Abstract
The association between moisture-related microbial growth (mesophilic fungi and bacteria) within insulated exterior walls and microbial concentrations in the indoor air was studied. The studied apartment buildings with precast concrete external walls were situated in a subarctic zone. Actinomycetes in the insulation layer were found to have increased concentrations in the indoor air. The moisture content of the indoor air significantly affected all measurable airborne concentrations.
Concrete sandwich facade panels have been commonly used in building frameworks in northern Europe since the 1960s. These panels consist of two reinforced concrete panels enclosing a layer of mineral wool thermal insulation (rock or fiberglass wool), essential in the subarctic climate. Mold growth has been reported on the internal concrete core, initiated by the condensation of indoor humidity due to serious panel cracks (3). In a previous study (16) it was shown that microbial growth is found infrequently in the insulation layer in structures of this type in Finland, but such growth is increased if the external wall is in poor condition. Microbial growth within the exterior walls has seldom been considered a risk factor for indoor air quality (10). Compared to microbial growth on internal structures, the envelope does not necessarily have similar direct contact with the indoor air. However, if the supply airflow drifts through a contaminated wall structure, it may affect the quality of the indoor air. In the present study, we evaluated whether indoor air quality may be influenced by microbial contamination in the insulation layer of precast concrete external walls.
Test buildings and insulation samples.
We studied 50 inhabited apartment buildings, 2 to 38 years in age, with precast concrete sandwich panels as their framework structure. The buildings were situated in the southern coastal area of Finland, in the Turku region (60°50"N, 22°05"E to 60°39"N 22°40"E) and in Salo (60°39"N, 23°12"E). Prior to sampling, the condition of the exterior walls was monitored visually by using five parameters (Table 1) with a three-step scale (bad, moderate, good). Insulation samples from the external wall were taken from the whole depth of the insulation layer through boreholes from the outside. The sampled sites of the panel were (i) the central upper edge, (ii) the upper corner, (iii) the side edge of the panel, (iv) below the window, (v) the lower edge, and (vi) the central area. A total of 364 concrete panels were sampled. For the microbiological analysis, a subsample of the insulation sample was suspended in peptone water (1.0 g of peptone, 0.85 g of NaCl, and 0.02% Tween 80 detergent in 1 liter of deionized water), and the concentrations were determined by dilution plating (14) on MEA agar (20.0 g of malt extract, 20.0 g of saccharose, 1.0 g of peptone, 20.0 g of agar, and 0.1 g of chloramphenicol in 1 liter of deionized water) and TYG agar (5.0 g of tryptone, 2.5 g of yeast extract, 1.0 g of glucose, 15.0 g of agar, and 0.5 g of cycloheximide in 1 liter of deionized water). Fungal colonies were counted after 7 days, and bacteria were counted after 10 days of incubation (at 25°C). The results are expressed as CFU per gram of insulation material. The fungal genera were identified microscopically, and bacteria were classified as actinomycetes and other bacteria. Detection limits ranged from 28 to 298 CFU g−1.
TABLE 1.
Variables tested and used to apply GLMMs to indoor air concentrationsa
| Variable | Indoor air concn ofb:
|
|||
|---|---|---|---|---|
| Act | Bact | Fungitot | Fungiref | |
| Microbial concn in insulation | ||||
| Maximum of samples taken from edge areas of the panelc | X | (X) | X | |
| Maximum of all samples per panelc | — | (X) | — | — |
| Other microbial sources | ||||
| Outdoor air concnc | — | — | X | X |
| Window ventilation on sampling dayd | — | X | — | X |
| Potted plantsd | — | X | X | — |
| Soil handling in the previous weekd | — | — | — | — |
| Petsd,e | — | X | — | — |
| Climatic parameters | ||||
| RH indoorsc | — | — | — | |
| Temp indoorsc | — | — | — | |
| Daily mean temp outdoorsc | — | — | ||
| Moisture content of indoor airc | X | X | X | X |
| Snow coverd | X | X | X | X |
| Night frostd | X | X | X | X |
| Condition of external panelf | ||||
| Panel curvingd | — | — | ||
| Elastic joint deterioration of paneld | X | — | ||
| Visible corrosion of reinforcementsd | — | — | ||
| Frost damage on paneld | — | — | ||
| Prevailing external moisture conditions on the walld | — | — | — | |
| Building agec | — | — | — | |
X, included in the model; (X), insignificant but included in the model; —, tested but excluded from the model.
Act, actinomycetes; Bact, other bacteria; Fungitot, total fungi.
Covariate.
Fixed class variable.
Dwellings with caged pets (birds or rodents) were excluded from sampling.
Based on visual monitoring prior to sampling.
Air samples and environmental data.
For air sampling we chose 18 of the original 50 buildings with different degrees of microbial contamination in the insulation. The ventilation system in each building consisted of an exhaust air fan with no mechanical air supply. Apartments with mold damage on the interior surfaces were excluded by visual survey and examination with a surface moisture probe. We sampled 88 dwellings, each two or three times in spring 1997 and from late autumn 1997 to spring 1998. At least one sampling per apartment was performed during the period of snow cover, as is recommended for subarctic areas (17). Air samples were taken with an Andersen 10-800 impactor (Graseby Andersen, Smyrna, Ga.) on MEA and TYG media. Outdoor samples (0.7 to 1.5 m above ground and ≥4 m from the building) were taken on each sampling day and in each area. If the temperature fell below −5°C, or in the case of snowfall, outdoor samples were not taken; in the statistical analyses the counts were set at zero. The plates were incubated and analyzed in a manner similar to that used for the insulation samples. Airborne concentrations are given in CFU per cubic meter after positive-hole correction (7). Before sampling, we advised the inhabitants to avoid activities (5) that might disperse spores into the air. Sources of error were surveyed with a questionnaire (handling of soil, moldy or soiled foodstuffs, organic household waste, or laundry; ventilation by open window; vacuuming and/or dusting; pets; occupation or hobby connected to agriculture or building renovation). Dwellings with caged pets (birds or rodents) were excluded from the sampling. The surveyor assessed the level of cleanliness and the number of potted plants and measured the RH (relative humidity) and temperature with an HM34 meter (Vaisala Oyj, Helsinki, Finland). The moisture content (in grams per cubic meter) was calculated from the RH and temperature using an approximate formula (12). Daily weather data originated from weather stations in Turku and Salo (Finnish Meteorological Institute, Helsinki, Finland).
Statistics.
We compared the microbial indoor air concentrations (actinomycetes, other bacteria, and total fungi) to the insulation contamination in the panel adjacent to the apartment measured. Since the sampling design forms a nested structure with random and repeated effects, and the airborne counts were considered to follow the Poisson distribution, a generalized linear mixed model (GLMM) was applied to the data (9). The fitted model predicts the probabilities that the event studied (indoor concentrations) will occur for covariates (e.g., outdoor concentration) and fixed class variables (e.g., snow cover). The insulation contamination was treated in the modeling as a covariate, not categorized as a treatment and control. Tested variables, both those included in the model and those tested but not improving it, are shown in Table 1. As insulation covariates, we tested both the maximum concentration of each microbial group in the panel and the maximum concentrations of the samples from the edge areas of the panel. We set concentrations below a detection limit at zero. Akaike's information criteria and the visual fit of the residuals were used as model-fitting criteria (SAS system for mixed models; SAS Institute, Inc., Cary, N.C.). Satterthwaite's approximation was used in determining degrees of freedom, and the Tukey-Kramer adjustment was used in pairwise comparisons. Analyses were performed with the GLIMMIX macro (SAS system for mixed models; SAS Institute, Inc.) in SAS statistical software (version 6.12; SAS Institute, Inc.). A log-linear model was fitted to test for associations between the occurrences of the most prevalent fungi in the insulation and their occurrences in the indoor air. The analysis was performed with the CATMOD procedure in SAS. An additional analysis with GLMM was carried out for Fungiref (reformed fungal values) in order to minimize the fungal background. The value was reformed by excluding Penicillium, Cladosporium, basidiomycetes, sterile mycelia, and Fusidium-like colonies from the total count. Of these excluded groups, Penicillium is the main genus found indoors in both damp and normal residences (15) and Cladosporium and basidiospores are the main fungal groups showing a similar periodicity in outdoor and indoor samples in the subarctic zone (6). Fusidium counts showed an outdoor peak on a few days of our sampling procedure.
Insulation contamination as an indoor air source.
The basic statistics for pooled data showed higher Poisson distribution means of airborne actinomycetes in dwellings with insulation contamination (>100 CFU per g of insulation material) than in other dwellings (Table 2). The counts of other bacteria were also somewhat higher in the test dwellings, but the fungal counts were lower (Table 2). These simple comparisons of weighted means did not deal with the various environmental factors and background sources affecting indoor air spores between the different samplings, but we took these factors into account in the modeling.
TABLE 2.
Statistics of measured airborne counts in dwellings with different microbial contamination in the insulation layera and in outdoor air
| Microbial category | Sampling site (concn) | n | Concn in insulation (CFU g−1)
|
Airborne concn (CFU m−3)
|
||
|---|---|---|---|---|---|---|
| Mean, Poisson distribution (95% CI) | Range | Mean, Poisson distribution (95% CI) | Range | |||
| Actinomycetes | Apartment (>100 CFU g−1) | 80 | 5,583.1 (4,282-7,281) | 191-57,477 | 4.0 (2.9-5.6) | 0-45 |
| Apartment (≤100 CFU g−1) | 137 | 9.7 —b | 0-72 | 2.5 (1.8-3.4) | 0-24 | |
| Outdoor air | 28 | 3.0 (1.6-5.6) | 0-14 | |||
| Other bacteria | Apartment (>10,000 CFU g−1) | 37 | 70,500 (52,430-94,790) | 10,930-583,330 | 1,001.0 (508.3-1,971.1) | 27-15,144 |
| Apartment (≤10,000 CFU g−1) | 186 | 1,580 (650-3,810) | 0-9,710 | 804.7 (574.5-1,127.3) | 2-20,407 | |
| Outdoor air | 28 | 195.1 (33.4-1,138.9) | 9-1,694 | |||
| Fungi (total) | Apartment (>1,000 CFU g−1) | 34 | 4,525.6 (3,854.1-5,314.0) | 1,257-7,959 | 112.1 (53.0-237.5) | 9-516 |
| Apartment (≤1,000 CFU g−1) | 188 | 106.5 (68.2-166.2) | 0-874 | 120.8 (88.8-164.3) | 2-1,784 | |
| Outdoor air | 29 | 321.5 (199.0-519.5) | 10-4,158 | |||
Pooled data of all replicate samplings.
—, overdispersed Poisson distribution, CI not presented.
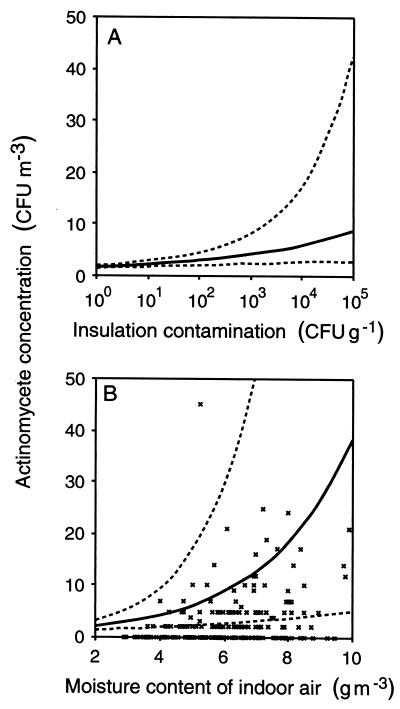
The modeling result showed that actinomycete growth within the building envelope significantly affects the indoor air (Table 3; Fig. 1A). A 10-fold increase in counts in the insulation increased counts in the indoor air 1.2-fold (95% confidence interval [CI], 1.09 to 1.32). According to our estimation, the limit of 10 CFU m−3 (a guideline value used in Finland to indicate an abnormal occurrence of actinomycetes in a dwelling [11]) is exceeded with relatively high counts in the insulation, over 10,000 CFU g−1.
TABLE 3.
Factors shown to influence the indoor air concentrations of actinomycetes and other bacteria
| Source(s) of variation | Statistic fora:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Actinomycetes
|
Other bacteria
|
||||||||
| Ndf | Ddf | F | P | Ndf | Ddf | F | P | ||
| Microbial concentration in insulation | 1 | 44.4 | 11.78 | 0.001 | 1 | 57.4 | 0.03 | 0.875 | |
| Ventilation by open window prior to sampling | 1 | 161.0 | 3.65 | 0.058 | |||||
| Potted plants | 3 | 154.0 | 3.09 | 0.029 | |||||
| Pets | 1 | 94.5 | 4.71 | 0.032 | |||||
| Moisture content of indoor air | 1 | 166.0 | 13.07 | <0.001 | 1 | 127.0 | 17.91 | <0.001 | |
| Snow coverage | 1 | 156.0 | 0.76 | 0.386 | 1 | 122.0 | 1.55 | 0.215 | |
| Night frost | 1 | 178.0 | 0.15 | 0.703 | 1 | 127.0 | 0.75 | 0.388 | |
| Snow coverage × night frost | 1 | 153.0 | 2.80 | 0.096 | 1 | 132.0 | 11.07 | 0.001 | |
| Joint deterioration of the panel | 3 | 89.6 | 3.52 | 0.018 | |||||
Ndf, numerator degrees of freedom; Ddf, denominator degrees of freedom. P values of <0.05 (in bold face type) are considered significant.
FIG. 1.
Expected actinomycete counts in indoor air as a function of insulation contamination (broken lines, 95% CI) (A) and the moisture content of indoor air (broken lines, 95% CI; ticks, actual data) (B).
We did not observe a significant indoor air effect of fungal contamination in the insulation, either for total counts or for modified Fungiref values (Table 4). A 10-fold increase in Fungiref in the insulation increased counts in the indoor air, but only 1.0001-fold. The most prominent fungi in the insulation layer occurred in the air of the test apartments somewhat more often than in that of the reference apartments (Table 5). The occurrence of Aureobasidium showed an increase of 17 percentage units between the test and reference dwellings, although the result was statistically only indicative (P = 0.085). However, the proportion of apartments with contaminated insulation was low (16). Therefore, the number of apartments with considerable insulation contamination, especially by fungi, was low in the data used (Table 2), which makes it difficult to observe any fungal biocontamination originating from the insulation.
TABLE 4.
Factors shown to influence the indoor air concentrations of total and reformed fungal counts
| Source(s) of variation | Statistic fora:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total fungi
|
Fungiref
|
||||||||
| Ndf | Ddf | F | P | Ndf | Ddf | F | P | ||
| Microbial concentration in insulation | 1 | 71.9 | 0.81 | 0.371 | 1 | 61.7 | 6.13 | 0.016 | |
| Outdoor airb | 1 | 88.9 | 28.53 | <0.001 | 1 | 104 | 6.12 | 0.015 | |
| Ventilationc | 1 | 104 | 0.02 | 0.898 | |||||
| Potted plants | 3 | 176.0 | 7.42 | <0.001 | |||||
| Moistured | 1 | 166 | 25.18 | <0.001 | |||||
| Snow coverage | 1 | 143.0 | 0.03 | 0.871 | 1 | 143 | 7.61 | 0.007 | |
| Night frost | 1 | 153.0 | 1.43 | 0.234 | 1 | 141 | 8.22 | 0.005 | |
| Snow coverage × night frost | 1 | 140.0 | 17.95 | <0.001 | 1 | 94.7 | 9.96 | 0.002 | |
| Outdoor airb × snow coverage × night frost | 3 | 101 | 2.52 | 0.062 | |||||
| Ventilationc × night frost | 1 | 138 | 8.24 | 0.005 | |||||
| Ventilationc × snow coverage | 1 | 143 | 7.52 | 0.007 | |||||
| Ventilationc × moistured × snow coverage | 2 | 119 | 5.5 | 0.005 | |||||
| Ventilationc × moistured × night frost | 2 | 117 | 4.25 | 0.017 | |||||
Ndf, numerator degrees of freedom; Ddf, denominator degrees of freedom. P values of <0.05 (in boldface type) are considered significant.
Microbial concentration in outdoor air.
Ventilation by open window prior to sampling.
Moisture content of indoor air.
TABLE 5.
Effects of the most prominent fungi in insulation on occurrences of the same organisms in indoor aira
| Organism | Occurrence in insulation | % Occurrence in air samplesb | Log likelihood resultc
|
||
|---|---|---|---|---|---|
| df | G2 | P | |||
| Acremonium | Not present | 7 (13/181) | 1 | 0.19 | 0.662 |
| Present | 10 (2/20) | ||||
| Aspergillus versicolor | Not present | 19 (33/171) | 1 | 0.01 | 0.929 |
| Present | 20 (6/30) | ||||
| Aureobasidium | Not present | 16 (29/183) | 1 | 2.97 | 0.085 |
| Present | 33 (6/18) | ||||
| Cladosporium | Not present | 78 (141/180) | 1 | 0.08 | 0.779 |
| Present | 81 (17/21) | ||||
| Penicilliumd | <100 CFU g−1 | 5 (7/131) | 1 | 0.01 | 0.903 |
| 100-1,000 CFU g−1 | 0 (0/37) | ||||
| ≥1,000 CFU g−1 | 6 (2/34) | ||||
Pooled data of all replicate samplings.
Values in parentheses represent the number of samples testing positive for the organism per the total number of samples.
G2, G statistic.
Percent occurrence in the air samples in concentrations of ≥100 CFU m−3.
Moisture content and other environmental factors.
Factors linked to seasonal environmental changes, such as the moisture content (in grams per cubic meter) of the indoor air or a wintry background (interaction, snow coverage × night frost), affected the counts of all microbial groups (Table 3 and 4). The moisture content was found to be far more useful than the RH, often used as a measure of air moisture. The moisture dependence was especially evident with actinomycetes; when the moisture content was below 3.5 g m−3, no airborne actinomycetes were observed (Fig. 1B). At a temperature of 20°C, this means an RH under 20%.
The release of dry actinomycete spores from cultures has been found to increase with a decrease in air humidity (19). Our results show a contrary trend at low humidities of <20%. In the northern climate, the indoor RH may fall below 20% for an extended period in the winter. The RH may rise much higher in dwellings with poor ventilation. The increased microbial counts associated with higher humidity may therefore be caused by insufficient ventilation. Low humidity may also interfere in the measurability of spores and bacteria, e.g., in viability (1), electrostatic adhesion (21), and size (for examples, see references 8 and 19). Thus, changes in the humidity may affect not only spore liberation or drift in and through the wall but also spore measurability.
Humidity and wintry conditions also explained the fungal counts, but this is due to a weak outdoor source in winter (Table 4). A moisture increase of 1 g m−3 enhanced indoor counts of total fungi 1.34-fold (95% CI, 1.18 to 1.52). The outdoor source, however, was not so notable with actinomycetes and other bacteria (Table 3).
We did not suspect bacteria other than actinomycetes to have originated from the insulation. The main bacterial sources in dwellings are normally the occupants, and increased levels of bacteria often indicate poor ventilation (18). As expected, other bacteria found in the insulation layer did not explain the airborne counts (Table 3). However, the effect of environmental factors (e.g., moisture and pets) in a similar fit analysis validated the applicability of the modeling method. An increase of 1 g of water/m3 of air enhanced indoor bacterial counts 1.46-fold (95% CI, 1.29 to 1.65).
Building-related factors were of little importance. The only variable to fit in the models was the effect of the deterioration of the elastic joints of the panel on actinomycete concentrations indoors (Table 3). The airborne counts were estimated to be higher in apartments without deterioration of elastic panel joints than in those with joints in poor condition. The number of potted plants in the dwelling affected fungal counts (Table 4). This finding agrees with those of Staib and others (20).
Conclusions.
In summary, our study showed that indoor air biocontamination originating from the envelope of precast concrete panel buildings in a subarctic climate is rare. However, the small-sized actinomycete spores do infiltrate from the wall structures. This confirmation is important because actinomycetes have been shown to cause various adverse health effects (4, 13), which are not necessarily associated with spore viability (2). Fungal contamination, infrequent in the insulation in this specific environment, was not found to affect indoor air. In a different climate or with a different structural design (thinner insulation layer, different ventilation system), buildings of the same type, for instance, those common in eastern Europe, may act differently. Biocontamination from the building envelope should not be ignored in future studies.
Acknowledgments
The study was supported financially by the TEKES National Technology Agency of Finland; the companies Rautaruukki, Fenestra, Partek-Paroc, and Isover; and the RTT Finnish Association of Building Product Industries.
We thank Kirsi Helkiö, Susanna Järvi, Sari Kiiski, Marjo Rantala, Hannu Lumivirta, and Erkki Helimo for assistance in this work and Ellen Valle for revising the English language version of the manuscript.
REFERENCES
- 1.Cox, C. S. 1995. Stability of airborne microbes and allergen, p. 77-99. In C. S. Cox and C. M. Wathes (ed.), Bioaerosols handbook. CRC Press, Boca Raton, Fla.
- 2.Hirvonen, M., M. Ruotsalainen, K. Savolainen, and A. Nevalainen. 1997. Effect of viability of Actinomycete spores on their ability to stimulate production of nitric oxide and reactive oxygen species in raw264.7 macrophages. Toxicology 124:105-114. [DOI] [PubMed] [Google Scholar]
- 3.Kaufhold, T., K. Fiedler, G. Jung, M. Lindner, and R. P. Gassel. 1997. Moisture and mould at the inner-walls of ‘Plattenbauten’ (prefabricated slabs)—examinations of a strange reason. Zentbl. Hyg. Umweltmed. 199:527-536. (In German.) [PubMed] [Google Scholar]
- 4.Lacey, J. 1997. Fungi and actinomycetes as allergens, p. 858-887. In A. B. Kay (ed.), Allergy and allergic diseases. Blackwell Scientific Publications, Oxford, United Kingdom.
- 5.Lehtonen, M., T. Reponen, and A. Nevalainen. 1993. Everyday activities and variation of fungal spore concentrations in indoor air. Int. Biodeterior. Biodegrad. 31:25-39. [Google Scholar]
- 6.Li, D. W., and B. Kendrick. 1995. A year-round comparison of fungal spores in indoor and outdoor air. Mycologia 87:190-195. [Google Scholar]
- 7.Macher, J. M. 1989. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am. Ind. Hyg. Assoc. J. 50:561-568. [DOI] [PubMed] [Google Scholar]
- 8.Madelin, T., and H. Johnson. 1992. Fungal and actinomycete spore aerosols measured at different humidities with an aerodynamic particle sizer. J. Appl. Bacteriol. 72:400-409. [DOI] [PubMed] [Google Scholar]
- 9.McCullagh, P., and J. A. Nelder. 1989. Generalized linear models, 2nd ed. Chapman & Hall, London, United Kingdom.
- 10.Miller, J. D., P. D. Haisley, and J. H. Reinhardt. 2000. Air sampling results in relation to extent of fungal colonization of building materials in some water-damaged buildings. Indoor Air 10:146-151. [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Social Affairs and Health in Finland. 1997. Sisäilmaohje. Guide 1997:1. Ministry of Social Affairs and Health, Helsinki, Finland.
- 12.Nevander, L., and B. Elmarsson. 1981. Fukthandboken. Svensk Byggtjänst, Helsingborg, Sweden.
- 13.Paananen, A., R. Mikkola, T. Sareneva, S. Matikainen, M. Andersson, I. Julkunen, M. S. Salkinoja-Salonen, and T. Timonen. 2000. Inhibition of human NK cell function by valinomycin, a toxin from Streptomyces griseus in indoor air. Infect. Immun. 68:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasanen, A.-L., T. Juutinen, M. J. Jantunen, and P. Kalliokoski. 1992. Occurrence and moisture requirements of microbial growth in building materials. Int. Biodeterior. Biodegrad. 30:273-283. [Google Scholar]
- 15.Pasanen A.-L., M. Niininen, P. Kalliokoski, A. Nevalainen, and M. J. Jantunen. 1992. Airborne Cladosporium and other fungi in damp versus reference residences. Atmos. Environ. Part A 26:121-124. [Google Scholar]
- 16.Pessi, A.-M., K. Helkiö, J. Suonketo, M. Pentti, and A. Rantio-Lehtimäki. 1999. Microbial growth inside exterior walls of precast concrete buildings as a possible risk factor for indoor air quality, p. 899-904. In G. Raw, C. Aizlewood, and P. Warren (ed.), Indoor air 99. Proceedings of the 8th International Conference on Indoor Air Quality and Climate, vol. 1. Construction Research Communications Ltd., London, United Kingdom. Edinburgh, Scotland. [Google Scholar]
- 17.Reponen, T., A. Nevalainen, M. Jantunen, M. Pellikka, and P. Kalliokoski. 1992. Normal range criteria for indoor air bacteria and fungal spores in a subarctic climate. Indoor Air 2:26-31. [Google Scholar]
- 18.Reponen, T., A. Nevalainen, and T. Raunemaa. 1989. Bioaerosol and particle mass levels and ventilation in Finnish homes. Environ. Int. 15:203-208. [Google Scholar]
- 19.Reponen, T. A., S. V. Gazenko, S. A. Grinshpun, K. Willeke, and E. C. Cole. 1998. Characteristics of airborne actinomycete spores. Appl. Environ. Microbiol. 64:3807-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staib, F., B. Tompak, and A. Blisse. 1978. Aspergillus fumigatus and Aspergillus niger in two potted ornamental plants, cactus (Epiphyllum truncatum) and clivia (Clivia miniata). Biological and epidemiological aspects. Mycopathologia 66:27-30. [DOI] [PubMed] [Google Scholar]
- 21.Vincent, J. H. 1989. Aerosol sampling: science and practice. John Wiley & Sons Ltd., Chichester, United Kingdom.