Abstract
A xylanase gene, xynX, of Clostridium thermocellum had one thermostabilizing domain (TSD) between the signal peptide sequence and the catalytic domain (CD). The TSD of a truncated xylanase gene, xynX′TSD-CD, was transpositioned from the N terminus to the C terminus of the CD by overlapping PCRs, and a modified product, xynX′CD-TSD, was constructed. XynX′TSD-CD had a higher optimum temperature (70°C versus 65°C) and was more thermostable (residual activity of 68% versus 46% after a 20-min preincubation at 70°C) than the one without the TSD, XynX′CD. However, the domain-transpositioned enzyme, XynX′CD-TSD, showed a lower optimum temperature (30°C) and thermostability (20%) than XynX′CD. Both XynX′TSD-CD and XynX′CD-TSD showed significantly higher binding capacity toward xylan than XynX′CD, and the domain transposition did not cause any change in the binding ability. XynX′TSD-CD and XynX′CD-TSD also showed considerable binding to lichenan but not to carboxymethyl cellulose and laminarin. XynX′TSD-CD and XynX′CD-TSD had higher activities for insoluble xylan than XynX′CD, while XynX′CD was more active against soluble xylan than XynX′TSD-CD and XynX′CD-TSD. These results indicate that the TSD of XynX has dual functions, xylan binding and thermostabilization, and the domain should also be classified as a xylan-binding domain (XBD). The binding capacity of the XBD was not affected by domain transpositioning within the gene.
Xylan, a highly branched β-1,4-linked d-xylose polymer, is a major component of hemicellulose which can be degraded by many xylanolytic bacteria and fungi. Clostridium thermocellum secretes xylanases in addition to cellulosome, which contains at least 14 to 26 different polypeptides, such as endoglucanases, exoglucanases, xylanases, and at least one noncatalytic scaffolding protein. Eight xylanase genes have been characterized from C. thermocellum: xynZ (8), xyn10B (formerly xynY) (3, 6), xynC (11), xynX (14, 16), xynA and xynB (12), and xynU and xynV (5).
Xylanases often exhibit a modular structure composed of catalytic domains linked to one or more noncatalytic domains such as cellulose-binding domains (CBDs), thermostabilizing domains (TSDs), and S-layer-like domains (9). The domains of the modular enzymes were often found to be able to function independent of each other (7, 29-31). The CBDs were found to retain their ability to bind to cellulosic materials even when the domains were fused to foreign proteins (1, 24).
Several xylanases have been reported to have TSDs, for example, Thermoanaerobacterium saccharolyticum xylanase A (XynA) (19, 20), Thermotoga maritima xylanase A (XynA) (31), Cellulomonas fimi xylanase C (XynC) (4), C. thermocellum xylanase Y (XynY) (6), and Bacillus sp. xylanase (2). The mutant xylanases lacking the domain were found to have significantly lower thermostability than the full-length enzymes. However, some recent reports indicate that the TSDs of some modular xylanases have functions other than thermostabilization and the major function might be xylan binding (3, 22, 27).
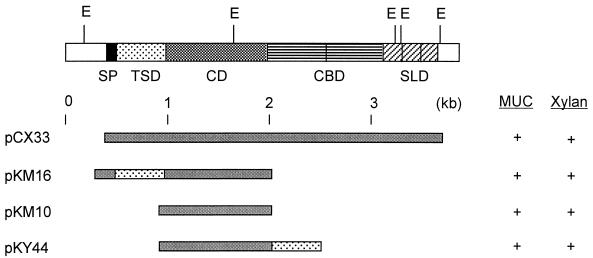
We have previously cloned C. thermocellum xynX and identified the TSD by making deletion mutants and comparing the sequence with those of other xylanase genes (16). The modular enzyme is composed of a signal peptide, a TSD, a catalytic domain (CD), two repeats of the CBD, and three repeats of an S-layer-like domain. The TSD of xynX was located between the signal peptide sequence and the CD, in the order TSD-CD.
For this study, we constructed a truncated and transpositioned xylanase, XynX′CD-TSD, with CD-TSD in that order. With the transpositioned enzyme, we tried to elucidate the major function of the domain and to investigate the influence of the transposition on thermostabilizing and xylan-binding functions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
C. thermocellum ATCC 27405 was used as a source of the xylanase gene (14). Plasmid pCX33 contains the entire xynX gene (16), and Escherichia coli DH5α (pCX33) was used for the production of the untruncated enzyme. Plasmids pKM16 and pKM10 (16) were used as the sources of TSD and CD, respectively. E. coli DH5α and BL21(DE3) were used as cloning hosts, and pUC19 was used as a cloning vector. Cells were grown in Luria-Bertani medium at 37°C and, if necessary, ampicillin (50 μg/ml) was added to the medium.
Construction of a TSD-transpositioned gene.
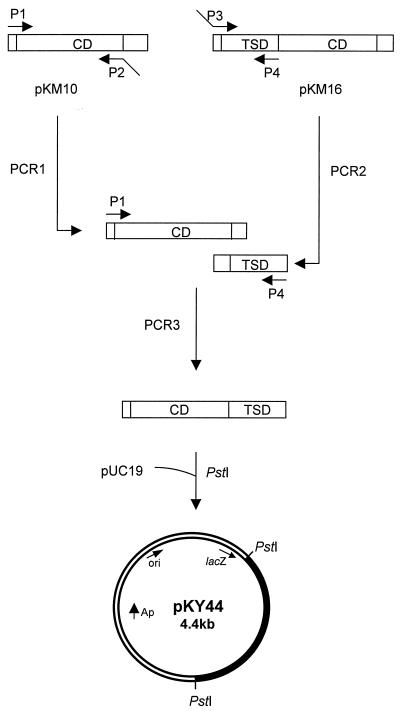
The TSD was transpositioned from the N terminus to the C terminus of the CD by overlapping PCRs (13). The primers used to produce the required mutation were as follows: the forward primer for the CD (P1), a 19-mer (5′-CTATGACCATGATTACGCC-3′); the reverse primer for the CD (P2), a 34-mer (5′-CAAATTCGCATTATTATAAGCTGGTTTTGCCTGT-3′); the forward primer for the TSD (P3), a 34-mer (5′-ACAGGCAAAACCAGCTTATAATAATGCGAATTTG-3′); and the reverse primer for the TSD (P4), a 32-mer (5′-GTCGACCTGCAGTACTTGTATTGGATTTTGTG-3′). A PstI restriction site (bold) and extra bases (italic) were introduced to the 5′ end of P4. The primers were prepared by Bioneer Co., Daejeon, Korea.
The conditions for 20 successive cycles were as follows: denaturation at 93°C for 1 min, annealing at 56°C for 1.5 min, and primer extension at 72°C for 1.5 min. One additional cycle was performed as follows: denaturation at 93°C for 1 min, primer extension at 72°C for 10 min, and cooling at 4°C (17). The products of the primary reactions were then combined and fused together by another round of PCR using P1 and P4 as the primers. The final PCR products were digested with PstI and then ligated into pUC19 as described by Sambrook and Russell (25). Restriction enzymes, T4 DNA ligase, and calf intestinal alkaline phosphatase were purchased from Gibco-BRL (Gaithersburg, Md.) and Boehringer Mannheim (Indianapolis, Ind.). Other chemicals were from Sigma Chemical Co. (St. Louis, Mo.). The ligation mixture was used to transform E. coli DH5α and BL21(DE3) (10). E. coli cells harboring the recombinant plasmids were then grown and selected on agar plates containing 0.5% xylan. The nucleotide sequence of the modified gene was determined by Bioneer Co. using the dideoxy chain termination method (26).
Enzyme assay and activity staining.
Cell extracts of E. coli cells carrying the recombinant plasmids were prepared as follows: cells were harvested, washed with 50 mM sodium citrate buffer, pH 5.5, resuspended in a minimal volume of the same buffer, and sonicated for 2 min with a sonicator (Model VCX600; Sonics & Materials) at 40% output. Cell debris was removed by centrifuging at 15,000 × g for 20 min at 4°C. Xylanase activity of the cell extract was determined at pH 5.5 and 50°C using 0.5% (wt/vol) oat spelt xylan (Sigma) as the substrate for 15 min as described previously (14, 23). Carboxymethyl cellulose (CMC) hydrolyzing activity was measured under the above conditions except with 0.8 U of enzyme, 0.5% CMC (Sigma; medium viscosity) as the substrate, and a 3-h incubation time. The enzyme activity toward p-nitrophenylcellobioside (Sigma) was determined by measuring the amount of p-nitrophenol released (14). For optimum temperature determination, the enzymes were reacted with the substrate for 30 min at designated temperatures ranging from 15 to 80°C. For thermostability studies, the enzymes were preincubated at 70°C in the absence of the substrate, samples were withdrawn at designated time intervals, and residual activities were determined. One unit of enzyme activity was defined as the amount of the enzyme that liberated 1 μmol of reducing sugar per min. Protein concentration was determined by the method of Lowry et al. (21). Activity staining for xylanase was carried out using 4-methylumbelliferyl-β-d-cellobioside (MUC) (Sigma) after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (14).
Affinity analysis with soluble and insoluble polysaccharides.
Soluble and insoluble fractions of xylan were prepared from 5% (wt/vol) oat spelt xylan solution in deionized water. After stirring the solution for 1 h at room temperature and centrifuging at 15,000 × g for 20 min at 4°C, the supernatant and the pellet were used as soluble and insoluble fractions, respectively. Binding abilities of the enzyme to soluble substrates were semiquantitatively assessed by denaturing SDS-PAGE using 11.5% gels containing the soluble fraction of oat spelt xylan, CMC, lichenan (Sigma), or laminarin (Sigma). Soluble substrates were added to the gels prior to polymerization at a final concentration of 0.1% (wt/vol). Denaturing gels without polysaccharides were used as a control. Activity staining with MUC was performed at 45°C since XynX′CD-TSD showed little activity at 55°C, the temperature at which activity determinations and stainings for XynX′CD and XynX′TSD-CD were routinely performed. The changes in the mobilities of XynX′CD-TSD and XynX′TSD-CD due to the inclusion of the soluble polysaccharides were compared to the change in the mobility of XynX′CD.
The binding capacities of the xylanases for insoluble polysaccharides were measured as follows: approximately 0.12 U of the enzymes was mixed with 1.5% (wt/vol) polysaccharides, insoluble xylan, or Avicel in 0.5 ml of 50 mM sodium citrate buffer, pH 5.5, by shaking for 40 min at 0°C, and then the mixtures were centrifuged at 15,000 × g for 20 min at 4°C to remove the insoluble polysaccharides and the enzymes bound to the polysaccharides. The amount of the unbound enzyme was determined by assaying xylanase activity in the supernatant.
RESULTS
Construction of a domain-transpositioned xylanase gene.
A modified xylanase gene encoding a domain-transpositioned protein, xynX′CD-TSD, was constructed by overlapping PCRs (Fig. 1). The backward primer for the CD was designed to have a sequence complementary to the 5′-TSD sequence at the 5′ end, and the forward primer for the TSD was designed to have a sequence complementary to the 3′-CD sequence at the 5′ end. A fused gene product of 1.7 kb was obtained by PCR using the separately amplified CD (1.1 kb) and TSD (0.6 kb), the forward primer for the CD, and the backward primer for the TSD. The product was found to have the domains in the order CD-TSD (Fig. 2) by restriction analyses and DNA sequencing (data not shown), and the plasmid containing the modified gene was named pKY44.
FIG. 1.
Construction of a domain-transpositioned truncated xylanase gene, xynX′CD-TSD, of C. thermocellum. The filled region in pKY44 represents xynX′CD-TSD.
FIG. 2.
Domain organization of XynX and its derivatives. SP, signal peptide; SLD, S-layer-like domain.
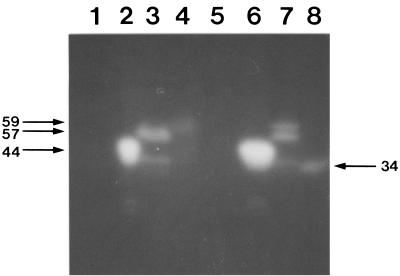
The molecular mass of XynX′CD-TSD expressed in E. coli DH5α was 34 kDa on an activity-stained gel after SDS-PAGE, significantly smaller than the expected size of 59 kDa, while the molecular masses of the largest XynX′TSD-CD and XynX′CD expressed in the same host corresponded well to the expected sizes, 57 and 44 kDa, respectively (Fig. 3). When xynX′CD-TSD was expressed in E. coli BL21(DE3), the size of the major protein, which was the largest of the proteins present, corresponded well to the expected size (Fig. 3). XynX′CD-TSD expressed in E. coli BL21(DE3) and XynX′TSD-CD and XynX′CD expressed in E. coli DH5α were used for further studies.
FIG. 3.
Activity staining of proteins produced from transformants after SDS-PAGE. Samples in lanes 1 to 4 were prepared from strain E. coli BL21(DE3) and in lanes 5 to 8 were from strain E. coli DH5α. Lanes 1 and 5, pUC19 was used as a control; lanes 2 and 6, XynX′CD; lanes 3 and 7, XynX′TSD-CD; and lanes 4 and 8, XynX′CD-TSD. Numerals on the left represent the molecular masses in kilodaltons of the largest proteins in lanes 4, 6, and 7. The numeral on the right represents the molecular mass in kilodaltons of the protein in lane 8.
Influence of the domain transposition on optimum temperature and thermostability of the enzyme.
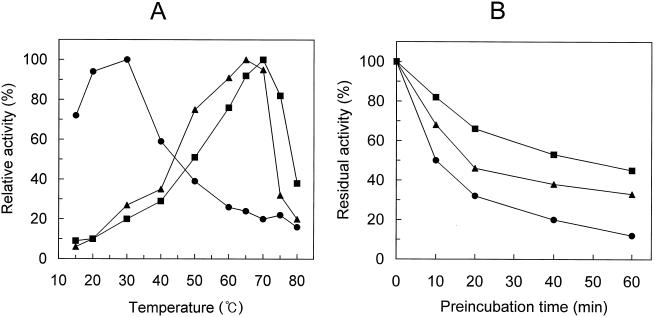
The optimum temperature of the enzyme with the TSD, XynX′TSD-CD, was 5°C higher than that of the one without the TSD, XynX′CD (Fig. 4A). XynX′TSD-CD also showed higher thermostability, and the residual activities of XynX′TSD-CD and XynX′CD after 20 min of preincubation at 70°C were 68 and 46%, respectively (Fig. 4B).
FIG. 4.
Influence of temperature on xylanase activities of the domain-transpositioned XynX. (A) Optimum temperatures of XynX derivatives were determined as described in Materials and Methods. The activity of each enzyme at its optimum temperature was considered to be 100%. (B) Thermostabilities of XynX derivatives were determined by preincubating the enzymes at 70°C for the designated times and then assaying the residual activities as described in Materials and Methods. The activity of nonpreincubated enzyme was considered to be 100%. ▴, XynX′CD; ▪, XynX′TSD-CD; •, XynX′CD-TSD.
The domain-transpositioned enzyme, XynX′CD-TSD, had an optimum temperature of 30°C, which was significantly lower than those of XynX′TSD-CD and XynX′CD (Fig. 4A). The activity of XynX′CD-TSD at 70°C, the optimum temperature for XynX′TSD-CD, was less than 20% of the activity at its optimum temperature. XynX′CD-TSD showed more than 70% of its maximal activity at 15°C. XynX′CD-TSD was significantly less thermostable than both XynX′TSD-CD and XynX′CD, and the residual activity of XynX′CD-TSD after 20 min of preincubation at 70°C was only about 30%.
Influence of domain transposition on affinity of the enzyme for soluble and insoluble polysaccharides.
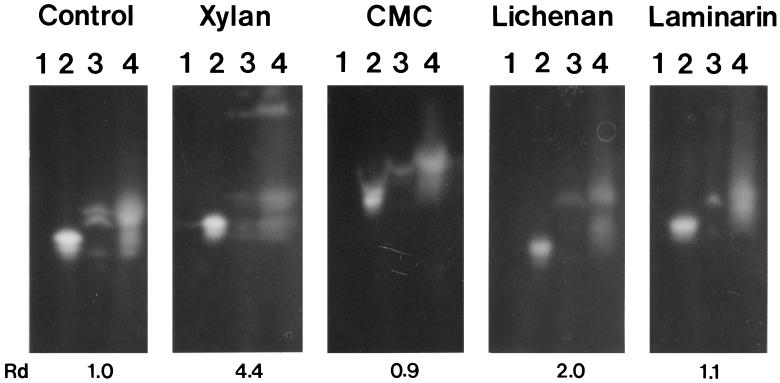
The electrophoretic mobility of the control protein, XynX′CD, was only slightly affected by the inclusion of soluble xylan in the gel. XynX′TSD-CD bound well to soluble xylan, as evidenced by the decrease in the relative mobility due to the inclusion of xylan (Fig. 5). The decrease in the relative mobility of XynX′TSD-CD was about four times greater than that of XynX′CD. The domain-transpositioned enzyme, XynX′CD-TSD, showed the same level of binding to soluble xylan as XynX′TSD-CD (Fig. 5). XynX′TSD-CD and XynX′CD-TSD showed negligible binding to CMC and laminarin (a β-1,3-glucan), although the inclusion of medium-viscosity CMC in the gel retarded the migrations of all the proteins, probably due to the viscosity of CMC (Fig. 5). XynX′TSD-CD and XynX′CD-TSD bound to lichenan, a mixed-linkage β-1,3/β-1,4-glucan, but the level of binding to lichenan was apparently lower than that to xylan (Fig. 5). The decrease in the relative mobility of XynX′TSD-CD and XynX′CD-TSD due to the inclusion of lichenan was about half of that with xylan.
FIG. 5.
Affinity denaturing gel electropherogram of truncated XynX enzymes. The control panel contained no polysaccharide, and the others contained 0.1% designated polysaccharide. Lanes 1, control; lanes 2, XynX′CD; lanes 3, XynX′TSD-CD; lanes 4, XynX′CD-TSD. Numerals below the gels represent relative retardedness calculated from the difference in relative mobility (Rd). Rd = (Rf of XynX′CD in the presence of the polysaccharide − Rf of XynX′TSD-CD in the presence of the polysaccharide)/(Rf of XynX′CD in the absence of the polysaccharide − Rf of XynX′TSD-CD in the absence of the polysaccharide), where Rf = distance migrated/length of gel.
The enzymes with the TSD, XynX′TSD-CD and XynX′CD-TSD, showed significantly higher binding capacities toward insoluble xylan than the one without the TSD, and no apparent difference in binding was observed between XynX′TSD-CD and XynX′CD-TSD (Table 1). Both XynX′TSD-CD and XynX′CD-TSD also bound well to Avicel, while XynX′CD showed little binding to the substrate.
TABLE 1.
Binding capacity of truncated XynX enzymes to insoluble xylan and Avicela
| Enzyme | Relative binding (%) (mean ± SD)
|
|
|---|---|---|
| Insoluble xylan | Avicel | |
| XynX′CD | 6 ± 4 | 9 ± 5 |
| XynX′TSD-CD | 52 ± 7 | 31 ± 6 |
| XynX′CD-TSD | 46 ± 4 | 53 ± 3 |
Final concentrations of the polysaccharides were 1.5% (wt/vol). The activity of the same amount of each enzyme mixed only with the buffer was considered to be 100%. For more details, refer to Materials and Methods.
XynX′CD-TSD showed a similar level of activity toward oat spelt xylan, birchwood xylan, and CMC as XynX′TSD-CD, and both enzymes showed higher activities toward oat spelt xylan than birchwood xylan (data not shown). The enzyme with only the CD, XynX′CD, was more active for the soluble form of xylan than the insoluble form, whereas the enzymes with TSD, XynX′CD-TSD and XynX′TSD-CD, showed higher activities for the insoluble form (Table 2). Both the enzymes were much less active toward CMC and p-nitrophenylcellobioside than toward the xylans, and no detectable activities were observed toward lichenan and laminarin (data not shown).
TABLE 2.
Relative activities of truncated XynX enzymes on soluble and insoluble xylan
| Enzyme | Relative activity (%) (mean ± SD) on xylana
|
|
|---|---|---|
| Soluble | Insoluble | |
| XynX′CD | 170 ± 7 | 50 ± 5 |
| XynX′TSD-CD | 105 ± 3 | 89 ± 7 |
| XynX′CD-TSD | 122 ± 6 | 79 ± 4 |
The activity of each enzyme on unfractionated xylan (1.0% [wt/vol]) was considered to be 100%. Preparations of soluble and insoluble fractions are described in Materials and Methods. Final concentrations of soluble and insoluble substrates were approximately 0.2 and 0.8%, respectively.
DISCUSSION
It was found that deletion of a certain domain(s) from several modular xylanases made the enzyme derivatives much more susceptible to thermal inactivation than the full-length enzymes, and these domains have often been called TSDs (4, 6, 19, 20, 31). We have previously found that XynX from C. thermocellum had a TSD and that the domain was important for thermostability (16). It was found that the deletion of a region, the 33rd to 194th amino acid residues of the modular enzyme, lowered the optimum temperature by 5 to 10°C. The truncated enzyme without the region, XynX′CD, was also found to be less stable than XynX′TSD-CD when preincubated at 70°C. From these observations, we had concluded that the deleted region of XynX could be a TSD.
Xylanases with a TSD(s) have the domain in front of, at the rear of, or on both sides of the CD, and TSDs exist as singular or repeated forms. It was found that about half of the 27 sequences with significant homology with the TSD region of XynX contained one copy of TSD, and the others had two copies of this domain in tandem (16). Most of the TSDs were N-terminal to the CD and associated only with the family 10 xylanases (mainly thermophilic xylanases), while a few of the TSDs were C-terminal to the CD and associated with the family 11 mesophilic xylanases (27). The CD of XynX of C. thermocellum is a member of the family 10 xylanases (7, 29), and the TSD of the enzyme was found to be in a singular form and to be N-terminal to the CD (16).
Similarly to the TSDs, the CBDs of glycosyl hydrolases have been found to be either N-terminal or C-terminal to the CDs (29). The CBDs showed their function regardless of their location within the gene and retained their function when the domain was added to foreign proteins (1, 17, 24). These results prompted us to study the effect of transpositioning the TSD of the family 10 xylanase on thermostability of the enzyme. While this study was being performed, it was proposed that TSDs should be designated xylan-binding domains (XBDs) since the primary function of xylanase-associated TSDs seemed to be xylan binding rather than thermostabilization (3, 22, 27). Here, we report the effect of transpositioning the TSD from the N terminus to the C terminus of the CD on thermostability and on carbohydrate binding capacity of a truncated xylanase.
A domain-transpositioned truncated xylanase gene, xynX′CD-TSD, was constructed by overlapping PCR with primer sets for the CD and TSD. When xynX′CD-TSD was expressed in E. coli DH5α, the produced enzyme of 34 kDa was significantly smaller than the expected size, 59 kDa, probably due to internal proteolytic cleavage. Proteolytic cleavage of the full-length XynX has been observed with the same host in a previous study (16), and proteolytic cleavage of the CBD of Bacillus subtilis endo-β-1,4-glucanase in Bacillus megaterium and E. coli has also been observed (15, 18). When the domain-transpositioned gene was expressed in a host deficient in a membrane protease, E. coli BL21(DE3), the size of the major protein produced corresponded well to the expected size. The transposition of the TSD domain within the gene resulted in a loss of thermal stability and lowered optimum temperature, indicating that the thermostabilizing function of the domain is not independent of the location of the domain within the gene.
XynX′TSD-CD bound well to both soluble and insoluble xylans. The domain transposition did not cause any change in the binding capacity of the enzyme toward the xylans. XynX′TSD-CD and XynX′CD-TSD also bound to lichenan, though the level of binding was apparently lower than that to xylan. The A2 domain of Thermotoga maritima XynA (22), the X6b domain of C. thermocellum Xyn10B (3), and the N-terminal TSD of XynA of Caldibacillus cellulovorans (27, 28) have been shown to bind well to xylan, and the A2 and X6b domains bind to lichenan. XynX′TSD-CD also showed considerable binding to Avicel, unlike the A2 and X6b domains, and XynX′CD-TSD showed a little higher binding capacity than XynX′TSD-CD.
The results of this study are summarized as follows. The truncated xylanases with the TSD bound to xylan better than the one without the domain, the binding capacities were not affected by the domain transposition, and the thermostability of the truncated enzyme was significantly lowered by the domain transposition. With these observations, we conclude that the TSD of XynX of C. thermocellum has dual functions, xylan binding and thermostabilization, and the domain should be classified as an XBD as well as a TSD. The facts that XynX′CD showed higher activity for soluble xylan than the enzymes with a TSD, XynX′TSD-CD or XynX′CD-TSD, and that XynX′TSD-CD and XynX′CD-TSD had higher activities for insoluble xylan than XynX′CD suggest that the domain may increase the availability of the insoluble substrate by binding to it. It should be interesting to study the effect of transferring the domain to other proteins since the domain transposition within the gene showed no noticeable influence on its binding capacity.
Acknowledgments
This work was supported by a Genetic Engineering Research Grant (project no. 1998-019-G30002) from the Korea Research Foundation.
REFERENCES
- 1.Ahn, D. H., H. Kim, and M. Y. Pack. 1997. Immobilization of β-glucosidase using the cellulose-binding domain of Bacillus subtilis endo-β-1,4-glucanase. Biotechnol. Lett. 19:483-486. [Google Scholar]
- 2.Blanco, A., P. Diaz, J. Zueco, P. Parascandola, and F. I. J. Pastor. 1999. A multidomain xylanase from a Bacillus sp. with a region homologous to thermostabilizing domains of thermophilic enzymes. Microbiology 145:2163-2170. [DOI] [PubMed] [Google Scholar]
- 3.Charnock, S. J., D. N. Bolam, J. P. Turkenburg, H. J. Gilbert, L. M. A. Ferreira, G. J. Davis, and C. M. G. A. Fontes. 2000. The X6 “thermostabilizing” domains of xylanases are carbohydrate-binding modules: structure and biochemistry of the Clostridium thermocellum X6b domain. Biochemistry 39:5013-5021. [DOI] [PubMed] [Google Scholar]
- 4.Clarke, J. H., K. Davidson, H. J. Gilbert, C. M. G. A. Fontes, and G. P. Hazlewood. 1996. A modular xylanase from mesophilic Cellulomonas fimi contains the same cellulose-binding and thermostabilizing domains as xylanases from thermophilic bacteria. FEMS Microbiol. Lett. 139:27-35. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes, A. C., C. M. G. A. Fontes, H. J. Gilbert, G. P. Hazelwood, T. H. Fernandes, and L. M. A. Ferreira. 1999. Homologous xylanases from Clostridium thermocellum: evidence for bi-functional activity, synergism between xylanase modules and the presence of xylan-binding domains in the enzyme complexes. Biochem. J. 342:105-110. [PMC free article] [PubMed] [Google Scholar]
- 6.Fontes, C. M. G. A., G. P. Hazlewood, E. Morag, J. Hall, B. H. Hirst, and H. J. Gilbert. 1995. Evidence for a general role for non-catalytic thermostabilizing domains in xylanases from thermophilic bacteria. Biochem. J. 307:151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilkes, N. R., B. Henrissat, D. G. Kilburn, R. C. Miller, Jr., and R. A. J. Warren. 1991. Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol. Rev. 55:303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grepinet, O., M. C. Chebrou, and P. Beguin. 1988. Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum. J. Bacteriol. 170:4582-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall, J., and H. J. Gilbert. 1988. The nucleotide sequence of a carboxymethyl-cellulase gene from Pseudomonas fluorescens subsp. cellulosa. Mol. Gen. Genet. 213:112-117. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-135. In D. M. Glover (ed.), DNA cloning, vol. 1. IRL Press, Oxford, United Kingdom.
- 11.Hayashi, H., K. I. Takagi, M. Fukumura, T. Kimura, S. Karita, K. Sakka, and K. Ohmiya. 1997. Sequence of xynC and properties of XynC, a major component of the Clostridium thermocellum cellulosome. J. Bacteriol. 179:4246-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi, H., M. Takehara, T. Hattori, T. Kimura, S. Karita, K. Sakka, and K. Ohmiya. 1999. Nucleotide sequences of two contiguous and highly homologous xylanase genes xynA and xynB and characterization of XynA from Clostridium thermocellum. Appl. Microbiol. Biotechnol. 51:348-357. [DOI] [PubMed] [Google Scholar]
- 13.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 14.Jung, K. H., K. M. Lee, H. Kim, K. H. Yoon, S. H. Park, and M. Y. Pack. 1998. Cloning and expression of a Clostridium thermocellum xylanase gene in Escherichia coli. Biochem. Mol. Biol. Int. 44:283-292. [DOI] [PubMed] [Google Scholar]
- 15.Kim, H., D. H. Ahn, and M. Y. Pack. 1993. Cleavage of Bacillus subtilis endo-β-1,4-glucanase by B. megaterium protease. Biotechnol. Lett. 15:127-132. [Google Scholar]
- 16.Kim, H., K. H. Jung, and M. Y. Pack. 2000. Molecular characterization of xynX, a gene encoding a multidomain xylanase with a thermostabilizing domain from Clostridium thermocellum. Appl. Miocrobiol. Biotechnol. 54:521-527. [DOI] [PubMed] [Google Scholar]
- 17.Kim, H., M. Goto, H. J. Jeong, K. H. Jung, I. Kwon, and K. Furukawa. 1998. Functional analysis of a hybrid endoglucanase of bacterial origin having a cellulose-binding domain of fungal exoglucanase. Appl. Biochem. Biotechnol. 75:193-204. [DOI] [PubMed] [Google Scholar]
- 18.Kim, H., S. F. Kim, D. H. Ahn, J. H. Lee, and M. Y. Pack. 1995. Internal cleavage of Bacillus subtilis BSE616 endo-β-1,4-glucanase expressed in Escherichia coli. J. Microbiol. Biotechnol. 5:26-30. [Google Scholar]
- 19.Lee, Y. E., S. E. Lowe, B. Henrissat, and J. G. Zeikus. 1993. Characterization of the active site and thermostability regions of endoxylanase from Thermoanaerobacterium saccharolyticum B6A-RI. J. Bacteriol. 175:5890-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, Y. E., S. E. Lowe, and J. G. Zeikus. 1993. Gene cloning, sequencing, and biochemical characterization of endoxylanase from Thermoanaerobacterium saccharolyticum B6A-RI. Appl. Environ. Microbiol. 59:3134-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry, O. H., N. J. Rosenbrough, A. L. Farr, and R. J. Randell. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 22.Meissner, K., D. Wassenberg, and W. Liebl. 2000. The thermostabilizing domain of the modular xylanase XynA of Thermotoga maritima represents a novel type of binding domain with affinity for soluble xylan and mixed-linkage β-1,3/β-1,4-glucan. Mol. Microbiol. 36:898-912. [DOI] [PubMed] [Google Scholar]
- 23.Miller, G. L. 1959. Use of dinitrosalicylic acid reagent for the determination of reducing sugar. Anal. Chem. 31:426-428. [Google Scholar]
- 24.Ong, E., N. R. Gilkes, R. C. Miller, Jr., A. J. Warren, and D. G. Kilburn. 1991. Enzyme immobilization using a cellulose-binding domain: properties of a beta-glucosidase fusion protein. Enzyme Microb. Technol. 3:59-65. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunna, A., M. D. Gibbs, and P. L. Bergquist. 2000. The thermostabilizing domain, XynA, of Caldibacillus cellulovorans xylanase is a xylan binding domain. Biochem. J. 346:583-586. [PMC free article] [PubMed] [Google Scholar]
- 28.Sunna, A., M. D. Gibbs, and P. L. Bergquist. 2000. A novel thermostable multidomain 1,4-β-xylanase from “Caldibacillus cellulovorans” and effect of its xylan-binding domain on enzyme activity. Microbiology 146:2947-2955. [DOI] [PubMed] [Google Scholar]
- 29.Tomme, P., R. A. J. Warren, and N. R. Gilkes. 1995. Cellulose hydrolysis by bacteria and fungi. Adv. Microb. Physiol. 37:1-81. [DOI] [PubMed] [Google Scholar]
- 30.Warren, R. A. J. 1996. Microbial hydrolysis of polysaccharides. Annu. Rev. Microbiol. 50:183-212. [DOI] [PubMed] [Google Scholar]
- 31.Winterhalter, C., P. Heinrich, A. Candussio, G. Wich, and W. Liebl. 1995. Identification of a novel cellulose-binding domain with the multidomain 120 kDa xylanase XynA of the hyperthermophilic bacterium Thermotoga maritima. Mol. Microbiol. 15:431-444. [DOI] [PubMed] [Google Scholar]