Phagocytic cells of the innate immune system, such as macrophages and neutrophils, are a primary line of defense against microbial infections. Patients with defects in innate immunity, such as those with chronic granulomatous disease or neutropenia, are extremely sensitive to a variety of infections. When a phagocyte recognizes the presence of an invading cell, it engulfs the microbe with its membrane to form the phagosome, an intracellular compartment containing the microbe. This compartment matures by fusion with lysosomes to create the phagolysosome, an organelle replete with antimicrobial compounds and an acidic pH. Internalization creates a hostile environment for the microorganism, which, of course, is the intent.
The phagolysosome is a precarious neighborhood even before the onslaught of antimicrobial compounds. Engulfment by the macrophage thrusts the microorganism into an alien milieu, one devoid of key nutrients necessary for metabolism and division. Surviving the antimicrobial assault in the phagolysosome depends on the microbe's ability to synthesize the proteins and other cellular components necessary to counteract these stresses. Thus, a pathogen must find the requisite nutrients to provide the building blocks for these complex macromolecules and the energy with which to synthesize them.
In this article we consider the initial responses of several microbes to nutrient deprivation inside the macrophage. The first of these, Mycobacterium tuberculosis, the bacterium that causes tuberculosis, resides for prolonged periods within the macrophage, in which it can proliferate and subsequently spread throughout the body. The second, the yeast Saccharomyces cerevisiae, is killed efficiently by the macrophage. The third, the opportunistic fungal pathogen Candida albicans, survives ingestion by changing rapidly from a yeast to a filamentous morphology, lysing the macrophage from the inside out. Once free, C. albicans cells are able to disseminate through the body. The interaction of C. albicans with the macrophage is transient, as opposed to the long-term persistence of M. tuberculosis. Although the outcomes of this macrophage capture are quite different among the three microbes, the initial responses of all three to the internal environment are remarkably similar: induction of the glyoxylate cycle, a pathway that permits the utilization of compounds with two carbons (C2 compounds), such as acetate, to satisfy cellular carbon requirements.
THE BACTERIUM: M. TUBERCULOSIS
In 1998, there were an estimated 6.7 million cases of tuberculosis resulting in 2.4 million deaths (15), making tuberculosis the most lethal infectious disease. While tuberculosis is infrequent in Western countries (though its prevalence is on the rise), it obviously remains a significant problem in the developing world. It is estimated to exist as a dormant infection in over 2 billion people, awaiting the proper conditions to become activated. At least part of this latency results from the ability of M. tuberculosis to reside for very long times within macrophages.
Sturgill-Koszycki and colleagues modeled this survival strategy by using a macrophage-resident population of the related bacterium Mycobacterium avium. The protein complement of these cells was compared by two-dimensional gel analysis to that of cells grown in vitro. One protein present at significantly higher levels in the phagocytosed population was identified by microsequencing as isocitrate lyase, a key enzyme of the glyoxylate cycle (22). A second group used a cDNA selection technique to identify genes upregulated upon phagocytosis. Isocitrate lyase was one of the 11 genes identified and was the only metabolic gene in the set (5). Thus, two different approaches point to the induction of isocitrate lyase as a key response to phagocytosis in Mycobacterium species.
Both M. avium and M. tuberculosis have two open reading frames, aceA and icl, whose products are homologous to isocitrate lyase, and it was initially unclear which one truly encoded isocitrate lyase. Several lines of evidence suggest that the relevant gene is icl. First, some Mycobacterium strains have mutations in aceA and/or express aceA differently. Moreover, only icl complements a Mycobacterium smegmatus icl mutant for growth on C2 carbon sources (7).
In work done in the Russell and Jacobs labs, Icl was fused to green fluorescent protein (GFP) to show that macrophage ingestion stimulates icl expression in M. tuberculosis as well as M. avium (14), as the cDNA selection method had suggested (5). The level of induction of the GFP fusion protein was even higher when the cells were inside macrophages stimulated with gamma interferon and lipopolysaccharide. This group constructed a strain lacking icl and showed that it had no growth defects in broth medium. Earlier studies of isocitrate lyase from M. tuberculosis suggested that the protein activity was induced upon entry into stationary phase and by hypoxic conditions (16, 26); however, the icl mutant strain constructed for this study had no adverse phenotypes under either of these conditions (14).
The importance of the glyoxylate pathway in disease progression was confirmed in several virulence assays. When tested in an in vitro model, the icl mutant had a slight survival defect when it was inside resting macrophages and a significant defect in activated macrophages. In a mouse model of tuberculosis, the icl mutant strain of M. tuberculosis grows at the same rate as the wild-type strain during the early, acute phase of infection. However, the icl mutant strain is much less persistent and is eventually cleared from the lungs of infected animals. This difference manifests itself as a dramatically reduced virulence of the icl mutant strain. The virulence defect of the icl mutant strain was eliminated in immunodeficient mice lacking gamma interferon. Taken together, these data indicate that the glyoxylate cycle is critical for the pathogenicity of M. tuberculosis but that the requirement for this pathway is partially dependent on the immune status of the host (14).
THE FUNGI: C. ALBICANS, S. CEREVISIAE, AND CRYPTOCOCCUS NEOFORMANS
Systemic fungal infections have increased dramatically in prevalence and severity over the last few decades, in concert with the number of patients living for extended periods with significant immune dysfunction. AIDS, cancer chemotherapy, and organ transplantation have all contributed to this rise, as has the widespread use of antibiotics. The most common systemic fungal infection is candidiasis, which accounts for well over half of these invasive mycoses (3). A single species, C. albicans, causes the majority of these infections. C. albicans, which also causes oropharyngeal thrush and vaginitis, is normally a commensal of the mammalian gastrointestinal tract, in which it lives without adverse effects on the host. C. albicans is a yeast related to the brewer's yeast S. cerevisiae. Brewer's yeast is not normally pathogenic, though it can be isolated from patients on rare occasions. Because of the plethora of molecular genetic tools available for this organism, it is frequently used to model various aspects of the behavior of C. albicans.
Both C. albicans and S. cerevisiae are readily phagocytosed by cultured macrophages in the presence of serum. While the macrophages efficiently kill S. cerevisiae, engulfment induces C. albicans cells to grow in a filamentous morphology. These hyphal filaments can penetrate through the membrane of the phagocytic cell, releasing the fungal cell back into the extracellular medium while killing the macrophage in the process. The different outcomes are not surprising; C. albicans is a common pathogen while S. cerevisiae is rarely found in human hosts.
We utilized a genomic approach to understand the fungal response to phagocytosis. To do this, we used S. cerevisiae as a model, since genomic microarrays have been available for S. cerevisiae for several years, whereas the genome sequence of C. albicans has only recently been completed (24). Using a coculture system, we isolated S. cerevisiae cells from the macrophage phagolysosome after 3 h of contact, a time at which their C. albicans counterparts, phagocytosed but not killed by the macrophage, were beginning to escape from the macrophage. RNA from this population was analyzed with genomic microarrays and compared to RNA from cells grown in medium alone (11).
The primary response to phagocytosis in S. cerevisiae was the induction of gene products related to the glyoxylate cycle. Both isocitrate lyase (the ICL1 product) and malate synthase (the MLS1 product), the key enzymes of the glyoxylate cycle, were highly induced (both were induced 22-fold above the levels in the control population), as were malate dehydrogenase and citrate synthase. Further, the products of genes with functions associated with the glyoxylate cycle were also induced, such as acetyltransferases and carrier proteins which transport metabolites between organelles, acetyl coenzyme A (acetyl-CoA) synthase, and the gluconeogenic enzyme fructose-1,6-bisphosphatase. In total, 11 of the 15 genes whose products were most highly induced in macrophages encode proteins whose function is related to the glyoxylate cycle. Other transcripts, most notably those of the tricarboxylic acid (TCA) cycle, were not induced under these conditions.
Based on these observations for Saccharomyces, we analyzed the glyoxylate pathway in C. albicans when this organism is inside the macrophage. The C. albicans homologs of isocitrate lyase (products of the CaICL1 and CaMLS1 genes) are also induced upon phagocytosis, as determined by Northern blotting. The C. albicans Δicl1/Δicl1 mutant strain has no in vitro phenotypes other than the expected inability to utilize C2 carbon sources, but it is markedly less virulent in a mouse model of systemic candidiasis (11). Further, the mutant strain is less persistent in internal organs such as the kidney and liver than is the wild-type strain (our unpublished observations). Thus, in these fungi and in M. tuberculosis, the glyoxylate cycle is both induced during phagocytosis by the macrophage and required for full virulence in C. albicans.
S. cerevisiae is not a pathogen in immunocompetent humans or in the mouse model described earlier. However, Goldstein and McCusker have developed an animal model that allows them to assess the ability of different Saccharomyces strains to survive in vivo (4). This assay uses immunodeficient mice lacking part of the complement cascade and is done as a competition, with two Saccharomyces strains being injected into the same mouse. The relative levels of abundance of the two strains in the tissues of the animal (they focused on the brain) at time points several weeks postinjection are a measure of the in vivo fitness of the two strains. Using this system, they determined that an Δicl1 mutant strain persists nearly as well in the brain as the wild-type strain; hence, the glyoxylate cycle does not significantly affect the ability of S. cerevisiae to survive in vivo (4).
The role of the glyoxylate cycle in one other fungal pathogen, the yeast C. neoformans, has been studied. This yeast, taxonomically quite distant from C. albicans and S. cerevisiae, can cause a fungal pneumonia, but its most serious manifestation is a central nervous system (CNS) meningitis. This infection is one of the leading causes of death in AIDS patients (8, 20). J. Perfect and colleagues found that isocitrate lyase (the product of ICL1) is upregulated in C. neoformans cells at the site of infection (the CNS), as determined by differential display technology. Unlike the earlier examples, this population was from an in vivo infection and therefore was not necessarily exposed to phagocytic cells. Mutations in ICL1, however, do not affect the virulence of this fungus in an animal model of cryptococcal meningitis (18a). As for S. cerevisiae, this finding raises the question of why the glyoxylate cycle is induced if it does not affect the organism's fitness in vivo.
GLYOXYLATE CYCLE
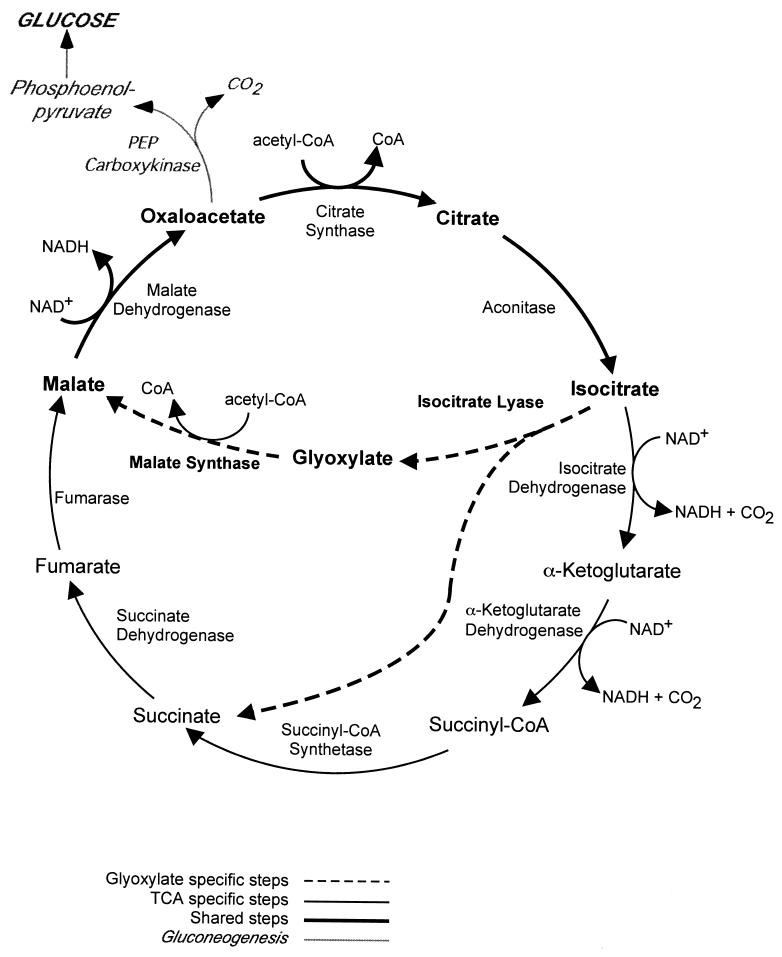
What is the glyoxylate cycle and why should such diverse organisms respond to contact with the immune system by inducing this pathway? The primary function of the glyoxylate cycle is to permit growth when C2 compounds, such as ethanol and acetate, are the only sources of carbon (9). Glucose, as the preferred carbon source in most organisms, can be both converted into five-carbon sugars (such as ribose and deoxyribose) via the pentose phosphate pathway and catabolized to acetyl-CoA via glycolysis. Acetyl-CoA enters the TCA cycle (Fig. 1), where eight enzymatic steps convert it into intermediates which feed numerous biosynthetic pathways, including those for amino acids, heme, fatty acids, and glucose. Without flux through the TCA cycle, an organism, whether a bacterium or a mammal, cannot survive.
FIG. 1.
TCA or glyoxylate cycle and gluconeogenesis. The schematic depicts the basic enzymatic steps in the TCA cycle (black lines), which is common to all organisms, those of the glyoxylate cycle (dashed lines), which is specific to microorganisms and plants, and those steps shared by both cycles (bold lines) and the initial reaction of gluconeogenesis (shaded lines). PEP, phosphoenolpyruvate.
In microorganisms, however, glucose is frequently not available, and simple carbon compounds provide the only accessible carbon. The TCA cycle, with its two decarboxylation steps (Fig. 1), does not permit assimilation of carbon and thus does not provide a route for the synthesis of macromolecules from C2 compounds. The glyoxylate pathway (also dubbed the glyoxylate shunt, for clear reasons) bypasses these decarboxylations, allowing C2 compounds to serve as carbon sources in gluconeogenesis and to be incorporated into glucose and, from there, into amino acids, DNA, and RNA.
The glyoxylate cycle has two critical steps. In the first, isocitrate (six carbons) is hydrolyzed to succinate (four carbons) and glyoxylate (two carbons) by isocitrate lyase. In the second step, acetyl-CoA (two carbons) is condensed with glyoxylate to produce malate (four carbons) by malate synthase. Malate, an intermediate of the TCA cycle, is converted to oxaloacetate, to citrate (by the addition of another molecule of acetyl-CoA), and then to isocitrate again. These steps are enzymatically identical to those of the TCA cycle, but there is regulatory specificity conferred both by dedicated glyoxylate isozymes and by compartmentalization. Thus, C2 compounds can replenish the intermediates of the TCA cycle via the glyoxylate cycle.
Most importantly, though, the TCA cycle is the origination of gluconeogenesis. The first committed step in gluconeogenesis (Fig. 1) is the conversion of oxaloacetate to phosphoenolpyruvate via the enzyme phosphoenolpyruvate carboxykinase (Pck1 in S. cerevisiae). This enzyme and fructose-1,6-bisphosphatase (Fbp1 in S. cerevisiae) serve as the two unique steps in gluconeogenesis (the only steps that are not simply glycolysis running in reverse) and as such are the key regulators of glucose production. FBP1 was one of the genes whose product was highly induced upon phagocytosis in the yeast experiment (11), supporting the idea that the primary purpose for the induction of the glyoxylate cycle is the production of glucose. Glucose, then, provides the energy and building blocks (such as nucleotides) to sustain the cell.
WHY IS THE GLYOXYLATE CYCLE INDUCED IN PHAGOCYTOSED MICROORGANISMS?
The most likely explanation for the induction of the glyoxylate cycle in bacteria and fungi upon phagocytosis is that activation of this pathway represents the response of these microorganisms to nutrient starvation in the phagosome. The composition of the phagolysosome, in terms of what nutrients may be available to phagocytosed microorganisms, is essentially unknown but is unlikely to include a significant amount of glucose or certain amino acids. Thus, the induction of the glyoxylate pathway may be an indication that the organism has identified simple carbon sources in this environment and is activating the pathways necessary to utilize them. Where might these simple compounds come from? An intriguing possibility is that they are derived from the breakdown of fatty acids via β oxidation, which results in acetyl-CoA, whose utilization would require the glyoxylate cycle. If fatty acid breakdown, whether of host lipids or lipids from the microorganism itself, occurs within the phagolysosome, it might provide a carbon source accessible to the pathogen while it is inside the macrophage.
There are several other examples of cellular processes that are not specifically essential in vitro but are required for full virulence in vivo. Amino acid metabolism contributes to survival in vivo in both Mycobacterium and fungi. In the Mycobacterium bovis bacillus Calmette-Guerin (BCG) strains used as vaccines, auxotrophy for leucine confers severe avirulence, even in immunodeficient SCID animals (6, 13). In C. albicans and C. neoformans, strains deficient in synthesis of uracil or adenine are avirulent (10, 17). Similarly, iron uptake is required for full virulence in many species, including M. tuberculosis and C. albicans (2, 12, 18). None of these processes is essential during growth on standard laboratory media and, for that reason, have historically been overlooked (inappropriately, it is now clear) in the search for antimicrobial agents.
As demonstrated by the studies described above, the presence (and induction) of the glyoxylate cycle, while required for full virulence in both M. tuberculosis and C. albicans, is clearly not sufficient to make an organism pathogenic. M. avium and S. cerevisiae, which are not common pathogens in mammals, induce isocitrate lyase in response to phagocytosis. Thus, as is the case with several other genes related to nutrient acquisition (such as amino acid metabolism), the glyoxylate cycle is a prerequisite for virulence but is not a virulence factor per se. Rather, the term “persistence factor” has been applied to the M. tuberculosis enzyme, which seems an appropriate description (21).
The loss of isocitrate lyase function in M. tuberculosis and C. albicans confers a significant virulence defect, whereas in C. neoformans and S. cerevisiae it does not. This difference could be explained if different organisms had distinct organ tropisms during an infection or if there was an inherent bias in the animal models. The brain or CNS is obviously of significant clinical interest, at least for C. neoformans, but is a different target organ from that used for tuberculosis (lung) or systemic candidiasis (liver and kidneys mostly) and may explain the differences seen in these studies. The differences in the glyoxylate findings with regard to the organ tropism of the pathogen and the animal model used provide a well-warranted caution in the use and interpretation of these models. Nevertheless, the glyoxylate cycle was induced in both S. cerevisiae and C. neoformans, suggesting that both organisms recognize nutrient starvation when they colonize a mammal.
GLYOXYLATE CYCLE AND PLANT PATHOGENS
A recent report suggests that the glyoxylate cycle is also important for the virulence of phytopathogens. An insertional mutagenesis screen for avirulence in the bacterium Rhodococcus fascians, which causes a gall in several plant species, identified a mutant defective in the glyoxylate pathway enzyme malate synthase (VicA). Strains containing this mutation were still able to persist on plant tissues (asymptomatically) but were unable to cause disease (25). Similarly, an insertional mutagenesis screen for avirulent mutants of Leptosphaeria maculans, a fungal pathogen of Brassica napus (canola), identified isocitrate lyase (7a).
Circumstantial evidence from other fungi support a role for the glyoxylate cycle in growth on the plant. A transgene fusing GFP to the promoter of the Neurospora crassa isocitrate lyase was expressed in Tapesia yallundae, a pathogen of wheat. This construct is properly regulated in vitro and is specifically activated during infection of the plant (1). Further, metabolism of intracellular stores of lipids appears to be important for the formation of the appresorium (infection structure) in the rice blast fungus Magnaporthe grisea (23). Conversely, the glyoxylate cycle was found by microarray analysis to be moderately induced in response to infection in the model plant Arabidopsis thaliana (19), which, like most plants, has a functional glyoxylate cycle. By this response, the plant may be using lipids for energy to combat the infection or it may be seeking to sequester lipids from the invading organism. In either case, it is clear that the glyoxylate cycle plays a role in mediating both the plant-pathogen and the animal-pathogen interactions.
GLYOXYLATE CYCLE AND VIRULENCE: IMPLICATIONS
The recent upswing in cases of tuberculosis in the Western world, combined with the advent of drug-resistant tuberculosis strains, underscores the need for novel pharmaceuticals. The phenotype of the isocitrate lyase mutant, a pronounced defect in long-term persistence, suggests that a drug targeting the enzymes of the glyoxylate cycle might be useful in treating the latent infection (affecting perhaps one-third of the planet) as well. The treatment of fungal infections such as candidiasis has always suffered from a paucity of therapeutic agents. Eukaryotic yeast cells are similar enough to mammalian cells that drug targets are difficult to develop. With the population of immunocompromised people on the rise, the frequency of invasive fungal infections continues to increase, making the need for effective treatments more imperative.
The enzymes of the glyoxylate cycle are valuable targets for the development of antimicrobial drugs because this pathway does not exist in the mammalian host. Mammals require sugars as a carbon source; therefore, they do not need the glyoxylate cycle (or perhaps the absence of a glyoxylate cycle is why we require glucose). Drugs targeted to the glyoxylate enzymes would be predicted to be specific and might have less host toxicity than drugs that inhibit more conserved processes. In addition, they might be effective against a wide range of infectious agents, including both fungi and bacteria. The crystal structure of isocitrate lyase from Mycobacterium has been determined recently, both alone and in complex with several small molecule inhibitors, providing hope that a pharmacologically suitable inhibitor can be developed (21).
More generally, the discovery of the role of the glyoxylate cycle in microbial virulence should refocus attention on the critical importance of basic metabolic pathways in the development of disease. If a pathogen is unable to synthesize the precursor nucleic and amino acids required for growth, it is unlikely to proliferate or persist. For this reason, elucidation of a pathogen's nutritional requirements in vivo as well as the mechanism by which microbes acquire these nutrients once inside a host are critical in understanding virulence and disease.
Acknowledgments
We thank B. Jacobs and R. Wheeler for critical comments on the manuscript.
G.R.F. is supported by NIH grant 5RO1-GM35010.
REFERENCES
- 1.Bowyer, P., E. Mueller, and J. Lucas. 2000. Use of an isocitrate lyase promoter-GFP fusion to monitor carbon metabolism of the plant pathogen Tapesia yallundae during infection of wheat. Mol. Plant Pathol. 1:253-262. [DOI] [PubMed] [Google Scholar]
- 2.De Voss, J. J., K. Rutter, B. G. Schroeder, H. Su, Y. Zhu, and C. E. Barry III. 2000. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 97:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29: 239-244. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein, A. L., and J. H. McCusker. 2001. Development of Saccharomyces cerevisiae as a model pathogen. A system for the genetic identification of gene products required for survival in the mammalian host environment. Genetics 159:499-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guleria, I., R. Teitelbaum, R. A. McAdam, G. Kalpana, W. R. Jacobs, Jr., and B. R. Bloom. 1996. Auxotrophic vaccines for tuberculosis. Nat. Med. 2:334-337. [DOI] [PubMed] [Google Scholar]
- 7.Höner Zu Bentrup, K., A. Miczak, D. L. Swenson, and D. G. Russell. 1999. Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J. Bacteriol. 181:7161-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Idnurm, A., and B. J. Howlett. 2002. Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryot. Cell 1:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan, J. E., D. Hanson, M. S. Dworkin, T. Frederick, J. Bertolli, M. L. Lindegren, S. Holmberg, and J. L. Jones. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30(Suppl. 1):S5-S14. [DOI] [PubMed] [Google Scholar]
- 9.Kornberg, H. L. 1966. The role and control of the glyoxylate cycle in Escherichia coli. Biochem. J. 99:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lay, J., L. K. Henry, J. Clifford, Y. Koltin, C. E. Bulawa, and J. M. Becker. 1998. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect. Immun. 66:5301-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83-86. [DOI] [PubMed] [Google Scholar]
- 12.Manabe, Y. C., B. J. Saviola, L. Sun, J. R. Murphy, and W. R. Bishai. 1999. Attenuation of virulence in Mycobacterium tuberculosis expressing a constitutively active iron repressor. Proc. Natl. Acad. Sci. USA 96:12844-12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAdam, R. A., T. R. Weisbrod, J. Martin, J. D. Scuderi, A. M. Brown, J. D. Cirillo, B. R. Bloom, and W. R. Jacobs, Jr. 1995. In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect. Immun. 63:1004-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinney, J. D., K. Höner zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 15.Murray, C. J., and J. A. Salomon. 1998. Modeling the impact of global tuberculosis control strategies. Proc. Natl. Acad. Sci. USA 95:13881-13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy, P. S., M. Sirsi, and T. Ramakrishnan. 1973. Effect of age on the enzymes of tricarboxylic acid and related cycles in Mycobacterium tuberculosis H37Rv. Am. Rev. Respir. Dis. 108:689-690. [DOI] [PubMed] [Google Scholar]
- 17.Perfect, J. R., D. L. Toffaletti, and T. H. Rude. 1993. The gene encoding phosphoribosylaminoimidazole carboxylase (ADE2) is essential for growth of Cryptococcus neoformans in cerebrospinal fluid. Infect. Immun. 61:4446-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanan, N., and Y. Wang. 2000. A high-affinity iron permease essential for Candida albicans virulence. Science 288:1062-1064. [DOI] [PubMed] [Google Scholar]
- 18a.Rude, T. H., D. L. Toffaletti, G. M. Cox, and J. R. Perfect. 2002. Relationship of the glyoxylate pathway to the pathogenesis of Cryptococcus neoformans. Infect. Immun. 70:5684-5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheideler, M., N. L. Schlaich, K. Fellenberg, T. Beissbarth, N. C. Hauser, M. Vingron, A. J. Slusarenko, and J. D. Hoheisel. 2002. Monitoring the switch from housekeeping to pathogen defense metabolism in Arabidopsis thaliana using cDNA arrays. J. Biol. Chem. 277:10555-10561. [DOI] [PubMed] [Google Scholar]
- 20.Selik, R. M., J. M. Karon, and J. W. Ward. 1997. Effect of the human immunodeficiency virus epidemic on mortality from opportunistic infections in the United States in 1993. J. Infect. Dis. 176: 632-636. [DOI] [PubMed] [Google Scholar]
- 21.Sharma, V., S. Sharma, K. Hoener zu Bentrup, J. D. McKinney, D. G. Russell, W. R. Jacobs, Jr., and J. C. Sacchettini. 2000. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol. 7:663-668. [DOI] [PubMed] [Google Scholar]
- 22.Sturgill-Koszycki, S., P. L. Haddix, and D. G. Russell. 1997. The interaction between Mycobacterium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 18:2558-2565. [DOI] [PubMed] [Google Scholar]
- 23.Thines, E., R. W. Weber, and N. J. Talbot. 2000. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 12:1703-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzung, K. W., R. M. Williams, S. Scherer, N. Federspiel, T. Jones, N. Hansen, V. Bivolarevic, L. Huizar, C. Komp, R. Surzycki, R. Tamse, R. W. Davis, and N. Agabian. 2001. Genomic evidence for a complete sexual cycle in Candida albicans. Proc. Natl. Acad. Sci. USA 98:3249-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vereecke, D., K. Cornelis, W. Temmerman, M. Jaziri, M. Van Montagu, M. Holsters, and K. Goethals. 2002. Chromosomal locus that affects pathogenicity of Rhodococcus fascians. J. Bacteriol. 184:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayne, L. G., and K.-Y. Lin. 1982. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect. Immun. 37:1042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]