Abstract
l-Threonine can be made by the amino acid-producing bacterium Corynebacterium glutamicum. However, in the course of this process, some of the l-threonine is degraded to glycine. We detected an aldole cleavage activity of l-threonine in crude extracts with an activity of 2.2 nmol min−1 (mg of protein)−1. In order to discover the molecular reason for this activity, we cloned glyA, encoding serine hydroxymethyltransferase (SHMT). By using affinity-tagged glyA, SHMT was isolated and its substrate specificity was determined. The aldole cleavage activity of purified SHMT with l-threonine as the substrate was 1.3 μmol min−1 (mg of protein)−1, which was 4% of that with l-serine as substrate. Reduction of SHMT activity in vivo was obtained by placing the essential glyA gene in the chromosome under the control of Ptac, making glyA expression isopropylthiogalactopyranoside dependent. In this way, the SHMT activity in an l-threonine producer was reduced to 8% of the initial activity, which led to a 41% reduction in glycine, while l-threonine was simultaneously increased by 49%. The intracellular availability of l-threonine to aldole cleavage was also reduced by overexpressing the l-threonine exporter thrE. In C. glutamicum DR-17, which overexpresses thrE, accumulation of 67 mM instead of 49 mM l-threonine was obtained. This shows that the potential for amino acid formation can be considerably improved by reducing its intracellular degradation and increasing its export.
Amino acids are of great economic interest (17), and Corynebacterium glutamicum is particularly well suited for producing these cellular metabolites. For instance, the synthesis pathways and the biochemical conditions for the production of l-glutamate (12), l-lysine (8), l-tryptophan (18), and l-isoleucine (11) have been studied in detail in this organism. However, in high cellular synthesis, competing reactions may lead to the degradation of the desired amino acid (11, 35) and thus limit product formation. In the same way, export from inside the cell to the outside into the medium may place a considerable limitation on product formation (4, 35, 39, 44). While the synthesis pathways are well studied and the general principles of increasing the flux via these pathways are established (7), this is only true to a lesser extent of the degradation reactions and the translocation of the intracellularly formed amino acids from the cell into the medium (9, 10).
In the present study we investigated these aspects in l-threonine formation with C. glutamicum. l-Threonine synthesis proceeds in three steps, starting from aspartate semialdehyde. The corresponding biosynthesis genes hom and thrB, which form an operon, were cloned (14), as was thrC (16). The hom gene codes for homoserine dehydrogenase, and alleles of this gene, such as HomG378E [hom(Fbr)], that code for a dehydrogenase which is no longer feedback-inhibited by l-threonine have been identified (34). The overexpression of hom and thrB with high-copy-number plasmids is possible (13, 29), whereas hom(Fbr) thrB can only be expressed at low levels (35). This is due to the resulting high internal l-threonine concentration of up to 100 mM, versus less than 1 mM in the wild type. Increased internal l-threonine concentration is associated with increased glycine formation (5, 35). Furthermore, the very high internal concentration of l-threonine indicates that its export is limited. This export is catalyzed by the recently identified ThrE carrier (39), which is present not only in C. glutamicum, but also in bacteria, archaea, and fungi (46).
The purpose of our present work was to determine how l-threonine is degraded to glycine in C. glutamicum. There are indications that a threonine 3-dehydrogenase may be responsible for such reaction in Corynebacteriaceae (3). In Corynebacterium sp. strain B6, an activity of as much as 2.04 μmol min−1 (mg of protein)−1 was detected (2). Furthermore, we wanted to reduce an identified activity in C. glutamicum and to examine whether improved l-threonine accumulation occurs. A further goal was to increase the ThrE-catalyzed export of l-threonine and again to assay for improved l-threonine accumulation.
MATERIALS AND METHODS
Bacteria, plasmids, and growth conditions.
The strains and plasmids used are listed in Table 1. Luria-Bertani (LB) was used as the standard medium for Escherichia coli, while C. glutamicum was precultivated on brain heart infusion medium (BHI; Difco). The minimal medium used for product accumulation and enzyme assays was CGXII (19). Cultures of strain DR-17 were additionally supplied with 300 mg of l-leucine liter−1. When appropriate, E. coli strains received carbenicillin or kanamycin (each at 50 μg ml−1) or tetracycline (10 μg ml−1). C. glutamicum received kanamycin (50 μg ml−1) or tetracycline (5 μg ml−1). A reduced concentration of kanamycin, 15 μg ml−1, was used to obtain recombinant cells of C. glutamicum after transformation (42). Strains with a chromosomally integrated vector received a concentration of 25 μg of kanamycin ml−1. E. coli was grown at 37°C, and C. glutamicum was grown at 30°C.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| DH5αMCR | Cloning strain | 15 |
| M15/pREP4 | K-12 derivative suitable for protein isolation, containing vector supplying lac repressor, Kmr | Qiagen |
| S17-1 | Mobilizable donor strain | 40 |
| C. glutamicum | ||
| ATCC 13032 | Wild type | |
| DR-17 | Threonine producer derived from lysine producer MH20-22B | 28, 35 |
| DM368-2 | Threonine producer with feedback-resistant aspartate kinase and homoserine dehydrogenase | Degussa |
| 13032::pK18mobglyA′ | Wild type with glyA under control of tac promoter | This work |
| DM368-2::pK18mobglyA′ | DM368-2 with glyA under control of tac promoter | This work |
| Plasmids | ||
| pK18mob | Mobilizable vector, nonreplicative in C. glutamicum, Kmr | 37 |
| pQE30 | Expression vector supplying 6× His tag coding sequence, Apr | Qiagen |
| pVWEx2 | C. glutamicum expression vector with tac promoter, Tetr | 45 |
| pUC18glyA | pUC18 with 1.7-kb PCR fragment containing glyA | This work |
| pUC18glyA-2 | pUC18 with 1.3-kb PCR fragment containing promoterless glyA | This work |
| pVWEx2glyA | pVWEx2 with 1.4-kb TfiI-EcoRI insert from pUC18glyA | This work |
| pK18mobglyA′ | pK18mob with 2.0-kb PCR fragment from pVWEx2glyA, containing lacIq and Ptac fused to 5′-terminal fragment of glyA | This work |
| pQE30glyA | pQE30 with 1.3-kb PCR fragment containing glyA | This work |
| pEC-T18mob2 | E. coli-C. glutamicum shuttle vector, Tetr | AF445081 |
| pUC18thrE | pUC18 with thrE of C. glutamicum | 39 |
| pEC-T18mob2thrE | pEC-T18mob2 with SacI-XbaI fragment of pUC18thrE | This work |
Construction of plasmids.
The degenerate primers given in the Results section were used to amplify and clone an internal fragment of glyA. This fragment, together with genome information, was used to amplify the entire glyA gene as a 1,722-bp fragment with the primers 5′-GCTTGCAGCGTTTTGCTCTGCC-3′ and 5′-ACCCGTAACCTCTTCCACATAGG-3′. The amplified fragment was blunted and ligated with SmaI-cleaved pUC18 to give pUC18glyA. This served as a template to amplify promoterless glyA with the primers 5′-GCGGATCCATGACCGATGCCCACCAAG-3′ and 5′-CCGTCGACTTAGACGATGGTCCAGTCTTC-3′, where a BamHI and a SalI site, respectively, were attached. The resulting structural gene together with the added restriction sites was ligated with SmaI-cleaved pUC18 to give pUC18glyA-2. The glyA gene was excised from this vector as a BamHI-SalI fragment and ligated with BamHI- and SalI-cleaved pQE30 to yield pQE30glyA, containing glyA with six His codons attached to its 5′ end. The entire construct was verified by sequencing.
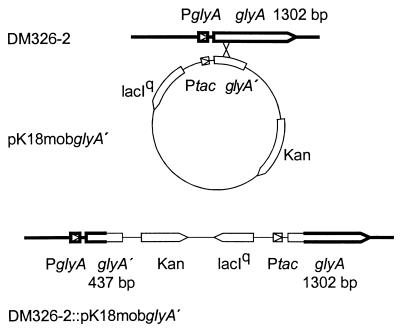
To place glyA under the control of the tac promoter, a 1,418-bp EcoRI-TfiI fragment of glyA was isolated which contained glyA without its own promoter. The fragment was blunted and ligated with BamHI-cleaved pVWEx2, made by V. Wendisch (45), which is a derivative of pKW0 (20), enabling isopropylthiogalactopyranoside (IPTG)-dependent expression. In the resulting construct, pVWEx2glyA, the glyA gene is fused with Ptac. With pVWEx2glyA as a template and the primer pair 5′-CCGGAATTCTCACTGCCCGCTTTCCAGTC-3′ and 5′-CGGGATCCCAGCTTTCCGGAGAAGTTCAAC-3′, a 2-kb fragment was amplified, containing a 437-bp fragment of the 5′ end of glyA together with the fused tac promoter and in addition the lac repressor lacIq, which is also present on pVWEx2glyA. By use of the attached BamHI and EcoRI sites, this fragment was ligated with the mobilizable and nonreplicative vector pK18mob to yield pK18mobglyA′ (see Fig. 3).
FIG. 3.
Depletion construct pK18mobglyA′ to reduce expression of SHMT. Shown is the construct itself with the inducible tac promoter and the 3′ part of glyA. Furthermore, the recombination of pK18mobglyA′ with the chromosomal glyA sequences of strain DM326-2 is shown, and at the bottom is shown the resulting genomic organization in the strain enabling IPTG-dependent Ptac-driven expression of glyA.
Plasmid pEC-T18mob2 was used to overexpress plasmid-encoded thrE. This plasmid confers tetracycline resistance to C. glutamicum and is based on pGA1 (41, 43). To insert thrE into this vector, the exporter gene was excised from pUC18thrE as a SacI-XbaI fragment and ligated with the correspondingly digested vector.
Construction of strains.
Intergeneric gene transfer was used to place glyA in the chromosome of C. glutamicum under the control of the IPTG-inducible tac promoter (37). For this purpose, E. coli S17-1 was transformed with the nonreplicative plasmid pK18mobglyA′ to kanamycin resistance. Conjugation and selection for kanamycin resistance were done in the presence of 0.1 mM IPTG. The correct integration into the chromosome via glyA sequences was verified with appropriate primer pairs and controls. The integration mutants DM368-2::pK18mobglyA′ and ATCC13032::pK18mobglyA′ were made by this procedure. These mutants carry one intact copy of glyA under the control of the inducible tac promoter and one incomplete copy under the natural promoter (see Fig. 3).
Isolation of SHMT.
Serine hydroxymethyltransferase (SHMT) was isolated from E. coli M15/pREP4 containing pQE30glyA. LB cultures containing 50 μg of carbenicillin and 25 μg of kanamycin per ml were grown for 2 h to give an optical density at 600 nm (OD600) of 0.6. Then 1 mM IPTG was added for the induction of His6-glyA expression, and incubation was continued for a further 3 h up to an OD600 of 2.5. Cells were harvested and lysed by sonication. The resulting extract was centrifuged, and the enzyme was isolated via Ni2+-nitrilotriacetic acid affinity chromatography.
Enzyme assays.
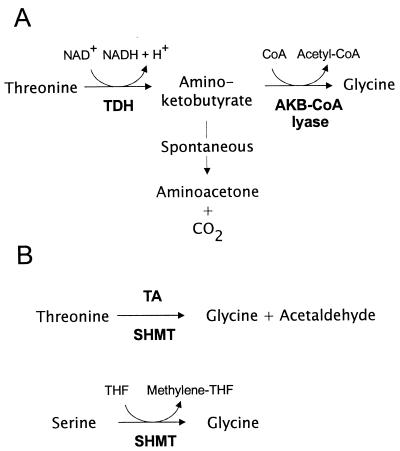
The threonine 3-dehydrogenase activity (EC 1.1.1.103) coupled to amino-ketobutyrate lyase (Fig. 1A) was assayed in a system containing (per 0.7 ml) 300 μl of Tris-acetate-EDTA-potassium phosphate buffer (200 mM Tris-acetate-EDTA, 25 mM potassium phosphate, pH 8.6), 100 μl of KCl (400 mM), 70 μl of NAD+ (100 mM), 70 μl of coenzyme A (CoA, 50 mM), 17.5 μl of l-threonine (200 mM), 42.5 μl of water, and 100 μl of crude extract. The reaction was started by the addition of substrate. Aliquots were removed after 0, 30, 60, and 90 min of incubation at 30°C, treated with 15% (wt/vol) trichloroacetic acid, and, after neutralization, used for glycine analysis by high-pressure liquid chromatography (HPLC).
FIG. 1.
Pathways for l-threonine degradation. (A) Pathway initiated by threonine-3-dehydrogenase (TDH) activity, with subsequent CoA-dependent conversion of 2-amino-3-ketobutyrate by amino-ketobutyrate lyase (AKB-CoA lyase) or spontaneous decarboxylation of 2-amino-3-ketobutyrate. (B) l-Threonine cleavage by transaldolase (TA) or SHMT activity and the main activity of SHMT generating 5,10-methylene tetrahydrofolate (THF).
An independent second assay made use of the fact that in the absence of CoA, amino-ketobutyrate spontaneously decarboxylates to aminoacetone, which can be quantified with Ehrlich's reagent (26). The system contained (per 1.6 ml) 800 μl of Tris-HCl (250 mM, pH 8.4), 50 μl of NAD+ (100 mM), 250 μl of l-threonine (500 mM, pH 8.4), 400 μl of water, and 100 μl of crude extract. The reaction was started by the addition of NAD+, stopped after incubation for 30 min (37°C) with 400 μl of 25% (wt/vol) trichloroacetic acid, and, after centrifugation, used for aminoacetone quantification. This was done by mixing 500 μl of sample, 500 μl of sodium acetate buffer (2 M, pH 4.6), 50 μl of NaOH (2.5 M), and 25 μl of acetylacetone. Assay mixtures were boiled for 10 min, and after cooling, 1 ml of Ehrlich's reagent was added (0.2 g of dimethylamino benzaldehyde dissolved in 8.4 ml of acetic acid) plus 1.6 ml of 70% (vol/vol) perchloric acid. After incubation for 15 min at room temperature, absorption readings were taken at 553 nm and compared with those of controls and standards.
The threonine aldolase activity generating glycine plus acetaldehyde (EC 4.1.2.5) (Fig. 1B) was assayed in the following system: 400 μl of equilibrated crude extract was passed over a PD10 column (Amersham Pharmacia) with Tris-acetate-EDTA-potassium phosphate buffer (200 mM Tris-acetate-EDTA, 25 mM potassium phosphate, pH 8.6), 100 μl of pyridoxal 5′-phosphate (2 mM), 100 μl of l-threonine (200 mM), 100 μl of l-isoleucine (10 mM), and 300 μl of water. Reaction mixtures were incubated for up to 120 min. The protein was precipitated with trichloroacetic acid and, after neutralization, used for glycine quantification via HPLC.
The same assay was also used to assay for formation of acetaldehyde. After termination of the reaction with trichloroacetic acid, 800 μl of supernatant was mixed with 200 μl of N-methylbenzothiazolon hydrazone (1%, wt/vol) and the pH was adjusted to 3 to 4 with 45 μl of neutralization buffer (31.8 g of K2CO3 in 100 ml of 20 mM Tris-HCl, pH 8.0). Assay mixes were boiled for 3 min, and after cooling, 2.5 ml of 0.2% (wt/vol) FeCl3 was added. After 5 min at room temperature they were mixed with 6.5 ml of acetone, and absorptions were read at 670 nm.
The SHMT (EC 2.1.2.1) was assayed with 300 μl of HEPES-NaOH (200 mM, pH 7.0), 200 μl of crude extract (equilibrated via passage through PD-10 columns with the same buffer), 100 μl of pyridoxal-5′-phosphate (2 mM), 50 μl of 5,10-methylene tetrahydrofolate (18 mM in 0.1% [wt/vol] dithiothreitol), 200 μl of substrate (100 mM, either l-threonine or l-serine), and 150 μl of water. Reaction mixes were incubated for up to 15 min, and 500-μl samples were mixed with 125 μl of 25% (wt/vol) trichloroacetic acid, placed on ice, and centrifuged in the cold. Then 480 μl of the resulting supernatant was neutralized with buffer (31.8 g of K2CO3 in 100 ml of 20 mM Tris-HCl, pH 8.0), and glycine was quantified via HPLC.
All enzyme activities are given in nanomoles per minute per milligram of protein, with protein determined with the biuret reaction.
Amino acid quantification.
Amino acids were quantified by automated precolumn derivatization with ortho-phthaldialdehyde (21), followed by reversed-phase chromatography with fluorometric detection as previously described (39).
Nucleotide sequence accession number.
The sequence data have been submitted to GenBank under accession number AF327063.
RESULTS
l-Threonine-degrading enzyme activities.
The l-threonine accumulation of C. glutamicum is accompanied by an undesirable glycine accumulation (35). Since it was reported that Corynebacterium species possess threonine dehydrogenase activity (3), yielding, together with 2-amino-3-ketobutyrate, CoA ligase glycine as one end product (Fig. 1A), we assayed for this activity in C. glutamicum. In extracts of the type strain ATCC 13032 and the l-threonine producer DR-17, neither NAD+ nor CoA-dependent glycine formation could be detected. Furthermore, no specific amino acetone formation occurred due to spontaneous decarboxylation of the intermediate 2-amino-3-ketobutyrate (26).
A second possible glycine-yielding reaction is the pyridoxal-5′-phosphate-dependent aldole cleavage of l-threonine. This can be catalyzed by an aldolase (22) or as a side reaction of SHMT (38) (Fig. 1B). In extracts of the same strains used previously, glycine formation with a specific activity of 2.2 nmol min−1 (mg of protein)−1 was detected. Also, the expected increase in formation of the second end product, acetaldehyde, could be detected. Therefore, a specific threonine aldolase or an aldolase activity due to SHMT is present in C. glutamicum. However, it is not possible to differentiate between these two threonine-degrading possibilities without genetic or biochemical work. Attempts to complement the aldolase mutant E. coli GS245 with gene banks used successfully for complementation of other E. coli mutations (19) failed, although E. coli GS245 was successfully used to isolate lta from a Pseudomonas strain (23). We therefore focused on glyA, the gene encoding SHMT.
Cloning of glyA and purification of SHMT.
The degenerate primers 5′-GAYATGGCNCACTTCGCNGG-3′ and 5′-ACNARGTGNACRTCNGTNCC-3′, designed to match conserved regions within known glyA genes, were used to clone a 372-bp PCR product. Based on this fragment, glyA of C. glutamicum was eventually cloned as a 1.7-kb fragment to give pUC18glyA. Sequence analysis identified glyA (1,305 nucleotides), which is preceded by two inverted repeats together with two possible promoter regions. This might indicate highly regulated transcriptional control of the gene, as is the case in E. coli (24). The deduced polypeptide has an Mr of 46,539 and exhibits the highest identities over its whole length with homologues from Mycobacterium tuberculosis (73%), Bacillus subtilis (53%), and E. coli (48%).
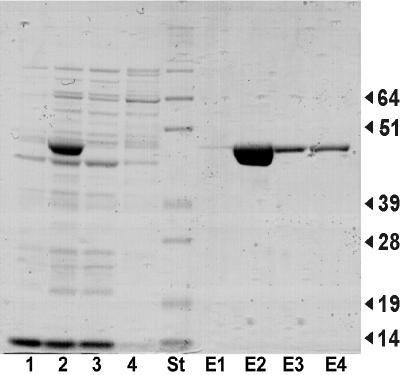
To further characterize the gene product, glyA was amplified together with attached BamHI and SalI sites to enable cloning in pQE30. This resulted in a fusion of glyA with (His)6-Gly-Thr codons attached to its 5′ end. Plasmid pQE30glyA was used to transform E. coli M15, and a culture of the recombinant strain was induced to enable isolation of the His-tagged SHMT. Figure 2 demonstrates the successful isolation of the polypeptide. Its molecular weight is fully consistent with that deduced from the glyA sequence. The protein (fraction E3) was used to assay l-serine- and l-threonine-dependent glycine formation. With l-serine as the substrate (and 5,10-methylene tetrahydrofolate [THF]), the specific activity was 31.0 μmol min−1 (mg of protein)−1, and with l-threonine it was 1.3 μmol min−1 (mg of protein)−1. This shows that glyA encodes functionally active SHMT and that this enzyme also converts l-threonine at a rate of about 1/25th that of l-serine.
FIG. 2.
Purification of SHMT. A sodium dodecyl sulfate gel is shown, containing an extract of E. coli without induced glyA expression (lane 1), with induced expression (lane 2), or the flowthrough (lanes 3 and 4). Lane St, size standards, with sizes indicated on the right (in kilodaltons). Lanes E1 to E4, eluted SHMT protein.
Construction of a GlyA depletion strain.
In order to study the consequences of reduced SHMT activity on product formation, we tried to inactivate glyA by use of an internal fragment in a mobilizable inactivation vector (37). However, even on complex medium supplemented with glycine, an appropriate integration mutant could not be obtained, indicating that glyA is essential (see below). This resembles the situation with E. coli, in which the glyA gene product is the major enzyme generating one-carbon units (24). Therefore, a strain in which the chromosomal wild-type glyA gene is controlled by the IPTG-inducible tac promoter was constructed.
For this purpose, promoterless glyA obtained as a 1,418-bp TfiI-BamHI fragment was cloned into pVWEx2, supplying the tac promoter as well as its repressor lacIq (Fig. 3). From this vector, together with the appropriate primers, lacIq, Ptac, and a 5′-glyA part of 437 bp was amplified and subsequently cloned into the nonreplicative vector pK18mob (37). By using conjugative transfer, C. glutamicum DM368-2 was transformed to kanamycin resistance in the presence of IPTG. One resulting transformant was designated DM368-2::pK18mobglyA′ and verified by PCR to have the full chromosomal copy of glyA under the control of Ptac (Fig. 3).
Growth and SHMT activity of the GlyA depletion strain.
As shown in Fig. 4, growth of the constructed strain DM368-2::pK18mobglyA′ was reduced without IPTG addition, whereas the addition of 10 μM IPTG restored growth comparable to that of the control. This is in accord with a reduced and limiting SHMT activity without IPTG being present. The remaining growth is probably due to SHMT activity in the cells used as the inoculum. This was made from washed cells grown in complex medium plus 100 μM IPTG. When we used cells as grown in Fig. 4 without IPTG addition and then used them as an inoculum for a new culture, growth was completely abolished in the absence of IPTG (not shown). This indicates that SHMT is essential for C. glutamicum and glyA is the sole relevant gene encoding this enzyme activity.
FIG. 4.
Growth of strain DM326-2::pK18mobglyA′ on minimal medium without IPTG (○) or with 10 μM (×) or 100 μM (▪) IPTG and the control strain DM326-2 pZ1 without IPTG (•).
To confirm this view, cells were grown as before on the minimal medium and harvested after 10 h of cultivation, and SHMT activity was determined with l-serine or l-threonine as the substrate. There was a clear relation of the IPTG concentration to SHMT activity (Table 2). The roughly 10% activity in the absence of IPTG is apparently too low to sustain normal growth of C. glutamicum. Identical experiments made with a depletion mutant of the type strain ATCC 13032 yielded almost identical results (not shown). The analysis confirms the Ptac-driven glyA expression and the fact that the cassette is suitable for assaying the consequences of reduced SHMT activity in an l-threonine producer strain.
TABLE 2.
IPTG-dependent SHMT activitya
| C. glutamicum strain | IPTG concn (μM) | SHMT sp act [nmol min−1 (mg of protein)−1]
|
|
|---|---|---|---|
| l-Serine | l-Threonine | ||
| DM368-2 pZ1 (control) | 0 | 25.8 | 1.6 |
| DM368-2::pK18mobglyA′ | 0 | 2.1 | <0.2 |
| DM368-2::pK18mobglyA′ | 10 | 5.1 | 0.8 |
| DM368-2::pK18mobglyA′ | 100 | 21.8 | 1.7 |
Assays were done with the respective amino acid at 20 mM as the substrate and are based on glycine formation quantified by HPLC.
l-Threonine accumulation at reduced glyA expression.
In order to quantify the effects of reduced glyA expression on product accumulation, cells of DM368-2::pK18mobglyA′ were grown as described above on minimal medium, and l-threonine and glycine accumulation was determined. As can be seen in Fig. 5A, the absence or a reduced concentration of IPTG resulted in increased l-threonine accumulation. At high glyA expression (100 μM IPTG) 7.5 mM l-threonine had accumulated after 72 h, whereas at low expression (no IPTG), an 11.2 mM concentration of this amino acid was present. This was inversely related to the glycine concentration, which was 6.8 and 4.2 mM, respectively, indicating the in vivo formation of glycine from l-threonine.
FIG. 5.
l-Threonine (solid bars) and glycine accumulation (open bars) with IPTG concentrations as indicated at 24, 48, and 72 h. (A) Product accumulation with DM326-2::pK18mobglyA′. (B) Productaccumulation with DM326-2::pK18mobglyA′/pEC-T18mob2thrE,overexpressing the exporter gene. Mean values and standard deviations for three independent cultures are presented.
l-Threonine accumulation at increased thrE and reduced glyA expression.
We tried to combine the positive effect of reduced glyA expression with thrE overexpression; the latter gene encodes the l-threonine exporter of C. glutamicum (39). For this purpose, plasmid pECT18mob2thrE was made (see Materials and Methods) and introduced into the same strain as used before to give DM368-2::pK18mobglyA′ pECT18mob2thrE. This strain was cultivated again with the three different IPTG concentrations, and the amino acid accumulation was quantified. As shown in Fig. 5B, at all time points and all IPTG concentrations, elevated levels of l-threonine were obtained. Together with reduced glyA expression (0 mM IPTG), the l-threonine accumulation increased from 11.2 to 12.5 mM. This indicates that due to the observed increased export rate of l-threonine (39), the limiting export for this amino acid in C. glutamicum (32, 35) was in part overcome. At the same time, the glycine concentration was further reduced.
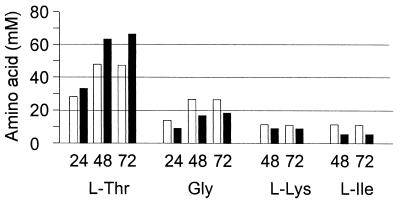
The effect of thrE expression on amino acid accumulation was also studied in strain DR-17 (35). This strain has three copies of the biosynthesis genes hom(Fbr) thrB integrated in its chromosome and is kanamycin resistant, preventing the use of pK18mobglyA′. Strain DR-17 was transformed with pECT18mob2thrE to tetracycline resistance, and cells were grown in minimal medium to follow product accumulation (Fig. 6). Without thrE, 48.6 mM l-threonine had accumulated after 72 h, whereas in its presence a 67.6 mM concentration was formed. In addition to glycine, strain DR-17 also accumulated l-isoleucine and l-lysine (35). Upon thrE overexpression, the concentration of all three accompanying amino acids was reduced (Fig. 6). The strongest relative reduction was found for l-isoleucine (11.6 to 6.0 mM), but not l-lysine (12.1 to 10.7 mM). This suggests a direct intracellular competition of threonine dehydratase (ilvA) and carrier (thrE), together with SHMT (glyA), for their common substrate l-threonine.
FIG. 6.
Amino acid accumulation at 24, 48, and 72 h as a function of thrE expression with strain DR-17. Solid bars, strain DR-17/pEC-T18mob2thrE; open bars, control DR-17/pEC-T18mob2. Each value represents the average of two independent experiments.
DISCUSSION
SHMT is essential in C. glutamicum and may thus be the only enzyme which makes activated C-1 units available in the form of 5,10-methylene tetrahydrofolate. Because a large fraction of about 15% of the glycolytic flux is diverted to satisfy this C-1 requirement during growth on glucose, glyA expression is strictly regulated. Thus, for example, at least two regulators, MetR and PurR, are involved in glyA expression control in E. coli in accordance with this central role of SHMT (25). It is therefore not surprising that in the promoter region of glyA in C. glutamicum, two pairs of inverted repeats are present which are candidates for the interaction of regulator proteins (not shown).
In general, the l-threonine-degrading enzymes are often difficult to differentiate. Among other reasons, this is due in part to overlapping enzyme activities or identical products (31). An l-threonine 3-dehydrogenase activity (EC 1.1.1.103) (tdh in E. coli) is considered responsible for the degradation of l-threonine in Corynebacterium sp. strain B6 (2). However, this enzyme activity is not detectable in C. glutamicum ATCC 13032. Furthermore, the degradation of l-threonine by l-threonine aldolase is possible, generating glycine plus acetaldehyde (EC 4.1.2.5). E. coli, for example, and also Pseudomonas spp. (23) have the corresponding lta gene. In E. coli, threonine aldolase can partially replace SHMT activity (22). In principle, there is also l-allo-threonine aldolase activity (EC 4.1.2.6), which has lower activity with l-threonine as the substrate. However, this enzyme is not very widespread.
We conclude that C. glutamicum does not have an aldolase because our genetic attempt to isolate this gene by complementation was unsuccessful. Analysis of the genome did not result in a corresponding gene either (not shown). A third argument is that the SHMT in C. glutamicum is essential, which would not be expected in a situation analogous to that of E. coli. We have no indications of a catabolic threonine dehydratase (EC 4.2.1.16) comparable to tdh in E. coli. All experimental findings were obtained not only with the wild type of C. glutamicum but also with strain DR-17, which originated from it. This also rules out specific degrading enzymes being expressed in C. glutamicum only at a high intracellular l-threonine concentration.
For the reasons discussed, we ascribe the l-threonine degradation in C. glutamicum to SHMT (EC 2.1.2.1). As shown with the isolated enzyme, the enzyme from C. glutamicum catalyzes the aldole cleavage of l-threonine with 4% of the activity of the cleavage of l-serine. It is thus similar to the rabbit enzyme, which also cleaves l-threonine with 6% of the activity of the cleavage of l-serine (39). However, this l-threonine cleavage is not a general property of SHMTs, since the rat enzyme is without detectable cleavage activity (27). This may be why there is such an intensive discussion of the in vivo participation of the enzyme in l-threonine degradation (31). Our experiments with l-threonine-producing strains on the product spectrum as a function of glyA expression, however, show that the enzyme can also cleave l-threonine in vivo.
An interesting question is, of course, how l-threonine cleavage by this essential enzyme can be further avoided in the case of l-threonine production by C. glutamicum. An attractive possibility would be the specific reduction of the enzyme's side activity. The three-dimensional structure of the protein is known (36). An essential structural element of the active site of the E. coli enzyme is 222-VVTTTTHKT-230, pyridoxal 5′-phosphate being bound to the ɛ-amino group of the lysine residue in position 229 as an internal aldimine (36). The motif with the string of T residues is strongly conserved and is present in an almost identical form in B. subtilis, Haemophilus influenzae, and Homo sapiens. As found in the present work, it is perceptibly modified in C. glutamicum, which has 230-VVSSTVHKT-239. Nevertheless, within the original string of T residues, the residue corresponding to T226 in the E. coli enzyme is retained, probably because this residue, as in the E. coli enzyme, is directly involved in the catalytic cycle.
As shown by systematic replacement of threonine residues with alanine, the E. coli enzyme tolerates substitutions of all the T residues except T226 (1). Most interestingly, the T230A substitution strongly reduces the activity with l-threonine as the substrate to less than 5% of the original activity with l-threonine, whereas more than 50% of that with l-serine is retained. This is an indication of the potential for altering the specificity of the enzyme by engineering residues within this catalytic site. In this connection, the question of how this problem of l-threonine cleavage might have been solved in the l-threonine producer of E. coli used industrially is naturally also of interest. This strain originated from undirected mutagenesis (12) and could therefore, for example, be mutated in the region of the SHMT discussed. Promoter mutations are, of course, also possible, and such mutations have also already been used successfully in C. glutamicum to improve l-lysine formation (6, 33).
In any case, reduced SHMT activity can reduce the undesirable in vivo aldole cleavage of l-threonine, so that less glycine is formed and more l-threonine is available for export. Even thrE overexpression alone boosts l-threonine accumulation. In this case, less glycine is also formed, i.e., glycine formation can be reduced via increased l-threonine export, probably due to reduced availability of this substrate for SHMT. The joint reduction of the internal aldole cleavage of l-threonine and the simultaneously increased export raises l-threonine accumulation from 7.5 to 11.2 mM. In strain DR-17, the l-threonine level was increased from 48.6 to 67.6 mM by thrE overexpression. The great increase obtained with this strain could mean that in DR-17, in which very high internal l-threonine concentrations of about 100 mM are present (35), the exporter is saturated with its substrate l-threonine. Alternatively, it is also conceivable that the permeability of the cell wall (9, 30) in strain DR-17 is different from that in the wild type, since, in addition to active export, another route contributing about 40% to total l-threonine efflux is diffusion (32, 39).
Acknowledgments
We thank A. Tauch and J. Kalinowski from the University of Bielefeld for pK18mob and pECT18mob2, K. Krumbach (this laboratory) for carrying out fermentations, and Degussa AG (Düsseldorf) for financial support.
REFERENCES
- 1.Angelaccio, S., S. Pascarella, E. Fattori, F. Bossa, W. Strong, and V. Schirch. 1992. Serine hydroxymethyltransferase: origin of substrate specificity. Biochemistry 31:155-162. [DOI] [PubMed] [Google Scholar]
- 2.Bell, S. C., and J. M. Turner. 1976. Bacterial catabolism of threonine. Threonine degradation initiated by l-threonine-NAD+ oxidoreductase. Biochem. J. 156:449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, S. C., and J. M. Turner. 1977. Bacterial catabolism of l-threonine. Threonine degradation initiated by l-threonine hydrolyase (deaminating) in a species of Corynebacterium. Biochem. J. 164:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmann, A., M. Vrljic, M. Pátek, H. Sahm, R. Krämer, and L. Eggeling. 2001. Regulation and specificity of the LysE-mediated export of amino acids by Corynebacterium glutamicum. Microbiology 147:1765-1774. [DOI] [PubMed] [Google Scholar]
- 5.Colon, G. E., M. S. Jetten, T. T. Nguyen, M. E. Gubler, M. T. Follettie, A. J. Sinskey, and G. Stephanopoulos. 1995. Effect of inducible thrB expression on amino acid production in Corynebacterium lactofermentum ATCC 21799. Appl. Environ. Microbiol. 61:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Graaf, A. A., L. Eggeling, and H. Sahm. 2001. Metabolic engineering for L-lysine production by Corynebacterium glutamicum. Adv. Biochem. Eng. Biotechnol 73:9-29. [DOI] [PubMed] [Google Scholar]
- 7.Eggeling, L., and H. Sahm. 1999. Amino acid production: principles of metabolic engineering, p. 153-176. In S. Y. Lee and E. T. Papoutsakis (ed.), Metabolic engineering. Marcel Dekker, Inc., New York, N.Y.
- 8.Eggeling, L., and H. Sahm. 1999. l-Glutamate and l-lysine: traditional products with impetuous developments. Appl. Microbiol. Biotechnol. 52:146-153. [Google Scholar]
- 9.Eggeling, L., and H. Sahm. 2001. The cell wall barrier of Corynebacterium glutamicum and amino acid efflux. J. Biosci. Bioeng. 92:201-213. [Google Scholar]
- 10.Eggeling, L., K. Krumbach, and H. Sahm. 1999. l-Glutamate efflux with Corynebacterium glutamicum: why is penicillin treatment or Tween addition doing the same? J. Mol. Microbiol. Biotechnol. 3:67-68. [PubMed] [Google Scholar]
- 11.Eggeling, L., S. Morbach, and H. Sahm. 1997. The fruits of molecular physiology: engineering the l-isoleucine biosynthesis pathway in Corynebacterium glutamicum. J. Biotechnol. 56:167-182. [Google Scholar]
- 12.Eggeling, L., W. Pfefferle, and H. Sahm. 2001. Amino acids, p. 281-303. In C. Radledge and B. Kristiansen (ed.), Basic biotechnology. Cambridge University Press, Cambridge, England.
- 13.Eikmanns, B. J., M. Metzger, D. Reinscheid, M. Kircher, and H. Sahm. 1991. Amplification of three biosynthesis genes in Corynebacterium glutamicum and its influence on carbon flux in different strains. Appl. Microbiol. Biotechnol. 34:617-622. [DOI] [PubMed] [Google Scholar]
- 14.Follettie, M. T., H. K. Shin, and A. J. Sinskey. 1988. Organization and regulation of the Corynebacterium glutamicum hom-thrB and thrC loci. Mol. Microbiol. 2:53-62. [DOI] [PubMed] [Google Scholar]
- 15.Grant, S. G. N., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han, K., J. A. Archer, and A. J. Sinskey. 1990. The molecular structure of the Corynebacterium glutamicum threonine synthase gene. Mol. Microbiol. 4:1693-1702. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson, J. 1994. Bulk amino-acid fermentation: technology and commodity trading. Bio/Technology 12:152-155. [Google Scholar]
- 18.Ikeda, M., and R. Katsumata. 1999. Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl. Environ. Microbiol. 65:2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keilhauer, C., L. Eggeling, and H. Sahm. 1993. Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J. Bacteriol. 175:5595-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronemeyer, W., N. Peekhaus, R. Krämer, H. Sahm, and L. Eggeling. 1995. Structure of the gluABCD cluster encoding the glutamate uptake system of Corynebacterium glutamicum. J. Bacteriol. 177:1152-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindroth, P., and K. Mopper. 1979. High performance liquid chromatographic detection of subpicomole amounts of amino acids by pecolumn fluorescence derivatization with o-phthdialdehyde. Anal. Chem. 51:1167-11174. [Google Scholar]
- 22.Liu, J. Q., T. Dairi, N. Itoh, M. Kataoka, S. Shimizu, and H. Yamada. 1998. Gene cloning, biochemical characterization and physiological characterization of a thermostable low-specificity l-threonine aldolase from Escherichia coli. Eur. J. Biochem. 255:220-226. [DOI] [PubMed] [Google Scholar]
- 23.Liu, J. Q., S. Ito, T. Dairi, N. Itoh, M. Kataoka, S. Shimizu, and H. Yamada. 1998. Gene cloning, nucleotide sequencing, and purification of the low-specificity l-threonine aldolase from Pseudomonas sp. strain NCIMB 10558. Appl. Environ. Microbiol. 64:549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz, E., and G. V. Stauffer. 1995. Characterization of the MetR binding sites for the glyA gene of Escherichia coli. J. Bacteriol. 177:4113-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenz, E., M. D. Plamann, and G. V. Stauffer. 1996. Escherichia coli cis- and trans-acting mutations that increase glyA gene expression. Mol. Gen. Genet. 250:81-88. [DOI] [PubMed] [Google Scholar]
- 26.Marcus, J. P., and E. E. Dekker. 1993. pH-dependent decarboxylation of 2-amino-3-ketobutyrate, the unstable intermediate in the threonine dehydrogenase-initiated pathway for threonine utilization. Biochem. Biophys. Res. Commun. 190:1066-1072. [DOI] [PubMed] [Google Scholar]
- 27.Masuda, T., M. Sakamoto, I. Nishizaki, H. Hayashi, M. Yamamoto, and H. Wada. 1987. Affinity purification and characterization of serine hydroxymethyltransferases from rat liver. J. Biochem. (Tokyo) 101:643-652. [DOI] [PubMed] [Google Scholar]
- 28.Menkel, E., G. Thierbach, L. Eggeling, and H. Sahm. 1989. Influence of increased aspartate availability on lysine formation by a recombinant strain of Corynebacterium glutamicum and utilization of fumarate. Appl. Environ. Microbiol. 55:684-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morinaga, Y., H. Takagi, M. Ishida, K. Miwa, T. Sato, S. Nakamori, and K. Sano. 1987. Threonine production by coexistence of cloned genes encoding homoserine dehydrogenase and homoserine kinase in Brevibacterium lactofermentum. Agric. Biol. Chem. 51:93-100. [Google Scholar]
- 30.Nampoothiri, K. M., C. Hoischen, B. Bathe, B. Möckel, W. Pfefferle, K. Krumbach, H. Sahm, and L. Eggeling. 2002. Expression of genes of lipid synthesis and altered lipid composition modulates l-glutamate efflux of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 58:89-96. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa, H., T. Gomi, and M. Fujioka. 2000. Serine hydroxymethyltransferase and threonine aldolase: are they identical? Int. J. Biochem. Cell. Biol. 32:289-301. [DOI] [PubMed] [Google Scholar]
- 32.Palmieri, L., D. Berns, R. Krämer, and M. Eikmanns. 1996. Threonine diffusion and threonine transport in Corynebacterium glutamicum and their role in threonine production. Arch. Microbiol. 165:48-54. [Google Scholar]
- 33.Pátek, M., B. J. Eikmanns, J. Pátek, and H. Sahm. 1996. Promoters from Corynebacterium glutamicum: cloning, molecular analysis and search for a consensus motif. Microbiology 142:1297-1309. [DOI] [PubMed] [Google Scholar]
- 34.Reinscheid, D. J., B. J. Eikmanns, and H. Sahm. 1991. Analysis of a Corynebacterium glutamicum hom gene coding for a feedback-resistant homoserine dehydrogenase. J. Bacteriol. 173:3228-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinscheid, D. J., W. Kronemeyer, L. Eggeling, B. J. Eikmanns, and H. Sahm. 1994. Stable expression of hom1-thrB in Corynebacterium glutamicum and its effect on the carbon flux to threonine and related amino acids. Appl. Environ. Microbiol. 60:126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarsdale, J. N., S. Radaev, G. Kazanina, V. Schirch, and H. T. Wright. 1999. Crystal structure at 2.8 Å resolution of E. coli serine hydroxymethyltransferase in complex with glycine substrate and 5-formyl tetrahydrofolate. J. Mol. Biol. 296:155-168. [DOI] [PubMed] [Google Scholar]
- 37.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 38.Schirch, V., and T. Gross. 1968. Serine transhydroxymethylase: Identification as the threonine and allothreonine aldolases. J. Biol. Chem. 243:5651-5668. [PubMed] [Google Scholar]
- 39.Simic, P., L. Eggeling, and H. Sahm. 2001. l-threonine export: use of peptides to identify a new translocator from Corynebacterium glutamicum. J. Bacteriol. 183:5317-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 11:784-791. [Google Scholar]
- 41.Tauch, A., A. Pühler, J. Kalinowski, and G. Thierbach. 2000. TetZ, a new tetracycline resistance determinant discovered in gram-positive bacteria, shows high homology to gram-negative regulated efflux systems. Plasmid 44:285-291. [DOI] [PubMed] [Google Scholar]
- 42.van der Rest, M. E., C. Lange, and D. Molenaar. 1999. A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl. Microbiol. Biotechnol. 52:541-545. [DOI] [PubMed] [Google Scholar]
- 43.Vašicová, P., Z. Abrhámová, J. Nešvera, M. Pátek, H. Sahm, and B. Eikmanns. 1998. Integrative and autonomously replicating vectors for analysis of promoters in Corynebacterium glutamicum. Biotechnol. Techniques 12:743-746. [Google Scholar]
- 44.Vrljić, M., H. Sahm, and L. Eggeling. 1996. A new type of transporter with a new type of cellular function: l-lysine export from Corynebacterium glutamicum. Mol. Microbiol. 22:815-826. [DOI] [PubMed] [Google Scholar]
- 45.Wendisch, V. F. 1997. Ph.D. thesis. Heinrich-Heine-Universität, Düsseldorf, Germany.
- 46.Yen, M., Y. Tseng, P. Simic, H. Sahm, L. Eggeling, and M. H. Saier, Jr. 2002. The ubiquitous ThrE family of putative transmembrane amino acid efflux transporters. Res. Microbiol. 153:19-25. [DOI] [PubMed] [Google Scholar]