Abstract
A raw-starch-digesting amylase (RSDA) gene from a Cytophaga sp. was cloned and sequenced. The predicted protein product contained 519 amino acids and had high amino acid identity to α-amylases from three Bacillus species. Only one of the Bacillus α-amylases has raw-starch-digesting capability, however. The RSDA, expressed in Escherichia coli, had properties similar to those of the enzyme purified from the Cytophaga sp.
Starch is the major storage carbohydrate of many plants, and enzymes digesting starch are widely distributed in nature (6). The substrate is completely hydrolyzed to glucose through the combined actions of enzymes including α-amylase, β-amylase, glucoamylase, and debranching enzyme. These amylolytic enzymes have been isolated and their biochemical properties have been studied for many years. In more recent years, numerous amylolytic enzymes have been cloned, with some produced in heterologous expression systems (11, 20, 23). Furthermore, the three-dimensional structures of several of these enzymes have been constructed (14, 16).
The major application of amylases in the food industry is the saccharification of starch in the manufacture of diverse starch-derived products. Gelatinization and liquefaction of starch slurry, a required pretreatment, are catalyzed by α-amylase at 70°C and above 95°C, respectively (1). Therefore, amylases capable of digesting raw starch (raw-starch-digesting amylases [RSDA]) have drawn the attention of researchers as a possible replacement for this energy-consuming and economically costly step (17). Certain fungi and bacteria are known to produce RSDA, and some of these amylases have been purified and characterized (3, 4, 13). We isolated from a soil bacterium, a Cytophaga sp., a new RSDA which is an endo-type, raw-starch-absorbable amylase with a molecular weight of 59,000 (9).
To further investigate this enzyme, we cloned, sequenced, and expressed in Escherichia coli the RSDA gene of the Cytophaga sp. The enzyme produced by E. coli was also purified and characterized. A comparison with genes of other starch-degrading enzymes revealed high identity of Cytophaga sp. RSDA to α-amylases from some Bacillus species.
Preparation of antibodies against Cytophaga sp. RSDA and rsda gene probe.
The Cytophaga sp. for harvesting RSDA was grown at 37°C for 24 h in medium consisting of 0.5% (wt/vol) polypeptone-S, 0.5% (wt/vol) beef extract, 0.2% (wt/vol) yeast extract, and 0.2% (wt/vol) raw corn starch (Sigma, St. Louis, Mo.). The supernatant was collected as crude enzyme solution by centrifugation at 6,000 × g for 5 min. The stepwise purification of RSDA from the crude enzyme solution, including ammonium sulfate fractionation, DEAE-Sepharose ion-exchange chromatography (Pharmacia, Uppsala, Sweden), and gel filtration, was described by Jeang et al. (9).
Antiserum of RSDA was prepared in Sprague-Dawley rats by means of weekly intravenous injection of emulsified pure RSDA (50 μg) with Freund's complete adjuvant (Sigma) for the first injection and with incomplete adjuvant for the three subsequent injections. Immunoglobulin G was obtained from the antiserum through subsequent ammonium sulfate precipitation and DEAE-Sepharose CL 6B ion-exchange chromatography. The immunoglobulin G eluted in the first protein peak was collected, pooled, and further purified by absorption with the cell lysate of E. coli DH5α.
The NH2-terminal amino acid sequences of the purified full-length RSDA and a partially CNBr-digested fragment (21) were determined with a protein sequencer (model 476A; Applied Biosystems), and forward and reverse nucleotide primers were designed based on this sequence. The primers were used to amplify by PCR a 350-bp fragment of the Cytophaga sp. DNA purified by the method of Saito and Miura (18). The deduced amino acid sequence of the cloned fragment was compared with those of related enzymes in the SWISS-PROT databank and found to resemble an α-amylase. It was reported earlier that there was only one amylase produced by the Cytophaga sp. (2). The PCR product, with digoxigenin-labeled dUTP (Nonradioactive in situ hybridization application manual, p. 36-40, Boehringer Mannheim GmbH, Mannheim, Germany, 1996), was applied as a probe to screen for the rsda gene from a genomic library of the Cytophaga sp.
Cloning the rsda gene from the Cytophaga sp.
A genomic library of the Cytophaga sp. DNA was constructed in λ-ZAP II, and E. coli XL1-Blue was infected with phage particles. Ten positive clones were obtained among 1.5 × 105 plaques screened by hybridization with digoxigenin-labeled DNA probe. All of the positive plaques reacted with antibodies raised against the Cytophaga sp. RSDA. In addition, a halo appeared around the positive plaques when a Luria-Bertani (LB) agar plate containing soluble starch was flooded with an iodine solution. These results indicate that the selected clones not only contained the rsda gene but also expressed an active enzyme. The clones expressed the foreign gene without IPTG (isopropyl-β-d-thiogalactopyranoside), suggesting the presence of promoter regions upstream of the rsda gene on the inserted DNA.
The inserted DNA on the positive recombinant phage was determined to be 7.5 kb in length by PCR with T3 and T7 primers on pBluescript SK-phagemid. A 3.2-kb fragment of DNA was subcloned into the baculovirus transfer vector pAcUW21 (PharMingen, San Diego, Calif.) to give the plasmid pARMH.
Characterization of the rsda gene.
An open reading frame predicted to encode a protein of 519 amino acids was identified by sequencing (19, 22) the DNA insert on pARMH. This gene was designated rsda. The 35th to 57th and 138th to 145th amino acid residues of the deduced ORF matched with the NH2- and internal NH2-terminal sequences of the Cytophaga sp. RSDA, respectively. Therefore, it is proposed that the gene product contains a signal peptide of 34 amino acid residues. In comparison with gene products in the SWISS-PROT databank, the identity of RSDA to α-amylases produced by Bacillus licheniformis, Bacillus amyloliquefaciens, and Bacillus stearothermophilus was 75, 74, and 67%, respectively. On the other hand, there were only low levels of identity, i.e., 30 and 24%, between the Cytophaga sp. RSDA and those from Bacillus sp. strain B1018 and Cryptococcus sp., respectively. While the Cytophaga sp. RSDA had all four conserved regions reported for α-amylases in general, only α-amylase of B. licheniformis was found to possess raw-starch-digesting capacity (7). His237, Glu263, and Asp330 are predicted to be the active sites, while Asp233, Lys236, Tyr264, Trp265, Gln266, and His329 may be related to substrate binding. A previous study showed that RSDA activity decreased after treatment with N-bromosuccinimide and dinitrofluorobenzene, the modifying agents for Trp and Lys, respectively (9). Lee et al. (12) reported that α-amylase from Thermococcus profundus was also inhibited by N-bromosuccinimide treatment. These results indicate that Trp and Lys residues are related to enzymatic activity. There is no Thr- and Ser-rich segment in the present sequence, though such a segment was suggested to be related to the raw-starch-digesting capabilities of amylases from Aspergillus awamori (5) and Bacillus subtilis (6).
Expression of the rsda gene in E. coli.
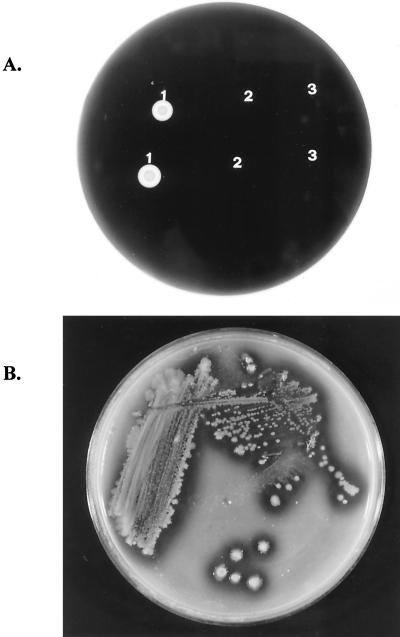
When grown on an LB agar plate containing soluble starch, the transformant E. coli (pARMH) showed a clear zone around the colony after iodine treatment. This was not observed for E. coli DH5α or E. coli DH5α(pAcUW21). The clear zone also appeared when the transformant was grown on medium containing raw starch (Fig. 1). These results indicate that the host cells harboring the rsda gene from the Cytophaga sp. expressed the gene product, RSDA, with bioactivity.
FIG. 1.
(A) Iodine staining of E. coli grown on an LB agar plate containing soluble starch. 1, E. coli (pARMH); 2, E. coli (pAcUW21); 3, E. coli DH5α, the host cells. (B) Growth of E. coli (pARMH) on an LB agar plate containing raw corn starch.
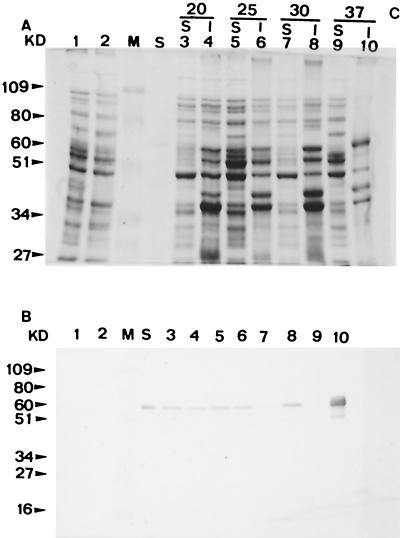
After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and immunoblotting of the cell lysate of E. coli (pARMH), a positive band appeared corresponding to the location of purified RSDA from the Cytophaga sp. Furthermore, the transformant was found to express the cloned gene product intracellularly by the method described in QIAexpress (QIAexpress, p. 40-45, Qiagen Inc. Press, Hilden, Germany, 1992) (data not shown). As mentioned above, the inserted DNA contained a signal peptide sequence, and RSDA is an extracellular enzyme in the Cytophaga sp. It was observed that most RSDA was produced in an insoluble form when E. coli (pARMH) was cultured at 37°C. The amount of soluble form increased as the bacteria were incubated at lower temperatures (Fig. 2). Production of insoluble protein products is often observed when genes are overexpressed in E. coli (10).
FIG. 2.
Effect of incubation temperature on the forms of RSDA expressed from E. coli (pARMH) as shown by Coomassie brilliant blue staining (A) and Western blotting (B). S, cytoplasmic soluble fraction; I, cytoplasmic insoluble fraction. Lanes M, low-molecular-weight standard; lanes S, purified RSDA from the Cytophaga sp.; lanes 1 and 2, cell lysates of E. coli DH5α and E. coli DH5α(pAcUW21), respectively; lanes 3, 5, 7, and 9, soluble fractions of the E. coli DH5α(pARMH) cell lysate at the indicated temperatures (in degrees Celsius); lanes 4, 6, 8, and 10, insoluble fractions of the E. coli (pARMH) cell lysate at the indicated temperatures.
Activity staining on a native PAGE gel of the cell lysate of E. coli (pARMH) resulted in a clear band on the top of the running gel at a location corresponding to that of RSDA purified from the Cytophaga sp.; the pI of RSDA revealed by isoelectric focusing was 8.5. The pI of the deduced amino acid sequence, as calculated with PC/GENE and SeqWbe 2001 (Accelrys, Inc.), was 5.5. This difference could have been due to surface charge changes caused by the folding of the polypeptide chain.
Biochemical properties of the recombinant RSDA from E. coli.
The raw-starch-digesting activity was assayed by using corn starch as a substrate according to the method of Itakor et al. (8) with slight modification. One unit of RSDA activity was defined as the amount of enzyme that produced reducing sugar equivalent to 1 mg of glucose per h. Two hundred forty units of RSDA activity were detected in the supernatant of the cell extract of a 1-ml culture. The yield of enzyme was similar to that of the Cytophaga sp. in properly designed cultural medium.
The purified enzyme displayed a single protein band on sodium dodecyl sulfate-PAGE gel stained with Coomassie brilliant blue R250 (data not shown). The NH2-terminal sequence of the purified enzyme was analyzed as AATNG, identical to the first five amino acids of the Cytophaga sp. RSDA.
The effects of pH on RSDA activity were determined for pH values from 3 to 10. The pH of the reaction solution was adjusted with 100 mM universal buffer (15) from pH 3 to 10 at intervals of 0.5. The profiles of the effect of pH on the authentic and recombinant RSDA toward soluble starch were similar. The results indicate that both enzymes have a broad range of pH optima, i.e., approximately pH 4 to 6.5 and 3.5 to 6, respectively. RSDA from E. coli (pARMH) shifts to the acidic region by a pH value of 0.5. To measure the effect of temperature on RSDA from both sources, the enzyme was assayed with soluble starch from ∼20 to 80°C at 5°C intervals. To measure the temperature stability of these two proteins, the enzyme solution was incubated from ∼20 to 80°C at 5°C intervals for 1 h before residual soluble-starch-digesting activity was measured. A temperature optimum of 50°C was observed for both authentic and recombinant RSDA. Incubation at 55°C for 1 h left less than 10% activity for both RSDA.
All of these experimental results point out that RSDA expressed in E. coli (pARMH) possessed the properties of the authentic enzyme. However, minor conformational changes in three-dimensional structure might have caused some variations in the pKa value of functional groups in the active site.
Conclusions.
The rsda gene of a Cytophaga sp. was cloned and expressed in E. coli. The enzyme was produced intracellularly by the transformant, although the signal peptide had been digested as in the Cytophaga sp. A larger amount of the mature soluble form of RSDA was produced when E. coli was cultured at lower temperatures. The transformant produced RSDA by using the promoter located in the inserted DNA. Cloning and expressing the rsda gene of the Cytophaga sp. in E. coli could facilitate further investigation of the relationship between the structure and function of this raw-starch-digesting amylase; transferring the rsda gene of a soil bacterium to microorganisms that are generally regarded as safe, such as B. subtilis and/or Lactobacillus sp., might find new applications of the gene product in the food and feed industry.
Nucleotide sequence accession number.
The nucleotide sequence of the rsda gene has been deposited in the GenBank database under accession no. AF067653.
REFERENCES
- 1.Bigelis, R. 1993. Carbohydrases, p. 124-125. In T. Nagodawithana and G. Reed (ed.), Enzymes in food processing, 3rd ed. Academic Press, Inc., San Diego, Calif.
- 2.Chiou, S. Y., and C. L. Jeang. 1995. Factor affecting production of raw-starch-digesting amylase by the soil bacterium Cytophaga sp. Biotechnol. Appl. Biochem. 22:377-384. [Google Scholar]
- 3.Hamilton, L. M., C. T. Kelly, and W. M. Fogarty. 1999. Purification and properties of the raw starch degrading α-amylase of Bacillus sp. IMD 435. Proc. Biotechnol. 35:27-33. [Google Scholar]
- 4.Hamilton, L. M., C. T. Kelly, and W. M. Fogarty. 1999. Purification and properties of the raw starch degrading α-amylase of Bacillus sp. IMD 434. Biotechnol. Lett. 21:111-115. [Google Scholar]
- 5.Hayashida, S., K. Nakahara, K. Kuroda, T. Miyata, and S. Iwanaga. 1989. Structure of raw-starch affinity site on the Aspergillus awamori var. kawachi glucoamylase I molecule. Agric. Biol. Chem. 53:135-141. [Google Scholar]
- 6.Hayashida, S., Y. Teramoto, T. Inoue, and S. Mitsuiki. 1990. Occurrence of an affinity site apart from the active site on the raw-starch-digesting but non-raw-starch-adsorbable Bacillus subtilis 65 α-amylase. Appl. Environ. Microbiol. 56:2584-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helbert, W., M. Schulein, and B. Henrissat. 1996. Electron microscopic investigation of the diffusion of Bacillus licheniformis α-amylase into corn starch granules. Int. J. Biol. Macromol. 19:165-169. [DOI] [PubMed] [Google Scholar]
- 8.Itakor, P., O. Shida, and N. Tsukadoshi. 1989. Screening for raw starch digesting bacteria. Agric. Biol. Chem. 53:53-60. [Google Scholar]
- 9.Jeang, C. L., Y. H. Lee, and L. W. Chang. 1995. Purification and characterization of a raw-starch digesting amylase from a soil bacterium-Cytophaga sp. Biochem. Mol. Biol. Int. 35:549-557. [PubMed] [Google Scholar]
- 10.Jeang, C. L., C. H. Wung, B. Y. Chang, S. H. Yen, and D. W. Lour. 1999. Characterization of the Bacillus macerans cyclodextrin glucanotransgerase overexpressed in Escherichia coli. Proc. Natl. Sci. Counc. R.O.C. B 23:62-68. [PubMed] [Google Scholar]
- 11.Konishi, H., T. Sato, H. Yamagata, and S. Udaka. 1990. Efficient production of human α-amylase by a Bacillus brevis mutant. Appl. Microbiol. Biotechnol. 34:297-302. [DOI] [PubMed] [Google Scholar]
- 12.Lee, J. T., H. Kanai, T. Kobayashi, T. Akiba, and T. Kudo. 1996. Cloning, nucleotide sequence and hyper-expression of α-amylase gene from an archaeon, Thermococcus profundus. J. Ferment. Bioeng. 82:432-438. [Google Scholar]
- 13.Mamo, G., and A. Gessesse. 1999. Purification and characterization of two raw-starch-digesting thermostable α-amylases from a thermophilic Bacillus. Enzyme Microb. Technol. 25:433-438. [Google Scholar]
- 14.Matsuura, Y., H. Kusunoki, W. Harada, and M. Kakuda. 1984. Structure and possible catalytic residues of Taka amylase. J. Biochem. (Tokyo) 95:695-702. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie, H. A. 1969. pH and buffers, p. 485. In R. M. C. Dawson, D. C. Elliott, W. H. Elliott, and K. M. Jones (ed.), Data for biochemical research, 2nd ed. Oxford University Press, Bristol, United Kingdom.
- 16.Mikami, B., E. D. Hegre, M. Sata, Y. Katsube, M. Hirose, Y. Morita, and J. C. Sacchettini. 1993. The 2.0 Å resolution structure of soybean α-amylase complex with α-cyclodextrin. Biochemistry 32:6836-6845. [DOI] [PubMed] [Google Scholar]
- 17.Mikuni, K., M. Monma, and K. Kainuma. 1987. Alcohol fermentation of corn starch digesting by Chalara paradoxa amylase without cooking. Biotechnol. Bioeng. 29:729-732. [DOI] [PubMed] [Google Scholar]
- 18.Saito, H., and L. Miura. 1963. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim. Biophys. Acta 72:619-626. [PubMed] [Google Scholar]
- 19.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain termination inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sogaard, M., J. S. Anderson, P. Roepstorff, and B. Svensson. 1993. Electrospray mass spectrometry characterization of post-translational modification of barley α-amylase 1 produced in yeast. Bio/Technology 11:1162-1165. [DOI] [PubMed] [Google Scholar]
- 21.Steers, E., Jr., G. R. Craven, and C. B. Anifinsen. 1965. Evidence for nonidentical chains in the beta-galactosidase of Escherichia coli K12. J. Biol. Chem. 240:2478-2484. [PubMed] [Google Scholar]
- 22.Studier, F. W. 1989. A strategy for high-volume sequencing of cosmid DNAs: random and directed priming with a library of oligonucleotides. Proc. Natl. Acad. Sci. USA 86:6917-6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson, B., and M. Sogaard. 1992. Protein engineering of amylases. Biochem. Soc. Trans. 20:34-42. [DOI] [PubMed] [Google Scholar]