recent radiation of Iberian barbel fish (Teleostei, Cyprinidae) inferred from cytochrome b genes. J. Hered. 91:283-288. [DOI] [PubMed] [Google Scholar]
- 7.Conroy, C. J., and J. A. Cook. 1999. MtDNA evidence for repeated pulses of speciation within arvicoline and murid rodents. J. Mammal. Evol. 6:221-245. [Google Scholar]
- 8.Conway, D. J., C. Fanello, J. M. Lloyd, B. M. A. Al-Joubori, A. H. Baloch, S. D. Somanath, C. Roper, A. M. J. Oduola, B. Mulder, M. M. Povoa, B. Singh, and A. W. Thomas. 2000. Origin of Plasmodium falciparum malaria is traced by mitochondrial DNA. Mol. Biochem. Parasitol. 111:163-171. [DOI] [PubMed] [Google Scholar]
- 9.Guillard, R. R., and J. H. Ryther. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve.) Gran. Can. J. Microbiol. 18:229-239. [DOI] [PubMed] [Google Scholar]
- 10.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. E vol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 11.Lin, S., and E. J. Carpenter. 1999. A PSTTLRE-form of cdc2-like gene in the marine microalga Dunaliella tertiolecta. Gene 239:39-48. [DOI] [PubMed] [Google Scholar]
- 12.Marshall, H. G., D. Seaborn, and J. Wolny. 1999. Monitoring results for Pfiesteria piscicida and Pfiesteria-like organisms from Virginia waters in 1998. Va. J. Sci. 50:287-298. [Google Scholar]
- 13.Oldach, D., C. F. Delwiche, K. S. Jakobsen, T. Tengs, E. G. Brown, J. W. Kempton, E. F. Schaefer, H. A. Bowers, H. B. Glasgow, Jr., J. M. Burkholder, K. A. Steidinger, and P. A. Rublee. 2000. Heteroduplex mobility assay-guided sequence discovery: elucidation of the small subunit (18S) rRNA sequences of Pfiesteria piscicida and related dinoflagellates from complex algal culture and environmental sample DNA pools. Proc. Natl. Acad. Sci. USA 97:4303-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson, O. P., and M. F. Smith. 1999. Genetic similarity between Akodon olivaceus and Akodon xanthorhinus (Rodentia: Muridae) in Argentina. J. Zool. 247:43-52. [Google Scholar]
- 15.Rublee, P. A., J. Kempton, E. Schaefer, J. M. Burkholder, H. B. Glasgow, and D. W. Oldach. 1999. PCR and FISH detection extends the range of Pfiesteria piscicida in estuarine waters. Va. J. Sci. 50:325-326. [Google Scholar]
- 16.Saccone, C., C. Gissi, C. Lanave, A. Larizza, G. Pesole, and A. Reyes. 2000. Evolution of the mitochondrial genetic system: an overview. Gene 261:153-159. [DOI] [PubMed] [Google Scholar]
- 17.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. E vol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 18.Utermohl, H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 9:1-38. [Google Scholar]
- 19.Zhang, H., N. Mikawa, Y. Yamada, N. Horie, A. Okamura, T. Utoh, S. Tanaka, and T. Motonobu. 1999. Detection of foreign eels in the natural waters of Japan by PCR. Fish. Sci. 65:684-686. [Google Scholar]