Abstract
The aim of this work was to modify the cell surface properties of Saccharomyces cerevisiae by expression of the HFBI hydrophobin of the filamentous fungus Trichoderma reesei on the yeast cell surface. The second aim was to study the immobilization capacity of the modified cells. Fusion to the Flo1p flocculin was used to target the HFBI moiety to the cell wall. Determination of cell surface characteristics with contact angle and zeta potential measurements indicated that HFBI-producing cells are more apolar and slightly less negatively charged than the parent cells. Adsorption of the yeast cells to different commercial supports was studied. A twofold increase in the binding affinity of the hydrophobin-producing yeast to hydrophobic silicone-based materials was observed, while no improvement in the interaction with hydrophilic carriers could be seen compared to that of the parent cells. Hydrophobic interactions between the yeast cells and the support are suggested to play a major role in attachment. Also, a slight increase in the initial adsorption rate of the hydrophobin yeast was observed. Furthermore, due to the engineered cell surface, hydrophobin-producing yeast cells were efficiently separated in an aqueous two-phase system by using a nonionic polyoxyethylene detergent, C12-18EO5.
Humans have long utilized the tendency of many microorganisms in nature to bind to solid surfaces in a number of biotechnological processes. For example, metabolites, such as amino acids and ethanol, have been produced on an industrial scale by using immobilized microbial cells. In the brewing industry, a continuous immobilized process is used side by side with traditional lagering, and primary beer fermentation with immobilized yeast reactors is under development. The idea behind immobilization is principally to improve the volumetric efficiency, shorten the process time, and achieve more efficient conversion of substrate into product instead of unnecessary biomass. Several factors are known to influence the attachment of microbial cells to solid carrier materials, although the direct interactions are still largely unknown. The chemical composition of the yeast cell surface, the age and the growth phase, and the surface charge and hydrophobicity have a strong impact on the attachment properties of the cell. Other important factors are the physicochemical properties of the support surface and the pH and ionic strength of the medium (11). Attachment of cells to carriers can be enhanced by the expression of molecules that modify cell wall properties.
Cell surface expression of proteins has been exploited in various ways, including the display of peptide or antibody libraries for rapid screening and engineering of ligands and binders or of enzyme activities and improvement of the immobilization capacity of cells. Native surface proteins may be used to target the protein of interest to the cell surface, and carrier proteins such as agglutinins and flocculin have generally been used for yeast (for reviews, see references 14 and 20). Among others, several glycosyl hydrolases, lipase, and antibody fragments have been expressed on the surface of yeast as α-agglutinin fusions (20; L. G. J. Frenken, P. de Geus, F. M. Klis, H. Y. Toschka, and C. T. Verrips, 1994, Int. Pat. Appl. WO 94/18330, and F. M. Klis, M. P. Schreuder, H. Y. Toschka, and C. T. Verrips, 1994, Int. Pat. Appl. WO 94/01567). Surface display with a flocculin anchor is, however, less well documented (Frenken et al., 1994, Int. Pat. Appl. WO 94/18330). The yeast flocculin protein, encoded by the dominant FLO1 gene (24), is involved in the cell-cell adhesion process generally known as flocculation, which consists of the aggregation of dispersed yeast cells into large clumps. The Flo1 protein is a large protein of over 150 kDa composed of a multimodular N-terminal lectin-like domain separated from the C-terminal part by a 1,100-amino-acid-long, highly repetitive and O-glycosylated stem-like structure (24). This suggests that the Flo1 protein is anchored in the plasma membrane via the C-terminal membrane-spanning region while the glycosylated stem takes at least a partly extended conformation, thus enabling the lectin domain to be exposed on the cell surface.
In this study, a variant of Flo1 protein containing 1,259 C-terminal amino acids was used as an anchor to express a hydrophobin protein, HFBI, from the filamentous fungus Trichoderma reesei (10) on the yeast cell surface. Hydrophobins are small amphipathic proteins found uniquely in filamentous fungi that show interesting physicochemical properties, such as surface activity and self-assembly (25, 27). Hydrophobins have been shown to adhere efficiently and stably to both natural and artificial surfaces. For example, SC3 hydrophobin coating Teflon can withstand treatment with the boiling detergent sodium dodecyl sulfate (29). Upon assembly on the surface, an amphipathic protein layer is formed that changes the wettability of the solid from hydrophobic to hydrophilic and vice versa (28, 29). On account of the specific properties of hydrophobins, several application possibilities have been suggested, ranging from protein immobilization and surface modification to their use as emulsifiers in food processing (13, 26). The idea of expressing hydrophobins on cell walls to enhance cell immobilization is challenging. Accordingly, the aim of this study was to improve the attachment capacity of Saccharomyces cerevisiae by hydrophobin surface display.
MATERIALS AND METHODS
Yeast strains and culture conditions.
The yeast strain H452 (a suc2 ade2-1 can1-100 his3-11 his3-15 leu2-3 leu2-112 trp1-1 ura3-1 kil−; wild type, W303-1A) (19), which was used as parental strain, was obtained from Hans Ronne, University of Uppsala, Uppsala, Sweden. The control strain H2155 (plasmid pYES2) and strains VTT-C-99315 (plasmid pTNS23) and H2749 (plasmid pTNS40), which produce HFBI-Flo1 fusion and truncated Flo1 proteins, respectively, are transformant strains originating from H452.
Yeast cells were grown either in YPD (1% yeast extract, 2% peptone, 2% glucose) or on synthetic complete medium (SC) (15). For plasmid selection and maintenance, SC-Ura medium lacking uracil was used. SC-Ura-Met medium lacking uracil and methionine was used for in vivo labeling of yeast cells. SC was complemented with either 2% galactose (SCG) or 2% glucose (SCD). The yeast cells were grown at 30°C to an A600 of 3 to 4 for cell surface characterization and binding assays.
Construction of yeast strains expressing HFBI-FloI fusion protein and truncated Flo1p on the cell surface.
For construction of an HFBI-Flo1 fusion protein expression cassette, the region coding for mature HFBI (from Ser-23 to the stop codon) of T. reesei was amplified by PCR with pARO1 (10) as a template, 5′ TCT AGC TCT AGA AGC AAC GGC AAC GGC AAT GTT 3′ as a 5′ primer, and 5′ TGC TAG TCG ACC TGC TAG CAG CAC CGA CGG CGG TCT G 3′ as a 3′ primer. The PCR fragment was ligated to pGEM-T vector (Promega), resulting in pTNS10. The hfb1 fragment was released from pTNS10 with XbaI and NheI and ligated to pTNS15 cut with the same restriction enzymes to remove the Flo1 lectin domain. Plasmid pTNS15 was essentially the same as pBR-ADH1-FLO1L (24), which contains the FLO1 gene in between the ADH1 promoter and terminator, except that a NheI site in the pBR322 backbone was replaced by a BglII site and a unique XbaI site was introduced at the unique AocI site preceding the putative signal sequence cleavage site. The resulting plasmid pTNS18 contained the complete expression cassette for HFBI-Flo1 fusion protein, in which HFBI replaced the putative lectin domain from Ser-26 to Ser-319 in the yeast flocculin Flo1.
In the next step, a pYES (Invitrogen)-based yeast expression vector for production of HFBI-Flo1 fusion protein was constructed. pYES2 carries GAL1 promoter and CYC1 terminator sequences which regulate transcription. The plasmid pTNS18 was digested with HindIII, and the released 3.95-kb fragment containing the expression cassette for HFBI-Flo1 was ligated to pYES2 digested with HindIII. This ligation mixture was concentrated by standard ethanol precipitation. In addition to unligated fragments and uncorrected ligation products, the ligation mixture should also contain molecules where the vector and insert are correctly ligated with each other to result in plasmid pTNS23, which carries the expression cassette for HFBI-Flo1 operably linked to GAL1 and CYC1 terminator sequences.
The cloning of the expression cassette into the GAL1 promoter containing yeast expression vector was unsuccessful in Escherichia coli. To circumvent this, the ligation mixture described above was transformed by using the LiAc method of Gietz et al. (5) on a laboratory S. cerevisiae strain, H452 (wild type, W303-1A) (19). Transformant colonies able to grow on SCD-Ura plates were picked and streaked on selective plates. Nitrocellulose replicas were taken from the plates and treated for colony hybridization according to the method of Sherman et al. (15). To find those yeast colonies containing the pTNS23 plasmid, replicas were hybridized with a digoxigenin-labeled hfb1 coding fragment, after which an immunological detection was performed according to the manufacturer's instructions (Boehringer Mannheim). Plasmids were recovered from several yeast colonies giving positive hybridization signals by isolating total DNA and using this in electroporation of E. coli. Restriction mapping and sequencing were carried out to confirm that the pTNS23 plasmid in the yeast transformants was correct. One of the transformants carrying plasmid pTNS23 was chosen for further studies and was designated VTT-C-99315. The control strain for this transformant is yeast strain H2155, which carries the plasmid pYES2 in an H452 background.
A yeast strain expressing the truncated Flo1p that was missing the amino acids from Ser-26 to Ser-319 was constructed by removing the corresponding codons by digestion of pTNS15 with XbaI and NheI followed by religation of the vector. The truncated Flo1 cassette was isolated after digestion with HindIII and cloned at the HindIII site of pYES2. The resulting plasmid pTNS40 was transformed by using the LiAc method of Gietz et al. (5) into strain H452 (wild type, W303-1A) (19).
Cell surface properties.
The surface tension of the yeast surface was determined by sessile drop contact angle measurements on yeast cell lawns prepared as described by Azeredo et al. (2). The measurements were carried out at room temperature with water, diiodomethane, and glycerol as reference liquids. Determination of contact angles was performed automatically with the aid of an image analysis system (Kruss-GmbH, Hamburg, Germany). The images were received by a video camera connected to a 486 DX4 100-MHz personal computer with an automatic measuring system (G2/G40). At least 20 measurements were taken for each liquid. The total surface tension values (γtot) and the relative contributions of Lifshits-van der Waals (LW) and the electron donor (γ−) and electron acceptor (γ+) parameters of the Lewis acid-base (AB) interactions were calculated by using the method of van Oss et al. (22).
For the zeta potential determinations, yeasts were cultivated in SCG-Ura medium. The cells were harvested by centrifugation (5,000 × g, 5 min) and washed two times in phosphate-buffered saline (PBS) (pH 7.0; 10 mM). Finally, the cells were resuspended to a final concentration with an A600 of 1 in PBS at different values of pH adjusted by adding HCl or NaOH. The zeta potential of the yeast cells was measured with a Zeta-Meter 3.0+.
Separation of yeast cells in ATPS.
Approximately 1.1 × 108 to 6.3 × 108 yeast cells in SCG culture medium were used to prepare the aqueous two-phase system (ATPS) with 7% (wt/vol) of the nonionic polyoxyethylene detergent C12-18EO5 (a gift from Henkel GmbH, Düsseldorf, Germany) in a total volume of 5 ml. After thorough mixing in an overhead shaker, the separation took place by gravity settling in a water bath at 30°C. After phase separation, samples were taken from the top detergent phases, and dilution series from 10−1 to 10−5 were prepared in 0.9% NaCl and plated on YPD plates. After incubation, the amount of yeast colonies was calculated.
Carrier materials.
Porous silicate glass beads (Siran) with granule and pore sizes of 2 to 3 mm and 60 to 300 μm, respectively, were obtained from Schott Glaswerke, Mainz, Germany. Siran carrier was made more hydrophobic by overnight treatment with trimethylchlorosilane (Sigma T-4252), whereafter excess silane was removed by washing with sterile water. Porous (pore size, 50 by 150 μm) ImmobaSil (FS) carrier (disks of 0.8 by 0.25 mm) of virgin USP class VI silicone was obtained from Ashby Scientific Ltd., Coalville, United Kingdom.
The surface tension of ImmobaSil was determined by contact angle measurements with water formamide and α-bromonaphthalene, performed as described above. The surface tensions of the other carriers were measured by means of the thin-layer wicking technique with decane, water, and formamide according to the methodology described elsewhere (17).
The zeta potentials of the carriers were measured by using fine particles of crushed materials immersed in PBS at different pH values. The measurements were performed with a Zeta-Meter 3.0+.
In vivo labeling of yeast cells.
The yeasts cultivated on SCG-Ura medium were harvested and washed with SCG-Ura-Met medium. Washed cells were resuspended to the initial volume with washing medium, and after 30 to 60 min, 50 to 80 μCi of [35S]methionine was added to each shake flask. The cells were incubated at 30°C for 60 min with shaking at 250 rpm. After labeling, the cells were washed with SCG-Ura and finally resuspended in the same medium to obtain a stock cell suspension of approximately 2 × 108 to 5 × 108 cells/ml. Dilutions of this cell suspension were used in the binding assays.
Binding assays with labeled yeast cells.
Binding assays with [35S]methionine-labeled yeast cells were carried out in tightly capped 2-ml Luer lock syringes containing 1 ml of yeast cell suspension in SCG-Ura medium and approximately 0.5 g of carrier. The number of yeast cells used in the various experiments varied between 104 and 108, and contact times varied between 10 min and 16 h. Binding was performed with overhead shaking at either 4 or 22°C. After the contact period, the syringes were emptied through a 100-μm-pore-size mesh filter unit (Millipore). The carrier remaining in the syringe was washed once with 1 ml of fresh binding medium, and the two filtrates were combined. The radioactivity of the filtrates (10-μl aliquots) and of the washed carriers taken out from the filter units was measured with a liquid scintillation counter. A competition assay was carried out similarly, except that 0.5 mg of pure HFBI hydrophobin was added to the yeast suspension of 107 cells, binding was carried out at 22°C, and contact times of 30 min and 2 h were used.
RESULTS
Yeast producing HFBI-Flo1 fusion.
To express hydrophobin on the yeast cell surface, the HFBI hydrophobin of the cellulolytic filamentous fungus T. reesei was fused with the yeast flocculin gene FLO1. The expression cassette for the fusion contained the native putative Flo1 signal sequence for secretion. The codons following the Flo1 signal peptide from Ser-26 to Ser-319, which encode the lectin domain and precede the highly repetitive glycosylated part of the flocculin, were replaced by the HFBI hydrophobin. The rest of the protein was unaltered, containing, e.g., the carboxy-terminal plasma membrane anchor. In addition, a strain was constructed which expressed a truncated form of Flo1p.
The retarded growth of the hydrophobin-producing yeast VTT-C-99315 on inducing medium containing galactose compared to that of the control strain H2155 harboring only the pYES2 plasmid indicated that the fusion protein was expressed. Both strains grew similarly on repressing glucose medium (data not shown). Expression and correct localization of the HFBI fusion protein on the cell surface were further verified by determining the surface characteristics of each yeast strain (see below). Expression of the hydrophobin fusion did not seem to alter the morphology of the yeast cells, nor did it cause abnormal cell aggregation (data not shown).
Surface characteristics of yeast cells.
To verify whether the expression of the HFBI hydrophobin on the yeast cell surface induces alterations in the cell wall physicochemical surface properties, the surface characteristics (surface tension, charge, and hydrophobicity) of yeast strains were determined.
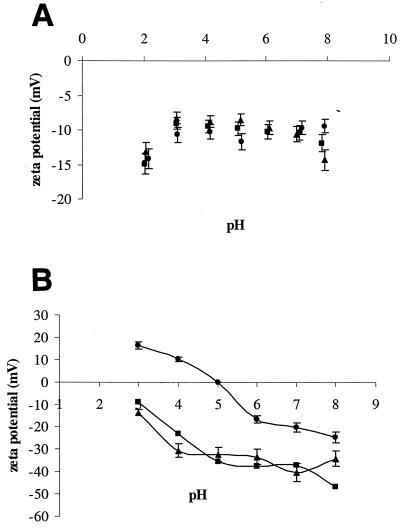
The surface charges of the three strains were evaluated by means of zeta potential values. These strains were negatively charged in the pH range assayed (Fig. 1A). Although they exhibited a similar profile of zeta potential versus pH, the hydrophobin-producing strain was slightly less negative than the control strain that did not express Flo1.
FIG. 1.
Values of the zeta potential as a function of pH. (A) The control strains H2155 (▪) and H2749 (•) and the strain VTT-C-99315 expressing HFBI-Flo1 fusion protein (▴); (B) the carrier materials unmodified Siran (▴), siliconized Siran (▪), and ImmobaSil (•).
The contact angles with three different liquids and the respective standard deviations are presented in Table 1. These values reveal that the hydrophobin-producing yeast has more affinity for the apolar liquid (lower contact angle with α-bromonaphthalene). This means that the surface of the hydrophobin-producing yeast is more apolar than the surface of the control strains. This is in accordance with the values of the apolar component (γLW) of the surface tension of the strains (Table 1).
TABLE 1.
Contact angles, surface tension, and hydrophobicity of yeast strains
| Yeast straina | Contact angle (°)
|
Surface tensionb
|
Hydrophobicity (ΔGiwi) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Glycerol | α-Bromonaphthalene | γLW | γ+ | γ− | γAB | γtot | ||
| H2155 | 20.7 ± 2.0 | 21.4 ± 1.8 | 45.5 ± 3.1 | 32.1 | 4.2 | 44.4 | 27.3 | 59.4 | 17.3 |
| H2749 | 30.0 ± 1.9 | 38.5 ± 2.2 | 41.9 ± 1.8 | 33.8 | 1.6 | 46.2 | 17.4 | 51.2 | 23.7 |
| VTT-C-99315 | 32.8 ± 2.1 | 27.2 ± 1.9 | 34.9 ± 2.1 | 36.7 | 3.1 | 34.7 | 20.7 | 57.4 | 7.2 |
H2155, control strain; H2749, truncated-Flo1p-expressing strain; VTT-C-99315, HFBI-Flo1-expressing strain.
Surface tension parameters include the apolar component (γLW) and the electron donor (γ−) and electron acceptor (γ+) parameters of the polar component (γAB) of the surface tension (γtot).
The hydrophobicity of the yeast strains was calculated with the surface tension values by using the method proposed by van Oss and Giese (23). According to these authors, the hydrophobicity of an entity (i) can be evaluated as the free energy of interaction between two such entities when immersed in water (w), represented as ΔGiwi. This expresses the degree to which the attraction of the solid molecules to water is greater (hydrophilicity) or smaller (hydrophobicity) than the attraction that water molecules have to each other. Thus, when the global free energy of interaction between the molecules of the solid surfaces immersed in water is repulsive (ΔGiwi > 0), the entity is considered hydrophilic. On the contrary, the more negative ΔGiwi is, the higher the hydrophobicity. According to this notation, the three strains were hydrophilic. However, the hydrophobin-producing yeast was much less hydrophilic than the control ones (Table 1). The control strain expressing the truncated Flo1 protein (without the HFBI or lectin part) was more hydrophilic than the other control, confirming the determinant role of the HFBI part on cell surface hydrophobicity and thus verifying that the HFBI-Flo1p was expressed and correctly localized on the cell surface. The higher hydrophilicity of the cells expressing truncated Flo1p is probably due to the fact that the region following the lectin domain is heavily glycosylated. If this part is exposed on the surface, then it confers a higher degree of hydrophilicity to the cell wall. Increased hydrophilicity is linked to an increase in cell wall protein glycosylation (9). This strain, on account of its higher hydrophilicity, had a smaller adhesion capability. Therefore, strain H2155 was used as a control in further experiments.
Separation of yeast cells in ATPS.
Cells can be separated by partitioning between two immiscible phases composed of aqueous polymer solutions (1). By this method, particles are separated according to their surface properties. Characterization of the HFBI-Flo1-producing yeast strain showed increased hydrophobicity of the cells, and we tested whether these cells partition to the C12-18EO5 detergent-rich phase in cloud point extraction. After phase separation by gravity settling, the top detergent phase was evidently turbid in the case of strain VTT-C-99315, indicating that the yeast cells had been partitioned from the aqueous phase to the detergent phase. The top detergent phase of the control strain was clear. To determine the separation of the cells, aliquots were taken from both phases and plated on YPD plates. Between 70 and 230 times more colonies of strain VTT-C-99315 were counted on the plates after incubation than for the control strain. The biggest differences between the strains were obtained with a small amount of cells. Thus, expression of the HFBI hydrophobin and its localization on the yeast cell surface clearly modify the surface properties of the cell in such a way that the yeast cells may be separated in ATPS by using detergents such as polyoxyethylenes. These results are in agreement with the information obtained with contact angles.
Attachment studies.
The surface characteristics of the supports used in the binding assays are presented in Table 2 and Fig. 1B. The data in Table 2 show that all of the carriers were hydrophilic (positive ΔGiwi values) with the exception of ImmobaSil, which was hydrophobic (negative ΔGiwi). All of the carrier materials tested were negatively charged at pH values above 5. Siran and siliconized Siran were negatively charged at pH values below 5, as well. ImmobaSil became positively charged at pH values of <5, i.e., under the conditions used for the binding assays and relevant also for, e.g., the brewing of beer.
TABLE 2.
Surface tension and hydrophobicity of the different carrier materials
| Support | Surface tensiona
|
Hydro- phobicity (ΔGiwi) | ||||
|---|---|---|---|---|---|---|
| γLW | γ+ | γ− | γAB | γtot | ||
| Siran | 56.1 | 0.0 | 138.6 | 0.0 | 56.1 | 119.8 |
| Siliconized Siran | 43.6 | 2.3 | 49.8 | 0.0 | 43.6 | 20.9 |
| ImmobaSil | 33.8 | 0.0 | 14.0 | 0.0 | 33.8 | −29.0 |
| ImmobaSil plus HFBI (30 min) | 30.7 | 0.0 | 18.1 | 0.0 | 30.7 | −17.5 |
| ImmobaSil plus HFBI (120 min) | 34.2 | 0.0 | 17.7 | 0.0 | 34.2 | −21.1 |
Surface tension parameters include the apolar component (γLW) and electron donor (γ−) and electron acceptor (γ+) parameters of the polar component (γAB) of the surface tension (γtot).
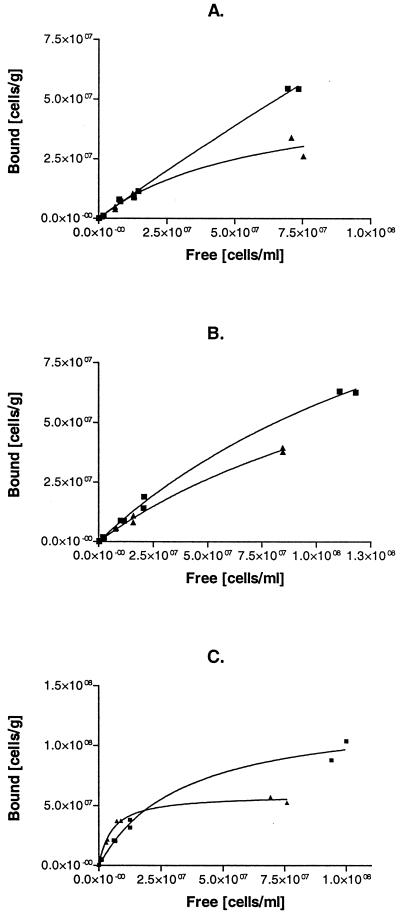
By using different concentrations of cells in the binding assays, it was possible to obtain the adsorption isotherms for each type of carrier, as presented in Fig. 2. Different contact times were also assayed. No significant differences between the strains were observed when a long contact time (16 h) was used (data not shown). With a shorter, 2-h contact time, improved adsorption on ImmobaSil was clearly demonstrated for the hydrophobin yeast strain at a relatively low cell concentration, whereas in systems overloaded with cells, the control yeast strain seemed to attach more efficiently (Fig. 2). Similar effects could not be obtained when siliconized or unmodified Siran beads were used as supports. In this case, the control yeast strain displayed a higher ability for attachment than the hydrophobin yeast strain for all of the concentrations tested (Fig. 2).
FIG. 2.
Adsorption isotherms of cells on different supports: Siran (A), siliconized Siran (B), and ImmobaSil (C). Results for the control strain H2155 (▪) and strain VTT-C-99315 expressing HFBI-Flo1 fusion protein (▴) are shown. The treatment time was 2 h at 4°C.
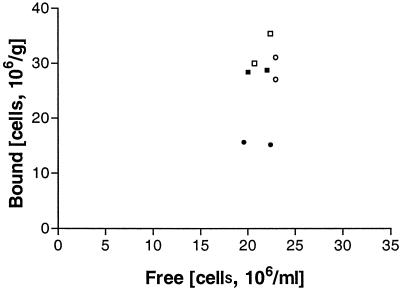
When pure HFBI protein was added to the binding assay and a treatment time of 30 min was used, adsorption of both the control and the hydrophobin yeast cells on ImmobaSil was decreased (Fig. 3). Table 2 presents the surface characteristics of untreated and HFBI-treated ImmobaSil and indicates that the hydrophobin can adsorb to the carrier material, modifying its hydrophobicity. The support became less hydrophobic when treated with HFBI, and it appears that the hydrophobicity of the carrier was reduced less with a longer treatment time.
FIG. 3.
Binding of the HFBI-Flo1-expressing strain VTT-C-99315 onto ImmobaSil with (filled symbols) and without (open symbols) the presence of purified HFBI protein at 4°C with a treatment time of 30 min (circles) or 120 min (squares).
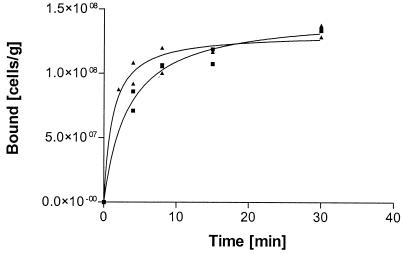
Results presented earlier in the text indicated that there might be differences in the binding rates of the two yeast strains. To study this, an experiment testing the kinetic binding to ImmobaSil was carried out in which a single concentration of cells was used (Fig. 4). The hydrophobin-producing yeast strain attached more significantly at early times up to 8 min, whereafter no differences between the two strains could be observed. This further confirms that hydrophobin protein on the yeast cell surface introduces extra binding capacity to the cell under suboptimal conditions, i.e., low cell concentration and short reaction time.
FIG. 4.
Adsorption of the control strain H2155 (▪) and the HFBI-Flo1-expressing strain VTT-C-99315 (▴) on ImmobaSil as a function of time.
The differences in the binding affinities of the yeast strains were quantified by calculating partition coefficients (Kp), considering cell attachment as an adsorption process (Table 3). The Kp is defined as the slope of the straight line through the first data points of an isotherm and describes the partitioning of the yeast cells between the liquid phase and the support at low surface coverage. As seen in Table 3, the initial slopes (K) of the isotherms show a twofold increase in the level of hydrophobin yeast binding to ImmobaSil compared with that of the control yeast. For the two glass-based supports (Siran and siliconized Siran), there were no significant differences in the adsorption of the two yeast strains.
TABLE 3.
Partition coefficients of the control strain H2155 and strain VTT-C-99315a
| Support | KH2155 | KVTT-C-99315 |
|---|---|---|
| Siran | 0.90 | 0.76 |
| Siliconized Siran | 0.80 | 0.62 |
| ImmobaSil | 3.4 | 6.6 |
The values for the partition coefficients (K) at 95% confidence level were obtained from the initial slopes of the curves shown in Fig. 2.
To be able to calculate true association and dissociation coefficients (Ka and Kd) for adsorption, the maximal amount of bound cells (Bmax) should be known (4, 6). The data presented in Fig. 2 do not, however, allow the determination of Bmax, due to a lack of points at higher cell densities. When the binding isotherms shown in Fig. 2, especially that of the ImmobaSil support, are looked at more closely, it can be approximated that Bmax of the control yeast on ImmobaSil is higher than that of the hydrophobin yeast. Since the dissociation coefficient, Kd, is directly dependent on Bmax as defined by the equation Kp = Bmax/Kd, it could be assumed that Kd is higher for the control yeast than for the hydrophobin yeast. Thus, the association constant, the inverse of Kd, is higher for the hydrophobin yeast.
The values of the free energy of adhesion of the yeast strains on the different supports in water are presented in Table 4. The thermodynamic approach considers that a negative ΔG value indicates favorable adhesion of yeast cells while a positive value indicates that adhesion is unfavorable. The lowest negative value was obtained for the hydrophobin yeast strain on unmodified ImmobaSil, followed by the values obtained for the interaction with hydrophobin-modified ImmobaSil, while adhesion of the hydrophobin yeast strain on Siran was thermodynamically unfavorable. The attachment of the control yeast strain was in all cases less favorable than that of the hydrophobin yeast strain. The calculated thermodynamic values are in accordance with the experimental results.
TABLE 4.
Values of the free energy of adhesion of the control strain H2155 and strain VTT-C-99315
| Yeast strain | ΔGadhesion (mJ/m2)
|
||||
|---|---|---|---|---|---|
| Siran | Siliconized Siran | ImmobaSil | ImmobaSil plus HFBI (30 min) | ImmobaSil plus HFBI (120 min) | |
| H2155 | 50.9 | 22.1 | 6.2 | 9.8 | 8.6 |
| VTT-C-99315 | 44.7 | 12.2 | −3.2 | 0.9 | −1.0 |
DISCUSSION
In nature, hydrophobins are commonly found in emergent structures of filamentous fungi, aerial hyphae, spores, and infection structures, where they form a hydrophobic, water-repellent layer (25). The hydrophobin coating protects the fungal structures from water and drying out. The role of hydrophobins in the attachment of plant pathogenic fungi on the surfaces of the plants has been discussed. Hydrophobin-mediated fungal attachment has been reported for the nonpathogenic basidiomycete Schizophyllum commune, which has been shown to adhere to Teflon via its cell wall hydrophobin, SC3 (29). In the present study, S. cerevisiae expressing the HFBI hydrophobin of T. reesei showed an increase in surface hydrophobicity (more precisely, a decrease in cell surface hydrophilicity). The significant difference in the cell surface properties of the hydrophobin yeast was manifested in the ability of the apolar yeast cells to partition to a polyoxyethylene detergent in aqueous two-phase separation. The HFBI-expressing strain also displayed slightly lower negative surface charge, which would contribute to a decrease in the electrostatic repulsion between the cell and the negatively charged supports (Siran and siliconized Siran). However, it was the control strain that was capable of attaching to these carriers to a larger extent. This indicates that the electrostatic interactions are not determinants in this process. Many studies have highlighted the importance of the hydrophobic interactions in microbial adhesion and yeast flocculation (12, 16, 18). In this case it seems that the increase in hydrophobicity of the VTT-C-99315 strain was the driving force in the attachment to the hydrophobic support. This is reinforced by the decrease in the attachment of the hydrophobin-expressing strain to the ImmobaSil carrier, which turned less hydrophobic after being coated with pure HFBI during a contact time of 30 min. An interesting point to note is that for longer periods of contact (120 min) with the hydrophobin, the hydrophobicity of ImmobaSil increases again. This can be explained by the self-assembly of the hydrophobin on the solid surface to form bilayered structures similar to those reported for SC3 of Schizophyllum commune (21).
For each strain, the number of attached cells increases with the decrease in the Gibbs free energy of interaction, which is in accordance with the thermodynamic theory of adhesion. However, the Gibbs free energies of adhesion of different strains on one carrier cannot be fully compared. As already stated by Busscher and Weerkamp (3), the thermodynamic approach describes the adhesion of a specific bacterial strain to various solid substrata but not the behavior of various bacterial strains with respect to one substratum, because the capacity to establish short-range interactions is very much strain dependent. In this report, the results strongly suggest that the HFBI hydrophobin plays no important role in the attachment of the yeast to hydrophilic supports (Siran and siliconized Siran). All of this reasoning applies to the case where adhesion takes place by contacting the carrier with suspensions of relatively low cell concentration. As the cell concentration rises, a surface saturation is attained for ImmobaSil, whereas the other two carriers have a different behavior. In other words, for Siran and siliconized Siran, the increase in the number of bound cells with higher cell concentrations is due to the occlusion of cells within the pores of the immobilization support and not to a direct interaction with the surface.
While no differences between the two strains were observed when prolonged contact times or high cell densities were used, improved adsorption to ImmobaSil was demonstrated for the hydrophobin yeast when relatively short contact times and low cell densities were used. Partition coefficients describing the partitioning of cells between the liquid and solid phases at low surface coverage indicate a twofold higher attachment of the hydrophobin yeast compared with that of the control yeast. The benefit of hydrophobin display for yeast immobilization also became evident in the early time points of the kinetic experiment with the hydrophobic support. Both the steady-state and kinetic experiments suggest that hydrophobin display gives additional advantages in the first steps of the adsorption event, when a large hydrophobic surface area is free for a small number of cells to adsorb through hydrophobic interactions. In later stages, fewer free binding sites are available and steric hindrance and the lateral interactions between the cells become more important.
Interestingly, hydrophobin-producing yeast cells did not form more or larger cell aggregates than the parent strain, and therefore, flocculation of the cells cannot be correlated with increased adsorption. Overexpression of an intact Flo1 has previously been shown to improve the immobilization potential of brewer's yeast due to increased flocculation (7). In an earlier report, yeast cells expressing another cell wall hydrophobin, QID3 of Trichoderma harzianum, were shown to form large cell flocs due to aberrant cytokinesis and cell separation (8).
We have described here the successful expression of a fungal hydrophobin on the yeast cell surface that leads to changes in the yeast cell surface properties with no effect on flocculation. However, it remains to be seen whether the yeast expressing HFBI described here would have any real value in industrial processes based on immobilization, such as brewing. Also, hydrophobins vary in their properties, e.g., in their abilities to adhere to and modify solids and to form stable assemblages, and once more is known of the properties and differences of these interesting proteins, another hydrophobin displayed on the yeast surface may have a benefit in immobilized high-cell-density industrial applications. Of particular interest concerning the expression of the hydrophobin is the possibility of using the modified yeast strain in processes based on two immiscible phases where the hydrophobic yeast cells are preferentially retained in the more apolar phase.
Acknowledgments
We thank Seija Nordberg and Riitta Nurmi for skilled technical assistance and Sirkka Keränen for useful discussions.
This research was financed by Oy Panimolaboratorio-Bryggerilaboratorium Ab and the Technology Development Centre (Tekes) of Finland.
REFERENCES
- 1.Albertsson, P.-Å. 1986. Partition of cell particles and macromolecules, 3rd ed. Wiley, New York, N.Y.
- 2.Azeredo, J., I. Ramos, L. Rodrigues, R. Oliveira, and J. A. Teixeira. 1997. Yeast flocculation: a new method for characterising cell surface interactions. J. Inst. Brewing 103:359-361. [Google Scholar]
- 3.Busscher, H. J., and A. Weerkamp. 1987. Specific and non-specific interactions in bacteria adhesion to solid substrata. FEMS Microbiol. Rev. 46:165-173. [Google Scholar]
- 4.Cowan, M. M. 1995. Kinetic analysis of microbial adhesion. Methods Enzymol. 253:179-189. [DOI] [PubMed] [Google Scholar]
- 5.Gietz, D., A. St. Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klotz, I. M. 1989. Ligand-protein affinities, p. 25-54. In T. E. Creighton (ed.), Protein function—a practical approach. IRL Press, Oxford, United Kingdom.
- 7.Linko, M., I. Virkajärvi, N. Pohjala, K. Lindborg, J. Kronlöf, and E. Pajunen. 1997. Main fermentation with immobilized yeast—a breakthrough?, p. 385-394. In Proceedings of the European Brewery Convention Congress, Maastricht, Belgium. IRL Press, Oxford, United Kingdom.
- 8.Lora, J. M., J. de la Cruz, T. Benitez, A. Llobell, and J. A. Pintor-Toro. 1994. A putative catabolite-repressed cell wall protein from the mycoparasitic fungus Trichoderma harzianum. Mol. Gen. Genet. 242:461-466. [DOI] [PubMed] [Google Scholar]
- 9.Masuoka, J., and K. C. Hazen. 1997. Cell wall protein mannosylation determines Candida albicans cell surface hydrophobicity. Microbiology 143:3015-3021. [DOI] [PubMed] [Google Scholar]
- 10.Nakari-Setälä, T., N. Aro, N. Kalkkinen, E. Alatalo, and M. Penttilä. 1996. Genetic and biochemical characterization of the Trichoderma reesei hydrophobin HFBI. Eur. J. Biochem. 235:248-255. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira, R. 1997. Understanding adhesion: a means for preventing fouling. Exp. Therm. Fluid Sci. 14:316-322. [Google Scholar]
- 12.Panagoda, G. J., A. N. Ellepola, and L. P. Samaranayake. 1998. Adhesion to denture acrylic surfaces and relative cell surface hydrophobicity of Candida parapsilosis and Candida albicans. APMIS 106:736-742. [PubMed] [Google Scholar]
- 13.Scholtmeijer, K., J. G. H. Wessels, and H. A. B. Wösten. 2001. Fungal hydrophobins in medical and technical applications. Appl. Microbiol. Biotechnol. 56:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Schreuder, M. P., A. T. A. Mooren, H. Y. Toschka, C. T. Verrips, and F. M. Klis. 1996. Immobilizing proteins on the surface of yeast cell. Trends Biotechnol. 14:115-120. [DOI] [PubMed] [Google Scholar]
- 15.Sherman, F., G. R. Fink, and J. B. Hicks. 1983. Methods in yeast genetics. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Teixeira, J. A., R. Oliveira, J. Azeredo, M. Sousa, and C. Sil. 1995. Cell wall surface properties and flocculence of a Kluyveromyces marxianus strain. Colloids Surf. B 5:197-203. [Google Scholar]
- 17.Teixeira, P., J. Azeredo, R. Oliveira, and E. Chibowski. 1998. Interfacial interactions between nitrifying bacteria and mineral carriers in aqueous media determined by contact angle measurements and thin layer wicking technique. Colloids Surf. B 12:69-75. [Google Scholar]
- 18.Teixeira, P., and R. Oliveira. 1999. Influence of surface characteristics on adhesion of Alcaligenes denitrificans to polymeric substrates. J. Adhesion Sci. Technol. 13:1287-1294. [Google Scholar]
- 19.Thomas, B. J., and R. Rothstein. 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56:619-630. [DOI] [PubMed] [Google Scholar]
- 20.Ueda, M., and A. Tanaka. 2000. Genetic immobilization of proteins on the yeast cell surface. Biotechnol. Adv. 18:121-140. [DOI] [PubMed] [Google Scholar]
- 21.van der Vegt, W., H. C. van der Mei, H. A. B. Wösten, J. G. H. Wessels, and H. J. Busscher. 1995. A comparison of the surface activity of the fungal hydrophobin SC3p with those of other proteins. Biophys. Chem. 57:253-260. [DOI] [PubMed] [Google Scholar]
- 22.van Oss, C. J., M. K. Chaudhury, and R. J.Good. 1987. Monopolar surfaces. Adv. Colloid Interface Sci. 28:35-64. [DOI] [PubMed] [Google Scholar]
- 23.van Oss, C. J., and R. F. Giese. 1995. The hydrophilicity and hydrophobicity of clay minerals. Clay Minerals 43:474-477. [Google Scholar]
- 24.Watari, J., Y. Takata, M. Ogawa, H. Sahara, S. Koshino, M.-L. Onnela, U. Airaksinen, R. Jaatinen, M. Penttilä, and S. Keränen. 1994. Molecular cloning and analysis of the yeast flocculation gene FLO1. Yeast 10:211-225. [DOI] [PubMed] [Google Scholar]
- 25.Wessels, J. G. H. 1994. Developmental regulation of fungal cell wall formation. Annu. Rev. Phytopathol. 32:413-437. [Google Scholar]
- 26.Wessels, J. G. H. 1997. Hydrophobins: proteins that change the nature of the fungal surface. Adv. Microb. Physiol. 38:1-45. [DOI] [PubMed] [Google Scholar]
- 27.Wösten, H. A. B., and M. L. de Vocht. 2000. Hydrophobins, the fungal coat unravelled. Biochim. Biophys. Acta 1469:79-86. [DOI] [PubMed] [Google Scholar]
- 28.Wösten, H. A. B., O. M. H. de Vries, and J. G. H. Wessels. 1993. Interfacial self-assembly of a fungal hydrophobin into a hydrophobic rodlet layer. Plant Cell 5:1567-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wösten, H. A. B., F. H. J. Schuren, and J. G. H. Wessels. 1994. Interfacial self-assembly of a hydrophobin into an amphipathic protein membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 13:5848-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]