Abstract
A total of 361 Escherichia coli O157 isolates, recovered from humans, cattle, swine, and food during the years 1985 to 2000, were examined to better understand the prevalence of antimicrobial resistance among these organisms. Based on broth microdilution results, 220 (61%) of the isolates were susceptible to all 13 antimicrobials tested. Ninety-nine (27%) of the isolates, however, were resistant to tetracycline, 93 (26%) were resistant to sulfamethoxazole, 61 (17%) were resistant to cephalothin, and 48 (13%) were resistant to ampicillin. Highest frequencies of resistance occurred among swine isolates (n = 70), where 52 (74%) were resistant to sulfamethoxazole, 50 (71%) were resistant to tetracycline, 38 (54%) were resistant to cephalothin, and 17 (24%) were resistant to ampicillin. Based on the presence of Shiga toxin genes as determined by PCR, 210 (58%) of the isolates were identified as Shiga toxin-producing E. coli (STEC). Among these, resistance was generally low, yet 21 (10%) were resistant to sulfamethoxazole and 19 (9%) were resistant to tetracycline. Based on latex agglutination, 189 (52%) of the isolates were identified as E. coli O157:H7, among which 19 (10%) were resistant to sulfamethoxazole and 16 (8%) were resistant to tetracycline. The data suggest that selection pressure imposed by the use of tetracycline derivatives, sulfa drugs, cephalosporins, and penicillins, whether therapeutically in human and veterinary medicine or as prophylaxis in the animal production environment, is a key driving force in the selection of antimicrobial resistance in STEC and non-STEC O157.
Escherichia coli is commonly found in human and animal intestinal tracts and, as a result of fecal contamination or contamination during food animal slaughter, is often found in soil, water, and foods. Shiga toxin-producing E. coli (STEC) O157 has emerged as a public health threat following its initial identification as a pathogen in a 1982 outbreak of illness associated with the consumption of undercooked ground beef (19). Specifically, E. coli O157:H7 and O157:NM (nonmotile) are recognized as major etiologic agents in hemorrhagic colitis (HC) and hemolytic-uremic syndrome (HUS) in humans (22). The U.S. Centers for Disease Control and Prevention estimates that E. coli O157:H7 causes approximately 73,400 illnesses and 60 deaths each year in the United States (11). Recent reports indicate that antimicrobial resistance of E. coli O157 is on the rise (1, 4, 6, 10, 13, 20, 33). Yet the extent to which different antimicrobial use practices have contributed to the increase in antimicrobial resistance is not clear.
The usefulness of antimicrobial therapy for STEC infections is unresolved. Because antimicrobials may lyse bacterial cell walls, thereby liberating Shiga toxins (9, 24, 29), and/or cause increased expression of Shiga toxin genes in vivo (32), they are not recommended for treating STEC O157 infections. However, recent studies suggest that some antimicrobials, if administered early in the course of infection, may prevent disease progression to HUS (5, 8, 21). Because STEC infections are not aggressively treated with antimicrobial therapy, many isolates may yet be susceptible to numerous antimicrobials.
In addition to their therapeutic use in human and veterinary medicine, antimicrobials are routinely used for disease prevention and growth promotion in animal production. This practice leads to the inevitable selection of antimicrobial resistance among commensals in the intestinal tracts of food animals, which poses a public health threat (27). For instance, antimicrobial-resistant bacteria from food animals may colonize the human population via the food chain, contact through occupational exposure, or waste runoff from animal production facilities (23, 27). Food animals, in particular mature cattle, which may be asymptomatic carriers of E. coli O157, including STEC (12), when exposed to antimicrobial agents in the animal production environment, may serve as a reservoir of antimicrobial-resistant bacteria.
This study was initiated to determine the prevalence of STEC and characterize antimicrobial resistance among 361 isolates of E. coli O157 recovered from humans, swine, cattle, and food during the past 15 years. The objective was to better understand the prevalence of antimicrobial resistance in STEC and non-STEC O157 from these different sources.
MATERIALS AND METHODS
Bacterial strains.
A total of 361 E. coli O157 isolates, collected from humans, cattle, swine, and food during the years 1985 to 2000, were used in this study (Table 1). The isolates were obtained from the collection of the E. coli Reference Center (ECRC) located at The Pennsylvania State University. The ECRC functions as a reference laboratory accepting clinical samples for diagnostic purposes; thus the samples were originally collected as suspected etiologic agents. One hundred thirty-one (36%) of the isolates were recovered from humans, 133 (37%) were recovered from cattle, 70 (19%) were recovered from swine, and 27 (8%) were recovered from food (17 isolates from beef, 2 from pork, and 8 from food sources not further specified). One hundred ninety-five (54%) of the isolates were collected during the years 1996 to 2000, 70 (19%) were collected during 1991 to 1995, and 96 (27%) were collected during 1985 to 1990. Forty-six (35.1%) of the human isolates were from Massachusetts; 26 (19.8%) were from Argentina; 20 (15.3%) were from Pennsylvania; 38 (29%) were from eight states within the United States, the District of Columbia, Brazil, Japan, and Saudi Arabia; and 1 (0.8%) was from an unreported location. Eighty-seven (65.4%) of the cattle isolates were from Virginia and 46 (34.6%) were from 13 other states within the United States. Twenty-six (37.1%) of the swine isolates were from Iowa, 15 (21.4%) were from Nebraska, and 29 (41.4%) were from seven other states within the United States, Puerto Rico, South Korea, and Hungary. Eight (29.6%) of the food isolates were from Maryland, 5 (18.5%) were from California, 13 (48.1%) were from four other states within the United States, and 1 (3.7%) was from an unreported location. Prior to antimicrobial susceptibility testing, each isolate was streaked for isolation on Trypticase soy agar supplemented with 5% defibrinated sheep's blood (Becton Dickinson, Sparks, Md.). Stock cultures were placed in brucella broth (Becton Dickinson)-glycerol (80:20) and stored at −80°C.
TABLE 1.
Source, phenotype, and year of isolation of E. coli O157 isolates
| Source of isolation | No. of isolates
|
||||||
|---|---|---|---|---|---|---|---|
| Total | With phenotype:
|
Isolated from:
|
|||||
| STECa | Non-STEC | 1985-1990 | 1991-1995 | 1996-2000 | |||
| Humans | 131 | 89 | 42 | 17 | 31 | 83 | |
| Cattle | 133 | 100 | 33 | 12 | 14 | 107 | |
| Swine | 70 | 4 | 66 | 61 | 5 | 4 | |
| Food | 27 | 17 | 10 | 6 | 20 | 1 | |
| Total | 361 | 210 | 151 | 96 | 70 | 195 | |
Determined by the presence of stx1 or stx2 (see Materials and Methods).
Serotyping.
The presence of O and H antigens was tested for by using the standard method as described by Orskov et al. (16). In brief, 20-μl portions of antisera for 181 O types and 52 H types were used for serotype determination in 96-well titer plates. Heat-treated bacteria were added to the antisera and incubated at 50°C for 24 h. Serotyping was based on a positive agglutination reaction.
DNA isolation and PCR amplification.
Cultures were grown overnight at 37°C on veal infusion agar (Becton Dickinson). A small amount of the culture was resuspended in 200 μl of distilled water, heated to 99°C for 15 min, and centrifuged for 2 min at 12,000 × g. The resulting supernatant was used as a template for PCR. Shiga toxin genes stx1 and stx2 were detected by multiplex PCR. Oligonucleotide primer sets for stx1 included forward primer 5" ACA CTG GAT GAT CTC AGT GG 3" and reverse primer 5" CTG AAT CCC CCT CCA TTA TG 3" (26). For stx2, the primer set included forward primer 5" GGC ACT GTC TGA AAC TGC TCC 3" and reverse primer 5" TCG CCA GTT ATC TGA CAT TCT G 3" (17). Reaction contents for each 11-μl PCR consisted of 50 ng of stx1 primers/μl, 20 ng of stx2 primers/μl, 0.18 mM each deoxyribonucleotide, 4.0 mM MgCl2, 0.4 U of Taq DNA polymerase (PGC Scientific, Gaithersburg, Md.), 50 mM Tris-HCl (pH 8.3), 250 μg of bovine serum albumin/ml, 2% sucrose, and 0.1 mM cresol red (Idaho Technologies, Salt Lake City, Utah). The PCR was done using the rapid-cycle DNA amplification method (28) and consisted of 30 cycles of template denaturation at 94°C, primer annealing at 54°C, and primer extension at 74°C for 30 s. All reactions were performed using an Idaho Technologies Rapid Cycler brand thermal cycler. Amplified products were electrophoresed in 1% agarose gels at 200 V for 30 s and visualized under UV light. Positive samples were identified based on the presence of bands of appropriate sizes compared to the STEC O157 positive control (ATCC 43895).
Antimicrobial susceptibility determination.
Antimicrobial susceptibility profiles were performed via broth microdilution in accordance with National Committee for Clinical Laboratory Standards guidelines (16) using the PASCO MIC/ID system (Becton Dickinson) and recommended quality control organisms. The following antimicrobial agents were included in the panels: chloramphenicol, ampicillin, amoxicillin-clavulanic acid, cephalothin, ceftiofur, ceftriaxone, gentamicin, sulfamethoxazole, trimethoprim-sulfamethoxazole, nalidixic acid, ciprofloxacin, tetracycline, and cefoxitin (Table 2).
TABLE 2.
Class, dilution range, and resistance breakpoints of tested antimicrobialsa
| Class and antimicrobial | Dilution range tested (μg/ml) | Resistance breakpoint (μg/ml) |
|---|---|---|
| Cephalosporins | ||
| Cefoxitin | 1-32 | 32 |
| Ceftiofur | 1-16 | 8 |
| Ceftriaxone | 0.06-64 | 64 |
| Cephalothin | 1-32 | 32 |
| Penicillins | ||
| Amoxicillin-clavulanic acid | 0.25/0.12-32/16 | 32/16 |
| Ampicillin | 0.25-32 | 32 |
| Sulfonamides and potentiated sulfonamides | ||
| Sulfamethoxazole | 32-512 | 512 |
| Trimethoprim-sulfamethoxazole | 0.06/1.19-4/76 | 4/76 |
| Quinolones and fluoroquinolones | ||
| Ciprofloxacin | 0.004-8 | 4 |
| Nalidixic acid | 2-256 | 32 |
| Phenicols | ||
| Chloramphenicol | 1-32 | 32 |
| Aminoglycosides | ||
| Gentamicin | 0.25-16 | 16 |
| Tetracycline | 1-16 | 16 |
All antimicrobial resistance testing was performed according to National Committee for Clinical Laboratory Standards guidelines (14) using E. coli ATCC 25922 and ATCC 35218, Enterococcus faecalis ATCC 51299, and Pseudomonas aeruginosa ATCC 27853 as controls.
Data analysis.
All data were entered into computer spreadsheets (Access and Excel; Microsoft, Bellevue, Wash.), which were used to generate descriptive statistics including graphs and charts of antimicrobial resistance prevalence.
RESULTS
Serotypes of E. coli O157 isolates.
Table 1 summarizes information regarding the 361 E. coli O157 isolates analyzed during this study. The majority (191; 53%) of the isolates were identified as H type H7, 61 (17%) were identified as NM (nonmotile), and 17 (5%) were identified as H43. Fifty-three (15%) of the isolates did not react to any of the 52 H antisera. The serotypes of 31 (9%) of the isolates were not assayed, whereas the remaining 14 (3%) were H types H4, H11, H12, H16, H19, H32, H42, H44, H54, and U (data not shown).
Prevalence of STEC.
Based on the presence of the stx1 and/or stx2 gene(s), 210 (58%) of the isolates were characterized as STEC (Table 1). The highest prevalence of STEC was found among isolates from cattle (75.2%; n = 133), humans (67.9%; n = 131), and food (63%; n = 27). Conversely, a small number (5.7%) of swine isolates (n = 70) were characterized as STEC.
Source of isolation versus antimicrobial resistance.
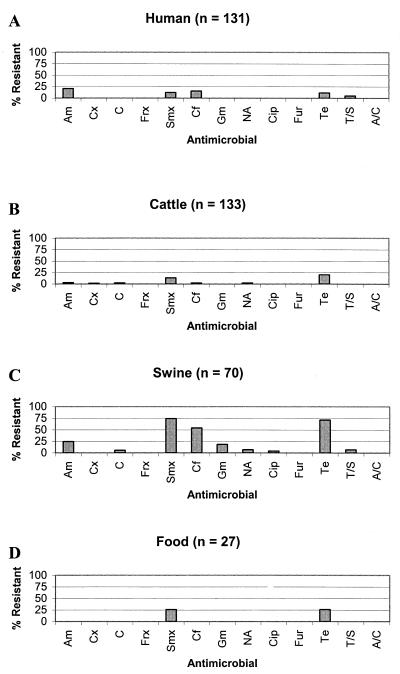
Prevalence of antimicrobial agent resistance among E. coli O157 isolates from humans, cattle, swine, and food is listed in Fig. 1. Among those isolates recovered from humans (n = 131), 27 (21%) were resistant to ampicillin, 16 (12%) were resistant to sulfamethoxazole, 20 (15%) were resistant to cephalothin, 15 (12%) were resistant to tetracycline, and 7 (5%) were resistant to trimethoprim-sulfamethoxazole (Fig. 1A). Similar to results for human O157 isolates, 18 (14%) of the cattle isolates (n = 133) were resistant to sulfamethoxazole. However, 27 (20%) of the isolates were resistant to tetracycline. A much smaller proportion of cattle isolates were resistant to ampicillin (3%); chloramphenicol, cephalothin, and nalidixic acid (2.3% each); cefoxitin (1.5%); and ciprofloxacin, trimethoprim-sulfamethoxazole, and amoxicillin-clavulanic acid (0.1% each) (Fig. 1B). Interestingly, the highest prevalence of resistance was observed among O157 isolates recovered from swine (Fig. 1C). Fifty-two (74%) of the swine isolates (n = 70) were resistant to sulfamethoxazole, 50 (71%) were resistant to tetracycline, 38 (54%) were resistant to cephalothin, and 17 (24%) were resistant to ampicillin. Lower prevalence of resistance to chloramphenicol (5.7%), gentamicin (18.6%), nalidixic acid (7.1%), ciprofloxacin (4.3%), and trimethoprim-sulfamethoxazole (7.1%) was observed. Among food isolates (n = 27), 7 (26%) were resistant to sulfamethoxazole and 7 (26%) were resistant to tetracycline. All food isolates were susceptible to each of the remaining 11 antimicrobials (Fig. 1D).
FIG. 1.
Antimicrobial resistance among E. coli O157 isolates recovered from humans (A), cattle (B), swine (C), and food (D). Am, ampicillin; Cx, cefoxitin; C, chloramphenicol; Frx, ceftriaxone; Smx, sulfamethoxazole; Cf, cephalothin; Gm, gentamicin; NA, nalidixic acid; Cip, ciprofloxacin; Fur, ceftiofur; Te, tetracycline; T/S, trimethoprim-sulfamethoxazole; A/C, amoxicillin-clavulanic acid.
Antimicrobial resistance among STEC versus non-STEC isolates.
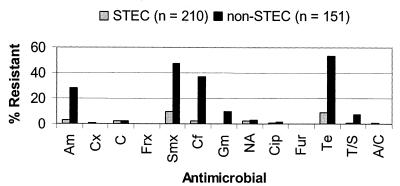
Figure 2 summarizes the prevalence of antimicrobial resistance among STEC and non-STEC O157 isolates. Resistance among non-STEC isolates was higher than among STEC isolates for ampicillin, sulfamethoxazole, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole. For cephalothin, chloramphenicol, nalidixic acid, ciprofloxacin, and amoxicillin-clavulanic acid, the prevalence of resistance among STEC isolates was similar to that among non-STEC isolates. All isolates, whether STEC or non-STEC, were susceptible to ceftriaxone and ceftiofur.
FIG. 2.
Comparison of levels of antimicrobial agent resistance among STEC and non-STEC isolates. STEC isolates were characterized by the presence of stx1 or stx2 (see Materials and Methods). Am, ampicillin; Cx, cefoxitin; C, chloramphenicol; Frx, ceftriaxone; Smx, sulfamethoxazole; Cf, cephalothin; Gm, gentamicin; NA, nalidixic acid; Cip, ciprofloxacin; Fur, ceftiofur; Te, tetracycline; T/S, trimethoprim-sulfamethoxazole; A/C, amoxicillin-clavulanic acid.
Antimicrobial resistance among E. coli O157:H7 isolates.
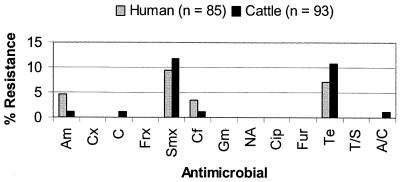
Serological analysis indicated that 191 (53%) of 361 isolates were E. coli O157:H7. Of these, 93 (49.2%) were isolated from cattle, 85 (45%) were isolated from humans, and 11 (5.8%) were isolated from food (9 isolates from beef, and 2 from food sources not further specified). E. coli O157:H7 was not identified from any of the 17 swine STEC isolates. All E. coli O157:H7 food isolates were susceptible to all of the antimicrobials tested in this study. Figure 3 presents the prevalence of antimicrobial resistance among E. coli O157:H7 isolates from humans and cattle. The prevalence of resistance among human O157:H7 isolates was similar to that among cattle isolates for ampicillin (5 versus 1%), cephalothin (4 versus 1%), chloramphenicol (0 versus 1%), sulfamethoxazole (9 versus 12%), tetracycline (7 versus 11%), and amoxicillin-clavulanic acid (0 versus 1%). All E. coli O157:H7 isolates, regardless of the source of isolation, were susceptible to cefoxitin, ceftriaxone, gentamicin, nalidixic acid, ciprofloxacin, ceftiofur, and trimethoprim-sulfamethoxazole.
FIG. 3.
Comparison of levels of antimicrobial resistance among E. coli O157:H7 isolates recovered from humans and cattle. Am, ampicillin; Cx, cefoxitin; C, chloramphenicol; Frx, ceftriaxone; Smx, sulfamethoxazole; Cf, cephalothin; Gm, gentamicin; NA, nalidixic acid; Cip, ciprofloxacin; Fur, ceftiofur; Te, tetracycline; T/S, trimethoprim-sulfamethoxazole; A/C, amoxicillin-clavulanic acid.
Multiple antimicrobial resistance.
Two hundred twenty (61%) of the isolates analyzed during this study were susceptible to all of the 13 antimicrobials assayed. However, 27 (7.5%) were resistant to one antimicrobial, 60 (17%) were resistant to two, 28 (8%) were resistant to three, 19 (5%) were resistant to four, 3 (2%) were resistant to five, and 2 (0.1%) were resistant to six (Table 3). Among non-STEC isolates (n = 152), 90 (59%) were resistant to two or more antimicrobials. One non-STEC swine isolate recovered in 1996 was resistant to eight antimicrobials (ampicillin, chloramphenicol, sulfamethoxazole, cephalothin, gentamicin, nalidixic acid, ciprofloxacin, and tetracycline). A much smaller number (24; 11%) of STEC isolates were resistant to two or more antimicrobial agents. One STEC isolate was resistant to six antimicrobial agents (ampicillin, sulfamethoxazole, nalidixic acid, ciprofloxacin, tetracycline, and trimethoprim-sulfamethoxazole), and one was resistant to seven (ampicillin, chloramphenicol, sulfamethoxazole, nalidixic acid, ciprofloxacin, tetracycline, and trimethoprim-sulfamethoxazole); both of these isolates were recovered from swine.
TABLE 3.
Multiple antimicrobial resistance among the 361 E. coli O157 isolates analyzed during this study
| No. of antimicrobials to which resistance was shown | No. of isolates
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 361) | With phenotype:
|
From:
|
||||||
| STECa(n = 210) | Non-STEC (n = 151) | Humans(n = 131) | Cattle(n = 133) | Swine(n = 70) | Food(n = 27) | |||
| 0 | 220 | 182 | 38 | 95 | 102 | 4 | 19 | |
| 1 | 27 | 4 | 23 | 7 | 9 | 9 | 2 | |
| 2 | 60 | 16 | 44 | 20 | 15 | 19 | 6 | |
| 3 | 28 | 6 | 22 | 3 | 6 | 19 | 0 | |
| 4 | 19 | 0 | 19 | 5 | 0 | 14 | 0 | |
| 5 | 3 | 0 | 3 | 1 | 0 | 2 | 0 | |
| 6 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | |
| 7 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 8 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | |
| >8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Determined by the presence of stx1 and/or stx2 (see Materials and Methods).
Among the 361 isolates tested, 99 (27%) were resistant to tetracycline, 93 (26%) were resistant to sulfamethoxazole, and 83 (23%) were resistant to both antimicrobials. Of these coresistant isolates, 47 (57%) were from swine, 16 (19%) were from cattle, 14 (17%) were from humans, and 6 (7%) were from food.
DISCUSSION
The microbial ecosystems of humans, swine, cattle, and food are undoubtedly inextricably connected; therefore it is difficult to pinpoint the origin of the antimicrobial resistance that we observed. However, our finding that among E. coli O157 isolates there was high prevalence of resistance to tetracycline, sulfamethoxazole, cephalothin, and ampicillin agrees with previous reports (6, 13, 33).
Because antimicrobial-resistant bacteria from food animals may colonize the human population via the food chain, contact through occupational exposure, or waste runoff from animal production facilities (23, 27), it is possible that resistant bacteria may be readily transferred from food animals to humans. Tetracycline is infrequently used to treat human enteric infections, yet a substantial number of human E. coli isolates were tetracycline resistant. One possibility is that tetracycline-resistant bacteria from food animals have colonized humans via one of the aforementioned routes of transmission. However, it is also possible that tetracycline resistance among human isolates may be attributable to coselection via genetic linkage of resistance determinants (31) or to the use of tetracycline to treat nonenteric bacterial infections in humans.
For STEC, E. coli O157:H7 is the classical serotype, causing approximately 73,500 illnesses, 2,000 hospitalizations, and 60 deaths per year in the United States (11). The fact that the isolates included in this study were suspected etiologic agents may explain the high prevalence of O157:H7 and other STEC isolates among them. There was a high prevalence of resistance to sulfamethoxazole and tetracycline among O157:H7 isolates recovered from humans and cattle. While sulfa drugs and tetracycline are approved for use in cattle (2; Food and Drug Administration, The FDA Approved Animal Drug List, theGreen Book [http://www.fda.gov/cvm/greenbook/greenbook.html], 2001), we cannot determine conclusively whether the high rates of resistance observed among O157 isolates may be attributed to the use of these drugs in cattle production. Interestingly, Galland et al. (6) found that among 57 putative E. coli O157:H7 isolates recovered from cattle, 27 (47%) were resistant to amoxicillin-clavulanic acid. We found that only 1 of 93 E. coli O157:H7 cattle isolates exhibited amoxicillin-clavulanic acid resistance. This difference may be because Galland et al. used a methodology different from ours as well as a resistance breakpoint (>4/2 μg/ml) that has since been increased (>32/16 μg/ml) (15). Alternatively, it may be the result of temporal and geographical differences between the two studies, Galland et al. having collected samples over an 11-month period from a specific region of southwestern Kansas.
Currently, treatment of STEC O157 infection with antimicrobials is regarded as controversial (7, 18, 30). In the United States, antimicrobial therapy is generally not recommended for treatment of these infections because of the potential for the release of Shiga toxin thereby leading to HUS. However, clinical trials in which a chemically synthesized analog of Shiga toxin receptor Gb3 is administered orally to patients in an effort to absorb toxin and curtail disease progression to HUS appear promising (3). Phase III trials for evaluating this therapeutic option are under way (14), and, if successful, antimicrobial therapy may be a viable option for the treatment of STEC infection. Thus, the finding that the majority of STEC isolates were susceptible to each of the antimicrobials tested is encouraging. Nevertheless, approximately 10% of STEC isolates were resistant to sulfamethoxazole and tetracycline. Because cattle are reservoirs of STEC (34), the use of sulfa drugs and tetracycline derivatives in cattle may select for resistance among these bacteria (23, 27). Interestingly, a small percentage of cattle isolates were resistant to cefoxitin, chloramphenicol, and nalidixic acid. Because none of these drugs are approved for cattle in the United States, the resistance phenotypes observed may be due to the use of related approved bovine antimicrobial agents such as florfenicol and enrofloxacin (25). Further research is needed to determine the impact of these frontline antimicrobials on resistance development among STEC O157:H7 and other STEC strains.
The highest prevalence of antimicrobial resistance occurred among swine O157 isolates, where greater than 50% of all isolates were resistant to sulfamethoxazole, cephalothin, or tetracycline and greater than 20% of all isolates were resistant to ampicillin or gentamicin. These data do not demonstrate a steadfast link between antimicrobial use in swine and development of antimicrobial resistance among swine E. coli O157 isolates. However, the findings that the highest prevalence of resistance occurred among swine isolates and that resistance was to drug classes approved for use in swine 2; Food and Drug Administration, [http://www.fda.gov/cvm/greenbook/greenbook.html]) suggest that antimicrobial use in these animals may be a factor in the emergence of antimicrobial resistance in E. coli O157. Because these agents are options for treating enteric infections in humans, the finding that a large number of swine isolates were resistant to them is a concern.
Multiple antimicrobial resistance in STEC and non-STEC may partly result from the spread of genetic elements including plasmids, transposons, and integrons (33) that may confer resistance to numerous antimicrobials. However, there is a paucity of data with regard to the mechanisms of antimicrobial resistance identified among STEC O157:H7 isolates from animals and humans. Further research to characterize the resistance phenotypes observed among the E. coli O157 isolates identified in this study is warranted.
A drawback of this study was that the numbers of swine isolates (high prevalence of resistance) and of cattle and human isolates (low prevalence of resistance) were unevenly distributed regarding year of isolation. Therefore, all attempts to analyze resistance as a function of time were confounded. Similar problems impeded analysis of resistance versus location of isolation.
The isolates examined in this study came from clinical cases and therefore may have been exposed to elevated concentrations of antimicrobials as a result of treatment efforts. Nonetheless, our findings suggest that use of antimicrobials, including tetracycline derivatives, sulfa drugs, and penicillins, has selected for antimicrobial resistance phenotypes in STEC and non-STEC O157. Because the emergence and dissemination of antimicrobial resistance in STEC may complicate future therapeutic options that are being developed for treatment of HUS and HC, continued surveillance of emerging antimicrobial resistance among zoonotic food-borne pathogens, including E. coli O157:H7, is required to ensure public health.
Acknowledgments
We thank Anna Nevius, Mary Bartholomew, and James Nataro for insightful comments.
This work was made possible by grants from the U.S. Department of Agriculture (grant NRI 2000-02600) and the Joint Institute for Food Safety and Applied Nutrition of the University of Maryland and the U.S. Food and Drug Administration.
REFERENCES
- 1.Aarestrup, F. M., and H. C. Wegener. 1999. The effects of antibiotic usage in food animals on the development of antimicrobial resistance of importance for humans in Campylobacter and Escherichia coli. Microbes Infect. 1:639-644. [DOI] [PubMed] [Google Scholar]
- 2.Animal Health Institute. 2001. Feed additive compendium. Miller Publishing Co., Minnetonka, Minn.
- 3.Armstrong, G. D., P. C. Rowe, P. Goodyer, E. Orrbine, T. P. Klassen, G. Wells, A. MacKenzie, H. Lior, C. Blanchard, F. Auclair, B. Thompson, D. J. Rafter, and P. N. McLaine. 1995. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J. Infect. Dis. 171:1042-1045. [DOI] [PubMed] [Google Scholar]
- 4.Farina, C., A. Goglio, G. Conedera, F. Minelli, and A. Caprioli. 1996. Antimicrobial susceptibility of Escherichia coli O157 and other enterohaemorrhagic Escherichia coli isolated in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 15:351-353. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima, H., T. Hashizume, Y. Morita, J. Tanaka, K. Azuma, Y. Mizumoto, M. Kaneno, M. Matsuura, K. Konma, and T. Kitani. 1999. Clinical experiences in Sakai City Hospital during the massive outbreak of enterohemorrhagic Escherichia coli O157 infections in Sakai City, 1996. Pediatr. Int. 41:213-217. [DOI] [PubMed] [Google Scholar]
- 6.Galland, J. C., D. R. Hyatt, S. S. Crupper, and D. W. Acheson. 2001. Prevalence, antibiotic susceptibility, and diversity of Escherichia coli O157:H7 isolates from a longitudinal study of beef cattle feedlots. Appl. Environ. Microbiol. 67:1619-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi, T., J. Inatomi, A. Wake, M. Takamizawa, H. Katayama, and T. Iwata. 1999. Failure of prediarrheal antibiotics to prevent hemolytic uremic syndrome in serologically proven Escherichia coli O157:H7 gastrointestinal infection. J. Pediatr. 135:768-769. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda, K., O. Ida, K. Kimoto, T. Takatorige, N. Nakanishi, and K. Tatara. 1999. Effect of early fosfomycin treatment on prevention of hemolytic uremic syndrome accompanying Escherichia coli O157:H7 infection. Clin. Nephrol. 52:357-362. [PubMed] [Google Scholar]
- 9.Karch, H., N. Stockbine, and A. O'Brien. 1986. Growth of Escherichia coli in the presence of trimethoprim-sulfamethoxazole facilitates detection of Shiga-like toxin producing strains by colony blot assay. FEMS Microbiol. Lett. 35:141-145. [Google Scholar]
- 10.Kim, H. H., M. Samadpour, L. Grimm, C. R. Clausen, T. E. Besser, M. Baylor, J. M. Kobayashi, M. A. Neill, F. D. Schoenknecht, and P. I. Tarr. 1994. Characteristics of antibiotic-resistant Escherichia coli O157:H7 in Washington State, 1984-1991. J. Infect. Dis. 170:1606-1609. [DOI] [PubMed] [Google Scholar]
- 11.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng, J., and M. P. Doyle. 1998. Emerging and evolving microbial foodborne pathogens. Bull. Inst. Pasteur 96:151-164. [Google Scholar]
- 13.Meng, J., S. Zhao, M. P. Doyle, and S. W. Joseph. 1998. Antibiotic resistance of Escherichia coli O157:H7 and O157:NM from animals, food, and humans. J. Food Prot. 61:1511-1514. [DOI] [PubMed] [Google Scholar]
- 14.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing. Tenth informational supplement M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa..
- 16.Orskov, I., F. Orskov, B. Jann, and K. Jann. 1977. Serology, chemistry and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 41:667-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proulx, F., J. P. Turgeon, G. Delage, L. Lafleur, and L. Chicoine. 1992. Randomized, controlled trial of antibiotic therapy for Escherichia coli O157:H7 enteritis. J. Pediatr. 121:299-303. [DOI] [PubMed] [Google Scholar]
- 19.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 24:681-685. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt, H., J. von Maldeghem, M. Frosch, and H. Karch. 1998. Antibiotic susceptibilities of verocytotoxin-producing Escherichia coli O157 and non-O157 strains isolated from patients and healthy subjects in Germany during 1996. J. Antimicrob. Chemother. 42:548-550. [DOI] [PubMed] [Google Scholar]
- 21.Shiomi, M., M. Togawa, K. Fujita, and R. Murata. 1999. Effect of early oral fluoroquinolones in hemorrhagic colitis due to Escherichia coli O157:H7. Pediatr. Int. 41:228-232. [DOI] [PubMed] [Google Scholar]
- 22.Thielman, N. M., and R. L. Guerrant. 1999. Escherichia coli, p. 188-200. In V. L. Yu, T. C. Merigan, Jr., and S. L. Barriere (ed.), Antimicrobial therapy and vaccines. The Williams & Wilkins Company, Baltimore, Md.
- 23.van den Bogaard, A. E., and E. E. Stobberingh. 1999. Antibiotic usage in animals: impact on bacterial resistance and public health. Drugs 58:589-607. [DOI] [PubMed] [Google Scholar]
- 24.Walterspiel, J., S. Ashkenazi, A. Morrow, and T. G. Cleary. 1992. Effect of subinhibitory concentrations of antibiotics on extracellular Shiga-like toxin 1. Infection 20:25-29. [DOI] [PubMed] [Google Scholar]
- 25.White, D. G., C. Hudson, J. J. Maurer, S. Ayers, S. Zhao, M. D. Lee, L. Bolton, T. Foley, and J. Sherwood. 2000. Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J. Clin. Microbiol. 38:4593-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witham, P. K., C. T. Yamashiro, K. J. Livak, and C. A. Batt. 1996. A PCR-based assay for the detection of Escherichia coli Shiga-like toxins in ground beef. Appl. Environ. Microbiol. 62:1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witte, W. 1998. Medical consequences of antibiotic use in agriculture. Science 279:996-997. [DOI] [PubMed] [Google Scholar]
- 28.Wittwer, C. T., G. B. Reed, and K. M. Ririe. 1994. Rapid cycle DNA amplification, p. 174-181. In K. B. Mullis, F. Ferré, and R. A. Gibbs (ed.), The polymerase chain reaction. Birkauser, Boston, Mass.
- 29.Wong, C. S., S. Jelacic, R. L. Habeeb, S. L. Watkins, and P. I. Tarr. 2000. The risk of hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoh, M., and T. Honda. 1997. The stimulating effect of fosfomycin, an antibiotic in common use in Japan, on the production/release of verotoxin-1 from enterohaemorrhagic Escherichia coli O157:H7 in vitro. Epidemiol. Infect. 119:101-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhanel, G. G., J. A. Karlowsky, M. H. Saunders, R. J. Davidson, D. J. Hoban, R. E. Hancock, I. McLean, and L. E. Nicolle. 1995. Development of multiple-antibiotic-resistant (Mar) mutants of Pseudomonas aeruginosa after serial exposure to fluoroquinolones. Antimicrob. Agents Chemother. 39:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, X., A. D. McDaniel, L. E. Wolf, G. T. Keusch, M. K. Waldor, and D. W. Acheson. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181:664-670. [DOI] [PubMed] [Google Scholar]
- 33.Zhao, S., D. G. White, B. Ge, S. Ayers, S. Friedman, L. English, D. Wagner, S. Gaines, and J. Meng. 2001. Identification and characterization of integron-mediated antibiotic resistance among Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 67:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]