Abstract
The aerosolization process of fungal propagules of three species (Aspergillus versicolor, Penicillium melinii, and Cladosporium cladosporioides) was studied by using a newly designed and constructed aerosolization chamber. We discovered that fungal fragments are aerosolized simultaneously with spores from contaminated agar and ceiling tile surfaces. Concentration measurements with an optical particle counter showed that the fragments are released in higher numbers (up to 320 times) than the spores. The release of fungal propagules varied depending on the fungal species, the air velocity above the contaminated surface, and the texture and vibration of the contaminated material. In contrast to spores, the release of fragments from smooth surfaces was not affected by air velocity, indicating a different release mechanism. Correlation analysis showed that the number of released fragments cannot be predicted on the basis of the number of spores. Enzyme-linked immunosorbent assays with monoclonal antibodies produced against Aspergillus and Penicillium fungal species showed that fragments and spores share common antigens, which not only confirmed the fungal origin of the fragments but also established their potential biological relevance. The considerable immunological reactivity, the high number, and the small particle size of the fungal fragments may contribute to human health effects that have been detected in buildings with mold problems but had no scientific explanation until now. This study suggests that future fungal spore investigations in buildings with mold problems should include the quantitation of fungal fragments.
Water damage in buildings is common and is often associated with mold problems. In North America, cross-sectional questionnaire studies have found that 27 to 36% of homes have mold problems (9, 51). Studies that included indoor air quality measurements have shown even higher numbers, from 42 to 56% (8, 11). In Europe, the prevalence of damp and moldy homes has been reported to be 17 to 46% for Great Britain (5, 23, 34, 42), 15 to 18% for The Netherlands (3, 55, 56), and 15% for Finland (41). Alarmingly, signs of present or previous moisture-related defects were found in 80% of randomly selected private homes investigated by civil engineers trained to recognize the signs of water leaks or condensation (37).
Increased prevalence of water-damaged buildings and subsequent fungal contamination may contribute to the noted increase in allergic diseases. Fungi can affect human health in a variety of ways. Possible reactions generally fall into one of three groups: allergic reactions (sensitization and immune responses, i.e., asthma, allergic rhinitis, or hypersensitivity pneumonitis), infections (growth of the fungus in or on the body, e.g., aspergillosis), and toxic responses (24, 30, 45). The toxic reactions are mainly connected with the secondary fungal metabolites, i.e., mycotoxins, but the role of cell wall components, such as β-(1→3)-d-glucans, has also been reported (4, 26, 29, 45, 47, 50). In addition, exposure to volatile organic compounds produced by fungi while growing on substrates and degrading them may be responsible for nonspecific symptoms, such as headaches; eye, nose, and throat irritation; and fatigue (4, 35).
An association between the existence of mold problems in buildings and adverse health effects has been found in several studies (24, 57). Although epidemiological studies show that people living or working in buildings with mold problems have more respiratory symptoms and diseases than people in nonproblem buildings, the relationship between inhaled fungal spores and induction of respiratory symptoms is still indeterminate and controversial in many aspects (7, 25, 35, 40, 45, 54). While the health problems in moldy buildings are associated with exposure to high concentrations of airborne fungal spores in a few studies (42, 58), several field studies show that the fungal spore concentration in problem buildings is not necessarily higher than that in nonproblem ones (14, 25, 36, 53). This suggests that airborne fungal spores may not be the only agents contributing to the health effects in damp indoor environments.
While some reports are available on the emission of fungal spores from moldy surfaces (16, 18, 39), the release of other types of fungal propagules has not been sufficiently explored. In our recent investigation on the release of fungal spores, performed in the laboratory under controlled environmental conditions (18), we found that parameters such as fungal species, air velocity above the surface, texture of the surface, and vibration of contaminated material affected the release of spores. The principal new finding reported here is that fungal fragments are released together with spores from contaminated surfaces. While the presence of fragments is documented with pollen exposures (43, 52), fungal fragments have gained much less attention. The role of fungal fragments is particularly interesting in the light of recent epidemiological studies on the relationship between outdoor air particulate pollution and health effects. These studies present evidence that fine particulates (size, <2.5 μm) are more strongly related to adverse health outcomes than coarse particles (10, 31, 48). The present paper characterizes the release of fungal fragments from contaminated surfaces and compares the results to those obtained in our previous study (18) on the release of fungal spores. The present study also reports data on the immunological reactivity of fungal fragments and spores.
MATERIALS AND METHODS
In the present study the same experimental setup was used and the same test materials and fungal species were selected as in our previous study on the release of fungal spores (18). A brief summary of the procedures is given below.
Experimental setup.
The aerosolization chamber and the experimental facility utilized for this study are depicted in Fig. 1. After incubation, the contaminated material (either an agar plate or a ceiling tile) was placed in a holder inside the aerosolization chamber. Fungal fragments and spores from the contaminated material were released by passing clean, HEPA-filtered air over the surface (12144 HEPA capsule filter; Pall Gelman Laboratory, Ann Arbor, Mich.) with controlled airflow rates. The entire setup was placed inside a class II biosafety cabinet (SterilchemGARD, Baker Company, Sanford, Maine). A HEPA filter in the exit flow collected all remaining released propagules to prevent contamination of the room environment.
FIG. 1.
Experimental setup (A) and its modification for testing the immunological reactivity of fungal propagules (B).
The experiments were conducted at four air velocities typical for the following environments: indoor air (0.3 m s−1), outdoor air (1.4 and 5.8 m s−1), and ventilation ducts (29.1 m s−1). These four velocities were adjusted through four combinations of two different orifice sizes for the air inlet and two different flow rates through the inlet. Two 400-orifice stages of the 6-stage Andersen impactor (Model 10-800; Andersen Instruments, Atlanta, Ga.) with an orifice diameter of 1.18 and 0.25 mm, respectively, were utilized as the air inlets, one at a time. By virtue of the pressure drop across the 400 nozzles, the airflow through each of the 400 air jets approaching the test surface was the same. The airflow rates were 7 and 35 liters min−1.
To investigate the influence of mechanical disturbance on fungal spore release, some tests were performed by applying vibration to the surface at a frequency of 1 Hz at a power level of 14 W. This frequency was selected because it is believed to cause a maximum vibration-induced structural response in buildings (27, 49). A simple electromagnet with an oscillating cylindrical hammer inside was used as the vibrator in the experiments. A sweep/function generator (Model 180; Wavetek, San Diego, Calif.) was connected to the electromagnet to generate the specific combination of frequency and power.
The concentration of released propagules was measured with an optical particle counter (Model 1.108; Grimm Technologies, Inc., Douglasville, Ga.). This device, based on light scattering, measures the concentration of particles in the (optical equivalent) size range of 0.3 to 20 μm. The duration of each experiment was 30 min. At the beginning of every experiment the system was operated in the absence of any test material in the chamber until the particle level was zero, as measured by the optical particle counter. In the next step, a noncontaminated agar plate or ceiling tile (incubated under the same conditions and times as the inoculated materials) was placed in the aerosolization chamber to establish the background level for particles released from the test surface when exposed to airflow and/or vibration. These levels were negligibly low, about 0.01% of the total released propagules. During all the release experiments in a biosafety cabinet, the temperature and relative humidity, measured with a humidity/temperature meter (Fisher Scientific Company, Pittsburgh, Pa.), were 20 to 24°C and 32 to 40%, respectively. This low humidity was chosen for the experiments to represent the worst-case scenario, as fungal propagules have been shown to be aerosolized more easily when the air is dry (in contrast to release into humid air) (16, 39). Each test was repeated three to six times. Before each test, the experimental system was purged by passing clean air through it.
Tested surface materials.
Two surface materials were tested for the release of fungal propagules (i.e., fragments and spores): agar plates filled with malt extract agar (Becton Dickinson Microbiology Systems, Sparks, Md.) and white ceiling tiles (Armstrong World Industries, Inc., Lancaster, Pa.). The latter material is commonly used in buildings in the United States and consists of human-made mineral fibers. The porous texture of this material was expected to support fungal growth (15). The tested agar and ceiling tile surfaces had the same round shape and the same dimensions as a plastic petri dish (diameter, 8.7 cm; height, 1.4 cm; area, 59.42 cm2). Both tested materials were sterilized before being prepared for the experiments. The agar plates were prepared according to the microbiological procedure recommended by the manufacturer (Becton Dickinson Microbiology Systems). Precut pieces of ceiling tiles were autoclaved at 121°C for 15 min. After sterilization, the agar plates and ceiling tiles were inoculated with specific fungal species.
Fungal species and growth conditions.
On the basis of earlier investigations (1, 44) and similar to our spore release study (18), three fungal species were selected for the tests: Aspergillus versicolor, Penicillium melinii, and Cladosporium cladosporioides. A. versicolor and C. cladosporioides are commonly present in indoor air (6, 17, 19, 28, 32, 38, 40). P. melinii is characteristic of soil environments. This species has, however, previously been isolated from contaminated building materials and was selected to represent fungi with large spores (44).
The fungal species were first grown on malt extract agar plates at 24°C at a relative humidity of 32 to 40% for 7 days before inoculation of the test materials. Fungal suspensions were prepared by washing fungal colonies from the agar plates with deionized and sterilized water (5 Stage Milli-Q Plus System; Millipore Corporation, Bedford, Mass.). The spore concentrations in the initial water suspensions were checked by using a bright line hemacytometer counting chamber (Model 3900; Hausser Scientific Company, Horsham, Pa.), and the concentration was adjusted to 106 spores per ml. The agar plates and ceiling tiles were inoculated with 0.1 and 1 ml of fungal suspensions, respectively. After inoculation, the agar plates and ceiling tiles were incubated in separate chambers at 24°C and a relative humidity of 97 to 99%. This humidity was achieved by placing saturated K2SO4 solution (150 g per liter) at the bottom of the incubation chambers (20). The agar plates were incubated for 7 days and the ceiling tiles were incubated for 6 (C. cladosporioides and P. melinii) or 12 (A. versicolor) months, which resulted in abundant fungal growth on both surfaces. Temperature and humidity in the chambers were monitored by a humidity/temperature meter.
After incubation, two samples of each of the two tested materials were used to determine the initial spore surface concentration. A 2-cm2 piece of the contaminated material was cut and suspended in 25 ml of deionized and sterilized water in a test tube. The spores were then extracted from the material by vortexing them for 10 min in a vortex touch mixer (Model 231; Fisher Scientific Company, Pittsburgh, Pa.). The spore concentrations in the resulting suspensions were determined with the bright line hemacytometer, which indicated about 107 spores per cm2 for both agar and ceiling tile samples. The hyphal structure of the fungal colonies on the agar and ceiling tile surfaces was observed by using both a light microscope (Model Labophot 2A; Nikon, Tokyo, Japan, available through Fryers Company, Inc., Carpentersville, Ill.) and a stereomicroscope (Model Stereomaster II; Fisher Scientific Company).
SEM analysis.
The presence of fragments was confirmed by scanning electron microscope (SEM) analysis. For this purpose, fungal propagules were aerosolized and sampled during 30-min experiments onto a 25-mm polycarbonate membrane filter with a pore size of 0.2 μm (Millipore Co.) with an in-line filter holder (Pall Gelman Laboratory), which replaced the HEPA filter in the outlet tube downstream of the aerosolization chamber (Fig. 1A). After sampling, the polycarbonate filters were coated with platinum (JEOL JFC-1300 auto fine coater metalliser; JEOL, Tokyo, Japan) and then analyzed by using low-vacuum SEM (Model JSM 5600LV; JEOL) paired with Oxford microanalysis. The secondary vacuum in the SEM operated at a pressure of 10−4 Pa. The images obtained during the analysis were digitized from an electron detector of the SEM and were passed to the computer.
Immunological reactivity of fungal propagules.
To test the immunological reactivity of A. versicolor and P. melinii propagules, the fragments and spores were simultaneously released from the agar plates at an air velocity of 24 m s−1 and were then collected onto separate filters by using a cascade impactor. For the purpose of these experiments, a 7-stage Andersen Cascade Impactor (Andersen Instruments Inc., Smyrna, Ga.) was added to the experimental setup (Fig. 1B). This device has particle cutoff sizes of 7.4, 4.7, 3.3, 2.1, 1.1, 0.65, and 0.43 μm for stages 1 through 7, respectively. The impaction plates of stages 3 to 7 were covered with a double-sided sticky tape (Manco Inc., Westlake, Ohio), which was discarded after each experiment. This was done to decrease particle bounce and to improve the separation of fragments and spores. Impaction stages 1 and 2 (which collected most of the spores) had 80-mm-diameter polyvinyl chloride (PVC) filters as substrates (Omega Specialty Instrument Co., Chelmsford, Mass.). The remaining propagules, most of which were fragments, were collected onto a 37-mm-diameter PVC filter with a pore size of 0.8 μm (SKC Inc., Eighty Four, Pa.). This filter was placed directly after the impactor outlet and is marked “After filter” in Fig. 1B.
Each set of samples was collected for 4 h. During this time the fungal propagules were released from 24 contaminated agar plates, which were changed every 10 min in order to attain sufficiently high concentration of released fungal propagules. The latter was measured simultaneously by using the Grimm optical particle counter and an ultrafine particle counter (P-Trak, Model 8525; TSI Incorporated, St. Paul, Minn.). The P-Trak is a condensation nuclei counter, which measures the concentration of particles in the size range of 0.02 μm to greater than 1 μm.
For the immunological reactivity tests, only the filter from stage 2 (collecting spores and their agglomerates in the 4.7- to 7.4-μm size range) and the after filter (collecting fragments) were used. After collection, each PVC filter was cut up, placed separately in a safe-lock Eppendorf micro test tube (Brinkmann Instruments, Inc., Westbury, N.Y.), and soaked with 1 ml of carbonate coating buffer at a pH of 9.6. The collected fungal propagules were suspended from the filters by vortexing for 0.5 min with a vortex mixer (Model Vortex-Genie 2; Scientific Industries, Bohemia, N.Y.). To guarantee purity (absence of spores) in the fragment samples, the fragment suspensions were filtered through 25-mm-diameter polycarbonate membrane filters with a pore size of 2 μm (Millipore Co.). The purity of the fragment suspensions as well as the spore concentrations present in the spore suspensions were checked by using a bright line hemacytometer counting chamber. The number of released fragments with diameters below 0.4 μm (collected on the after filter) was estimated by subtracting the number of particles within the 0.4- to 1-μm size range (recorded by the Grimm optical particle counter) from the number of particles within the 0.02- to 1-μm size range (recorded by the P-Trak ultrafine particle counter).
The immunological reactivity of the fungal propagules was tested by using a modified enzyme-linked immunosorbent assay (ELISA). The fungal fragment and spore suspensions (100 μl) were pipetted into the wells of ELISA MicroWell plates (Nalge Nunc International, Naperville, Ill.) and were incubated overnight at room temperature. After incubation the wells were washed twice with 200 μl of phosphate-buffered saline containing 0.05% Tween 20 (PBST). The ELISA plates were then processed according to the following five sequential steps, each step being separated from the next by two washing steps with PBST: (i) incubation for 1 h at room temperature in 200 μl of PBST containing 1% nonfat milk powder (PBSTM); (ii) incubation for 1 h at 37°C in 100 μl of monoclonal antibody (MAb) culture supernatant diluted five times into PBSTM; (iii) incubation for 1 h at 37°C in 100 μl of Biotin-SP-conjugated AffiPure goat anti-mouse immunoglobulin G plus immunoglobulin M secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) at a dilution of 1/5,000 in PBSTM; (iv) incubation for 1 h at 37°C in 100 μl of alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc.) at a dilution of 1/5,000 in PBSTM; and (v) incubation for 30 min at room temperature in 100 μl of substrate buffer (97 ml of diethanolamine, 100 mg of MgCl2 [both from Sigma Chemical Co., St. Louis, Mo.] in 1 liter of distilled water; pH was adjusted to 9.8 with HCl) containing one 5-mg p-nitrophenyl phosphate tablet (Sigma Chemical Co.) in 10 ml of buffer. Three different MAbs were used in the tests: MAb 14F7 produced against A. versicolor but cross-reacted with P. melinii, MAb 5F7 produced against Penicillium brevicompactum but cross-reacted with A. versicolor and P. melinii, and MAb 12G2 produced against Penicillium chrysogenum but cross-reacted with A. versicolor and P. melinii. After incubation, the absorbance of the prepared samples was read spectrophotometrically at a wavelength of 405 nm (UltraMicroplate Reader, Model ELx800; BIO-TEK Instruments, Inc., Winooski, Vt.). Two independent sets of samples for each tested fungal species of A. versicolor and P. melinii were tested in triplicate (one set is a pair of one spore and one fragment sample). Two blank PVC filters used as negative controls were tested in parallel with the sample filters.
Data analysis.
The data were statistically analyzed by analysis of variance and t test by using the software package STATISTICA for Windows (StatSoft, Inc., Tulsa, Okla.).
RESULTS
The optical particle size distribution was measured for each tested fungal species at an air velocity of 29.1 m s−1 with both agar and ceiling tile samples. The results are presented in Fig. 2. Previous reports indicate that the physical size of spores (measured under a microscope) is 2 to 3.5 μm for A. versicolor (close to spheres), 3 to 2 μm by 7 to 4 μm for C. cladosporioides (ellipsoidal shape), and 5 to 6 μm for P. melinii (close to spheres). The respective aerodynamic equivalent sizes are 2.5, 1.8, and 3.0 μm (44). The optical size distributions of the released fungal propagules, shown in Fig. 2, were the first ones to reveal that particles significantly smaller than the single spore size are released from the cultures of all three tested fungal species. This is clearly seen as an additional peak in the submicrometer size range. The peak in the number of released particles corresponding to the spore size for all test organisms is seen between 1.6 and 3.0 μm in equivalent optical diameter. Therefore, the particle size of 1.6 μm was selected as the lower counting limit separating spores from fungal fragments.
FIG. 2.
Optical size distribution of A. versicolor, C. cladosporioides, and P. melinii propagules released from both agar and ceiling tile surfaces (composite values) during 30-min experiments.
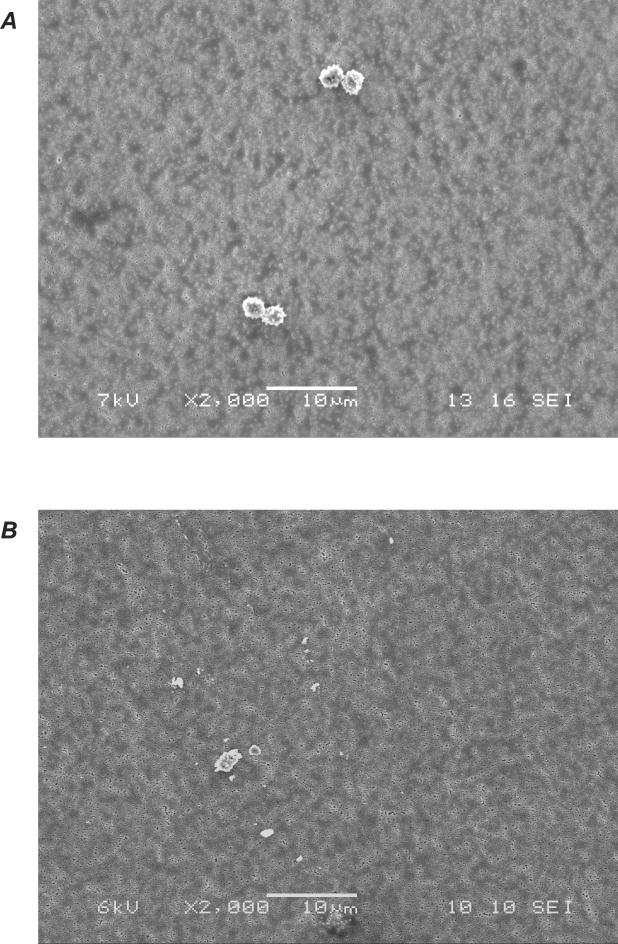
The presence of fungal fragments was confirmed by collecting filter samples and investigating them under the SEM. Figure 3 displays an example of fragments and spores released from A. versicolor culture. Simultaneous release of intact spores (Fig. 3A) as well as fragmented spores and/or hyphal fragments (Fig. 3B) was observed.
FIG. 3.
SEM pictures of propagules released from a ceiling tile contaminated with A. versicolor (panel A, intact spores; panel B, fragments).
Figure 4 shows a comparison between the number of fragments and spores released from agar surfaces at four air velocities. The number of released fragments ranged from 160 to 1,400 particles per cm2, and the number of spores ranged from 1 to 70 per cm2. Although the optical particle counter used in this study detects and counts particles only down to 0.3 μm, the data indicate that concentrations of released fragments were 11 to 320 times higher than those for spores of A. versicolor, 17 to 170 times higher than those for spores of C. cladosporioides, and 7 to 270 times higher than those for spores of P. melinii. All these differences were statistically significant (P < 0.05 for A. versicolor and C. cladosporioides and P < 0.000001 for P. melinii by t test). The lowest fragment/spore ratio was usually observed for an air velocity (ν) of 29.1 m s−1, and the highest ratio varied depending on the fungal species (v = 5.8 m s−1 for A. versicolor, v = 1.4 m s−1 for C. cladosporioides, and v = 0.3 m s−1 for P. melinii).
FIG. 4.
Number of fungal fragments and spores released simultaneously from agar surfaces at four different air velocities during 30-min experiments. The error bars indicate the standard deviation of 6 repeats for A. versicolor, 4 repeats for C. cladosporioides, and 10 repeats for P. melinii. The spore data are taken from Górny et al. (18).
Our recent findings (18) reveal that the spore release from agar increased with increasing air velocity. In this study, air velocity did not affect the number of released fragments (P > 0.05 by analysis of variance), and therefore further experiments were performed at the two extreme velocities, 0.3 m s−1 (typical air velocity in indoor environments) and 29.1 m s−1 (typical air velocity in ventilation ducts).
Table 1 compares the results on the release of fragments and spores from agar and ceiling tile surfaces at the two air velocities indicated above. Similar to what was determined about the release from agar, the number of aerosolized fungal fragments from ceiling tile surfaces was always higher than the number of released intact spores. At an air velocity of 29.1 m s−1 the release of fragments from ceiling tiles was much higher than that from agar surfaces, reaching its maximum of 5.7 × 105 particles per cm2 for P. melinii (P < 0.000001). At a lower air velocity of 0.3 m s−1 the release rate reached 2.4 × 103 fragments per cm2, but no significant differences were observed for the number of fragments released from these two surfaces. In contrast to agar, there was a noticeable increase in the number of released fragments for all tested fungal species with increased air velocity from the ceiling tile surfaces. The t test statistically confirmed these differences for fragments of A. versicolor (P < 0.05) and P. melinii (P < 0.001). Similar release trends were noted for fungal spores (18).
TABLE 1.
Average number of fungal fragments and spores released simultaneously from agar plates and ceiling tiles during 30-min experiments
| Species | Type of surface | No. of fragments and spores (cm−2) released at the following air velocitya:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.3 m s−1
|
29.1 m s−1
|
||||||||
| Fragment
|
Sporeb
|
Fragment
|
Sporeb
|
||||||
| Avg | SD | Avg | SD | Avg | SD | Avg | SD | ||
| A. versicolor | Agar plate without vibration | 1,240c | 2,270c | 26c | 60c | 441c | 491c | 18c | 14c |
| Ceiling tile without vibration | 2,390c | 229c | 101c | 69c | 129,000c | 103,000c | 44,800c | 40,600c | |
| Ceiling tile with vibration | 1,150 | 875 | 361 | 315 | 144,000 | 52,000 | 46,400 | 17,100 | |
| C. cladosporioides | Agar plate without vibration | 426 | 473 | 4 | 8 | 331 | 375 | 20 | 19 |
| Ceiling tile without vibration | 85 | 41 | 2 | 2 | 19,300 | 16,600 | 4,590 | 3,950 | |
| Ceiling tile with vibration | 429 | 175 | 70 | 66 | 129,000 | 99,100 | 42,400 | 34,300 | |
| P. melinii | Agar plate without vibration | 604c | 355c | 2c | 2c | 487c | 343c | 74c | 52c |
| Ceiling tile without vibration | 36c | 23c | 5c | 3c | 571,000c | 146,000c | 420,000c | 170,000c | |
| Ceiling tile with vibration | 1,550 | 690 | 463 | 98 | 646,000 | 53,200 | 509,000 | 53,700 | |
The numbers represent the average value and standard deviation of three repeats, unless indicated otherwise.
Fungal spore release data are from Górny et al. (18).
Average value for four repeats.
Data on the effect of surface vibration on the release of fungal fragments are also shown in Table 1. Vibration of ceiling tiles at the lower air velocity of 0.3 m s−1 increased the release of Cladosporium (P < 0.05) and Penicillium fragments (P < 0.01). For Aspergillus fragments this difference was not statistically significant. At v = 29.1 m s−1 no statistically significant effect of vibration on the release of fragments was observed. The same observation was previously concluded for the spore release experiments (18). Similar to the tests conducted without vibration, the augmentation of air velocity from 0.3 to 29.1 m s−1 resulted in an increase in the number of released fungal fragments from ceiling tiles when vibration was applied. Statistical analysis (t test) confirmed this trend for fragments of A. versicolor (P < 0.01) and P. melinii (P < 0.0001).
The percentage of released fungal fragments and spores during the first 10 min of the 30-min experiments is presented in Table 2. These data were obtained at airflow velocities of 0.3 and 29.1 m s−1 for agar and ceiling tiles without vibration applied and for ceiling tiles when these two air flows were accompanied by vibration. For all these species, the percentage of released fragments was 30 to 53% and of released spores was 27 to 45% at the air velocity of 0.3 m s−1 when no vibration was applied. When vibration was applied to the ceiling tile surfaces the respective mean percentage increased to 51 to 53% for the release of fragments and to 59 to 76% for the release of spores. At the air velocity of 29.1 m s−1 the mean percentage of released fungal propagules increased to 66 to 86% for fragments and to 71 to 88% for intact spores. Applying the surface vibration at this air velocity did not affect the fragment and spore release (the same mean values of 76 and 81%, respectively).
TABLE 2.
Percentage of released fungal fragments and spores during the first 10 min of 30-min experimentsa
| Species | Type of surface | % of released fragments and spores at the following air velocity:
|
|||
|---|---|---|---|---|---|
| 0.3 m s−1
|
29.1 m s−1
|
||||
| Fragment | Sporeb | Fragment | Sporeb | ||
| A. versicolor | Agar plate without vibration | 32 | 29 | 84 | 87 |
| Ceiling tile without vibration | 35 | 45 | 86 | 88 | |
| Ceiling tile with vibration | 51 | 59 | 77 | 79 | |
| C. cladosporioides | Agar plate without vibration | 30 | 27 | 66 | 71 |
| Ceiling tile without vibration | 53 | 30 | 79 | 80 | |
| Ceiling tile with vibration | 53 | 76 | 81 | 82 | |
| P. melinii | Agar plate without vibration | 37 | 32 | 66 | 76 |
| Ceiling tile without vibration | 41 | 30 | 77 | 86 | |
| Ceiling tile with vibration | 53 | 72 | 71 | 83 | |
After 30 min, spore and fragment release were 100%.
Fungal spore release data are from Górny et al. (18).
The correlation between the numbers of released fungal propagules was analyzed for the three tested fungal species. For each species, all the data on the number of released fungal fragments (from agar and ceiling tiles with and without vibration) at an air velocity of 0.3 m s−1 were grouped into one category and were correlated with the respective numbers of released spores. The data obtained at an air velocity of 29.1 m s−1 were grouped into another category and were tested separately. The results are summarized in Table 3. As shown, strong correlations were observed at the air velocity of 29.1 m s−1; for all three species the correlation coefficients were close to 1 (P < 0.05). At the air velocity of 0.3 m s−1, the correlation between fragments and spores was found to be statistically significant (r2 = 0.508, P < 0.05) only for P. melinii.
TABLE 3.
Correlation between the numbers of released spores and fragments
| Species | Correlation at the following air velocity:
|
|||
|---|---|---|---|---|
| 0.3 m s−1
|
29.1 m s−1
|
|||
| r2 | P | r2 | P | |
| A. versicolor | 0.084 | >0.05 | 0.986 | <0.05 |
| C. cladosporioides | 0.104 | >0.05 | 0.996 | <0.05 |
| P. melinii | 0.508 | <0.05 | 0.956 | <0.05 |
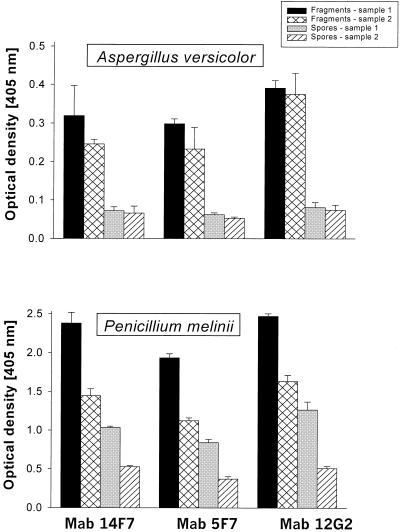
The immunological reactivities of A. versicolor and P. melinii fragments and spores are shown in Fig. 5. The total number of fragments versus spores in A. versicolor samples were as follows: sample 1, 4.7 × 106 versus 1.4 × 104; sample 2, 1.7 × 107 versus 1.3 × 104. The respective numbers for P. melinii were as follows: sample 1, 1.8 × 108 versus 1.6 × 105; sample 2, 1.0 × 107 versus 1.0 × 105. The immunological reactivity was expressed as the optical density of the respective fungal propagule sample incubated with a MAb (λ = 405 nm after 30 min of substrate incubation time). The fragment and spore samples always showed significant immunological reactivity independent of the type of MAb used. For the tested A. versicolor fragment samples the optical densities were 3.7 to 5.1 times higher than those for the spore samples (P value in t test varied from <0.05 to <0.01). For the P. melinii fragment samples the respective values were 2.0 to 3.2 times higher than those for the spore samples (always with a P value of <0.01). The blank filter samples (negative controls) tested simultaneously with the fragment and spore samples showed no activity in ELISA.
FIG. 5.
ELISA reactivity (defined as optical density) of fungal fragments and spores with three MAbs: MAb 14F7, MAb 5F7, and MAb 12G2. The error bars indicate the standard deviation of three repeats.
DISCUSSION
The most interesting finding of this study was that a significant amount of immunologically reactive particles having sizes considerably smaller than those of the spores was released from surfaces contaminated with fungi. Even with the accuracy of the Grimm optical size spectrometer, which allows measurement of particles as small as 0.3 μm in size, these fragments outnumbered the aerosolized spores by up to 320 times. The presence of fragments was confirmed by SEM observations.
The presence of airborne fragments is clearly documented with pollen exposures, as the onset of seasonal allergies is shown to start several weeks before the respective pollen grains are detected in the air (43, 52). In contrast, the role of fragments in fungal exposures has not been sufficiently recognized. The reason for this may be that fine and ultrafine fragment particles cannot be detected with traditional bioaerosol sampling and analysis methods. However, previous reports on mycelium pieces (that were large enough to be detected by light microscopy) indicate the possibility of the presence of fragments. Li and Kendrick (32) and Robertson (46) showed that the concentrations of fungal fragments in indoor air can reach an average level of 29 to 146 pieces per m3, i.e., up to 6.3% of all fungal propagules indoors. Madelin and Madelin (33) reported that pieces of mycelium are often blown away from contaminated surfaces, and some of these pieces remain viable and capable of initiating new growth. It is also possible that the fragments are pieces of spores and fruiting bodies or are formed through nucleation from secondary metabolites of fungi, such as semivolatile organic compounds.
Some researchers have compared the allergic responses of spore and mycelial extracts and have found that they share common allergens but that their reactivities vary and, in some cases of extract comparison, the intensities of the reactions from mycelium can exceed those obtained from the comparable spore extract studies (2, 12, 13). Our findings seem to be in good agreement with these results. All three tested MAbs revealed reactivity with the examined fungal propagules. The finding that the fragment samples had fungal antigens even after filtering for the remaining spores confirms that the fragments were indeed of fungal origin. Furthermore, the activity of the fungal fragment samples always exceeded that obtained for the spore samples. The reported numbers of fungal fragments and spores were released from the same area of contaminated surfaces during the same sampling time and thus represent a true exposure situation. The high number and reactivity of the fungal fragments are striking, suggesting that these fragments may significantly influence the health of exposed individuals. This factor has, to the best of our knowledge, been overlooked so far in studies evaluating indoor air quality. The specificity and the cross-reactivity of the tested MAbs should be taken into consideration when evaluating the above results. In our studies, the activity of P. melinii fragments and spores was higher than that of A. versicolor propagules. The high optical density values were probably caused by the higher number of antibody-specific fungal antigens present in the tested propagule suspensions. However, the high reactivity of fungal fragments itself appears to be of great significance from an exposure assessment point of view. As seen from the data given above, the high number of small particles being immunologically reactive and penetrating into the human respiratory tract can potentially be the cause of adverse health effects and can, at least in part, be responsible for unexplained cases of respiratory symptoms in damp buildings.
The results of previous studies (18, 21, 39, 59) indicate that the release of fungal spores generally increased when the air velocity above the contaminated surface increased. In the present study, however, the release of fragments from smooth agar surfaces was not affected by the air velocity. The different trend in fragment release compared to that of intact spores indicates that the fragments are aerosolized through a process different from that for spores. It is hypothesized that the fragments are already liberated from the mycelium or spores before the air currents carry them away. Thus, all the fragments are aerosolized at low air velocity, and an increase in the velocity does not increase their release. The increased release from rough ceiling tile surfaces appears to be related to the higher air turbulence effect above the surface cavities (18). The particular components of fungi (hyphae, conidiophores, and spore chains) overgrew almost the entire surface on both materials. Stereomicroscopic observations revealed that for ceiling tiles, growth occurs not only on the top surface, but the fungal colonies grow in each of the surface cavities as well. Fungal mycelium rises vertically upward, creating a mesh-like structure in the recesses of the ceiling tile surface. The higher air velocity with increased turbulence is more likely than the lower air velocity to release fungal propagules from the surface cavities. The difference in the fragment release between agar and ceiling tile can be partially caused by the differences in the moisture conditions. Moisture from agar can penetrate the thick layer of fungal growth and thus can increase the adhesion forces and reduce the release of fungal propagules. It should also be noted that the adhesion forces are higher for fungal fragments than for fungal spores due to the smaller size of the fragments.
On the basis of microscopic observations it can also be concluded that the morphology of fungal colonies may play an important role in the fragment release mechanism. A. versicolor and P. melinii colonies have longer, thinner conidiophores and longer spore chains than the colonies of C. cladosporioides. During exposure to the air currents, elongated Aspergillus and Penicillium colony parts (conidiophores, metulas, and phialides) as well as other structural elements (e.g., joint areas between the spores) are much more susceptible to desiccation stress (because of the larger exposed area) than the respective Cladosporium structures, and they probably become much more brittle when subjected to air turbulence.
In the real world ceiling tiles are probably under constant influence from different sources of vibration. This mechanical disturbance can originate both from the indoors and outdoors (operating home appliances, heating or air conditioning units, residents' activities, wall vibrations caused by road traffic, ground movement, etc.) and generally may lead to particle release from surfaces into the air (22). The vibration parameters selected for this study, i.e., a frequency of 1 Hz at a power level of 14 W, reflect the disturbances caused by, e.g., closing or slamming a door or children jumping. Similar to results obtained for spores (18), the vibration was shown to increase the release of fungal fragments at the low air velocity. This indicates that vibration is an important mechanism affecting the release of fungal propagules in indoor air environments and may partly explain the sporadic release of fungal propagules discussed below. High-velocity air currents appeared to have released all the fragments that were capable of being aerosolized, and therefore additional forces applied by vibration did not increase the release.
Regarding the colony morphology, mechanical stress caused by vibration may also cause additional release of fungal fragments from the elongated colony structures. Shorter structures of C. cladosporioides are more compact and, thus, are probably more resistant to the forces created by vibration of the surface. Elongated parts of A. versicolor and P. melinii colonies appear to be more easily affected by this mechanical force, which results in higher aerosolization of fragments, especially when the air current is low (indoor conditions).
The air currents were able to release up to 86% of the fungal fragments at v = 29.1 m s−1 and up to 53% of the fungal fragments at v = 0.3 m s−1 from contaminated surfaces during the first 10 min. This is almost the same percentage as that determined earlier for intact spore release (18). On the basis of these results it can be concluded that a significant portion of fungal propagules can be aerosolized from contaminated surfaces during a very short time interval. Such a high concentration of particles generated during a relatively short time period can significantly contribute to the indoor air quality. It is well known that fungal spore concentrations in indoor air have a wide temporal variation. Our study indicates that a similar variation is likely to occur with airborne fungal fragments in contaminated buildings.
This study showed that the number of released fragments was always higher than the number of intact spores released from contaminated surfaces. This trend seemed to be the same regardless of air velocity, surface material, and the presence or absence of vibration applied to the surface. On the basis of the correlation analysis of the above-described results it can be concluded that the number of fragments released at high air velocities can be predicted if the number of spores present in the air is known. At low air velocities such predictions could be burdened with significant error.
Conclusions.
This study revealed that fungal fragments are aerosolized simultaneously with spores from contaminated surfaces. The released fungal fragments consistently outnumber the spores and can exceed 6 × 105 particles per cm2. Such changes in the number of potentially immunologically relevant particles should be taken into consideration when performing exposure assessments in indoor environments.
The tests performed with MAbs produced against Aspergillus and Penicillium fungal species revealed that the fungal fragment and spore suspensions both had immunological reactivity. ELISA tests showed that fragments and spores share common antigens which not only confirmed the fungal origin of the fragments but also established their potential biological relevance. The fragment fraction of released fungal propagules, not previously measured in water-damaged buildings, may contribute to adverse health effects that have been detected among building inhabitants.
While the spore release from surfaces increased with increased air velocity, the release of fragments from smooth agar surfaces was not affected by the magnitude of the air velocity, indicating different release mechanisms for spores and fragments, respectively. At the low air velocity (typical for indoor environments), the application of vibration to the contaminated surface increased the number of released particles. Furthermore, our results show that up to 86% of aerosolizable fungal fragments can be rendered airborne during the first 10 min of exposure to airflow. Thus, this study indicates that the concentration of airborne fungal fragments is likely to vary widely, similar to the wide variations in spore concentration in contaminated buildings.
On the basis of the correlation analysis results, we conclude that in indoor environments the number of released spores is generally not a reliable indicator for the number of released fragments. Thus, future studies on mold problem buildings should include the measurement of fungal fragments in addition to intact spores.
REFERENCES
- 1.Aizenberg, V., T. Reponen, S. A. Grinshpun, and K. Willeke. 2000. Performance of Air-O-Cell, Burkard, and Button samplers for total enumeration of airborne spores. Am. Ind. Hyg. Assoc. J. 61:855-864. [DOI] [PubMed] [Google Scholar]
- 2.Aukrust, L., S. M. Borch, and R. Einarsson. 1985. Mold allergy—spores and mycelium as allergen sources. Allergy 40:43-48. [PubMed] [Google Scholar]
- 3.Brunekreef, B. 1992. Damp housing and adult respiratory symptoms. Allergy 47:498-502. [DOI] [PubMed] [Google Scholar]
- 4.Burge, H. A., and H. M. Ammann. 1999. Fungal toxins and β-(1→3)-d-glucans, p. 24.1-24.13. In J. Macher (ed.), Bioaerosols: assessment and control. American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio.
- 5.Burr, M. L., J. Mullins, T. G. Merret, and N. C. H. Stott. 1985. Asthma and indoor mould exposure. Thorax 40:688.4060112 [Google Scholar]
- 6.Chanda, S. 1996. Implications of aerobiology in respiratory allergy. Ann. Agric. Environ. Med. 3:157-164. [Google Scholar]
- 7.Cooley, J. D., W. C. Wong, and D. C. Straus. 1998. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 55:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall, M. S., and W. K. Sieber. 1996. The National Institute of Occupational Safety and Health indoor environmental evaluation experience. Part one: building environmental evaluations. Appl. Occup. Environ. Hyg. 11:533-539. [Google Scholar]
- 9.Dales, R. E., R. Burnett, and H. Zwanenburg. 1991. Adverse health effects among adults exposed to home dampness and molds. Am. Rev. Respir. Dis. 143:505-509. [DOI] [PubMed] [Google Scholar]
- 10.Dockery, D. W., C. A. Pope III, X. Xu, J. D. Spengler, J. H. Ware, M. E. Fay, B. G. Ferris, and F. E. Speizer. 1993. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 329:1753-1759. [DOI] [PubMed] [Google Scholar]
- 11.Ellringer, P. J., K. Boone, and S. Hendrickson. 2000. Building materials used in construction can affect indoor fungal levels greatly. Am. Ind. Hyg. Assoc. J. 61:895-899. [DOI] [PubMed] [Google Scholar]
- 12.Fadel, R., S. Paris, C. Fitting, R. Rassemont, and B. David. 1986. A comparison of extracts from Alternaria spores and mycelium. Allergy Clin. Immunol. 77:242. [Google Scholar]
- 13.Fadel, R., B. David, S. Paris, and J. L. Guesdon. 1992. Alternaria spore and mycelium sensitivity in allergic patients: in vivo and in vitro studies. Ann. Allergy 69:329-335. [PubMed] [Google Scholar]
- 14.Flannigan, B., E. M. McCabe, and R. McGarry. 1991. Allergenic and toxigenic microorganisms in houses. J. Appl. Bacteriol. 70:61-73. [PubMed] [Google Scholar]
- 15.Foarde, K., P. Dulaney, E. Cole, D. VanOsdel, D. Ensor, and J. Chang. 1993. Assessment of fungal growth on ceiling tiles under environmentally characterized conditions, p. 357-362. In P. Kalliokoski, M. Jantunen, and O. Seppänen (ed.), Particles, microbes, radon, vol. 4. Proceedings of Indoor Air 93 Conference. Gummerus Oy, Jyväskylä, Finland.
- 16.Foarde, K. K., D. W. VanOsdel, M. Y. Menetrez, and J. C. S. Chang. 1999. Investigating the influence of relative humidity, air velocity, and amplification on the emission rates of fungal spore, p. 507-512. In G. Raw, C. Aizlewood, and P. Warren (ed.), Proceedings of Indoor Air 99 Conference, vol. 1. CRC Ltd., London, United Kingdom.
- 17.Górny, R. L., E. Krysińska-Traczyk. 1999. Quantitative and qualitative structure of fungal bioaerosol in human dwellings of Katowice province, Poland, p. 873-878. In G. Raw, C. Aizlewood, and P. Warren (ed.), Proceedings of Indoor Air 99 Conference, vol. 1. CRC Ltd., London, United Kingdom.
- 18.Górny, R. L., T. Reponen, S. A. Grinshpun, and K. Willeke. 2001. Source strength of fungal spore aerosolization from moldy building material. Atmos. Environ. 35:4853-4862. [Google Scholar]
- 19.Gravesen, S., J. C. Frisvad, and R. A. Samson. 1994. Microfungi. Munksgaard, Copenhagen, Denmark.
- 20.Greenspan, L. 1977. Humidity fixed points of binary saturated aqueous solutions. J. Nat. Res. Bur. Stand. (A Phys. Chem.) 81:89-96. [Google Scholar]
- 21.Gregory, P. H., and M. E. Lacey. 1963. Liberation of spores from mouldy hay. Trans. Br. Mycol. Soc. 46:73-80. [Google Scholar]
- 22.Harney, J., M. Trunov, S. Grinshpun, K. Willeke, K. Choe, S. Trakumas, and W. Friedman. 2000. Release of lead-containing particles from a wall enclosure. Am. Ind. Hyg. Assoc. J. 61:743-752. [DOI] [PubMed] [Google Scholar]
- 23.Hunter, C. A., and R. G. Lea. 1994. The airborne fungal population of representative British homes, p. 141-153. In R. A. Samson, B. Flannigan, M. E. Flannigan, A. P. Verhoeff, O. C. G. Adan, and E. S. Hoekstra (ed.), Air quality monographs. Health implications of fungi in indoor environments, vol. 2. Elsevier Science B.V., Amsterdam, The Netherlands.
- 24.Husman, T. 1996. Health effects of indoor-air microorganisms. Scand. J. Work Environ. Health 22:5-13. [DOI] [PubMed] [Google Scholar]
- 25.Hyvärinen, A., T. Reponen, T. Husman, J. Ruuskanen, and A. Nevalainen. 1993. Characterizing mold problem buildings—concentrations and flora of viable fungi. Indoor Air 3:337-343. [Google Scholar]
- 26.Johanning, E., R. Biagini, D. Hull, P. Morey, B. Jarvis, and P. Landsbergis. 1996. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int. Arch. Occup. Environ. Health 68:207-218. [DOI] [PubMed] [Google Scholar]
- 27.Key, D. 1988. The calculation of structure response, p. 41-68. In Earthquake design practice for buildings. Thomas Telford, London, United Kingdom.
- 28.Kuo, Y. M., and C. S. Li.1994. Seasonal fungus prevalence inside and outside of domestic environments in the subtropical climate. Atmos. Environ. 28:3125-3130. [Google Scholar]
- 29.Lacey, J., and J. Dutkiewicz. 1994. Bioaerosols and occupational lung disease. J. Aerosol Sci. 25:1371-1404. [Google Scholar]
- 30.Levetin, E. 1995. Fungi, p. 87-120. In H. Burge (ed.), Bioaerosols. Lewis Publishers/CRC Press, Inc., Boca Raton, Fla.
- 31.Levy, J. I., J. K. Hammit, and J. D. Spengler. 2000. Estimating the mortality impacts of particulate matter: what can be learned from between-study variability? Environ. Health Perspect. 108:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, D. W., and B. Kendrick. 1995. A year-round comparison of fungal spores in indoor and outdoor air. Mycologia 87:190-195. [Google Scholar]
- 33.Madelin, T. M., and M. F. Madelin. 1995. Biological analysis of fungi and associated molds, p. 361-386. In C. S. Cox and C. M. Wathes (ed.), Bioaerosol handbook. Lewis Publishers/CRC Press, Inc., Boca Raton, Fla.
- 34.Martin, C. J., S. D. Platt, and S. M. Hunt. 1987. Housing conditions and ill health. Br. Med. J. 294:1125-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, J. D. 1992. Fungi as contaminants of indoor air. Atmos. Environ. 26:2163-2172. [Google Scholar]
- 36.Nevalainen, A., A.-L. Pasanen, M. Niininen, T. Reponen, M. J. Jantunen, and P. Kalliokoski. 1991. The indoor air quality in Finnish homes with mold problems. Environ. Int. 17:299-302. [Google Scholar]
- 37.Nevalainen, A., P. Partanen, E. Jääskeläinen, A. Hyvärinen, O. Koskinen, T. Meklin, M. Vahteristo, J. Koivisto, and T. Husman. 1998. Prevalence of moisture problems in Finnish houses. Indoor Air 4:45-49. [Google Scholar]
- 38.Pasanen, A.-L. 1992. Airborne mesophilic fungal spores in various residential environments. Atmos. Environ. 26:2861-2868. [Google Scholar]
- 39.Pasanen, A.-L., P. Pasanen, M. J. Jantunen, and P. Kalliokoski. 1991. Significance of air humidity and air velocity for fungal spore release into the air. Atmos. Environ. 25:459-462. [Google Scholar]
- 40.Piecková, E., and Z. Jesenská. 1999. Microscopic fungi in dwellings and their health implications in humans. Ann. Agric. Environ. Med. 6:1-11. [PubMed] [Google Scholar]
- 41.Pirhonen, I., A. Nevalainen, T. Husman, and J. Pekkanen. 1996. Home dampness, moulds and their influence on respiratory infections and symptoms in adults in Finland. Eur. Respir. J. 9:2618-2622. [DOI] [PubMed] [Google Scholar]
- 42.Platt, S. D., C. J. Martin, S. M. Hunt, and C. W. Lewis. 1989. Damp housing, mold growth, and symptomatic health state. Br. Med. J. 298:1673-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rantio-Lehtimäki, A., M. Viander, and A. Koivikko. 1994. Airborne birch pollen antigens in different particle sizes. Clin. Exp. Allergy 24:23-28. [DOI] [PubMed] [Google Scholar]
- 44.Reponen, T., K. Willeke, V. Ulevicius, A. Reponen, and S. A. Grinshpun. 1996. Effect of relative humidity on the aerodynamic diameter and respiratory deposition of fungal spores. Atmos. Environ. 30:3967-3974. [Google Scholar]
- 45.Robbins, C. A., L. J. Swenson, M. L. Nealley, R. E. Gots, and B. J. Kelman. 2000. Health effects of mycotoxin in indoor air: a critical review. Appl. Occup. Environ. Hyg. 15:773-784. [DOI] [PubMed] [Google Scholar]
- 46.Robertson, L. D. 1997. Monitoring viable fungal and bacterial bioaerosol concentrations to identify acceptable levels for common indoor environments. Indoor Environ. 6:295-300. [Google Scholar]
- 47.Rylander, R., K. Persson, H. Goto, K. Yuasa, and S. Tanaka. 1992. Airborne β-1,3-glucan may be related to symptoms in sick buildings. Indoor Environ. 1:263-267. [Google Scholar]
- 48.Schwartz, J., D. W. Dockery, and L. M. Neas. 1996. Is daily mortality associated specifically with fine particles? J. Air Waste Management Assoc. 46:927-939. [PubMed] [Google Scholar]
- 49.Skipp, B. O. 1984. Dynamic ground movement-man-made vibrations, p. 381-434. In P. B. Attewell and R. K. Taylor (ed.), Ground movement and their effects on structures. Surrey University Press, Chapman and Hall, New York, N.Y.
- 50.Sorenson, W. G. 1999. Fungal spores: hazardous to health? Environ. Health Perspect. 107:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spengler, J. D., L. Neas, S. Nakai, D. Dockery, F. Speizer, J. Ware, and M. Raizanne. 1993. Respiratory symptoms and house characteristics, p. 165-171. In P. Kalliokoski, M. Jantunen, and O. Seppänen (ed.), Health effects. Proceedings of Indoor Air 93 Conference, vol. 1. Gummerus Oy, Jyväskylä, Finland.
- 52.Spieksma, F. T. M., J. A. Kramps, A. C. van der Linden, B. E. P. H. Nikkels, A. Plomp, H. K. Koerten, and J. H. Dijkman. 1990. Evidence of grass-pollen allergenic activity in the smaller micronic atmospheric aerosol fraction. Clin. Exp. Allergy 20:273-280. [DOI] [PubMed] [Google Scholar]
- 53.Strachan, D. P., B. Flannigan, E. M. McCabe, and F. McGarry. 1990. Quantification of airborne moulds in the homes of children with and without wheeze. Thorax 45:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarlo, S. M., A. Fradkin, and R. S. Torbin. 1988. Skin testing with extracts of fungal species derived from the homes of allergy clinic patients in Toronto. Clin. Allergy 18:42-52. [DOI] [PubMed] [Google Scholar]
- 55.van der Laan, P. C. H. 1994. Moisture problems in the Netherlands: a pilot project to solve problem in social housing, p. 507-516. In R. A. Samson, B. Flannigan, M. E. Flannigan, A. P. Verhoeff, O. C. G. Adan, and E. S. Hoekstra (ed.), Air quality monographs. Health implications of fungi in indoor environments, vol. 2. Elsevier Science B.V., Amsterdam, The Netherlands.
- 56.Verhoeff, A. P., J. H. van Wijnen, J. S. M. Boleij, B. Brunekreef, E. S. van Reenen-Hoekstra, and R. A. Samson. 1990. Enumeration and identification of airborne viable mould propagules in houses. Allergy 45:275-284. [DOI] [PubMed] [Google Scholar]
- 57.Verhoeff, A. P., and H. A. Burge. 1997. Health risk assessment of fungi in home environments. Ann. Allergy Asthma Immunol. 78:544-556. [DOI] [PubMed] [Google Scholar]
- 58.Waegemaekers, M., N. van Wageningen, B. Brunekreef, and J. S. M. Boleij. 1989. Respiratory symptoms in damp houses. Allergy 44:192-198. [DOI] [PubMed] [Google Scholar]
- 59.Zoberi, M. H. 1961. Take-off of mold spores in relation to wind speed and humidity. Ann. Botany 25:53-64. [Google Scholar]