Abstract
Sensitive and specific routine detection of Ralstonia solanacearum in symptomless potato tubers was achieved by efficient enrichment followed by a reliable double-antibody sandwich indirect enzyme-linked immunosorbent assay based on the specific monoclonal antibody 8B-IVIA. This monoclonal antibody reacted with 168 typical R. solanacearum strains and did not recognize 174 other pathogenic or unidentified bacteria isolated from potato. The optimized protocol included an initial enrichment step consisting of shaking the samples in modified Wilbrink broth for 72 h at 29°C. This step enabled specific detection by the enzyme-linked immunosorbent assay of 1 to 10 CFU of R. solanacearum per ml of initial potato extract. Analysis of 233 commercial potato lots by this method provided results that coincided with the results of conventional methods.
Ralstonia solanacearum (E. F. Smith) Yabuuchi et al. (27) is the causal agent of potato bacterial wilt. It is a quarantine organism in many countries, including all of the European Union. Biovar 2 or race 3 of R. solanacearum is found mainly in temperate potato-growing areas and is the only biovar that has been described in Europe (13). Movement of seed potato stocks with symptoms or latently infected tubers is the main way that the disease spreads (11), and quarantine regulations have been introduced to avoid dissemination of the disease in the European Union (Directive 98/57 EC). Several detection methods, including isolation, indirect immunofluorescence (IIF) analysis, enzyme-linked immunosorbent assays (ELISAs), and bioassays, have been used for analysis of potato lots (5, 7, 8, 15). However, an ELISA (6) performed with polyclonal antibodies (PAbs) is suitable for large-scale certification of potato tubers and has been used for R. solanacearum detection (7, 8, 24). The specificity problems encountered, related to the high risk of cross-reactions, could be significantly reduced by using specific monoclonal antibodies (MAbs) (21, 22), which also represent an indefinite and constant source of antibodies of known specificity. In the last 15 years a number of MAbs have been raised against R. solanacearum (3, 12, 14, 24), but none has been used for large-scale testing. The high efficiency of an enrichment step prior to ELISA has been demonstrated for several plant-pathogenic bacteria (10, 20, 21, 22) and also for R. solanacearum, but only when PAbs were used for final detection (7, 8, 23; M. T. Gorris, P. Caruso, M. Cambra, and M. M. López, Proc. X Congr. Asoc. Latinoam. Fitopatol., abstr. 131, 1999; M. T. Gorris, S. Priou, L. Gutarra, E. R. French, M. Cambra, and M. M. López, Fitopatología 33:32, 1997). Consequently, this study was performed to provide an efficient, easy, and low-cost tool for sensitive and specific routine detection of small populations of R. solanacearum in potato stocks based on selective enrichment and an ELISA that uses specific MAbs.
A total of 171 previously characterized strains of R. solanacearum (168 typical strains and 3 atypical nonfluidal colony-forming strains according to Kelman's description [16]) were obtained from different collections (Table 1). In addition, 134 strains of the potato microbiota were isolated. We also analyzed 30 strains of other potato-pathogenic bacteria belonging to the genera Erwinia and Clavibacter and 10 strains of plant-pathogenic bacteria from other hosts. R. solanacearum strain PD 2762 from The Netherlands and R. solanacearum strain IVIA 1632.2 from La Palma, Spain, both belonging to biovar 2, were used for immunization and as positive controls. Chryseobacterium indologenes strain P 27 isolated from potato was used as a negative control in ELISAs. R. solanacearum strains were cultured on Wilbrink medium (19) at 29°C, and the optical density at 600 nm (OD600) of a culture containing 1 × 109 CFU of R. solanacearum strain PD 2762 per ml was 0.5.
TABLE 1.
Typical and atypical R. solanacearum strains, origins, and patterns of reactions with MAb 8B-IVIA and PAbs in ELISA
| Strain(s)a | Geographic origin | Hosts | Reaction withb:
|
||
|---|---|---|---|---|---|
| MAb 8B-IVIAc | PAb IVIA-1632.2d | PAb IVIA-2762e | |||
| Typical R. solanacearum strains | |||||
| CIP 001, CIP 057, CIP 067, CIP 068, CIP 074, CIP 078, CIP 079, CIP 086, CIP 096, CIP 097, CIP 102, CIP 103, CIP 146, CIP 161, CIP 182, CIP 188, CIP 204, CIP 232, CIP 429, CIP 434, IVIA 1642, PD 1446, PD 1455, SMT 41, SMT 42, SMT 43, SMT 44, SMT 45, SMT 46f | Americas (Argentina, Bolivia, Brazil, Chile, Columbia, Costa Rica, Mexico, Panama, Peru, United States, Uruguay, Venezuela) | Solanum tuberosum, Solanum melongena, Cyphomandia betacea, Heliconia spp., Lycopersicon esculentum, soil, Capsicum spp. | + | + | + |
| CIP 335, CIP 357, PD 1434, SMT 47g | Australia | Lycopersicon esculentum, Solanum tuberosum | + | + | + |
| CIP 170, CIP 171, IVIA 1496.1, IVIA 1496.6, IVIA 1532.4, IVIA 1546, IVIA 1600.4.1, IVIA 1600.8, IVIA 1600.9.1, IVIA 1600.10.4, IVIA 1600.11.2, IVIA 1600.12.1, IVIA 1600.15.1, IVIA 1602.1, IVIA 1602.10, IVIA 1602.11.1, IVIA 1602.14, IVIA 1602.15, IVIA 1602.17, IVIA 1620.1.1, IVIA 1620.2.1, IVIA 1620.3.1, IVIA 1620.4.1, IVIA 1634, IVIA 1635, IVIA 1671, IVIA 1672, IVIA 1673, IVIA 1678, IVIA 1692, IVIA 1760.1.1, IVIA 1760.2.1, IVIA 1760.3.1, IVIA 1778.1, IVIA 1778.2, IVIA 1778.3, IVIA 1805.1, IVIA 1805.2, IVIA 1805.8, IVIA 1805.9, PD 2763, PD 2764, PD 2779, PD 2781, SMT 2, SMT 3, SMT 4, SMT 5, SMT 6, SMT 7, SMT 8, SMT 9, SMT 10, SMT 11, SMT 12, SMT 13, SMT 14, SMT 15, SMT 16, SMT 17, SMT 18, SMT 19, SMT 20, SMT 21, SMT 22, SMT 23, SMT 24, SMT 25, SMT 26, SMT 27, SMT 28, SMT 29, SMT 30, SMT 31, SMT 32, SMT 33, SMT 35, SMT 36, SMT 37, SMT 38, SMT 39, SMT 40, SMT 66, SMT 68, SMT 69 (= IVIA 1632.2), SMT 70, SMT 71 (= PD 2762), SMT 72, SMT 73, SMT 74, SMT 76 | Europe (The Netherlands, Belgium, Cyprus, Slovenia, France, Germany, Greece, Portugal, Spain, Sweden, United Kingdom) | Solanum tuberosum, Solanum dulcamara, Lycopersicon esculentum, river water | + | + | + |
| CIP 110, CIP 112, CIP 115, CIP 165, CIP 169, CIP 180, CIP 265, CIP 271, CIP 289, CIP 393, CIP 399, PD 1410, PD 1424, PD 1454, SMT 48, SMT 49, SMT 50h | Asia (People's Republic of China, India, Indonesia, Iran, Japan, Malaysia, Nepal, The Philippines, Sri Lanka) | Morus alba, Solanum tuberosum, Arachis hypogaea, Zingiber officinale, Capsicum spp. | + | + | + |
| CIP 036, CIP 114, CIP 117, CIP 257, CIP 258, CIP 259, CIP 260, CIP 291, CIP 359, CIP 360, CIP 368, CIP 378, CIP 423, CIP 424, CIP 425, IVIA 1550.2, IVIA 1550.3, IVIA 1837.3, IVIA 1841.1, IVIA 1844.04, IVIA 1844.07, IVIA 1850.19, IVIA 1851.2, IVIA 1851.3, IVIA 1851.4, IVIA 1851.7, IVIA 1851.8, IVIA 1861 | Africa (Burundi, Cameroon, Egypt, Kenya, Nigeria, Réunion, Rwanda) | Solanum tuberosum, Portulaca oleracea | + | + | + |
| Atypical R. solanacearum strainsi | |||||
| SMT 1, SMT 34 | Europe (United Kingdom, France) | Solanum tuberosum, Solanum melongena | − | + | + |
| SMT 48j | Asia (Sri Lanka) | Solanum tuberosum | − | + | + |
CIP, International Collection, Centro Internacional de la Papa, Lima, Peru; IVIA, Collection of Plant Pathogenic Bacteria, Instituto Valenciano de Investigaciones Agrarias, Moncada, Spain; PD, Collection of Plant Protection Service, Wageningen, The Netherlands; SMT, Collection of EC-SMT-4-CT97-2179 project (DGXII-EGAA). All strains were biovar 2 strains unless indicated otherwise.
+, positive; −, negative.
Serological reaction with MAb 8B-IVIA (homologous strain PD 2762).
Serological reaction with antiserum PAb IVIA-1632.2 (homologous strain IVIA-1632.2).
Serological reaction with antiserum PAb IVIA-2762 (homologous strain PD 2762).
Strains PD 1446, SMT 41, and SMT 42 are biovar 1 strains; strain PD 1455 is a biovar 4 strain; and strain SMT 46 is a biovar 3 strain.
Strains PD 1434 and SMT 47 are biovar 3 strains.
Strains PD 1410, PD 1424, PD 1454, SMT 48, and SMT 49 are biovar 4 strains, and strain SMT 50 is a biovar 5 strain.
Strains that produced nonfluidal colonies and atypical symptoms when they were inoculated in tomato.
Strain SMT 48 is a biovar 4 strain.
Production of antibodies.
Six antisera were produced by the method of Alarcón et al. (2), and purified immunoglobulins from the following two antisera were selected because of their high performance in coating double-antibody sandwich indirect (DASI) ELISA plates: IVIA-1632.2/WC and IVIA-2762/Glu, obtained with whole cells and glutaraldehyde-fixed cells of strains IVIA 1632.2 and PD 2762, respectively. Ten MAbs specific for R. solanacearum were produced by using conventional hybridoma technology (18), and antibody isotype was determined by an indirect ELISA (Serva Feinbiochemica GmbH & Co). The specificity of the MAbs was evaluated by an indirect ELISA (1) with all characterized strains of R. solanacearum, unclassified strains of the potato microbiota, and other plant-pathogenic bacteria. The level of serological relationship (1, 26), expressed as a percentage, was calculated, and the optical density of each strain was compared with the ELISA value for R. solanacearum strain PD 2762, which was considered 100%.
MAb 8B-IVIA (isotype immunoglobulin M, K) was selected because of its specificity and its ability to react. This MAb, which reacted with all typical R. solanacearum strains tested belonging to biovars 1, 2, 3, 4 and 5 (Table 1), showed a close and homogeneous serological relationship with most R. solanacearum strains analyzed (data not shown). This is an indication of the close affinity of the selected MAb to an epitope that is highly conserved in typical strains of R. solanacearum. MAb 8B-IVIA did not recognize three atypical, nonfluidal colony-forming strains of R. solanacearum (SMT 1, SMT 34, and SMT 48) that caused only mild atypical symptoms when they were inoculated onto tomato (data not shown). The lack of reaction could be related to the lack of the epitope and a partial loss of virulence. Similar results were previously reported by other authors (3). MAb 8B-IVIA did not react with 134 unknown bacteria isolated from potato or with 40 other bacteria pathogenic for potato or for other hosts.
Enrichment DASI-ELISA (E-DASI-ELISA).
A DASI-ELISA that used purified immunoglobulins from PAbs IVIA-1632.2/WC and IVIA-2762/Glu at a final concentration of 1.5 μg/ml for coating the plates and MAb 8B-IVIA at a concentration of 0.1 μg/ml for the specific reaction was performed basically as described by Gorris et al. (10). Samples were analyzed before and after enrichment (see below). The test was carried out routinely in duplicate wells for each sample and included negative and positive enrichment broth controls and a negative potato extract control. Absorbance at 405 nm was measured from 30 min to 2 h. OD405 values greater than twice those of the negative control were considered positive. The low sensitivity of the DASI-ELISA without prior enrichment (data not shown) was close to that observed by other authors (7, 8). In order to improve the sensitivity of detection, seven liquid media were tested for the ability to increase R. solanacearum populations; these media included the modified SMSA broth (7, 9) proposed by European Directive 98/57/EC, CCSF (1.25 g of NaCl per liter, 0.05 g of MgSO4 per liter, 0.25 g of NH4H2PO4 per liter, 0.25 g of K2HPO4 per liter, 1.25 g of sodium citrate per liter), modified Wilbrink broth (MWB) (19) (10 g of sucrose per 900 ml, 5 g of proteose peptone per 900 ml, 0.5 g of K2HPO4 per 900 ml, 0.25 g of MgSO4 per 900 ml, 0.25 g of NaNO3 per 900 ml), potato broth (PB), potato dextrose broth (PDB), and two modifications of SMSA broth, SMSA-PB (1:1, vol/vol) (23) and SMSA-PDB (1:1, vol/vol). The MWB, PB, and PDB were prepared in two steps. After sterilization a 100-ml second part that contained the same antibiotics and inhibitors as SMSA broth (7, 9) at the same concentrations (but that did not contain tetrazolium chloride) was added. Suspensions of R. solanacearum PD 2762 whose concentrations ranged from 10 to 107 CFU/ml were prepared and added (1:10, vol/vol) to 4.5-ml portions of all liquid enrichment media. Negative enrichment controls were prepared by adding phosphate-buffered saline to the media. All the inoculated media were incubated with and without constant shaking for 72 h at 29°C in tubes with loosely fitting caps to permit aeration (17). The whole experiment was repeated twice, and each repetition was considered a block. OD600 data were analyzed by four-way factorial analysis of variance (mode of enrichment [with or without shaking], liquid medium, concentration, and block). Results of the comparison of different liquid enrichment media tested with pure R. solanacearum cultures are shown in Table 2. No three-way interaction was significant, but shaking by medium was significant. Hence, means at the shaking-by-medium level are shown in Table 2. Incubation with constant shaking rather than static incubation significantly increased the population of R. solanacearum, and the population was largest with MWB and smallest with SMSA. MWB allowed enrichment of the bacterial population from all the initial concentrations, including a single cell.
TABLE 2.
Efficiencies of enrichment in seven liquid media for increasing R. solanacearum populations (phosphate-buffered saline suspensions) after incubation at 29°C for 72 h with or without constant shaking
| Mediuma | Global mean OD600b
|
|
|---|---|---|
| Shaken | Not shaken | |
| SMSA | 0.064 i | 0.047 e, f, g |
| SMSA-PB (1:1, vol/vol) | 1.181 r | 0.101 e, f |
| SMSA-PDB (1:1, vol/vol) | 1.509 f | 0.218 d |
| PB | 0.636 h | 0.018 g |
| PDB | 0.855 g | 0.036 f, g |
| CCSF | 0.208 h | 0.127 e |
| MWB | 1.882 c | 0.534 c |
Compositions of the media are described in the text.
Average OD600 (two tubes) for two repetitions with initial bacterial concentrations ranging from 1 to 106 CFU/ml. The residual standard errors were 0.1509 and 0.1013 for the samples that were shaken and not shaken, respectively, while the P value was 0.0001 in both cases. Means followed by the same letter are not statistically different as determined by the Duncan method.
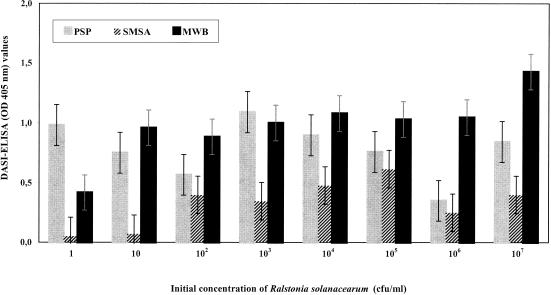
To compare several selected enrichment media with potato extracts, spiked samples of 200 tubers were prepared according to European Directive 98/57/EC from potato seed tubers that were not infected with R. solanacearum and were inoculated to obtain samples with final concentrations of 1 to 107 R. solanacearum cells per ml of extract. A negative potato extract control was also prepared by adding sterile phosphate-buffered saline. Eight enrichment assays were performed with the spiked potato extracts by using seed potato tubers of different origins and ages. The same media that were previously used to enrich pure cultures of R. solanacearum were used (data not shown). In addition, a modification of MWB with potato extract (PSP) was assayed (2 g of potato extract per liter, 10 g of sucrose per liter, 10 g of peptone per liter); this medium was prepared with the same second part as MWB. Aliquots (0.5 ml) of the spiked potato samples were added to 4.5-ml portions of the liquid enrichment media and incubated for 72 h at 29°C with constant shaking (125 rpm) in a Lab-line orbital shaker (model 4628). Most of the enrichment media assayed in this work contained PB and/or potato extract, which despite improving the growth of R. solanacearum, induced a high background in DASI-ELISA. The background observed with the media was also very dependent on the cultivar and postharvest age of the tubers used to prepare the spiked potato extract. For this reason only data obtained with three selected media (MWB, PSP, and SMSA) as the standard media were included in the analysis. Optical density data were analyzed by three-way factorial analysis of variance (liquid enrichment medium, concentration, and cultivar). The corrected optical density values obtained by subtracting the average optical density of the negative potato extract control from the optical density of each observation were analyzed. Means were compared by the Duncan procedure. Figure 1 shows the DASI-ELISA values after enrichment of spiked potato extract from initial concentrations of R. solanacearum ranging from 1 to 107 CFU/ml of potato extract in the three selected liquid media (MWB, PSP, and SMSA). Wilbrink broth (19) after modification was improved (MWB), and this medium proved to be the best liquid medium for enrichment of R. solanacearum in potato extract prior to DASI-ELISA analysis.
FIG. 1.
DASI-ELISA (OD405) values after enrichment of spiked potato extract (spiked with 1 to 107 CFU of R. solanacearum per ml) in three liquid media (PSP, SMSA, and MWB) incubated for 72 h at 29°C with constant shaking. Data from eight repetitions of experiments performed with two wells were compared by the Duncan test. The P value was <0.0001. The compositions of the media are described in the text.
Additional experiments were performed with similar spiked potato extracts in order to optimize the enrichment conditions. Different relative volumes of potato extract and enrichment medium (1:10, 1:50, and 1:100) and different incubation times (48 and 72 h) were compared. The experiments were repeated twice. After enrichment, the samples were examined by DASI-ELISA and the data were analyzed as described above. There were significant differences between enrichment results obtained with different proportions of potato extract and liquid enrichment medium, and the 1:10 dilution and incubation with constant agitation for 72 h were the most efficient conditions (data not shown).
Preincubation of samples in MWB (and in PSP if no background was expected) not only reduced the multiplication of other bacteria but also stimulated the growth of R. solanacearum when the preparations were combined with nutrients from potato extract.
Analysis of commercial potato lots.
The method developed was validated by analysis of 233 asymptomatic commercial lots of potatoes routinely performed in two different laboratories of the Spanish Plant Protection Service (Table 3). Extracts were also prepared by using the European protocol. They were plated on SMSA, analyzed by IIF using PAb IACR-PS-278 (IACR, Rothamsted, United Kingdom), and enriched for 72 h at 29°C with shaking in MWB (ratio of extract to broth, 1:10) before DASI-ELISA was performed with a kit developed for E-DASI-ELISA (Plant Print Diagnostics, Valencia, Spain). Additional PCR analysis (25) was performed only with samples that gave positive IIF results. R. solanacearum was detected in only 1.28% of the samples analyzed (Table 3) by E-DASI-ELISA, probably due to the Spanish origin of most of the lots. A generalized linear model with a binomial distribution was used to test the differences among diagnostic techniques, and no differences at the 5% level were found. The high level of positive and negative diagnostic results (97.4%) that coincided with the results of other techniques conventionally used for R. solanacearum detection, including SMSA plating and IIF, shows the high efficiency and reliability of the method developed. Six potato lots tested positive by IIF but were negative by ELISA and plating and were confirmed to be negative by PCR analysis, demonstrating that false-positive results can be obtained when only PAbs are used for routine detection of R. solanacearum.
TABLE 3.
Comparison of E-DASI-ELISA with other diagnostic techniques for detection of R. solanacearum in 233 commercial potato lots
| Techniquea | No. of positive samples | No. of negative samples | Total no. |
|---|---|---|---|
| SMSA plating | 2 | 231 | 233 |
| IIF | 8 | 225 | 233 |
| E-DASI-ELISA | 2 | 231 | 233 |
No significant differences at the 5% level were found when a generalized linear model was used.
The larger number of R. solanacearum and potato microbiota strains assayed in this study than in other studies (3, 12, 14, 23) and the pattern of reactions observed suggest that MAb 8B-IVIA has been well characterized and is different from the MAbs obtained previously. Furthermore, the E-DASI-ELISA protocol developed, including incubation of 1 volume of potato extract in 9 volumes of MWB for 72 h at 29°C with shaking, enabled detection of 1 to 10 CFU of R. solanacearum per ml. It increased the R. solanacearum concentration from very few cells to an OD600 of more than 1.8. This E-DASI-ELISA protocol successfully combines the high sensitivity of a postenrichment ELISA with the specificity and high affinity for R. solanacearum provided by MAb 8B-IVIA. The method is sensitive and reliable, and we are confident that it can be used in routine testing, in certification programs, and in epidemiological studies.
Acknowledgments
We are especially grateful to J. Elphinstone for careful editing of the manuscript and for useful suggestions. We thank participants in project SMT-4-CT-97-2179 for providing SMT strains, E. Carbonell for statistical analysis, E. French for supplying CIP strains, E. Bertolini and C. Morente for assistance, J. Carbonell for providing potato samples, and P. García Benavides for useful advice.
This work was supported by projects INIA 97-110C2-1, FAIR 5-CT97-3632, SMT-4-CT-97-2179, and FEDER 1FD1997-2279 and by agreement IVIA-7002 (IVIA-Plant Print Diagnostics S. L., Valencia, Spain).
REFERENCES
- 1.Alarcón, B., M. M. López, M. Cambra, M. T. Gorris, and J. Guerri. 1990. Differentation of Erwinia carotovora subsp. carotovora and Erwinia carotovora subsp. atroseptica isolated from potato by Western blot and subsequent indirect ELISA. J. Appl. Bacteriol. 69:17-20. [Google Scholar]
- 2.Alarcón, B., M. T. Gorris, M. Cambra, and M. M. López. 1995. Serological characterization of potato isolates of Erwinia carotovora subsp. atroseptica and subsp. carotovora using polyclonal and monoclonal antibodies. J. Appl. Bacteriol. 79:592-602. [Google Scholar]
- 3.Alvarez, A. M., J. I. Berestecky, J. I. Stiles, S. A. Ferreira, and A. A. Benedict. 1992. Serological and molecular approaches to identification of Pseudomonas solanacearum strains from Heliconia, p. 62-69. In G. L. Hartman and A. C. Hayward (ed.), Bacterial wilt. Proceedings of the International Conference. ACIAR Proceedings no. 31. Australian Centre for International Agricultural Research, Canberra, Australia.
- 4.Anonymous. 1998. Ralstonia solanacearum race 3, p. 276. In I. M. Smith and L. M. F. Charles (ed.), Distribution maps of quarantine pests for Europe. CAB International, Wallingford, United Kingdom.
- 5.Caruso, P., P. Llop, J. L. Palomo, P. García, C. Morente, and M. M. López. 1998. Evaluation of methods for detection of potato seed contamination by Ralstonia solanacearum, p. 128-132. In P. Prior, C. Allen, and J. Elphinstone (ed.), Bacterial wilt disease. Molecular and ecological aspects. Springer Verlag, Heidelberg, Germany.
- 6.Clark, M. F. 1981. Immunosorbent assays in plant pathology. Annu. Rev. Phytopathol. 19:83-106. [Google Scholar]
- 7.Elphinstone, J. G., J. Hennessy, J. K. Wilson, and D. E. Stead. 1996. Sensitivity of different methods for the detection of Ralstonia solanacearum in potato tubers extracts. Bull. OEPP 26:663-678. [Google Scholar]
- 8.Elphinstone, J. G., D. E. Stead, D. Caffier, J. D. Janse, M. M. López, U. Mazzucchi, P. Müller, P. Persson, E. Rauscher, E. Schiessendoppler, M. Sousa Santos, E. Stefani, J. Van. Vaerenbergh et al. 2000. Standardization of methods for detection of Ralstonia solanacearum in potato. Bull. OEPP (Organ. Eur. Mediterr. Prot. Plant) 30:391-395. [Google Scholar]
- 9.Englebrecht, M. C. 1994. Modifications of a semi-selective medium for the isolation of Pseudomonas solanacearum. Bact. Wilt Newsl. 10:3-5. [Google Scholar]
- 10.Gorris, M. T., B. Alarcón, M. M. López, and M. Cambra. 1994. Characterization of monoclonal antibodies specific for Erwinia carotovora subsp. atroseptica and comparison of serological methods for its selective detection on potato tubers. Appl. Environ. Microbiol. 60:2076-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham, J., D. A. Jones, and A. B. Lloyd. 1979. Survival of Pseudomonas solanacearum race 3 in plant debris and latently infected potato tubers. Phytopathology 69:1100-1103. [Google Scholar]
- 12.Griep, R. A., C. van Twisk, J. R. C. M. van Beckhoven, J. M. van der Wolf, and A. Schots. 1998. Development of specific recombinant monoclonal antibodies against the lipopolysaccharide of Ralstonia solanacearum race 3. Phytopathology 88:795-803. [DOI] [PubMed] [Google Scholar]
- 13.Hayward, A. C., J. Elphinstone, D. Caffier, J. Janse, E Stefani, E. R. French, and A. J. Wright. 1998. Round table on bacterial wilt (brown rot) of potato, p 420-430. In P. Prior, C. Allen, and J. Elphinstone (ed.), Bacterial wilt disease. Molecular and ecological aspects. Springer Verlag, Heidelberg, Germany.
- 14.He, L. Y. 1986. Bacterial wilt in the People's Republic of China, p. 40-48. In G. J. Persley (ed.), Bacterial wilt disease and the South Pacific. ACIAR Proceedings no. 13. Australian Centre for International Agricultural Research, Camberra, Australia.
- 15.Janse, J. D. 1988. A detection method for Pseudomonas solanacearum in symptomless potato tubers and some data on its sensitivity and specificity. Bull. OEPP (Organ. Eur. Mediterr. Prot. Plant) 18:343-351. [Google Scholar]
- 16.Kelman, A. 1954. The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathology 44:693-695. [Google Scholar]
- 17.Kelman, A., and J. Hruschka. 1973. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth culture of Pseudomonas solanacearum. J. Gen. Microbiol. 76:177-188. [DOI] [PubMed] [Google Scholar]
- 18.Köhler, G., and C. Milstein. 1975. Continuous cultures of fused cell secreting antibodies of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 19.Koike, H. 1965. The aluminum-cap methods for testing sugarcane varieties against leaf scald disease. Phytopathology 55:317-319. [Google Scholar]
- 20.López, M. M., M. Cambra, M. T. Gorris, and M. D. M. Pérombelon. 1998. Enrichment ELISA (E-ELISA), p. 28-38. In M. C. M. Perombelon and J. M. van der Wolf (ed.), Methods for the detection and quantification of Erwinia carotovora subsp. atroseptica on potatoes. Occasional publication no. 10. Scottish Crop Research Institute, Dundee, Scotland.
- 21.López, M. M., M. T. Gorris, P. Llop, J. Cubero, B. Vicedo, and M. Cambra. 1997. Selective enrichment improves isolation, serological and molecular detection of plant pathogenic bacteria, p. 117-121. In H.-W. Dehne, G. Adam, M. Diekmann, J. Frahm, A. Mauler-Machnik, and P. van Halteren (ed.), Diagnosis and identification of plant pathogens. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 22.López, M. M., P. Llop, J. Cubero, R. Peñalver, P. Caruso, E. Bertolini, M. T. Gorris, and M. Cambra. 2000. Strategies for improving serological and molecular detection of plant pathogenic bacteria, p. 83-87. In S. H. De Boer (ed.), Proceedings of the 10th International Conference on Plant Pathogenic Bacteria, Charlottetown, Canada. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 23.Priou, S., L. Gutarra, and P. Alley. 1999. Highly sensitive detection of Ralstonia solanacearum in latently infected potato tubers by postenrichment enzyme-linked immunosorbent assay on nitrocellulose membrane. EPPO Bull. (Organ. Eur. Mediterr. Prot. Plant) 29:117-125. [Google Scholar]
- 24.Robinson-Smith, A., P. Jones, J. G. Elphinstone, and S. M. D. Forde. 1995. Production of antibodies to Ralstonia solanacearum, the causative agent of bacterial wilt. Food Agric. Immunol. 7:67-79. [Google Scholar]
- 25.Seal, S. E., L. A. Jakson, J. P. W. Young, and M. J. Daniels. 1993. Differentiation of Pseudomonas solancearum, Pseudomonas syzygii, Pseudomonas pickettii, and blood disease bacterium by partial 16S sequencing: construction of oligonucleotide primers for sensitive detection by polymerase chain reaction. J. Gen. Microbiol. 139:1587-1594. [DOI] [PubMed] [Google Scholar]
- 26.Siverio, F., M. Cambra, M. T. Gorris, J. Corzo, and M. M. López. 1993. Lipopolysaccharides as determinants of serological variability in Pseudomonas corrugata. Appl. Environ. Microbiol. 59:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yabuuchi, E., Y. Kosako, I. Yano, H. Hotta, and Y. Nishiuchi. 1995. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov. Proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol. Immunol. 39:897-904. [DOI] [PubMed] [Google Scholar]