Abstract
The filamentous fungus Penicillium cyclopium conidiates in the presence of calcium ions in submerged culture without nutrient limitation according to a precisely timed program. Conidiation could be prematurely induced in a nutritionally sufficient medium which had previously supported growth, suggesting that a metabolite which influenced the process was produced. A diterpenoid with conidiation-inducing activity, conidiogenone, was purified from the culture medium, along with conidiogenol, a putative derivative with delayed activity. Contrary to previous thought, the presence of calcium was demonstrated to only decrease the threshold concentration of conidiogenone required for the induction to proceed. In light of these results, a mechanism of conidiation induction is presented according to which the mycelium produces a conidiation inducer (conidiogenone) that accumulates extracellularly. When a threshold concentration is reached, induction likely takes place by interaction with a specific cellular receptor. The results indicate that conidiogenone is both sufficient and necessary to induce conidiation.
Conidiogenesis is a widespread morphogenetic process that filamentous fungi undertake for dispersal. The cellular mechanisms underlying conidiation have been extensively studied in the model organisms Aspergillus nidulans and Neurospora crassa (1, 21). The strongest and most common stimulus for conidiation among filamentous fungi is the emergence of hyphae to the air (16). However, the precise mechanism by which conidiation induction is triggered under such a complex environmental change for the hypha has proven to be an elusive question.
Conidiation may be provoked in liquid culture under controlled conditions (7, 9, 20). In the 1940s, it was demonstrated that Penicillium notatum growing in submerged liquid culture could be induced to conidiate when calcium ions (0.5 to 5.0%, wt/vol) were present in the medium (6). Calcium-induced conidiation was later described in detail (9) as a process consisting of two phases, an initial growth phase in which the fungus attained competence for conidiation induction and a second phase, after induction by calcium, consisting of a sequence of synchronous and precisely timed morphogenetic changes leading to conidium formation. The attainment of competence was proposed to be a process related to the accumulation of fungal metabolites by the culture (10), although the nature of such metabolites has remained unknown to date.
Penicillium cyclopium displays a behavior pattern concerning the timing and pattern of conidiation as well as susceptibility to calcium induction very similar to but more consistent than that reported for P. notatum (22). A number of studies have shown that the cation is rapidly adsorbed to the cell surface (23, 24) and that calcium removal within the first 2 h of contact with the cells results in the reversal of the induction of conidiation (24). The precise role of the cation has not yet been accurately determined, but the accumulated evidence favors the hypothesis that the mechanism by which it exerts its action is related to binding rather than to its intracellular accumulation.
In this report, we describe the isolation of two closely related molecules produced by P. cyclopium which induce conidiation under nonnutrient limitation conditions in the presence and absence of calcium ions. The finding is relevant in the context of conidiation in liquid culture but also provides a plausible explanation for the process of conidiation induction on emergence to the aerial environment.
MATERIALS AND METHODS
Organism and culture conditions.
A single-spore isolate from P. cyclopium Westling (IMI 229034) was employed throughout this study. Batch cultures containing 10 liters of F medium with an inoculum load of 2.5 × 106 conidia ml−1 (if not stated otherwise) and an aeration rate of 6 liters min−1 at 25°C were performed as previously described (22).
Bioassay for conidiation-inducing activity.
A conidiation induction bioassay was carried out in shaken flask cultures, prepared as previously described (22) but instead using 25-ml Erlenmeyer flasks containing 5 ml of fresh F medium or mature F medium (i.e., medium in which the fungus had previously grown for 36 h in the absence of added calcium [mF medium], using the same notation as Hadley and Harrold [10]). Media were adjusted to pH 4.6 and supplemented to 10 mM CaCl2. Flasks were inoculated with 5 × 104 conidia ml−1 unless stated otherwise, and cultures were grown at 25°C with rotary shaking at 150 rpm. At the time of inoculation, extracts or fractions to be assayed for conidiation-inducing activity were added as methanolic or ethanolic solutions to fresh F medium.
The presence of conidiation-inducing activity was determined (by microscopic observation of mycelium samples taken at different times) according to the shortening of the period required for P. cyclopium to conidiate relative to that occurring in a fresh F medium. Conidiation time was considered to be the time at which 50% of the hyphal tips attained the final stage of conidiation (conidia-bearing tips). Conidiation induction was considered to have taken place 7 h earlier, according to previously established criteria (22).
Purification of the conidiation-inducing molecules.
Batches of 48-h-old mF medium (10 liters) were filtered (0.45-μm-diameter pore size) and adjusted to pH 7.0, and each filtrate was subjected to solid-phase extraction by passage through a 10-g Supelclean LC-phenyl column (Supelco). After column washing with 50 ml of 50% (vol/vol) aqueous methanol, retained activity was eluted with 150 ml of 100% methanol and kept at −20°C. Pooled extracts arising from 30 batches were evaporated to dryness, rendering 595 mg of a brown-yellowish oily residue. This residue was then dissolved in a minimum volume of methanol and further purified by reverse-phase semipreparative high-performance liquid chromatography (HPLC) on a Hypersil octyldecyl silane column (10 by 250 mm, 5-μm-diameter particle size), using an acetonitrile-water linear gradient (starting mobile phase, 45% [vol/vol] acetonitrile; final mobile phase, 100% acetonitrile; gradient time, 30 min; flow rate, 2.2 ml min−1; UV detector, 210 nm). Two fractions (A and B), peaking at 26.8 and 28.8 min, respectively, with conidiation-inducing activity were collected which, after solvent evaporation, yielded two yellowish oily residues of 16.3 and 4.9 mg, respectively. Both active fractions were further purified by normal-phase chromatography on a silica gel column (Matrex LC gel, 60 A, 35 to 70 μm [Grace]; 3 by 50 mm), with diethyl ether as the eluent. At the end of the purification procedure, 0.70 mg of conidiogenol and 0.64 mg of conidiogenone were obtained as colorless oils.
Analytical techniques and chemical synthesis.
Techniques concerning nuclear magnetic resonance and mass spectra have been described elsewhere (17a) Diacetylconidiogenol was obtained by treatment of conidiogenol (0.35 mg) with acetic anhydride (50 μl) in pyridine (200 μl) at room temperature overnight. Evaporation of the volatiles with a stream of nitrogen gas yielded 0.38 mg of diacetylconidiogenol, whose structure was confirmed by spectroscopic techniques.
Mycelial dry-weight determination.
Samples (100 ml) from cultures of P. cyclopium, prepared as described above and taken at different times, were filtered through a 0.45-μm-diameter-pore-size filter. After washing thoroughly with distilled water, the mycelium was resuspended in a minimal volume of water and dried at 105°C to a constant weight.
RESULTS
Conidiation induction requires the accumulation of a fungal metabolite in the medium.
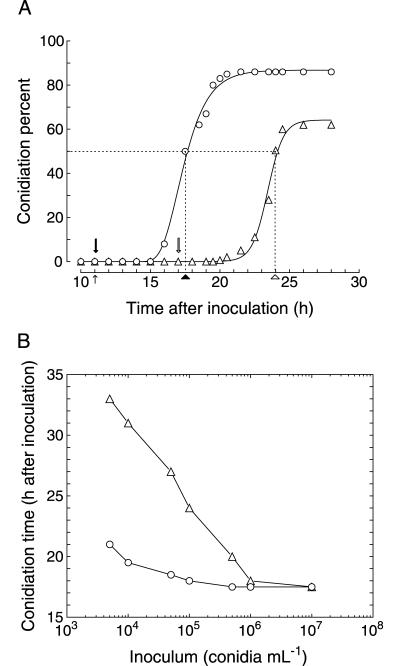
In a culture growing in Ca2+-supplemented fresh F medium (inoculum, 105 conidia ml−1), conidia germinate after 11 h of culture and conidiation follows at 24 h (Fig. 1A). The morphogenetic program leading to conidium formation invariably requires 7 h (22). Thus, induction can be accurately estimated to have occurred at 17 h of culture, after a 6-h period of vegetative growth following germination.
FIG. 1.
(A) Temporal appearance of conidium-bearing tips in cultures of P. cyclopium. The percentage of conidiating tips is expressed as the conidiation percent. Assays were carried out in shaken flask cultures inoculated with 105 conidia ml−1. The points of 50% conidiation, taken as the reference point at which conidiation was considered to have been reached, are indicated by dotted lines. The occurrence of germination (small arrow), conidiation in F medium (hollow arrowhead) and mF medium (filled arrowhead), and conidiation induction in F medium (hollow arrow) and mF medium (filled arrow) are indicated in the figure. (B) Effect of the inoculum load on conidiation time in cultures of P. cyclopium. Assays were carried out in shaken flasks with cultures inoculated with increasing inoculum loads. In both figures, triangles represent growth in fresh F medium and circles represent growth in mF medium. The mF medium was prepared by growing the fungus in a 10-liter fermenter for 36 h with an inoculum of 106 conidia ml−1. Values are expressed as the means of three independent experiments. Standard deviations (not shown) are lower than ±10%.
When the same experiment was conducted using mF medium, conidiation could be observed at 18 h (Fig. 1A). Hence, conidiation induction should have coincided in time with germination (11 h) and the culture did not require any period of vegetative growth for the attainment of competence.
The time period required for conidiation induction to occur after germination in fresh medium depended on the amount of the initial inoculum (Fig. 1B). The higher the level of inoculum, the earlier the conidiation induction occurred, reaching a lower limit of temporal coincidence with germination for inoculum loads higher than 106 conidia ml−1. In mF medium, this relation was also observed (Fig. 1B) with the same lower limit, but it was reached at lower inoculum loads (about 105 conidia ml−1).
The results obtained in both experiments indicate that the growing mycelium caused a modification in the surrounding medium that accounted for conidiation induction. The possibility was dismissed that nutrient starvation was the inducing stimulus, since the levels of nutrient depletion observed for mature and fresh medium were irrelevant in terms of growth kinetics. Moreover, when mF medium was supplemented with nutrients, the resulting time and pattern of conidiation remained identical to those with the unsupplemented medium (not shown).
Further experiments showed that the conidiation-inducing activity present in mF medium disappeared after autoclaving, was protease insensitive, was dialyzable through a 3,500-Da-cutoff dialysis membrane, and was extractable by liquid-liquid- or -solid-phase extraction procedures, as shown below. This evidence supported the view that the medium modification accounting for the observed conidiation-inducing activity was due to the presence of a metabolic product or inducer secreted by the mycelium.
Purification and structure elucidation of the conidiation-inducing molecules.
The medium extraction and fractionation procedure was used in combination with the conidiation induction bioassay to track the active compounds. Initial extractions from both mycelium and medium indicated that the conidiogenic activity was only localized in the latter. Thus, the medium alone was used for further purification steps. Of all the fractions obtained after reverse-phase HPLC separation, only two adjacent fractions (A and B) displayed conidiation-inducing activity, which after normal-phase chromatography separation, yielded submilligram quantities of the pure active compounds (0.70 and 0.64 mg for A and B, representing about 0.12% and 0.11%, respectively, of the starting crude extract arising from 30 fermentation batches). The overall yield of the purification method was about 2.3 and 2.1 μg per liter, respectively, of mF medium for compounds A and B.
The elucidation of the structures of compounds A and B was performed by means of spectroscopic techniques, including nuclear magnetic resonance and mass spectrometry, as reported elsewhere (T. Roncal, S. Cordobés, U. O. Ugalde, and O. Sterner, submitted for publication).
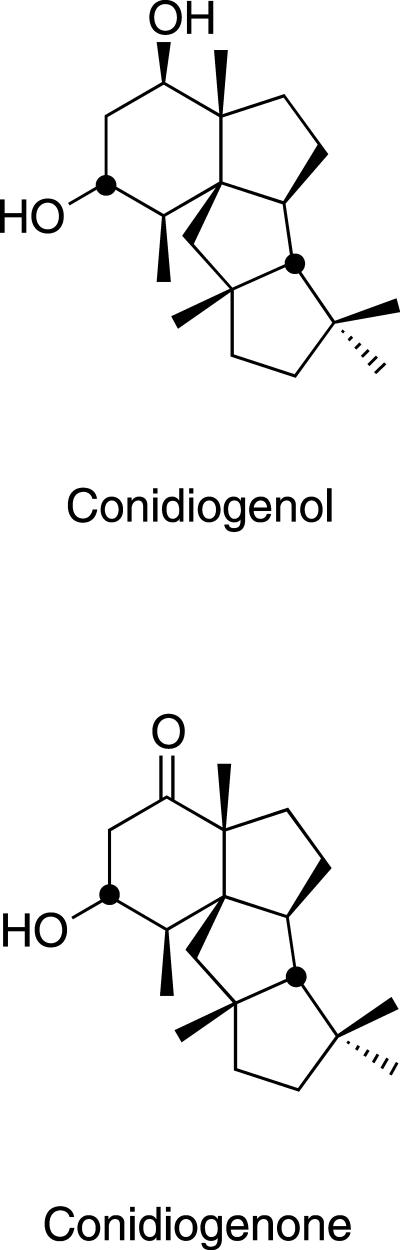
Based upon spectral data, compounds A and B were identified as two new diterpenes with novel terpenoid skeletons, as depicted in Fig. 2. Compound A (molecular formula, C20H34O2) and compound B (molecular formula, C20H32O2) have been assigned the trivial names conidiogenol and conidiogenone, respectively. Both molecules show tetracyclic hydrocarbon skeletons containing only two functional groups (two hydroxyls in conidiogenol, with one of them oxidized to a ketone group in conidiogenone). One (or both) of the functional groups appeared to be involved in the inducing activity, as suggested by the delayed activity displayed by the diacetylated derivative of conidiogenol relative to that of the unsubstituted molecule. The hydrolysis of the derivative to conidiogenol is probably catalyzed by esterases previously reported to be present in P. cyclopium (18).
FIG. 2.
Chemical structures of conidiogenol and conidiogenone, two novel diterpenes isolated from the P. cyclopium culture medium which showed conidiation-inducing activity.
Determination of conidiogenol and conidiogenone threshold concentrations.
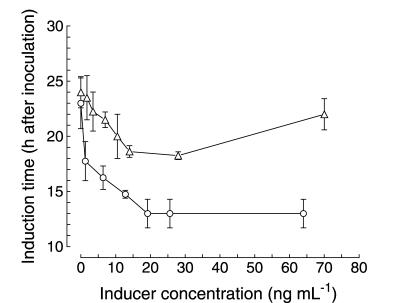
The concentration at which conidiogenone and conidiogenol were active was defined by determining the conidiation time (which determines the induction time) achieved at different doses of inducer when added to fresh F medium. As shown in Fig. 3, the highest increase in conidiation induction was reached for both compounds at concentrations of 20 to 25 ng ml−1 (6 × 10−8 to 8 × 10−8 mol liter−1), which indicated that their active concentration ranges were approximately the same. In the case of conidiogenone, since the lowest conidiation induction time was around 13 h after inoculation, very shortly after germination, it could be reasonably concluded that the contribution of the newly synthesized inducers would likely be negligible (see also below). Thus, the lowest conidiogenone concentration added to the medium which caused the earliest possible induction time was designated as the actual threshold value, i.e., 20 to 25 ng ml−1 (6 × 10−8 to 8 × 10−8 mol liter−1, or 60 to 80 nM).
FIG. 3.
Effect of inducer concentration on conidiation induction time. Increasing concentrations of conidiogenol (triangles) and conidiogenone (circles) were added to fresh F medium. The time at which conidiation induction occurred was determined by subtracting the 7-h period required for completion of morphogenesis from the measured conidiation time. Assay conditions were as described in Materials and Methods. The threshold concentrations required to induce conidiation were determined as the lowest concentrations which allowed induction to occur at the earliest time. Values are expressed as the means ± standard deviations of three independent experiments.
Two major differences arose in the action of the two molecules. First, conidiation induced by conidiogenol was always delayed (by 5 h) relative to that induced by equivalent conidiogenone levels. As their activity concentration ranges were the same, this result suggests that the truly active compound is conidiogenone and that conidiogenol may be an inactive derivative or precursor which requires oxidative transformation into the active form. Second, an inhibitory effect of conidiogenol was repeatedly observed for concentrations higher than the threshold concentration (concentrations up to 350 ng ml−1 were assayed), resulting in a dose-dependent delay in conidiation. This phenomenon was not observed for conidiogenone, which showed inducing activity only, irrespective of the amount by which its concentration exceeded the threshold concentration.
Time course of conidiogenone and conidiogenol production.
Conidiogenone and conidiogenol concentrations in the culture medium were estimated by means of the bioassay as a function of their resulting conidiation-inducing activity, using the threshold level as a standard for quantification. This method could not distinguish between the two molecules but rather measured the whole activity, which may depend on the concentrations of both agents.
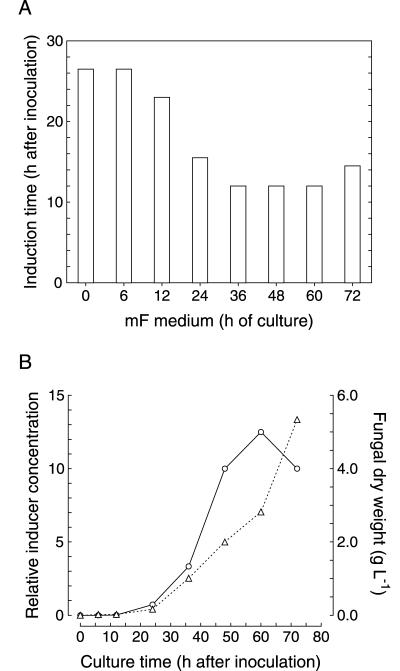
Mature media prepared from 36-, 48-, and 60-h-old cultures contained conidiation inducer levels equal to or higher than that of the threshold concentration, as deduced from the lowest conidiation time reached by them (Fig. 4A). Culture media from shorter incubations presented lower inducer concentrations, which resulted in increased conidiation times. Finally, a 72-h-old mature medium caused some delay in conidiation compared to that of the 60-h-old medium.
FIG. 4.
Time course of the production of conidiation inducers by P. cyclopium. The organism was cultured as explained in Materials and Methods, and samples of the culture medium were taken at different times. (A) Mature media from different culture times were directly assayed for conidiation-inducing activity, and the resulting induction times were determined. (B) Relative concentrations of conidiation inducers (compared to the threshold value) measured in the mature medium at different culture times (circles). Determination was done according to the procedure described in the text. The threshold concentration was assigned a relative value of 1 and is equal to 20 to 25 ng ml−1 in absolute terms. Fungal growth is expressed as dry weight (triangles). Values are expressed as the means of three independent experiments. Standard deviations (not shown) are lower than ±10%.
To determine more accurately the conidiation inducer concentrations in those media containing levels higher than the threshold concentration, the activity of several serial dilutions with fresh F medium was measured. In this way, the highest dilution showing the earliest conidiation time was determined as a measure of the concentration contained in that medium relative to the threshold concentration. The values obtained indicated that the 36-, 48-, and 60-h-old mature media contained inducer concentrations 3.3-, 10-, and 12.5-fold higher than threshold, respectively (Fig. 4B).
On the other hand, of cultures showing inducer levels lower than threshold (i.e., at 24 h and earlier) were estimated by comparing the time at which conidiation was reached to identical conidiation time. Using this methodology, we could establish that the inducer concentration after 24 h of culture was slightly below the threshold concentration and that by germination time it was barely detectable, possibly accounting for less than 2% of the threshold concentration (Fig. 4B).
Although conidiation in a 72-h-old mF medium was delayed relative to that found in the 60-h-old medium, surprisingly, dilution of the medium (up to 10-fold) caused an advancement in conidiation. The inducer levels were thus estimated to be 10-fold higher than that of the threshold. A delayed conidial germination was observed in the original 72-h-old mF medium which likely accounted for the partial inhibition, although an additional effect, arising from conidiogenol levels over that of the threshold concentration, could not be discounted.
The results indicate that conidiation inducers were first produced as soon as conidial germination took place, as a slight activity could be detected by 12 h, only 1 h after germination. Activity thereafter accumulated in the medium, with an initial period of exponential increase between 24 and 48 h, concomitant with biomass production, followed by a phase of deceleration up to the 60-h time point and a decrease thereafter.
The conidiation inducer levels calculated using this methodology would indicate that the overall recovery of the molecules from the medium was very low (about 2%). This is likely due to an inefficient extraction of the large aqueous volumes used for preparative purposes, since extraction of small volumes of mF medium rendered eluate inducer levels between 0.2- and 1-fold of that of the threshold concentration (see below) and consequent recovery values around 90%.
Conidiogenone is necessary and sufficient to induce conidiation.
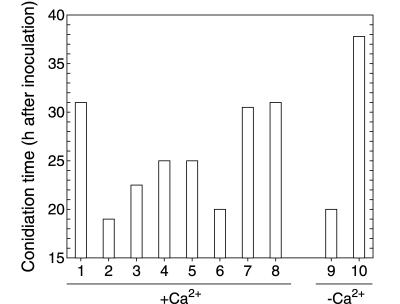
Solid-phase extraction of small volumes (100 ml) of mF medium effectively removed its conidiation induction potential, as shown in Fig. 5, without any detectable influence on growth rate (data not shown). It was therefore concluded that the extraction step eliminated substances responsible for the conidiation induction.
FIG. 5.
Effect of different media, extracts, and purified compounds on the conidiation time of P. cyclopium. Assays 1 to 8 were carried out according to the established procedure, with 10 mM calcium present in the medium. Assays 9 and 10 were performed with no calcium added to the medium. Lane 1, fresh F medium; lane 2, 48-h-old mF medium; lane 3, fresh F medium plus crude extract of the 48-h-old mF medium; lane 4, 48-h-old mF medium after extraction (100 ml extracted); lane 5, fresh F medium plus conidiogenol (25 ng ml−1); lane 6, fresh F medium plus conidiogenone (25 ng ml−1); lane 7, fresh F medium plus pooled fractions other than those containing conidiogenol and conidiogenone after HPLC separation of the crude extract; lane 8, 48-h-old mF medium subjected to autoclaving (121°C for 15 min); lane 9, fresh F medium (without calcium) plus conidiogenone (125 ng ml−1); lane 10, fresh F medium (without calcium). Values are expressed as the means of three independent experiments. Standard deviations (not shown) are lower than ±10%.
Further reverse-phase HPLC fractionation of the crude extract revealed conidiation-inducing activity in only two adjacent fractions, containing conidiogenol and conidiogenone, respectively. When the crude extract was deprived of both active fractions (that is, when the HPLC effluent fractions other than the two active fractions were pooled and assayed), no conidiation induction activity was detected (Fig. 5).
The evidence strongly suggested that conidiogenone was the only conidiation inducer present in mF medium and that its presence was necessary for the induction of conidiogenesis. This evidence is summarized as follows: (i) the time required to conidiate is directly related to the conidiogenone concentration in the medium; (ii) conidiation is unrelated to limitation in any of the nutrients; and (iii) apart from conidiogenone (and conidiogenol), no additional molecules have been detected with conidiogenic activity.
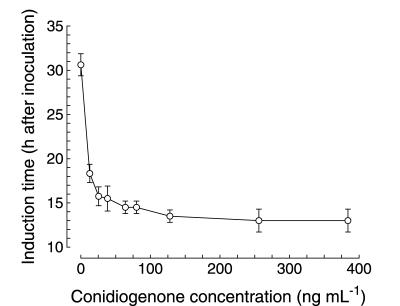
In all experiments carried out up to this point, calcium was always included in the culture medium in the belief that it was a necessary constituent for morphogenesis to be manifested. But when increased concentrations of conidiogenone in fresh F medium were assayed in the absence of added calcium, conidiation also took place (Fig. 5 and 6), reaching conidiation induction times identical to the earliest times obtained in calcium-supplemented cultures.
FIG. 6.
Effect of conidiogenone concentration on the conidiation induction time in the absence of calcium in the culture medium. Increasing concentrations of conidiogenone were added to fresh F medium (with no calcium added), and the time at which conidiation induction occurred was determined. All assay conditions were as described in Materials and Methods. The threshold concentration required to induce conidiation was determined as the lowest concentration which allowed induction to occur at the earliest time. Values are expressed as the means ± standard deviations of three independent experiments.
The concentration range within which conidiogenone acted was considerably higher in the absence of calcium, however (Fig. 6), showing a threshold concentration of about 125 ng ml−1 (4 × 10−7 mol liter−1), a level five- to sixfold higher than that found if calcium was present in the medium. This result indicated that calcium was unnecessary for the induction step and rather acted indirectly, by decreasing the conidiogenone threshold concentration required to efficiently induce conidiation.
Finally, both experiments indicate that the presence of conidiogenone is necessary and sufficient to induce conidiation in P. cyclopium growing in liquid culture and that calcium or other factors do not play a direct role in the induction of conidiation.
DISCUSSION
The exposure of the mycelium to the aerial medium is the most effective and widespread stimulus of conidiation (16). Nutrient starvation (16, 20), oxidative stress (11), light (15), and chemical signals (3, 4, 5, 13) have been proposed to have a role in this complex environmental change for the emerging hypha.
Following the line of evidence involving chemical signals, the existence of an extracellular factor responsible for (programmed) conidiation induction in the model organism Aspergillus nidulans has also been proposed, the synthesis of which would be related to an active fluG gene (13). The putative metabolite remains to be identified, although it appears that it would not be the same as psiAα (13), an endogenous molecule that also promotes asexual development in A. nidulans and acts as an antagonist of the other psi factors and related molecules which induce sexual development (2, 3, 4, 5, 14).
The action of fungal metabolites in the attainment of competence for conidiation in P. notatum was proposed by Hadley and Harrold (10) at a time when the inducer of the process was believed to be calcium ions.
We have developed this work further with the isolation from mature P. cyclopium-harboring medium of two molecules with conidiation-inducing activity: namely, conidiogenol and conidiogenone. The two molecules appear to be closely related, as evidenced by their chemical structures, which differ only in the oxidation state of one functional group (hydroxyl or ketone). It is therefore very likely that they are contiguous in a biosynthetic pathway and that one of them is the product of a reaction of oxidoreduction of the other. In addition, both molecules act at approximately the same threshold concentration. The experiment with the diacetylated derivative of conidiogenol shows that one or both functional groups present in the molecules are involved in their biological activity, which is probably mediated by binding to a cellular receptor. Thus, the existence of two molecules which differ in their key functional groups and are able to cause the same biological response at approximately the same threshold concentration (the affinity of the receptors for their ligands is highly specific), in addition to the fact that the response induced by conidiogenol is always delayed relative to that induced by conidiogenone at the same concentration (the delay would be the time required to accomplish such a conversion), implies that conidiogenol is converted into conidiogenone and that the latter is the actual inducer.
Conidiogenone and conidiogenol are produced from the very earliest stages of growth and are continuously released to the culture medium, where they accumulate until a threshold concentration is reached, triggering conidiation. Conidiogenone would therefore act as a hormone-like molecule at extremely low concentrations (around 10−7 to 10−8 mol liter−1), which is proof of its high specificity and potency.
A relevant finding of this study is that conidiogenone plays a central role in the induction of conidiation in submerged culture. The only demonstrated effect of calcium is that of reducing the conidiogenone threshold concentration required to induce conidiation, in a manner not yet understood but probably related to the binding of this cation to the extracellular hyphal surface (23, 24). Indeed, a strain of P. notatum, which essentially behaves like P. cyclopium with respect to conidiation induction, with a reduced capacity of calcium binding also showed a very reduced conidiation response in submerged liquid culture (17), suggesting a relationship between surface binding and conidiation induction.
The findings that conidiogenone accumulation is necessary for induction of conidiation and that calcium is not necessary at the induction step are extremely relevant to our understanding of conidiation induction in a general context. In submerged culture, conidiogenone diffuses through the fungal cell wall towards the bulk medium, with a dilution effect which prevents the threshold concentration from being reached. The conidiogenone dilution level may indicate the absence of aerial conditions. Conversely, on emergence to the aerial medium, diffusion is greatly reduced and secreted conidiogenone rapidly accumulates up to threshold levels in the vicinity of the plasma membrane and cell wall of aerial hyphae.
Under this new perspective, attainment of competence, the concept mentioned earlier (8, 10), would therefore represent the attainment of threshold concentrations of conidiogenone.
The mechanism of conidiation induction postulated here would resemble those already proposed for some prokaryotes (19), among which the A-factor from Streptomyces griseus, directing aerial mycelium formation and hence sporulation and synthesis of several secondary metabolites, is one of the best known (12).
Besides contributing to the clarification of some basic aspects concerning conidiation induction in P. cyclopium, the finding also poses new questions to be clarified, such as the relationship between conidiogenol and conidiogenone. The conversion of conidiogenol to conidiogenone and/or their resulting concentration ratio might be a final regulatory point controlling conidiation induction. Indeed, the inhibitory effect of conidiogenol at levels surpassing the threshold concentration could bear physiological significance.
Acknowledgments
We thank the Spanish Ministerio de Ciencia y Tecnología (MCYT) for financial support through grant BIO1999-0260, the University of the Basque Country (UPV/EHU) through grant UPV 221.215-EB179/98, and the Diputación Foral de Gipuzkoa. T.R. held a contract and a postdoctoral fellowship of MCYT and the University of the Basque Country, respectively. S.C. held a predoctoral fellowship of the University of the Basque Country.
REFERENCES
- 1.Adams, T. H., J. K. Wieser, and J.-H. Yu. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo, A. M., H. W. Gardner, and N. P. Keller. 2001. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 276:25766-25774. [DOI] [PubMed] [Google Scholar]
- 3.Calvo, A. M., L. L. Hinze, H. W. Gardner, and N. P. Keller. 1999. Sporogenic effect of polyunsaturated fatty acids on development of Aspergillus spp. Appl. Environ. Microbiol. 65:3668-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champe, S. P., and A. A. E. El-Zayat. 1989. Isolation of a sexual sporulation hormone from Aspergillus nidulans. J. Bacteriol. 171:3982-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champe, S. P., P. Rao, and A. Chang. 1987. An endogenous inducer of sexual development in Aspergillus nidulans. J. Gen. Microbiol. 133:1383-1387. [DOI] [PubMed] [Google Scholar]
- 6.Foster, J. W., L. E. McDaniel, H. B. Woodruff, and J. L. Stokes. 1945. Microbiological aspects of penicillin. V. Conidiospore formation in submerged cultures of Penicillium notatum. J. Bacteriol. 50:365-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galbraith, J. C., and J. E. Smith. 1969. Sporulation of Aspergillus niger in submerged liquid culture. J. Gen. Microbiol. 59:31-45. [DOI] [PubMed] [Google Scholar]
- 8.Gealt, M. A., and D. E. Axelrod. 1974. Coordinate regulation of enzyme inducibility and developmental competence in Aspergillus nidulans. Dev. Biol. 41:224-232. [DOI] [PubMed] [Google Scholar]
- 9.Hadley, G., and C. E. Harrold. 1958. The sporulation of Penicillium notatum Westling in submerged liquid culture. I. The effect of calcium and nutrients on sporulation. J. Exp. Bot. 9:408-417. [Google Scholar]
- 10.Hadley, G., and C. E. Harrold. 1958. The sporulation of Penicillium notatum Westling in submerged liquid culture. II. The initial sporulation phase. J. Exp. Bot. 9:418-425. [Google Scholar]
- 11.Hansberg, W., and J. Aguirre. 1990. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J. Theor. Biol. 142:201-221. [DOI] [PubMed] [Google Scholar]
- 12.Horinouchi, S., and T. Beppu. 1994. A-factor as a microbial hormone that controls cellular differentiation and secondary metabolism in Streptomyces griseus. Mol. Microbiol. 12:859-864. [DOI] [PubMed] [Google Scholar]
- 13.Lee, B. N., and T. H. Adams. 1994. The Aspergillus nidulans fluG gene is required for production of an extracellular developmental signal and is related to prokaryotic glutamine synthetase I. Genes Dev. 8:641-651. [DOI] [PubMed] [Google Scholar]
- 14.Mazur, P., K. Nakanishi, A. A. E. El-Zayat, and S. P. Champe. 1991. Structure and synthesis of sporogenic psi factors from Aspergillus nidulans. J. Chem. Soc. Chem. Commun. 20:1486-1487. [Google Scholar]
- 15.Mooney, J. L., and L. N. Yager. 1990. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 4:1473-1482. [DOI] [PubMed] [Google Scholar]
- 16.Morton, A. G. 1961. The induction of sporulation in mould fungi. Proc. R. Soc. Lond. B 153:548-569. [Google Scholar]
- 17.Pitt, D., J. C. Barnes, and U. O. Ugalde. 1988. Differential uptake of calcium by strains of Penicillium notatum and relationships to calcium-induced conidiation. Trans. Br. Mycol. Soc. 91:489-499. [Google Scholar]
- 17a.Roncal, T., S. Cordobés, U. Ugalde, Y. He, and O. Sterner. 2002. Novel diterpenes with potent conidiation inducing activity. Tetrahedron Lett. 43:6799-6802. [Google Scholar]
- 18.Roncal, T., U. O. Ugalde, and A. Irastorza. 1993. Calcium-induced conidiation in Penicillium cyclopium: calcium triggers cytosolic alkalinization at the hyphal tip. J. Bacteriol. 175:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmond, G. P. C., B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1995. The bacterial ′enigma': cracking the code of cell-cell communication. Mol. Microbiol. 16:615-624. [DOI] [PubMed] [Google Scholar]
- 20.Skromne, I., O. Sanchez, and J. Aguirre. 1995. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology 141:21-28. [DOI] [PubMed] [Google Scholar]
- 21.Springer, M. L. 1993. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays 15:365-374. [DOI] [PubMed] [Google Scholar]
- 22.Ugalde, U., and D. Pitt. 1983. Morphology and calcium-induced conidiation of Penicillium cyclopium in submerged culture. Trans. Br. Mycol. Soc. 80:319-325. [Google Scholar]
- 23.Ugalde, U. O., and D. Pitt. 1986. Calcium uptake kinetics in relation to conidiation in submerged cultures of Penicillium cyclopium. Trans. Br. Mycol. Soc. 87:199-203. [Google Scholar]
- 24.Ugalde, U. O., M. D. Virto, and D. Pitt. 1990. Calcium binding and induction of conidiation in protoplasts of Penicillium cyclopium. Antonie Leeuwenhoek 57:43-49. [DOI] [PubMed] [Google Scholar]