Abstract
The gene for an enantioselective amidase was cloned from Rhodococcus erythropolis MP50, which utilizes various aromatic nitriles via a nitrile hydratase/amidase system as nitrogen sources. The gene encoded a protein of 525 amino acids which corresponded to a protein with a molecular mass of 55.5 kDa. The deduced complete amino acid sequence showed homology to other enantioselective amidases from different bacterial genera. The nucleotide sequence approximately 2.5 kb upstream and downstream of the amidase gene was determined, but no indications for a structural coupling of the amidase gene with the genes for a nitrile hydratase were found. The amidase gene was carried by an approximately 40-kb circular plasmid in R. erythropolis MP50. The amidase was heterologously expressed in Escherichia coli and shown to hydrolyze 2-phenylpropionamide, α-chlorophenylacetamide, and α-methoxyphenylacetamide with high enantioselectivity; mandeloamide and 2-methyl-3-phenylpropionamide were also converted, but only with reduced enantioselectivity. The recombinant E. coli strain which synthesized the amidase gene was shown to grow with organic amides as nitrogen sources. A comparison of the amidase activities observed with whole cells or cell extracts of the recombinant E. coli strain suggested that the transport of the amides into the cells becomes the rate-limiting step for amide hydrolysis in recombinant E. coli strains.
Acylamide amidohydrolases (amidases) are used in biocatalysis for the chemoselective, regioselective, or enantioselective hydrolysis of various amides (17, 59). The chemo- and regioselectivities of amidases are utilized for the production of antibiotics (penicillin acylase), the hydrolysis of C-terminal amide groups in peptides (peptide amidase), the analysis of glycoproteins [peptide-N4-(N-acetyl-β-d-glucosaminyl)asparagine amidase F], or the transformation of cyclic imides (half-amidase, imidase) (5, 25, 50, 55, 57, 59). Enantioselective amidases are used for the production of optical active d- or l-α-amino acids, hydroxycarboxylic acids, or α-methylarylacetic and α-methoxyarylacetic acids. l-specific aminoamidases have been reported for Pseudomonas putida, Mycobacterium neoaurum, and Stenotrophomonas maltophilia, and a d-specific amino acid amidase has been found in Ochrobactrum anthropi. These enzymes usually also convert certain peptides and are therefore referred to as aminopeptidases (3, 21, 22, 41).
An evolutionarily different group of amidases has been found which enantioselectively converts 2-methylphenylacetamide (2-phenylpropionamide) and other α-methylarylacetamides. This group of amidases has been found in different rhodococci but also in gram-negative organisms, such as Pseudomonas chlororaphis B23 or Agrobacterium tumefaciens d3 (6, 31, 44, 45, 49).
One of the best-characterized amidases with the ability to enantioselectively hydrolyze various α-methylarylacetamides that has been described is from Rhodococcus erythropolis MP50. This isolate was obtained from an enrichment with naproxen nitrile as sole nitrogen source and produced almost pure S-naproxen [S-2-(6-methoxy-2-naphthyl)propionic acid] from racemic naproxen nitrile [2-(6-methoxy-2-naphthyl)propionitrile] or racemic naproxen amide [2-(6-methoxy-2-naphthyl)propionamide] (37, 39). The conversion of racemic naproxenamide to S-naproxen with this strain was also studied with immobilized whole cells in the presence of organic solvents (15, 16). The strain converted naproxen nitrile and other nitriles by the combined action of a nitrile hydratase and an amidase. The amidase was purified, characterized, and shown to be responsible for the high degree of enantioselectivity. The purified enzyme converted racemic 2-phenylpropionamide, naproxen amide, and ketoprofen amide [2-(3′-benzoylphenyl)propionamide] to the corresponding S-acids with enantiomeric excess of >99% at almost 50% conversion of the racemic amides (23, 38, 39). The amidase also demonstrated enantioselective acyl-transferase activity in the presence of hydroxylamine and was used to produce optical active 2-phenylpropionhydroxamate from racemic 2-phenylpropionamide (24). In order to allow a genetic manipulation of this interesting biocatalyst in the present study, the corresponding gene was cloned and characterized.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids.
R. erythropolis MP50 (DSMZ 9675) (37, 39) cells were routinely grown at 30°C in a mineral medium with succinate (10 mM), phenylacetonitrile (1 mM), Nutrient Broth (NB; Difco) (240 mg/liter), and NaCl (150 mg ml−1). For the isolation of genomic DNA, the strain was cultivated in Luria-Bertani (LB) medium with glycine (1.5% [wt/vol]) and 1.5% (wt/vol) saccharose. E. coli DH5α and E. coli JM109 cells were used as host strains for recombinant DNA work. E. coli strains were routinely cultured at 37°C in LB medium which was supplied with ampicillin (100 μg/ml), if appropriate.
The plasmid pBluescript II KS(+) (1) was used for most cloning experiments, and the l-rhamnose-inducible plasmid vector pJOE2702 was used for high levels of expression (62).
Analytical methods.
Amides and acids were analyzed by high-pressure liquid chromatography (HPLC) as previously described (60).
Preparation of cell extracts.
The cells of R. erythropolis MP50 were harvested by centrifugation (30 min, 8,000 rpm), resuspended in Tris-HCl buffer (30 mM, pH 7.5), and disintegrated by being ground with glass beads (0.3-mm diameter) in a Dyno-Mill type KDL homogenizer (Fa Willy A. Bachofen, Basel, Switzerland). The cells of recombinant E. coli strains were disrupted by using a French press as previously described (60). Unbroken cells and cell debris were removed by centrifugation at 100,000 × g for 30 min at 4°C. Protein was determined by the method of Bradford (10) using bovine serum albumin as a standard.
Expression of amidase in E. coli.
E. coli JM109(pST1WT) cells were grown in LB medium (120 ml) plus ampicillin (100 μg/ml) in 1-liter Erlenmeyer flasks at 37°C. When an optical density at 600 nm (OD600) of 0.2 to 0.3 was reached, 0.2% (wt/vol) rhamnose was added and the cells were cultivated at 30°C for another 6 h before the cells were harvested by centrifugation.
For the comparison of the amidase activities of cell extracts and whole cells of E. coli JM109(pST1WT), a bacterial culture was grown as described above and harvested by centrifugation and the cells were resuspended in Na+/K+ phosphate buffer (50 mM, pH 7.4). This cell suspension was split into two equal parts, and from one of them a cell extract was prepared. The amidase activities of both preparations were determined. The protein content of the cell extract was determined using the Bradford method. The protein concentration of the whole-cell suspension was estimated by assuming that the protein content which was determined for the cell extract was identical to the protein content of the whole cells from which the cell extract was prepared.
Standard assay for determination of enzyme activities with cell extracts and purified enzyme preparations.
The amide hydrolyzing activity was assayed routinely in reaction mixtures (0.5 ml) composed of 15 μmol of Tris-HCl buffer (pH 7.5) or 25 μmol of sodium potassium phosphate buffer (pH 7.4), 2.5 μmol of phenylacetamide (stock solution: 100 mM in methanol), and different amounts of protein (1 to 400 μg). The reaction was performed at room temperature in a plastic reaction tube. After different time intervals, aliquots were taken (100 μl each), the reaction was stopped by the addition of 10 μl of 1 M HCl, and the precipitated protein was removed by centrifugation (5 min, 20,800 × g). The hydrolysis of amides and the formation of the corresponding acids was determined by HPLC. One unit of enzyme activity was defined as the amount of enzyme that catalyzes the formation of 1 μmol of product per min.
Enzyme purification.
The amidase from R. erythropolis MP50 was purified at room temperature by use of a fast-performance liquid chromatography system (FPLC) system as previously described (23).
Protein cleavage, isolation of peptides, and sequencing of peptides and N termini.
The digestion of the purified amidase by trypsin and the subsequent separation of tryptic digests by reversed-phase HPLC were performed as described previously (60). The amino-terminal sequence of the amidase and the amino acid sequences of internal peptides from the amidase were determined by automated Edman degradation.
Isolation of genomic DNA.
Cell suspensions (1.5 ml) from R. erythropolis MP50 grown on LB were centrifuged (14,000 rpm, 3 min), and the cells were resuspended in 1 ml of 10% (wt/vol) saccharose containing 10 mg of lysozyme. Cells were incubated for 2 h at 37°C and harvested by centrifugation. Genomic DNA was prepared as described by Ausubel et al. (4).
Isolation of plasmid DNA.
Plasmid DNA from E. coli DH5α was isolated with the Flexi-Prep kit (Amersham Pharmacia Biotech) or the Qiaprep Spin Miniprep kit (Qiagen, Hilden, Germany). The preparation of plasmids for the detection of megaplasmids was basically performed as previously described (60).
DNA manipulation techniques.
Digestion of DNA with restriction endonucleases (Gibco BRL, New England Biolabs), electrophoresis, and ligation with T4 DNA ligase (Gibco BRL) were performed according to the standard procedures (52). Transformation of E. coli was done by the method described by Inoue et al. (28). PCR, cloning of PCR products, DNA sequencing, and nucleotide sequence analysis were performed as described previously (43, 60).
Hybridization procedures.
A digoxigenin (DIG) DNA labeling and detection kit was used according to the instructions of the supplier (Boehringer Mannheim). The hybridization temperature was set to 68°C.
Construction of plasmid pST1WT for overexpression of amidase in E. coli.
For expression in E. coli, the amidase gene was inserted in the plasmid vector pJOE2702 (62) under the control of an l-rhamnose-inducible promoter by using a PCR amplification strategy with simultaneous introduction of NdeI and HindIII sites as described previously (60).
Chemicals.
The synthesis and sources of all chemicals used have been described before (6, 7, 23, 60).
Nucleotide sequence accession number.
The sequence data reported in this article will appear in the GenBank nucleotide sequence database under the number AY026386.
RESULTS
Cloning of amidase gene.
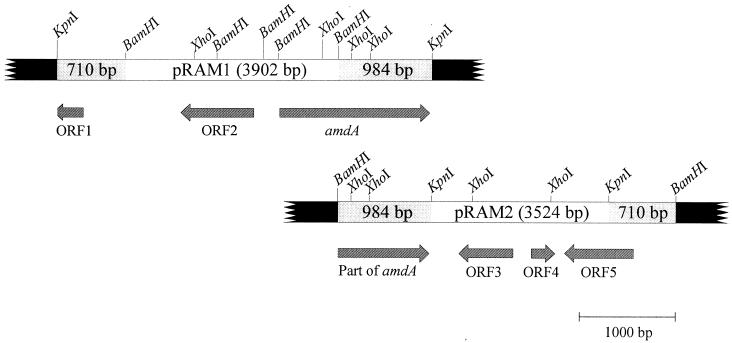
Amidase was purified from cell extracts of R. erythropolis MP50 basically as described previously by Hirrlinger et al. (23), and the amino-terminal amino acid sequence was determined (Table 1). The purified amidase was digested with trypsin, several peptides were isolated by HPLC, and the sequences of three fragments were determined (Table 1). The amino-terminal amino acid sequence and the sequences of the peptides P5329 and P6616 served for the design of oligonucleotide primers (Table 1) for PCR experiments. Using genomic DNA of strain MP50 as template and primers derived from the amino-terminal sequence and one of the internal peptides P6616 or P5329, DNA fragments with sizes of 0.7 or 1.5 kb, respectively, were amplified. The amplified 1.5-kb fragment was DIG labeled and used as a probe to identify an approximately 4-kb KpnI fragment from the total DNA of strain MP50, which was subsequently cloned into pBluescript II KS(+). This plasmid was designated pRAM1. The sequence of the inserted DNA fragment in pRAM1 demonstrated that the amidase gene was at one end of the cloned DNA fragment. In order to identify genes located downstream of the amidase gene in the genome of strain MP50, a probe was constructed by PCR from the amidase gene carried on plasmid pRAM1 and used to clone an about-3.5-kb BamHI fragment from the genomic DNA into pBluescript II KS(+). This construct was designated pRAM2.
TABLE 1.
Sequences of the amino terminus, tryptic peptides, and deduced oligonucleotides
| Protein or peptide | Amino acid sequencea | Deduced oligonucleotide sequences |
|---|---|---|
| Amino terminus | MRPNRPFGHVRPPTAEQLQEYSARHHFDLD | GC(GC)GAGCAG(CT)T(GCT)CA(AG)GA(AG)TA |
| P5329 | FEESTLYR | TA(GC)AG(GC)GT(GC)(GC)(AT)(CT)TC(CT)TC(AG)AA |
| P6616 | LPEPENYGSALGEGVSGLR | CCGTAGTT(CT)TC(GCT)GG(CT)TC(AGCT)GG |
| P8137 | V(N/S)VPL?TAAWPIQSGVM |
Segments used for the design of oligonucleotides for PCR are underlined.
Nucleotide sequence of amidase gene and surrounding DNA fragments.
Plasmids pRAM1 and pRAM2 contained inserts of 3,902 and 3,524 bp, respectively. The GC contents of these inserts were 68.0 and 67.4%, respectively, and were therefore within the typical range for the chromosomal DNA of R. erythropolis (67 to 71%) (19). In the insert in pRAM1, three tentative open reading frames (ORFs) were identified. The deduced amino acid sequence of one of these ORFs contained all of the amino acid sequences determined for the amino terminus and the internal peptides of the amidase, and the gene was therefore designated amdA. The knowledge of the amino-terminal amino acid sequence unequivocally proved that the start codon was a GTG triplet. The gene encoded a protein of 525 amino acids which corresponded to a protein with a molecular mass of 55.5 kDa. This value agreed sufficiently with the molecular mass of the amidase subunits (61 kDa) determined earlier by sodium dodecyl sulfate (SDS) gel electrophoresis (23). The deduced complete amino acid sequence showed the highest degree of sequence identity (34%) to an amidase from P. chlororaphis B23 (49).
The DNA fragment which was inserted in pRAM1 contained one more complete putative ORF (ORF2) and one fragmentary putative ORF (ORF1) which were transcribed in the opposite direction as amdA (Fig. 1). These ORFs showed the highest degree of sequence similarities to a regulatory protein from the GntR family and a transposase from the insertion element ISRh1, which was previously found in Rhizobium “hedysari” (Table 2).
FIG. 1.
Genetic organization of the 6.4-kb DNA fragment sequenced. Plasmids pRAM1 and pRAM2 carried the 3.9-kb KpnI fragment and the 3.5-kb BamHI fragment indicated. The positions and orientations of the different ORFs detected within the locus are shown by large arrows.
TABLE 2.
Putative genes and gene products from sequenced DNA fragments
| Gene or ORF | Position in sequence | Probable function of product | Size (amino acids) | Source | Region of sequence homology (aa according to BLAST search) | % Identitya | Reference for homologous proteinsb |
|---|---|---|---|---|---|---|---|
| 1 | 1-267 | Transposase | 89 | Rhizobium “hedysari” | ND | AAB81600 | |
| 2 | 1279-2028 | Regulator (GntR family) | 249 | Streptomyces coelicolor A3(2) | 22-235 | 32 | CAB61304 |
| amdA | 2306-3883 | Amidase | 525 | Pseudomonas chlororaphis B23 | 13-513 | 34 | P27765 |
| 3 | 4171-4740 | Hypothetical | 189 | Propionibacterium acidipropionici | 2-183 | 33 | CAB88395.1 |
| 4 | 4936-5193 | Hypothetical | 85 | Escherichia coli | 1-85 | 43 | P46147 |
| 5 | 5206-5220 | Transposase | 236 | Rhizobium “hedysari” | 13-230 | 55 | AAB81600 |
Percentage of amino acids that are identical when sequences were aligned with sequences listed in the GenBank database of the NCBI facilities. ND, not determined.
Accession number in the GenBank format.
The sequence analysis of the DNA fragment which was cloned in pRAM2 demonstrated that both sequences overlapped for 984 bp. Apart from the fragmentary amdA gene, three more putative ORFs were identified downstream from amdA (Fig. 1). The proteins encoded by ORF3 and ORF4 showed the highest degree of sequence identities to proteins with unknown functions from Propionibacterium acidpropionici and E. coli (Table 2). Surprisingly, it was found that the incomplete ORF1 which had been identified on pRAM1 was part of ORF5, which was identified on pRAM2. The protein encoded by ORF5 showed 53% sequence identity with the transposase from ISRh1. This suggested that amdA was part of a transposable element which was flanked by two direct repeats of an insertion element resembling ISRh1. This was further substantiated by the observation that ORF5 (the gene for the putative transposase) was flanked by two identical inverted repeats of 15 bp (5′-GGCACTGTCACGTTG-3′), which were very similar to the inverted repeats described for ISRh1 (both sequences contained one additional base pair each at different positions, but were otherwise identical). The same 15-bp repeat was also identified upstream of ORF1. No direct repeats were observed at the ends of the putative insertion elements containing ORF1 and ORF5. This has also been described for ISRh1 (46).
The presence of only a single copy of the amidase gene amdA in the genome of strain MP50 was confirmed by the results of the hybridization experiments which were performed with a 1.5-kb probe of amdA and preparations of genomic DNA digested with different restriction enzymes. In these experiments, consistently only one hybridizing band was observed with all restriction enzymes that did not cut within the amidase gene.
Location of amidase gene on plasmid in strain MP50.
It was previously shown that the enantioselective amidase of A. tumefaciens d3 is encoded by a plasmid (60). The analyses of cell lysates from strain MP50 by pulsed-field gel electrophoresis demonstrated the presence of two (presumably linear) plasmids with sizes of 180 and 230 kb. (Two linear plasmids with similar sizes had been previously identified in this strain [36]). After an additional S1 nuclease treatment of the cell lysates, two more plasmid bands with masses of about 5 and 40 kDa were detected. Because the S1 nuclease treatment linearizes cyclic plasmids, it can be assumed that these two smaller plasmids are present in a cyclic form in strain MP50. The plasmid DNAs were blotted and hybridized with the labeled 1.5-kb fragment of the amidase gene initially obtained by PCR (see above). This resulted in an hybridization signal with the 40-kb plasmid.
Expression of amidase gene in E. coli.
The amidase gene was amplified by PCR from plasmid pRAM1 using a set of primers which created new NdeI and HindIII restriction sites and also replaced the GTG start codon with an ATG start codon. The amplified fragment was then ligated into the l-rhamnose-inducible expression vector pJOE2702 (62) to yield plasmid pST1WT, which was used to transform E. coli JM109. The amidase gene was induced in the recombinant E. coli cells at 30°C by the addition of l-rhamnose. The amidase activity of these cell extracts with phenylacetamide (5 mM) as substrate was 0.69 U/mg.
Conversion of different α-substituted phenylacetamides by amidase from strain R. erythropolis MP50.
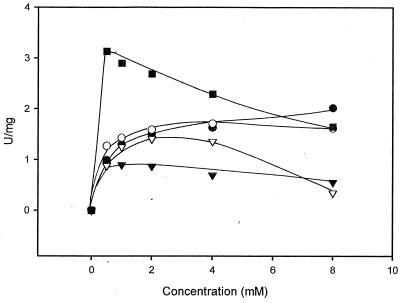
The purified amidase was incubated with 2-phenylpropionamide, mandeloamide, O-acetylmandeloamide, α-methoxyphenylacetamide, α-chlorophenylacetamide, or 2-methyl-3-phenylpropion-amide. The amidase showed good to excellent enantioselectivitieswith α-methylphenylacetamide, α-methoxyphenylacetamide, α-chlorophenylacetamide, and 2-methyl-3-phenylpropionamide (Table 3). These experiments were performed at a substrate concentration of 0.5 mM to allow a comparison with the results obtained earlier for the amidase from A. tumefaciens d3. In order to obtain some more information about the enzyme kinetics, different concentrations (0.5 to 8 mM) of the respective substrates were converted with the purified amidase. The enzyme obeyed traditional Michaelis-Menten kinetics in this substrate range only during the conversion of 2-phenylpropionamide and 2-methyl-3-phenylpropionamide (Fig. 2). In contrast, more or less-pronounced substrate inhibition effects were observed with mandeloamide, 2-methoxyphenylacetamide, and 2-chlorophenylacetamide.
TABLE 3.
Hydrolysis of different amides by enantioselective amidases from R. erythropolis MP50 and A. tumefaciens d3a
| Substrate | Structure | Relative activity (%)
|
Enantiomeric ratio
|
||
|---|---|---|---|---|---|
| MP50 | d3b | MP50 | d3b | ||
| 2-Phenylpropionamide | 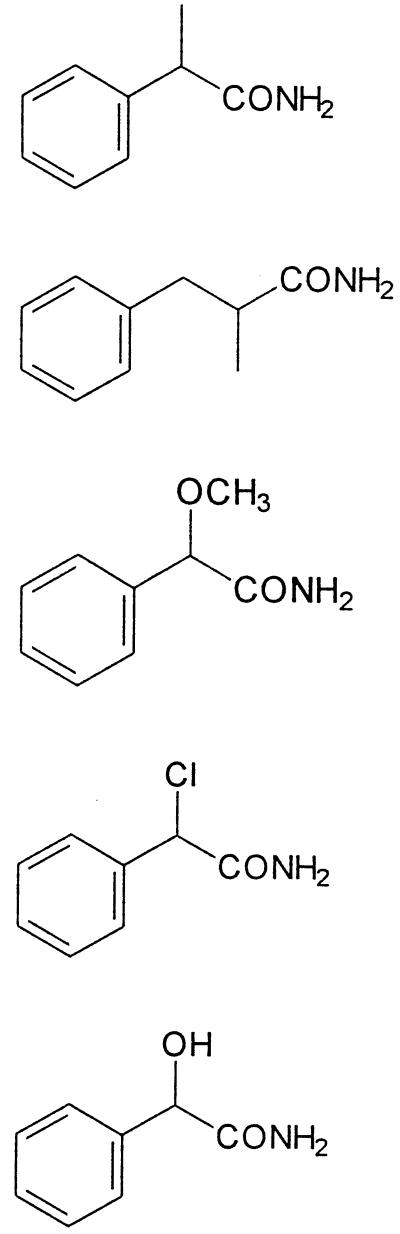 |
100 | 100 | >100 | >100 |
| 2-Methyl-3-phenylpropionamide | 104 ± 21 | 37 | 15 | >100 | |
| 2-Methoxyphenylacetamide | 20 ± 1 | 3 | >100 | 36 | |
| 2-Chlorophenylacetamide | 112 ± 6 | 56 | >100 | >100 | |
| Mandeloamide | 25 ± 6 | 15 | 8 | 6 | |
The reaction mixtures were 0.5 ml and contained 15 μmol of Tris-HCl (pH 7.5), 0.25 μmol of the respective amide, and purified amidase (1.4 to 3.2 mg) from R. erythropolis MP50. The reference data for the amidase from A. tumefaciens d3 were determined in 25 μM Na phosphate or K phosphate buffer (pH 7.4) with cell extracts (0.01 to 0.6 mg) from E. coli JM109 (pST2WT) (60). The specific activities of the purified amidase from strain MP50 and the cell extract from E. coli JM109 pST2WT with 2-phenylpropionamide as substrate were 3.4 ± 0.7 and 0.39 U/mg of protein, respectively. The tests were performed in duplicate, and the standard deviations are given.
Data were taken from a previous report (60), with permission.
FIG. 2.
Conversion of various concentrations of different amides by enantioselective amidase from R. erythropolis MP50. The reaction mixtures were 0.5 ml and contained 1.4 to 2.8 μg of the purified amidase, 15 μmol of Tris-HCl (pH 7.5), and a 0.5 to 8 mM concentration of 2-methyl-3-phenylpropionamide (•), 2-phenylpropionamide (○), 2-methoxyphenylacetamide (▾), mandeloamide (▿), or 2-chlorophenylacetamide (▪). The reaction mixtures were incubated at room temperature. After 5, 10, 15, 25, and 35 min, aliquots (80 μl each) were taken and the reaction was stopped by the addition of 8 μl of 1 M HCl. The precipitated protein was removed by centrifugation (3 min at 15,800 × g), and the supernatants were reneutralized with 1 M NaOH and analyzed by HPLC for the formation of the respective acids.
Growth of recombinant E. coli strains with amides as nitrogen source.
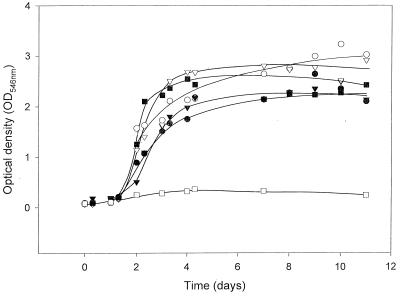
The availability of cloned enantioselective amidases should allow the improvement of these enzymes by evolutionary strategies, which require potent selection or screening techniques. The release of ammonia from the organic amides in the course of the amidase reaction should exert a strong selective pressure for the selection of amide-converting strains. It was therefore tested if the presence of the cloned amidase gene allowed the recombinant E. coli strains to grow with amides and if the growth rate was dependent from the substrate specificity of the amidase for the respective amides. These growth experiments demonstrated that the presence of the amidase gene indeed enabled E. coli JM109 cells to grow with different amides which were not used by the parental strain (Fig. 3). The growth rates of the recombinant E. coli strain did not correlate with the known substrate specificity of the purified amidase previously determined (23). These experiments suggested that in the recombinant strain, the activity of the amidase was not the growth-limiting factor in vivo. It was therefore tested if the uptake of the amides could be the rate-limiting step. Therefore, the conversion of 2-phenylpropionamide (0.5 mM) was compared with resting cells and a cell extract prepared thereof and it was found that the amidase activity of the cell extract (4 U mg of protein−1) was more than 10 times higher than the activity observed with resting cells (0.3 U mg of protein−1). In a control experiment with the wild-type strain R. erythropolis MP50, the cell extract demonstrated less than twice as much activity as the whole cells. These results suggested that the uptake of the amides may be the rate-limiting step in the recombinant organism and furthermore indicated that the genes encoding putative amide transport systems which have been found adjacent to the amidase genes in A. tumefaciens d3, Methylophilus methylotrophus, Pseudomonas aeruginosa, or Rhodococcus sp. strain R312 (12, 47, 60, 66, 68) may indeed be necessary for the optimal uptake of amides into the bacterial cells.
FIG. 3.
Growth of E. coli JM109 (pST1WT) with succinate as carbon source and different amides as nitrogen sources. The growth experiments were performed in 100-ml Erlenmeyer flasks with baffles in a medium which contained in 25 ml of Na phosphate or K phosphate buffer (50 mM, pH 7.4), succinate (25 mM), proline (0.5 mM), thiamine (1 μM), ampicillin (100 μg/ml), and acetamide (•), hexaneamide (○), isovalerianamide (▾), pivalamide (▿), or mandeloamide (▪) (5 mM each) or no additional nitrogen source (□). The flasks were inoculated with 100 μl of a preculture grown in the same growth medium with succinate and hexanamide. The bacterial cultures were grown at 37°C and growth monitored photometrically at 546 nm.
DISCUSSION
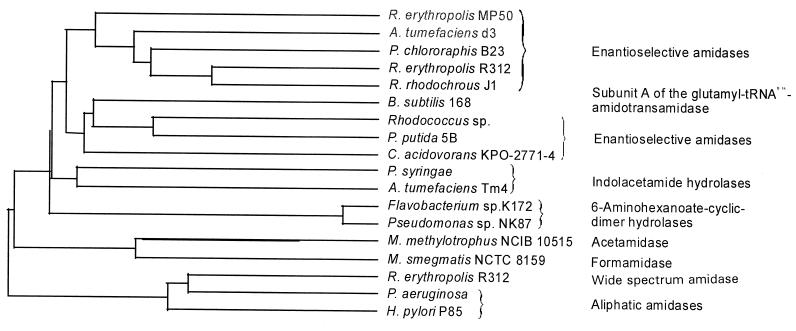
The ability of amidases to enantioselectively hydrolyze α-methylphenylacetamides and α-methoxyphenylacetamides was first demonstrated by Mayaux et al. for the enzymes from two rhodococcus strains (26, 44, 45). This ability was later shown for some other bacterial amidases from different gram-positive and gram-negative isolates (13, 32, 60). The sequence information which was obtained during these earlier studies, and the present work clearly demonstrated that these enantioselective amidases form a group of evolutionarily related enzymes, which differ according to their sequences significantly from other acylamide amidohydrolases, such as indolacetamide hydrolases, acetamide hydrolases, formamide hydrolases, and the so-called wide-spectrum amidases, which hydrolyze short-chain amides (basically acetamide and similar compounds) (11) (Fig. 4). Furthermore, these enantioselective amidases are, according to their amino acid sequences, fundamentally different from the aminopeptidases, which are used for the enantioselective synthesis of d- or l-amino acids from the corresponding racemic amides (3, 21, 22, 41). The BLAST searches for related enzymes surprisingly demonstrated that the subunit A of the glutamyl-tRNA(Gln)-amidotransamidase from Bacillus subtilis (and presumably various other organisms) also clustered within the group of the enantioselective amidases. This enzymatic activity is involved in gram-positive bacteria (and also Archaea and cyanobacteria) in the transamidation of misacylated Glu-tRNAGln to Gln-tRNAGln which functionally replaces the lack of a glutaminyl-tRNA synthetase in these organisms (14, 27).
FIG. 4.
Dendrogram resulting from pairwise alignments of amino acid sequences by using the program CLUSTAL. Agrobacterium tumefaciens d3 (AF315580 [60]); Agrobacterium tumefaciens (P03868 [30]); Bacillus subtilis 168 (O06491 [14]); Comamonas acidovorans KPO-2771-4 (20); Flavobacterium sp. strain K172 (P13397 [61]); Helicobacter pylori 85P (CAA72932 [53]); Methylophilus methylotrophus (Q50228 [67]); Mycobacterium smegmatis NCTC 8159 (Q07838 [42]), Pseudomonas aeruginosa (P11436 [2]), Pseudomonas chlororaphis B23 (P27765 [49]); Pseudomonas putida 5B (O69768 [67]); Pseudomonas sp. strain NK87 (P13398 [61]); Pseudomonas syringae EW2009 (P06618 [69]); Rhodococcus rhodochrous J1 (S38270 [32]); Rhodococcus sp. (A41326 [45]); Rhodococcus sp. strain R312 (enantioselective amidase) (P22984 [44]); and Rhodococcus sp. strain R312 (“wide-spectrum” amidase) (Q01360 [56]).
The genetic localization of the amidase from R. erythropolis MP50 resembled the situation observed earlier for the amidase from A. tumefaciens d3 and clearly differentiated both enzymes from other microorganisms, because previously no indications for the localization of amidase genes on plasmids have been found. Furthermore, all other genes encoding S-specific enantioselective amidases were physically connected to nitrile hydratase genes (32, 34, 44, 45, 49, 51, 67). The reason(s) for these differences is currently unclear, but it may be connected with the different enrichment conditions which were applied for the isolation of the respective organisms: R. erythropolis MP50 and A. tumefaciens d3 have been enriched with 2-arylpropionitriles. In contrast, all other well-studied strains possessing nitrile hydratase activity have been enriched with small aliphatic nitriles.
A further peculiarity of the localization of the amidase gene from R. erythropolis MP50 was the observation that it was surrounded by two copies of a putative insertion element. Recently, some examples for the presence of insertion elements in rhodococci have been described, and there is also one example known for the presence of an insertion element within a gene cluster which contains nitrile hydratase and amidase genes (33). Surprisingly, the sequence alignments demonstrated that the putative transposase from strain MP50 was much more closely related to the transposase from a gram-negative Rhizobium strain (IsRh1: 53% sequence identity) than to known insertion elements from other rhodococci (as IS1415, IS1676, IS1164, or IS2112; < 22% sequence identity) (33, 35, 40, 46, 48). The presence of two copies of the putative insertion element, which surround the amidase gene (and two putative regulatory genes) suggest that the amidase gene is part of a transposon structure. This hypothesis was further substantiated by the observation that according to Southern blotting experiments, the amidase gene was lost from the genome of the strain after growth under nonselective conditions and that these mutant strains still maintained a plasmid with a size of approximately 40 kb (unpublished results).
Amidases with the ability to enantioselectively hydrolyze 2-arylacylamides (such as 2-phenylpropionamide or 2-phenylbutyramide) have been found in several rhodococci and pseudomonads (8, 13, 18, 20, 23, 29, 32, 44, 45). The major aim in these previous studies was the preparation of S-2-arylpropionic acids (such as S-ibuprofen, S-naproxen, and S-ketoprofen), which are the pharmacologically active enantiomers in these nonsteroidal anti-inflammatory drugs produced in large quantities by the pharmaceutical industry (54). Only recently, some information was accumulating which suggested that this group of amidases is also able to enantioselectively convert phenylacetamide derivatives which carry substituents other than methyl groups in the α-position of the phenylacetamide core structure. Thus, it has been found that racemic α-aminophenylacetamide (phenylglycinamide) and also α-aminophenylacetonitrile (phenylglycinnitrile) can be converted to l-phenylglycin and d-phenylglycinamide with rather large enantiomeric excesses by various bacteria with nitrile hydratase/amidase activities (9, 37, 58, 63, 64, 65). The results of the present study about the enantioselective amidase from R. erythropolis MP50 and our previous study about the enzyme from A. tumefaciens d3 clearly demonstrated that other substituents in the α-position of phenylacetamide are also able to induce a highly enantioselective conversion by this group of amidases. This significantly increases the possible applications of this group of enzymes for biotransformation reactions.
REFERENCES
- 1.Alting-Mees, M. A., J. A. Sorge, and J. M. Short. 1992. pBlueskriptII: multifunctional cloning and mapping vectors. Methods Enzymol. 216:483-495. [DOI] [PubMed] [Google Scholar]
- 2.Ambler, R. P., A. D. Auffret, and P. H. Clarke. 1987. The amino acid sequence of the aliphatic amidase from Pseudomonas aeruginosa. FEBS Lett. 215:285-290. [DOI] [PubMed] [Google Scholar]
- 3.Asano, Y., T. Mori, S. Hanamoto, Y. Kato, and A. Nakazawa. 1989. A new D-stereospecific amino acid amidase from Ochrobactrum anthropi. Biochem. Biophys. Res. Commun. 162:470-474. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 5.Barsomian, G. D., T. L. Johnson, M. Borowski, J. Denman, J. F. Ollington, S. Hirani, D. S. McNeilly, and J. R. Rasmussen. 1990. Cloning and expression of peptide-N4-(N-acetyl-β-D-glucosaminyl)asparagine amidase F in Escherichia coli. J. Biol. Chem. 265:6967-6972. [PubMed] [Google Scholar]
- 6.Bauer, R., B. Hirrlinger, N. Layh, A. Stolz, and H.-J. Knackmuss. 1994. Enantioselective hydrolysis of racemic 2-phenylpropionitrile and other (R,S)-2-aryl-propionitriles by a new bacterial isolate, Agrobacterium tumefaciens strain d3. Appl. Microbiol. Biotechnol. 42:1-7. [Google Scholar]
- 7.Bauer, R., H.-J. Knackmuss, and A. Stolz. 1998. Enantioselective hydration of 2-arylpropionitriles by a nitrile hydratase from Agrobacterium tumefaciens d3. Appl. Microbiol. Biotechnol. 49:89-95. [Google Scholar]
- 8.Beard, T., M. A. Cohen, J. S. Parratt, N. J. Turner, J. Crosby, and J. Moilliet. 1993. Stereoselective hydrolysis of nitriles and amides under mild conditions using a whole cell catalyst. Tetrahedron: Asymmetry 4:1085-1104. [Google Scholar]
- 9.Beard, T. M., and M. I. Page. 1998. Enantioselective biotransformations using rhodococci. Antonie Leeuwenhoek 74:99-106. [DOI] [PubMed] [Google Scholar]
- 10.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 11.Chebrou, H., F. Bigey, A. Arnaud, and P. Galzy. 1996. Study of the amidase signature group. Biochim. Biophys. Acta 1298:285-293. [DOI] [PubMed] [Google Scholar]
- 12.Chebrou, H., F. Bigey, A. Arnaud, and P. Galzy. 1996. Amide metabolism: a putative ABC transporter in Rhodococcus sp. R312. Gene 182:215-218. [DOI] [PubMed] [Google Scholar]
- 13.Ciskanik, L. M., J. M. Wilczek, and R. D. Fallon. 1995. Purification and characterization of an enantioselective amidase from Pseudomonas chlororaphis B23. Appl. Environ. Microbiol. 61:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curnow, A. W., K.-W. Hong, R. Yuan, S.-I. Kim, O. Martins, W. Winkler, T. M. Henkin, and D. Söll. 1997. Glu-tRNAGln amidotransferase: A novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. USA 94:11819-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effenberger, F., B. W. Graef, and S. Oβwald. 1997. Preparation of (S)-naproxen by enantioselective hydrolysis of racemic naproxen amide with resting cells of Rhodococcus erythropolis MP50 in organic solvents. Tetrahedron: Asymmetry 8:2749-2755. [Google Scholar]
- 16.Effenberger, F., and B. W. Graef. 1998. Chemo- and enantioselective hydrolysis of nitriles and acid amides, respectively, with resting cells of Rhodococcus sp. C3II and Rhodococcus erythropolis MP50. J. Biotechnol. 60:165-174. [Google Scholar]
- 17.Fournand, D., and A. Arnaud. 2001. Aliphatic and enantioselective amidases: from hydrolysis to acyl transfer activity. J. Appl. Microbiol. 91:381-393. [DOI] [PubMed] [Google Scholar]
- 18.Gilligan, T., H. Yamada, and T. Nagasawa. 1993. Production of S-(+)-2-phenylpropionic acid from (R,S)-2-phenylpropionitrile by the combination of nitrile hydratase and stereoselective amidase in Rhodococcus equi TG328. Appl. Microbiol. Biotechnol. 39:720-725. [DOI] [PubMed] [Google Scholar]
- 19.Goodfellow, M. 1989. Genus Rhodococcus, p. 2369. In S. T. Williams, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 4. Williams & Wilkins, Baltimore, Md.
- 20.Hayashi, T., K. Yamamoto, A. Matsuo, K. Otsubo, S. Muramatsu, A. Matsuda, and K.-I. Komatsu. 1996. Characterization and cloning of an enantioselective amidase from Comamonas acidovorans KPO-2771-4. J. Ferment. Bioeng. 83:139-145. [Google Scholar]
- 21.Hermes, H. F. M., L. M. Croes, W. P. H. Peeters, P. J. H. Peters, and L. Dijkhuizen. 1993. Metabolism of amino acid amides in Pseudomonas putida ATCC 12633. Appl. Microbiol. Biotechnol. 40:519-525. [Google Scholar]
- 22.Hermes, H. F. M., R. F. Tandler, T. Sonke, L. Dijkhuizen, and E. M. Meijer. 1994. Purification and characterization of an L-amino amidase from Mycobacterium neoaurum ATCC 25795. Appl. Environ. Microbiol. 60:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirrlinger, B., A. Stolz, and H.-J. Knackmuss. 1996. Purification and properties of an amidase from Rhodococcus erythropolis MP50 which enantioselectively hydrolyzes 2-arylpropionamides. J. Bacteriol. 178:3501-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirrlinger, B., and A. Stolz. 1997. Formation of a chiral hydroxamic acid using an amidase from Rhodococcus erythropolis MP 50 and subsequent chemical Lossen-rearrangement to a chiral amine. Appl. Environ. Microbiol. 63:3390-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoople, D. W. T. 1998. Cleavage and formation of amide bonds, p. 243-275. In H.-J. Rehm, G. Reed, A. Pühler, P. Stadler, D. R. Kelly, and J. Peters (ed.), Biotransformations I, vol. 8a. Biotechnology, 2nd ed. Wiley-VCH, Weinheim, Germany.
- 26.Huang, W., J. Jia, J. Cummings, M. Nelson, G. Schneider, and Y. Linquist. 1997. Crystal structure of NHase reveals a novel iron centre in a novel fold. Structure 5:691-699. [DOI] [PubMed] [Google Scholar]
- 27.Ibba, M., A. W. Curnow, and D. Söll. 1997. Aminoacyl-tRNA synthesis: divergent routes to a common goal. Trends Biochem. Sci. 22:39-42. [DOI] [PubMed] [Google Scholar]
- 28.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 29.Kakeya, H., N. Sakai, T. Sugai, and H. Ohta. 1991. Microbial hydrolysis as a potent method for the preparation of optically active nitriles, amides and carboxylic acids. Tetrahedron Lett. 32:1343-1346. [Google Scholar]
- 30.Klee, H. J., M. B. Hayford, K. A. Kretzmer, G. F. Barry, and G. M. Kishore. 1991. Control of the ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell 3:1187-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi, M., Y. Fujiwara, M. Goda, H. Komeda, and S. Shimizu. 1997. Identification of active sites in amidases: evolutionary relationship between amide bond- and peptide-bond cleaving enzymes. Proc. Natl. Acad. Sci. USA 94:11986-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi, M., H. Komeda, T. Nagasawa, M. Nishiyama, S. Horinouchi, T. Beppu, H. Yamada, and S. Shimizu. 1993. Amidase coupled with low-molecular-mass nitrile hydratase from Rhodococcus rhodochrous J1. Eur. J. Biochem. 217:327-336. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi, M., H. Komeda, S. Shimizu, H. Yamada, and T. Beppu. 1997. Characterization and distribution of IS1164 that exists in the high molecular mass nitrile hydratase gene cluster of the industrial microbe Rhodococcus rhodochrous J1. Proc. Jpn. Acad. B 73:104-108. [Google Scholar]
- 34.Kobayashi, M., M. Nishiyama, T. Nagasawa, S. Horinouchi, T. Beppu, and H. Yamada. 1991. Cloning, nucleotide sequence and expression in Escherichia coli of two cobalt-containing nitrile hydratases from Rhodococcus rhodochrous J1. Biochim. Biophys. Acta 129:23-33. [DOI] [PubMed] [Google Scholar]
- 35.Kulakov, L. A., G. J. Poelarends, D. B. Janssen, and M. J. Larkin. 1999. Characterization of IS2112, a new insertion sequence from Rhodococcus, and its relationship with mobile elements belonging to the IS110 family. Microbiology 145:561-568. [DOI] [PubMed] [Google Scholar]
- 36.Küper, R. 1995. Das lineare Plasmid pBD2 aus Rhodococcus erythropolis BD2: Untersuchungen zur Konjugation und Replikation. Thesis. University of Göttingen, Göttingen, Germany.
- 37.Layh, N., B. Hirrlinger, A. Stolz, and H.-J. Knackmuss. 1997. Enrichment strategies for nitriles hydrolysing bacteria. Appl. Microbiol. Biotechnol. 47:668-674. [Google Scholar]
- 38.Layh, N., H.-J. Knackmuss, and A. Stolz. 1995. Enantioselective hydrolysis of ketoprofen amide by Rhodococcus sp. C3II and Rhodococcus erythropolis MP 50. Biotechnol. Lett. 17:187-192. [Google Scholar]
- 39.Layh, N., A. Stolz, J. Böhme, F. Effenberger, and H.-J. Knackmuss. 1994. Enantioselective hydrolysis of racemic naproxen nitrile and naproxen amide to S-naproxen by new bacterial isolates. J. Biotechnol. 33:175-182. [DOI] [PubMed] [Google Scholar]
- 40.Lessard, P. A., X. M. O'Brien, N. A. Ahlgren, S. A. Ribich, and A. J. Sinskey. 1999. Characterization of IS1676 from Rhodococcus erythropolis SQ1. Appl. Microbiol. Biotechnol. 52:811-819. [DOI] [PubMed] [Google Scholar]
- 41.Liese, A., K. Seelbach, and C. Wandrey. 2000. Aminopeptidase, p. 236-242. In A. Liese, K. Seelbach, and C. Wandrey (ed.), Industrial biotransformations. Wiley-VCH, Weinheim, Germany.
- 42.Mahenthiralingam, E., P. Draper, E. O. Davis, and M. J. Colston. 1993. Cloning and sequencing of the gene which encodes the highly inducible acetamidase of Mycobacterium smegmatis. J. Gen. Microbiol. 139:575-583. [DOI] [PubMed] [Google Scholar]
- 43.Marchuk, D., M. Drumm, A. Saulino, and F. S. Collins. 1991. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 19:1154.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayaux, J.-F., E. Cerbelaud, F. Soubrier, D. Faucher, and D. Pétré. 1990. Purification, cloning, and primary structure of an enantiomer-selective amidase from Brevibacterium sp. strain R312: structural evidence for genetic coupling with nitrile hydratase. J. Bacteriol. 172:6764-6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayaux, J.-F., E. Cerbelaud, F. Soubrier, P. Yeh, F. Blanche, and D. Pétré. 1991. Purification, cloning, and primary structure of a new enantiomer-selective amidase from a Rhodococcus strain: structural evidence for a conserved genetic coupling with nitrile hydratase. J. Bacteriol. 173:6694-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meneghetti, F., S. Alberghini, E. Tola, A. Giacomini, F. J. Ollero, A. Squartini, and M. P. Nuti. 1996. Presence of unique repeated insertion sequences in nodulation genes of Rhizobium “hedysari.” Plant Soil 186:113-120. [Google Scholar]
- 47.Mills, J., N. R. Wyborn, J. A. Greenwood, S. G. Williams, and C. W. Jones. 1998. Characterization of a binding-protein-dependent, active transport system for short-chain amides and urea in the methylotrophic bacterium Methylophilus methylotrophus. Eur. J. Biochem. 251:45-53. [DOI] [PubMed] [Google Scholar]
- 48.Nagy, I., G. Schoofs, J. Vanderleyden, and R. de Mot. 1997. Transposition of the IS21-related element IS1415 in Rhodococcus erythropolis. J. Bacteriol. 179:4635-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiyama, M., S. Horinouchi, M. Kobayashi, T. Nagasawa, H. Yamada, and T. Beppu. 1991. Cloning and characterization of genes responsible for metabolism of nitrile compounds from Pseudomonas chlororaphis B23. J. Bacteriol. 173:2465-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogawa, J., C.-L. Soong, M. Ito, T. Segawa, T. Prana, and S. Shimizu. 2000. 3-Carbamoyl-α-picolinic acid production by imidase-catalyzed regioselective hydrolysis of 2,3-pyridinedicarboximide in a water-organic solvent, two phase system. Appl. Microbiol. Biotechnol. 54:331-334. [DOI] [PubMed] [Google Scholar]
- 51.Payne, M. S., S. Wu, R. D. Fallon, G. Tudor, B. Stieglitz, I. M. Turner, Jr., and M. J. Nelson. 1997. A stereoselective cobalt-containing nitrile hydratase. Biochemistry 36:5447-5454. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 53.Skouloubris, S., A. Labigne, and H. de Reuse. 1997. Identification and characterization of an aliphatic amidase in Helicobacater pylori. Mol. Microbiol. 25:989-998. [DOI] [PubMed] [Google Scholar]
- 54.Snell, D., and J. Colby. 1999. Enantioselective hydrolysis of racemic ibuprofen amide to S-(+)-ibuprofen by Rhodococcus AJ270. Enzyme Microb. Technol. 24:160-163. [Google Scholar]
- 55.Soong, C.-L., J. Ogawa, and S. Shimizu. 2000. A novel amidase (half-amidase) for half-amide hydrolysis involved in the bacterial metabolism of cyclic imides. Appl. Environ. Microbiol. 66:1947-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soubrier, F., S. Lévy-Schil, J.-F. Mayaux, D. Pétré, A. Arnaud, and J. Crouzet. 1992. Cloning and primary structure of the wide-spectrum amidase from Brevibacterium sp. R312: high-homology to the amiE product from Pseudomonas aeruginosa. Gene 116:99-104. [DOI] [PubMed] [Google Scholar]
- 57.Stelkes-Ritter, U., G. Beckers, A. Bommarius, K. Drauz, K. Günther, M. Kottenhahn, M. Schwarm, and M.-R. Kula. 1997. Kinetics of peptide amidase and its application for the resolution of racemates. Biocatal. Biotrans. 15:205-219. [Google Scholar]
- 58.Stolz, A., S. Trott, M. Binder, R. Bauer, B. Hirrlinger, N. Layh, and H.-J. Knackmuss. 1998. Enantioselective nitrile hydratases and amidases from different bacterial isolates. J. Mol. Catal. B 5:137-141. [Google Scholar]
- 59.Sugai, T., T. Yamazaki, M. Yokoyama, and H. Ohta. 1997. Biocatalysis in organic synthesis. The use of nitrile- and amide hydrolysing microorganisms. Biosci. Biotech. Biochem. 61:1419-1427. [Google Scholar]
- 60.Trott, S., R. Bauer, H.-J. Knackmuss, and A. Stolz. 2001. Genetic and biochemical characterization of an enantioselective amidase from Agrobacterium tumefaciens strain d3. Microbiology 147:1815-1824. [DOI] [PubMed] [Google Scholar]
- 61.Tsuchiya, K., S. Fukuyama, N. Kanzaki, K. Kanagawa, S. Negoro, and H. Okada. 1989. High homology between 6-aminohexanoate-cyclic-dimer hydrolases of Flavobacterium and Pseudomonas strains. J. Bacteriol. 171:3187-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volff, J. N., C. Eichenseer, P. Viell, W. Piendl, and J. Altenbuchner. 1996. Nucleotide sequence and role in DNA amplification of the direct repeats composing the amplifiable element AUD1 of Streptomyces lividans 66. J. Mol. Microbiol. 21:1037-1047. [DOI] [PubMed] [Google Scholar]
- 63.Wang, M.-X., G. Lu, G.-J. Ji, Z.-T. Huang, O. Meth-Cohn, and J. Colby. 2000. Enantioselective biotransformations of racemic α-substituted phenylacetonitriles and phenylacetamides using Rhodococcus sp. AJ270. Tetrahedron: Asymmetry 11:1123-1135. [Google Scholar]
- 64.Wegman, M. A., U. Heinemann, A. Stolz, F. van Randwijk, and R. A. Sheldon. 2000. Stereoretentive nitrile hydratase catalysed hydration of D-phenylglycine nitrile. Org. Process Res. Develop. 4:318-322. [Google Scholar]
- 65.Wegman, M. A., U. Heinemann, F. van Randwijk, A. Stolz, and R. A. Sheldon. 2001. Hydrolysis of D,L-phenylglycine nitrile by new bacterial cultures. J. Mol. Catal. B 11:249-253. [Google Scholar]
- 66.Wilson, S. A., R. J. Williams, L. H. Pearl, and R. E. Drew. 1995. Identification of two new genes in the Pseudomonas aeruginosa amidase operon, encoding an ATPase (AmiB) and a putative integral membrane protein (AmiS). J. Biol. Chem. 270:18818-18824. [DOI] [PubMed] [Google Scholar]
- 67.Wu, S., R. D. Fallon, and M. S. Payne. 1998. Cloning and nucleotide sequence of amidase gene from Pseudomonas putida. DNA Cell Biol. 17:915-920. [DOI] [PubMed] [Google Scholar]
- 68.Wyborn, N. R., J. Mills, S. G. Willaims, and C. W. Jones. 1996. Molecular characterization of formamidase from Methylophilus methylotrophus. Eur. J. Biochem. 240:314-322. [DOI] [PubMed] [Google Scholar]
- 69.Yamada, T., C. J. Palm, B. Brook, and T. Kosuge. 1985. Nucleotide sequence of the Pseudomonas savastanoi indoleacetic acid genes show homology with Agrobacterium tumefaciens T-DNA. Proc. Natl. Acad. Sci. USA 82:6522-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]