Abstract
We used metabolic engineering to produce wine yeasts with enhanced resistance to glucose deprivation conditions. Glycogen metabolism was genetically modified to overproduce glycogen by increasing the glycogen synthase activity and eliminating glycogen phosphorylase activity. All of the modified strains had a higher glycogen content at the stationary phase, but accumulation was still regulated during growth. Strains lacking GPH1, which encodes glycogen phosphorylase, are unable to mobilize glycogen. Enhanced viability under glucose deprivation conditions occurs when glycogen accumulates in the strain that overexpresses GSY2, which encodes glycogen synthase and maintains normal glycogen phosphorylase activity. This enhanced viability is observed under laboratory growth conditions and under vinification conditions in synthetic and natural musts. Wines obtained from this modified strain and from the parental wild-type strain don't differ significantly in the analyzed enological parameters. The engineered strain might better resist some stages of nutrient depletion during industrial use.
The yeast Saccharomyces cerevisiae is an important microorganism in many industrial processes, including beer, bread, and wine making. In all of these processes, stresses applied to the yeast may affect fermentation efficiency (1). During wine making, yeast cells are subject to a hyperosmotic stress caused by sugar in must. As the fermentation progresses, the ethanol concentration may rise to levels that are toxic for yeast cells, and nitrogen starvation may also occur. Supraoptimal temperatures and deprivation of nutrients other than nitrogen are also possible in uncontrolled fermentations. During production of dry yeasts commonly used in the wine industry, cells are grown under aerobic conditions to increase biomass production. When cell density is high enough, the supply of carbon is interrupted to force the respiratory use of ethanol, and then cells are desiccated (14). In addition to the mixed stresses of the desiccation process, dry yeast cells suffer deprivation of glucose and other nutrients. Several of these stressing situations involve glucose deprivation and lead to loss of viability and fermentation power.
Glycogen is the main carbon source and energy reserve in many organisms, including yeast (12). Glycogen plays an important role during starvation, during adaptation to respiratory metabolism, in emergence from stationary phase, and during cell sporulation and spore germination (12).The amount of glycogen accumulated by a yeast cell depends on environmental conditions and on the cell growth phase (26). Glycogen also is linked to yeast viability (26), suggesting that it also has a function during the yeast stress response (8).
Present knowledge of the molecular mechanisms that underlie glycogen metabolism allows the design of genetic strategies to modify the level and/or the accumulation pattern of this compound in industrial yeast strains. Regulation of glycogen metabolism in yeast is mediated by cyclic AMP-dependent phosphorylation of glycogen synthase (EC 2.4.1.11) and glycogen phosphorylase (EC 2.4.1.1) (4) and by transcriptional (3, 10, 15, 24) and allosteric (9) regulators. Both GSY2 and GPH1 are transcriptionally regulated under stress conditions by Msn2p/Msn4p (27), the transcription factors binding the stress response element (STRE) present in the promoter of many stress-inducible genes in yeast (13, 25). These transcription factors are negatively regulated by cyclic AMP-dependent protein kinase A under optimal growth conditions (27).
Our objectives in this work were as follows: (i) to metabolically engineer wine yeast strains for glycogen overaccumulation, (ii) to test the effects of glycogen overaccumulation on resistance to glucose deprivation, and (iii) to evaluate the suitability of modified strains for wine production. The commercial wine yeast strain T73 (20) has been used as a host for different manipulations of genes coding for enzymes of glycogen metabolism. All engineered strains show an increased glycogen content in stationary phase under any tested growth condition. However, only the strain TG, overexpressing GSY2 and carrying the natural copies of GPH1, has an enhanced viability under glucose deprivation conditions, indicating the relevance of glycogen mobilization for resistance. Due to the industrial use of T73 and its derivative strain TG, glycogen content and resistance to glucose deprivation have been determined during growth in several media, including synthetic and natural musts. In the last case, the assay of some critical enological parameters indicates the suitability of the engineered strain for wine making.
MATERIALS AND METHODS
Plasmids and yeast strains.
Yep352 is an episomal yeast plasmid carrying the selectable marker URA3. S. cerevisiae industrial strain T73 is a natural diploid strain isolated from Alicante, Spain, musts (20). We constructed three different derivatives of strain T73. Strain TG was obtained by overexpressing the GSY2 yeast gene in a T73ura3-derivative strain (19). Overexpression was achieved by inserting a 3.8-kb SalI fragment containing the gene into the multicopy vector YEp352. Strain TΔg was obtained by sequential deletion of the two copies of the GPH1 gene in strain T73ura3. Disruption was carried out by homologous recombination at both ends of the GPH1 open reading frame of an integration cassette carrying a kanR marker gene flanked by loxP sites. Excision of the marker is inducible by expression of Cre recombinase introduced in the same strain (7), allowing repeated disruptions. Integration of the cassette at the GPH1 locus and further excision of the kanR marker were confirmed by PCR and Southern blot analysis. Uracil prototrophy was restored by introducing a 1.1-kb HindIII linear fragment containing the URA3 gene. Strain TGΔg was obtained by introducing the YEp352 plasmid containing GSY2 into the TΔgura3 strain (without restoration of uracil prototrophy by insertion of the 1.1-kb HindIII fragment). Strain TY was constructed by introducing the Yep352 vector in the T73ura strain. All the strains are, then, prototrophic.
Yeast transformation.
The T73ura3 strain was transformed with yeast plasmid YEp352 or its derivative carrying the GSY2 gene following the procedure of lithium acetate described by Ito et al. (11) as modified by Gietz et al. (5). Uracil prototrophy was the selectable marker. The T73 wine strain was transformed with integrative cassettes for the disruption of the GPH1 gene as described by Güldener et al. (7). The kanR marker gene present in the cassette was used to select transformants resistant to Geneticin.
Culture conditions.
Cultures grown under laboratory conditions were prepared in YPD medium (1% yeast extract, 2% bacteriological peptone, 2% glucose) or SD medium (0.67% yeast nitrogen base, 2% glucose) and incubated at 30°C with shaking (250 rpm). Solid media were prepared by adding 2% (wt/vol) agar. Selection of integrative transformants was carried out on YPD plates supplemented with 200 mg of Geneticin (G418 sulfate; GIBCO BRL, Rockville, Md.)/liter. For microvinification experiments, cells were grown in synthetic or natural musts. Synthetic must [200-g/liter glucose, 5-g/liter KH2PO4, 2-g/liter (NH4)2SO4, 0.4-g/liter MgSO47H2O, 1-g/liter yeast extract (pH 3.8; adjusted with H2SO4)] was sterilized at 0.5 atm for 30 min (6, 22). Natural musts from Bobal red grapes (Requena, Spain) were sterilized with 0.2% (vol/vol) dimethyl dicarbonate for 48 h at 4°C to allow decomposition. In both cases, 1-liter glass bottles were completely filled and capped to reproduce the anaerobic conditions of wine fermentation. The initial yeast inoculum consisted of 2.5 × 105 cells/ml from YPD overnight cultures. Bottles were incubated at 20 to 24°C with gentle shaking (125 rpm). To easily collect samples, screw caps were drilled and a glass tube was introduced to allow aspiration of the medium. Clamped rubber tubing connected to the end of the glass tube allowed the maintenance of anaerobic conditions. To follow fermentation progress, reducing sugar concentrations were recorded. Culture growth was observed by measuring optical density at 600 nm (OD600). Vinifications in natural must were maintained for 20 days after the end of fermentation.
Determination of reducing sugar concentration, ethanol, and enological parameters.
Reaction with DNS (10-g/liter 3,5-dinitrosalicylate, 16-g/liter sodium hydroxide, 300-g/liter potassium sodium tartrate 4-hydrate) and measuring of the developed OD540 value were used for determination of reducing sugar levels (23). A glucose standard curve was established with concentrations between 0 and 2 g/liter. Alcoholic grade (percent vol/vol) was determined by infrared spectrometry (Infrascan; Alliance Instruments). Other enological parameters (acetic acid, malic acid, glycerol, and acetaldehyde) were quantified by automated enzymatic determination (ECHO; Logotech).
Determination of glycogen content.
The method of Parrou and Francois (16) for the determination of glycogen content was used as follows. Cells (10 mg [dry weight]) were collected by centrifugation (3,000 × g; 2 min) from laboratory cultures or microvinification experiments at several growth stages and were washed with cold distilled water. Cells were stored at −20°C. Then, they were thawed, resuspended in 0.25 ml of 0.25 M Na2CO3, transferred to screw-cap tubes, and incubated at 95°C for 4 h. Cell extracts were neutralized by adding 0.15 ml of 1 M acetic acid and 0.6 ml of 0.2 M Na acetate (pH 5.2). One half of the sample was used to determine glycogen content by enzymatic breakage with a commercial Aspergillus niger amyloglucosidase (1.2 U/ml) (Boehringer Mannheim GmbH) at 57°C overnight under rotation in a hybridization oven. Controls were prepared with the other half of the sample without enzyme and treated as described above. Samples were centrifuged at 12,000 × g for 30 s, and the released glucose in supernatants was determined with glucose oxidase-peroxidase (Boehringer Mannheim GmbH).
Analysis and quantification of mRNA.
Cultures were inoculated at 3 × 105 cells/ml. Total RNA from yeast cells was obtained with an automated device for multisample processing (Fast-Prep; Savant, Farmingdale, N.Y.), and then 5-μg samples were analyzed by electrophoresis with formaldehyde-containing agarose gels and Northern blots. An EcoRI-BamHI 0.6-kb fragment from the GSY2 gene was used as the probe and was labeled by standard random priming with [α-32P]dCTP. mRNA quantification was carried out by direct measurement of radioactivity of the filters with an Instant Imager (Packard, Canberra, Australia).
Determination of cell viability.
Cultures were adjusted to OD600 = 1, and serial dilutions (1/100, 1/200, 1/400, 1/800, and 1/1,600) were prepared. Five microliters of each dilution was spotted on YPD plates and then incubated at 30°C for 1 day.
RESULTS
Determination of glycogen content in engineered strains.
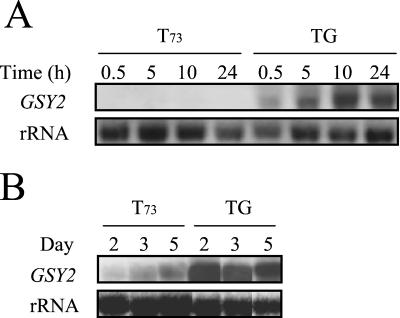
As already stated in the introduction, glycogen accumulation in yeast cells depends on the regulated function of two enzymes: glycogen synthase, mainly encoded by the GSY2 gene, and glycogen phosphorylase, encoded by the GPH1 gene. Theoretically, a larger amount of glycogen would be obtained by increasing the activity of glycogen synthase, by reducing or eliminating the activity of glycogen phosphorylase, or by combining both strategies. Overexpression of the GSY2 gene in strain TG was confirmed by Northern blot analysis (Fig. 1). A very high level of expression in strain TG growing in YPD medium was detected compared to that of T73 (Fig. 1A). During growth in synthetic must, a difference of two- to fourfold in the amount of GSY2 mRNA was found during the first days of vinification (Fig. 1B), and the difference remained significant until the end of fermentation. We analyzed the glycogen content of engineered strains (described in Materials and Methods) grown in a rich YPD medium at both exponential and stationary phases. The regulation of glycogen accumulation was maintained in all the strains, and glycogen content was low and very similar in cells at the exponential growth phase. The level of glycogen in stationary phase was higher in modified strains than in the T73 control strain. Glycogen accumulation was highest in TGΔg (75.6 ± 0.7 μg of glucose/mg [dry weight] of cells), followed by TG (60.2 ± 0.6 μg of glucose/mg [dry weight] of cells), TΔg (47.9 ± 0.4 μg of glucose/mg [dry weight] of cells), and T73 (33.9 ± 0.4 μg of glucose/mg [dry weight] of cells).
FIG. 1.
Overexpression of GSY2 in strain TG. Northern blot analysis of 5 μg of total mRNA from strains T73 and TG grown on YPD medium (A) and synthetic must (B).
Glycogen accumulation under vinification conditions.
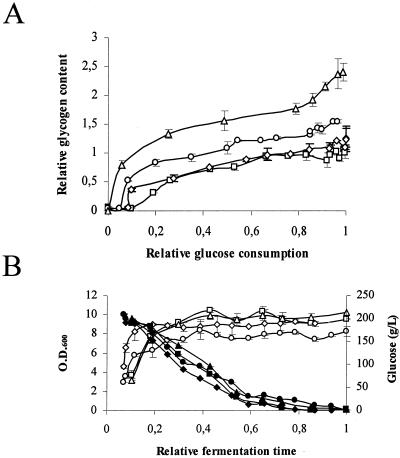
We determined the accumulation of glycogen during vinification experiments with synthetic must for all the modified strains (Fig. 2). For each strain, data are shown at different glucose consumption stages (Fig. 2A). Although the amount of glycogen accumulated was slightly different between experiments, the largest difference in glycogen accumulation was always that between T73 and TGΔg (Fig. 2A), even though the glucose consumption rates were similar (Fig. 2B).
FIG. 2.
Glycogen accumulation in engineered strains during microvinifications in synthetic must: T73 (□), TΔg (⋄), TG (○), and TGΔg (▵). Relative glucose consumption is the fraction of initial glucose concentration consumed at every point [1−(sugar/sugar0)], where sugar represents the glucose concentration. Relative fermentation time is the fraction of the total fermentation time (9 to 15 days) at every point (t/tf). Data correspond to two independent experiments. Error bars represent ranges. (A) For each strain, glycogen content is shown at different stages of glucose consumption and relative to the highest glycogen content of T73 strain in the corresponding control experiment. (B) Glucose consumption (black symbols) and cell density (open symbols) profiles along fermentations.
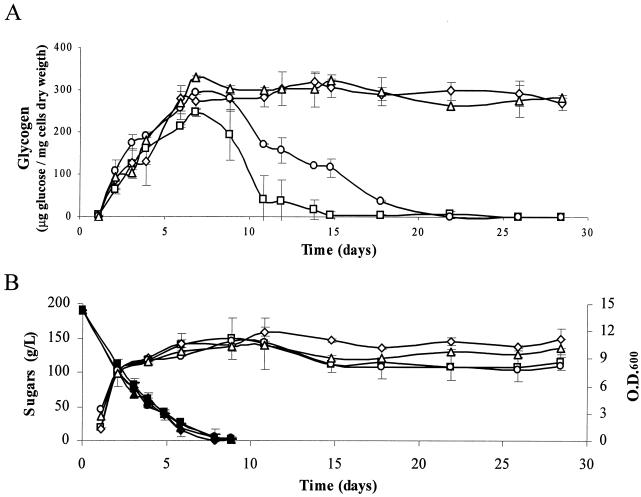
We also conducted vinification experiments with natural Bobal red grape must (Requena, Spain) (Fig. 3). All of the strains consumed all of the sugars present in the natural must in approximately 9 days (Fig. 3B). The behavior of the strains in natural musts was comparable to that with YPD and synthetic must (Fig. 3A). The quantification of several relevant enological parameters in the wines obtained in this experiment indicated no significant differences between the wines obtained with the natural strain and those obtained with their manipulated derivatives with respect to ethanol, acetic acid, malic acid, glycerol, and acetaldehyde content (data not shown).
FIG. 3.
Microvinification in natural must of strains T73 (□), TΔg (⋄), TG (○), and TGΔg (▵). Data correspond to two independent experiments. Error bars represent ranges. (A) Glycogen accumulation. (B) Profiles of growth (open symbols) and glucose consumption (black symbols).
Glycogen mobilization in manipulated strains.
We maintained the vinifications on natural must described above for 20 days after the exhaustion of sugars to monitor the fate of glycogen. Glycogen was quickly mobilized when fermentable sugar was exhausted in both T73 and TG (Fig. 3A), but it was completely degraded in T73 sooner than in TG. However, TΔg and TGΔg, which lack glycogen phosphorylase activity, maintained high levels of glycogen even 20 days after the end of the fermentation.
Impact of glycogen level and mobilization on resistance to glucose deprivation.
Two days after sugar exhaustion (day 11) (Fig. 4), strains TΔg and TGΔg had a lower viability (27 and 8%, respectively) than the parental strain; little or no growth was observed 3 days later (day 14) (Fig. 4). Strain TG showed higher viability than T73 (fourfold at day 14, after 5 days without a carbon source) and still maintained high viability 10 days after the exhaustion of sugars (day 18) (Fig. 4), correlating very well with its glycogen content and ability to mobilize it (Fig. 3A).
FIG. 4.
Viability of strains T73, TΔg, TG, and TGΔg after microvinification in natural must. Cell densities were adjusted to OD600 = 1; 5 μl (each) of 1/100, 1/200, 1/400, 1/800 and 1/1,600 dilutions was spotted onto YPD plates. Experiments were carried out in duplicate. Results shown correspond to one of these experiments.
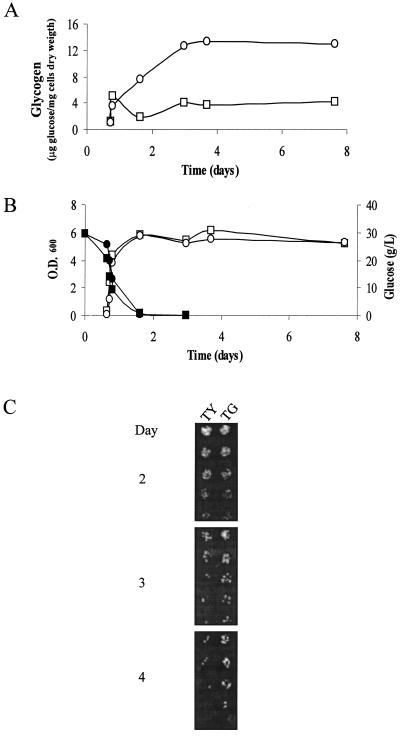
We also grew strain TG on SD medium and monitored glycogen accumulation and viability after glucose consumption (Fig. 5). Strain TY, transformed with the empty vector YEp352, was used as a control due to the selective medium (SD medium lacking uracil) used in this experiment These conditions are more similar to those generally used in experiments of glucose deprivation and also to the growth conditions during biomass production. Under these conditions, less glycogen was accumulated (Fig. 5A) than with natural or synthetic musts (Fig. 2 and 3), and glucose disappeared quickly (Fig. 5B). After glucose exhaustion, glycogen is not mobilized, likely due to the arrested state of cells caused by the lack of nutrients other than glucose. The transient decrease observed for the control strain is probably due to the action of glycogen phosphorylase, which is less relevant for strain TG, due to the overexpression of glycogen synthase. However, the viability data for strain TG once glucose was exhausted (Fig. 5C) were similar to those obtained under vinification conditions. TG strain showed 9.8-fold more viability than the control strain.
FIG. 5.
(A) Glycogen accumulation of strains TY (□) and TG (○) in SD medium. (B) Growth (open symbols) and glucose consumption (black symbols) profiles of these cultures. (C) Viability at different days for both strains. Experiments were carried out in duplicate. Viability results shown correspond to one of these experiments.
DISCUSSION
Metabolic engineering of industrial yeasts could be used to improve the performance of industrial fermentative processes. However, the complexity of many metabolic pathways and their regulatory mechanisms make it difficult to predict the physiological consequences of genetic modifications. We engineered a wine yeast for enhanced viability under glucose deprivation conditions, due to the lack of a carbon source during different stages of industrial processes.
Our results allow us to conclude that glycogen synthase activity, even when overexpressed, is still properly regulated and that glycogen accumulation reaches higher levels, but only in the normal physiological stages of growth. Larger amounts of glycogen in the cells of the modified strains do not affect the normal development of the fermentative process, as demonstrated by the standard profiles of cell density and sugar consumption (Fig. 2B and 3B), although small differences can be detected. Total fermentation times and cell growth in the wine industry are not exactly repetitive, and little variations are not relevant if glucose consumption is complete. Metabolically engineered strains constructed in this work are suitable for industrial wine making, and wines show normal enological parameters very similar to those produced by the commercial T73 strain used as a control. Some differences are detected in the behavior of modified strains when grown with synthetic or natural musts. A clear influence of the must in several aspects of the physiology of wine yeasts has been described, including gene expression patterns (17, 18, 21, 22) and accumulation of trehalose (6).
Increased resistance to glucose deprivation conditions has been tested under two different experimental conditions. The main conclusion is that only one of the engineered strains displays the industrially interesting property of increased resistance under glucose deprivation conditions, although all of them show a higher glycogen content. This phenotype of strain TG indicates the importance of glycogen as an energy reserve and the requirement of glycogen mobilization for displaying higher viability after glucose exhaustion. It is worth noting that overexpression of the GSY2 gene was achieved in both TG and TGΔg by introducing the gene into an episomal multicopy plasmid. The degree of plasmid loss was determined with several experiments and was always about 20%. Even higher levels of glycogen might be obtained by stable integration of the GSY2 gene under the control of a strong promoter.
The main potential biotechnological interest of the engineered strains constructed here is to provide starters for wine-making fermentations. During the production process, yeast cells are grown in molasses with continuous addition of medium and high aeration to obtain a high biomass production (2). When cell density is high enough, cells are maintained without glucose for variable time periods to force the respiration of the small amount of ethanol present at the end of the process, and finally they are dehydrated. This final period without glucose must affect the viability and fermentative performance of cells negatively when growth is reinitiated and the conversion of must to wine is carried out. As molasses is a natural medium with a very high sugar concentration, cells of strain TG should accumulate very large amounts of glycogen and maintain an enhanced viability. When these cells are used to inoculate must, fermentation is carried out as properly as when the wild-type strain is used, producing wines of the same enological quality. Moreover, cells from a finished fermentation that could be used to inoculate a new one, a common technology in traditional wineries, would also be more viable, as the enhanced resistance to glucose deprivation is also displayed under vinification conditions when the physiological state of the cells is much more compromised.
Experiments to test the behavior of the TG strain under industrial growth conditions are in progress, as is the construction of a stable strain carrying a genomic integration of the GSY2 gene under the control of a suitable yeast promoter.
Acknowledgments
We thank F. Estruch for the gift of the cloned GSY2 gene. We also thank M. del Olmo and A. Querol for critically reading the manuscript and helpful suggestions.
This work has been funded by grants ALI98-1041 from the Comisión Interministerial de Ciencia y Tecnología to J. E. Pérez-Ortín and GV99-105-1-13 from Generalitat Valenciana and ALI99-1224-CO2-02 from the Comisión Interministerial de Ciencia y Tecnología to E.M. R.P-T. and J.V.G-A. were recipients of fellowships from Conselleria de Cultura, Educació y Ciència (Generalitat Valenciana).
REFERENCES
- 1.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 2.Degre, R. 1993. Selection and commercial cultivation of wine yeast and bacteria, p. 249-282. In G. H. Fleet (ed.), Wine microbiology and biotechnology. Harwood Academic Publishers, New York, N.Y.
- 3.Francois, J. M., S. Thompson-Jaeger, J. Skroch, U. Zellenka, W. Spevak, and K. Tatchell. 1992. GAC1 may encode a regulatory subunit for protein phosphatase type 1 in Saccharomyces cerevisiae. EMBO J. 11:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francois, J., M. E. Villanueva, and H.-G. Hers. 1988. The control of glycogen metabolism in yeast. I. Interconversion in vivo of glycogen synthase and glycogen phosphorylase induced by glucose, a nitrogen source or uncouplers. Eur. J. Biochem. 174:551-559. [DOI] [PubMed] [Google Scholar]
- 5.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11:355-360. [DOI] [PubMed] [Google Scholar]
- 6.Gimeno-Alcañiz, J. V., J. E. Pérez-Ortín, and E. Matallana. 1999. Differential pattern of trehalose accumulation in wine yeast strains during the microvinification process. Biotechnol. Lett. 21:271-274. [Google Scholar]
- 7.Güldener, U., S. Heck, T. Fiedler, J. Beinhauer, and J. H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hottiger, T., P. Schmutz, and A. Wiemken. 1987. Heat-induced accumulation and futile cycling of trehalose in Sacharomyces cerevisiae. J. Bacteriol. 169:5518-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, K.-P., and E. Cabib. 1974. Yeast glycogen synthase in the glucose-6-phosphate dependent form. I. Purification and properties. J. Biol. Chem. 249:3851-3857. [PubMed] [Google Scholar]
- 10.Hwang, P. K., S. Tugendreich, and R. J. Fletterick. 1989. Molecular analysis of GPH1, the gene encoding glycogen phosphorylase in Saccharomyces cerevisiae. Mol. Cell. Biol. 9:1659-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lillie, S. H., and J. R. Pringle. 1980. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J. Bacteriol. 143:1384-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Pastor, M. T., G. Marchler, C. Schuller, A. Marchler-Bauer, H. Ruis, and F. Estruch. 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress-response element (STRE). EMBO J. 15:2227-2235. [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews, T. M., and C. Webb. 1991. Culture systems, p. 421-447. In M. F. Tuite and S. G. Oliver (ed.), Biotechnology handbooks 4: Saccharomyces. Plenum Press, Singapore.
- 15.Ni, H.-T., and D. C. LaPorte. 1995. Response of a yeast glycogen synthase gene to stress. Mol. Microbiol. 16:1197-1205. [DOI] [PubMed] [Google Scholar]
- 16.Parrou, J. L., and J. Francois. 1997. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal. Biochem. 248:186-188. [DOI] [PubMed] [Google Scholar]
- 17.Puig, S., and J. E. Pérez-Ortín. 2000. Stress response and expression patterns in wine fermentations of yeast genes induced at the diauxic shift. Yeast 16:139-148. [DOI] [PubMed] [Google Scholar]
- 18.Puig, S., A. Querol, D. Ramón, and J. E. Pérez-Ortín. 1996. Evaluation of the use of phase-specific gene promoters for the expression of enological enzymes in an industrial wine yeast strain. Biotechnol. Lett. 18:887-892. [Google Scholar]
- 19.Puig, S., D. Ramón, and J. E. Pérez-Ortín. 1998. Optimized method to obtain stable food-safe recombinant wine yeast strains. Agric. J. Food Chem. 46:1689-1693. [Google Scholar]
- 20.Querol, A., T. Huerta, E. Barrio, and D. Ramón. 1992. Dry yeast strain for use in fermentation of Alicante wines: selection and DNA patterns. J. Food Sci. 57:183-185. [Google Scholar]
- 21.Rachidi, N., P. Barre, and B. Blondin. 2000. Examination of the transcriptional specificity of an enological yeast. A pilot experiment on the chromosome-III right arm. Curr. Genet. 37:1-11. [DOI] [PubMed] [Google Scholar]
- 22.Riou, C., J. M. Nicaud, P. Marre, and C. Gaillardin. 1997. Stationary-phase gene expression in Saccharomyces cerevisiae during wine fermentation. Yeast 13:903-915. [DOI] [PubMed] [Google Scholar]
- 23.Robyt, J. F., and W. J. Whelan. 1972. Reducing value methods for maltodextrins. I. Chain-length dependence of alkaline 3,5-dinitrosalicylate and chain-length independence of alkaline copper. Anal. Biochem. 45:510-516. [DOI] [PubMed] [Google Scholar]
- 24.Rowen, D. W., M. Meinke, and D. C. LaPorte. 1992. GLC3 and GHA1 of Saccharomyces cerevisiae are allelic and encode the glycogen branching enzyme. Mol. Cell. Biol. 12:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt, A. P., and K. McEntee. 1996. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5777-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silljé, H. H. W., J. W. G. Paalman, E. G. Schure, S. Q. B. Olsthoorn, A. J. Verkleij, J. Boonstra, and C. T. Verrips. 1999. Function of trehalose and glycogen in cell cycle progression and cell viability in Saccharomyces cerevisiae. J. Bacteriol. 181:396-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, A., M. P. Ward, and S. Garrett. 1998. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]