Abstract
An isolate of L. monocytogenes Scott A that is tolerant to high hydrostatic pressure (HHP), named AK01, was isolated upon a single pressurization treatment of 400 MPa for 20 min and was further characterized. The survival of exponential- and stationary-phase cells of AK01 in ACES [N-(2-acetamido)-2-aminoethanesulfonic acid] buffer was at least 2 log units higher than that of the wild type over a broad range of pressures (150 to 500 MPa), while both strains showed higher HHP tolerance (piezotolerance) in the stationary than in the exponential phase of growth. In semiskim milk, exponential-phase cells of both strains showed lower reductions upon pressurization than in buffer, but again, AK01 was more piezotolerant than the wild type. The piezotolerance of AK01 was retained for at least 40 generations in rich medium, suggesting a stable phenotype. Interestingly, cells of AK01 lacked flagella, were elongated, and showed slightly lower maximum specific growth rates than the wild type at 8, 22, and 30°C. Moreover, the piezotolerant strain AK01 showed increased resistance to heat, acid, and H2O2 compared with the wild type. The difference in HHP tolerance between the piezotolerant strain and the wild-type strain could not be attributed to differences in membrane fluidity, since strain AK01 and the wild type had identical in situ lipid melting curves as determined by Fourier transform infrared spectroscopy. The demonstrated occurrence of a piezotolerant isolate of L. monocytogenes underscores the need to further investigate the mechanisms underlying HHP resistance of food-borne microorganisms, which in turn will contribute to the appropriate design of safe, accurate, and feasible HHP treatments.
Listeria monocytogenes is a gram-positive facultative anaerobic bacterium which can cause listeriosis, a serious disease with high mortality in immunocompromised individuals, unborn children, and neonates (4). Numerous food-borne outbreaks and sporadic cases of listeriosis have been reported, mostly in North America and Europe (4, 14). L. monocytogenes is able to grow at temperatures as low as −0.4°C (25), withstand osmotic stress, and survive mild preservation treatments (12, 14). These features make this bacterium a difficult but very important target organism to be eliminated from the food chain.
For thousands of years conventional thermal processing has been the most common method used to ensure the microbiological safety of foods. However, this type of processing can have detrimental effects on the nutritional value of certain foods. Recent trends in processing are aimed at producing more healthy, nutritious, and convenient food. As a result, new food preservation techniques and new concepts have been developed and are currently being used in the food industry. One of these concepts is hurdle technology, which refers to the application of a combination of different (minimal) hurdles with antimicrobial activities in food, with additive or synergistic antimicrobial effects. One of these newly used techniques is high hydrostatic pressure (HHP) treatment. The antimicrobial effects of HHP treatment were first demonstrated in 1899 by Hite (6), who proposed the use of this method for the pasteurization of milk. It took about a century until high pressure-treated foods became commercially available, first in Japan in 1990 and 6 years later in Europe and the United States (11). The fundamental basis of all pressure effects stems from the changes in volume which accompany biochemical and physiological processes (3, 26). In contrast to thermal processing, HHP treatment can inactivate microorganisms and unfavorable enzymes at ambient or low temperatures without greatly affecting flavor, color, or nutritional constituents within a food system (20).
Pressures of between 600 and 700 MPa for 15 min (18) or 350 MPa for 40 min (16) are able to inactivate vegetative cells of fungi and bacteria, including most infectious food-borne pathogens (17, 21). Pressure treatments applied in the food industry can vary in that range, depending on several factors, such as the processing time and temperature, the kind of food and its constituents, and the microorganisms or enzymes to be inactivated (17, 20). By combining HHP with other treatments, it may be possible to reduce costs and extend the range of products to which this technique can be applied (3, 10, 17).
A wide variety of HHP-induced phenomena in living cells have been reported and reviewed, including changes in cellular morphology, biochemical reactions, and membrane integrity (3, 5, 17). High pressures manifest their effects on cellular processes in many ways, including disruption of protein and DNA synthesis, membrane-associated processes, and macromolecular quaternary structures (e.g., protein denaturation) (3, 17, 22, 27). Survival of bacteria upon pressure depends on the species and medium composition (17). In general, gram-positive bacteria are more HHP tolerant (or piezotolerant) (28) than gram-negative bacteria (17, 20), and different strains of a species can differ widely in their resistance to HHP (1). Bacterial growth is inhibited at pressures in the range of 20 to 130 MPa, while higher pressures (above 130 MPa) cause cell death (5). In Escherichia coli, the syntheses of DNA, proteins, and RNA stop at 50, 58, and 77 MPa, respectively, while cell death occurs at pressures of above 200 MPa (27).
A problem observed in HHP treatments is that a small portion of a bacterial population can be relatively resistant to a certain pressure applied (16). This phenomenon is called tailing, and it is of great importance for the food industry because of its possible implications for food safety and the design of HHP treatment. In 1989, Metrick et al. (16) observed tailing effects when Salmonella enterica serovar Typhimurium was pressurized at 340 MPa, revealing a subfraction of the population with higher pressure resistance. However, when isolates derived from this subfraction were cultured, they displayed the normal pressure resistance. In addition, Hauben et al. (5) succeeded in isolating high-pressure-resistant E. coli mutants after numerous repeated cycles of selective HHP treatments. The mutants showed increased resistance to other stresses, such as heat (but only below 62°C) and superoxide stress generated by plumbagin, a naphthoquinone occurring in the plants belonging to Plumbago spp. The existence of mutants like these could be an explanation for the tailing phenomenon. So far, there is limited information regarding the mechanisms of bacterial survival and adaptation to high pressure. Research focused on these phenomena could increase our understanding and contribute to a more sophisticated use of HHP in food processing.
In this study we isolated a piezotolerant strain of L. monocytogenes following a single selection step of 400 MPa. We determined its growth characteristics, its HHP resistance at different pressures, and the effect of growth phase and temperature on its piezotolerance. Furthermore, we determined the resistance of this strain to heat, acid, and hydrogen peroxide. Finally, its survival in artificially contaminated semiskim milk was determined to evaluate its behavior in a food matrix.
MATERIALS AND METHODS
Bacterial strains, culturing conditions, and selection of a piezotolerant strain.
L. monocytogenes Scott A (Department of Food Science, Wageningen Agricultural University, Wageningen, The Netherlands) was used throughout this study. The stock culture was kept at −80°C in 15% (vol/vol) glycerol. Stock cultures were transferred to 9 ml of sterile brain heart infusion (BHI) broth (Oxoid, Hampshire, England), using a 0.3% (vol/vol) inoculum, and incubated at 30°C overnight before experiments. The piezotolerant isolate, designated L. monocytogenes AK01, was isolated from a population of L. monocytogenes Scott A that survived HHP treatment of 400 MPa for 20 min. The cells of L. monocytogenes Scott A from which AK01 was isolated were grown in BHI at 30°C as described above, harvested at mid-exponential phase, and resuspended in ACES [N-(2-acetamido)-2-aminoethanesulfonic acid] buffer before HHP treatment (for details, see “HHP treatment” below). We used an initial level of 109 CFU of wild-type (wt) L. monocytogenes ml of ACES buffer−1. After HHP treatment, viable numbers were reduced by reduced by ∼6 log units. Cells from a single colony of AK01 were cultured in BHI broth overnight, using a 0.3% (vol/vol) inoculum, and then kept as a stock culture at −80°C in 15% (vol/vol) glycerol. Several tests were carried out to confirm the identity of AK01 as L. monocytogenes, namely, growth on Pal-Cam Listeria selective medium (Merck, Darmstadt, Germany) and carbohydrate utilization determined with the API zym identification system (Bio Mérieux, Marcy-l'Etoile, France). Ribotyping (TNO Food, Zeist, The Netherlands) also showed identical rRNA sequences for the wt strain and strain AK01.
To conduct experiments, stock cultures of wt L. monocytogenes Scott A and strain AK01 were inoculated in BHI broth and subcultured at 30°C with 0.3% (vol/vol) inocula, and subsequently a 0.3% (vol/vol) inoculum of each culture was added to 100 ml of BHI broth. Cultures were then incubated in a shaking incubator (160 rpm) at 8, 22, or 30°C, and growth was monitored by measuring the optical density at 660 nm (OD660). Viable counts were also determined at regular time intervals by preparing 10-fold serial dilutions of samples in peptone-physiological salt (1.5 g of peptone liter−1 and 8.5 g of NaCl liter−1) and plating these on BHI agar (1.2%, wt/vol). Plates were incubated at 30°C for 3 days. The mid-exponential or stationary phase of growth of the wt and AK01 was determined at the different temperatures, based on the OD660 and the viable count measurements. The maximum specific growth rates (μmax) for the exponential phase were calculated using the equation μmax = (ln Nt − ln N0) × (t − t0)−1, where Nt is the cell population at time t (19).
Electron microscopy.
Ten microliters of a mid-exponential-phase culture of AK01 and the wt was placed in a sterile petri dish, and a Formvar-coated grid was put on top and left for 1 min. The grid was removed, the excess of liquid was drained off, and grid was placed on a drop of 2% phosphotungstic acid for 1 min. Subsequently, excess phosphotungstic acid was removed, and the grid was inspected with the use of an electron microscope (Jem-1200 EX II; Jeol Ltd., Tokyo, Japan).
HHP treatment.
The L. monocytogenes wt strain and AK01 were cultured at 8 or 30°C and harvested by centrifugation (10,000 × g, 10 min) at mid-exponential phase (OD660 of 0.3 and 0.2 for the wt and AK01, respectively). The cells were washed twice in 50 mmol of ACES liter−1 (ACES buffer; Sigma-Aldrich, Steinheim, Germany), pH 7.0. The pellet was resuspended in semiskim milk (Friesche Vlag, Ede, The Netherlands) or in ACES buffer to an OD660 of 0.1. Aliquots of 10 ml were transferred into sterile plastic tubes (Greiner, Kremsmünster, Austria). ACES buffer was selected because this buffer maintains pH 7.0 during high pressure treatments (21). Suspensions were placed in sterile plastic stomacher bags (Seward, London, United Kingdom) that were sealed while avoiding an excess of air bubbles. These pouches were submerged in glycol, which was the fluid medium through which the pressure was transferred (Resato, Roden, Holland). Subsequently, cell suspensions were exposed to pressures of 150, 200, 250, 300, 350, 400, 450, or 500 MPa in a high pressure unit (Resato) at 20°C for 20 min. The viability of L. monocytogenes was determined before and after pressure treatment. Serial 10-fold dilutions of samples were prepared in peptone-physiological salt (1.5 g of peptone liter−1 and 8.5 g of NaCl liter−1) and plated in triplicate onto BHI agar (1.2% [wt/vol] agar). Plates were incubated at 30°C for 5 days.
Stability of HHP resistance.
To test the stability of the HHP-resistant phenotype of L. monocytogenes AK01, cells were subcultured during five consecutive days, using 0.3% (vol/vol) inocula in 9 ml of fresh BHI medium (∼70 generations). The wt L. monocytogenes was cultured the same way and used as a control. On days 2 and 5 (∼30 and ∼70 generations, respectively), overnight cultures were inoculated (0.3%, vol/vol) in 100 ml of BHI broth, incubated at 30°C with shaking (160 rpm) to mid-exponential phase (OD660 of 0.3 and 0.2 for the wt and AK01, respectively), and harvested. These cells were tested for resistance to 250 or 300 MPa for 20 min.
Phase transition temperature.
Cells from wt and AK01 L. monocytogenes were incubated at 30°C, harvested at mid-exponential phase, and washed in ACES buffer as described above. The pellet was placed between two circular CaF2 windows (13 by 2 mm) and fitted in a liquid-nitrogen-cooled, temperature-controlled brass cell. The temperature of the sample was recorded and controlled by using two PT-100 elements that were near the sample windows. Fourier transform infrared spectra were recorded on a Fourier transform infrared spectrometer (model 1725; Perkin-Elmer, Beaconsfield, Buckinghamshire, United Kingdom) equipped with an external beam facility to which a Perkin-Elmer infrared microscope was attached. The microscope was equipped with a narrow-band mercury-cadmium-telluride LN2-cooled infrared detector. The sample was cooled to −40°C and subsequently heated to 70°C at a rate of 1.5°C min−1. During the heating, spectra were recorded every minute. Spectral analysis and display were carried out using the Infrared Data Manager Analytical Software, version 3.5 (Perkin-Elmer). Band positions were calculated as the averages of central positions in 20 slices between 75 and 90% of the total peak height. The peak positions of the CH2-symmetric stretching vibration bands were analyzed to estimate the extent of interaction between the acyl chains of the membrane lipids as a direct indication of the membrane fluidity. Thus, transitions in membranes from the gel to the liquid-crystalline phase can be observed in vivo from plots of the vibrational frequency of the absorption peaks versus the temperature at which spectra were recorded (7). Estimation of the temperature at which half of the hydrocarbon containing compounds had been melted (Tm) was made by probit analysis. Using this technique, we were able to monitor possible differences in the membrane fluidities of these two strains.
Heat treatment.
BHI broth (100 ml) was inoculated (0.1%, vol/vol) with an overnight culture of wt or AK01 L. monocytogenes and incubated with shaking (160 rpm) at 22 or 30°C. Cells were harvested at mid-exponential phase (OD660 of 0.3 and 0.2 for the wt and AK01, respectively), washed twice, and resuspended in ACES buffer. Suspensions were placed in 10-ml plastic tubes (Greiner) and incubated in a water bath at 55°C for a maximum of 20 min. At regular time intervals, samples were taken and viability was determined.
Acid treatment.
The L. monocytogenes wt strain and AK01 were cultured at 30°C and harvested as described above. Cells were resuspended in BHI broth adjusted to pH 2.5 with HCl. Viable counts were determined immediately after resuspension of cells in the low-pH BHI broth and then at regular time intervals.
H2O2 treatment.
The resistance of wt and AK01 L. monocytogenes to hydrogen peroxide (H2O2) was determined for cells cultured at 30°C to mid-exponential phase, by adding H2O2 (Merck, Hohenbrunn, Germany) to a concentration of 0.2%, wt/vol. Viable counts were determined just before and at regular time intervals after the addition of H2O2.
Growth in the presence of NaCl.
To determine the maximum NaCl concentration at which wt L. monocytogenes and AK01 were able to grow, cells were inoculated (0.3%, vol/vol) in BHI broth with final NaCl concentrations ranging from 8 to 14% (wt/vol), increasing by 0.5% (wt/vol). Growth and final population densities were monitored by measuring the OD665 at regular time intervals for a maximum of 50 h.
RESULTS
Characterization of strain AK01 as L. monocytogenes.
The identity of AK01 was confirmed as L. monocytogenes by growth on Pal-Cam Listeria selective medium, demonstration of characteristic metabolic activities of L. monocytogenes in the API zym system, and ribotyping (94% similarity with L. monocytogenes DUP-1042).
Microorganism and growth.
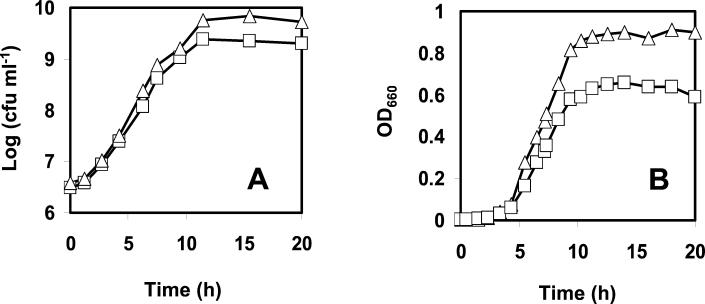
The OD660 and the viable counts of wt and AK01 L. monocytogenes were determined at 8, 22, or 30°C at regular time intervals. At all temperatures, the μmax of the wt was higher than the μmax of strain AK01. The respective μmaxs of the wt and AK01 were 0.97 and 0.87 h−1 at 30°C (Fig. 1A) and 0.68 and 0.60 h−1 at 22°C and 0.10 h and 0.09 h−1 at 8°C (data not shown). Furthermore, AK01 showed lower final OD660s than the wt (∼0.6 versus ∼0.9) (Fig. 1B) and lower final population densities (∼2.3 × 109 compared to ∼6 × 109 for the wt) at all temperatures.
FIG. 1.
Growth of wt L. monocytogenes (▵) and the piezotolerant isolate AK01 (□) monitored by viable counts (A) and OD660 (B). Cells were grown in BHI broth at 30°C with shaking (160 rpm).
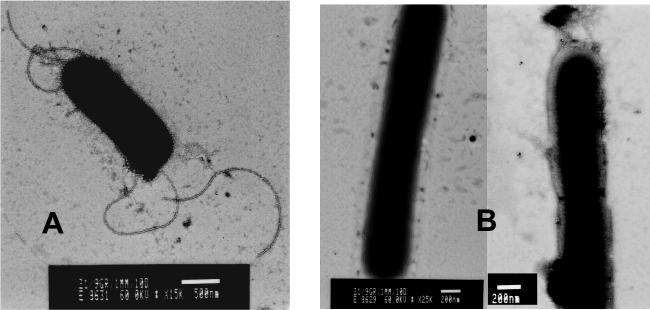
Microscopic examination showed immobility of strain AK01, while wt L. monocytogenes was motile. In addition, we observed that cells of strain AK01 were roughly twofold longer than wt cells. Further examination using electron microscopic analysis confirmed that the cells of AK01 were elongated, and in addition, flagella were not detected in strain AK01 (Fig. 2).
FIG. 2.
Visualization of exponentially grown cells of the wt (A) and AK01 (B) with electron microscopy. Bars, 500 nm (A) and 200 nm (B).
HHP resistance.
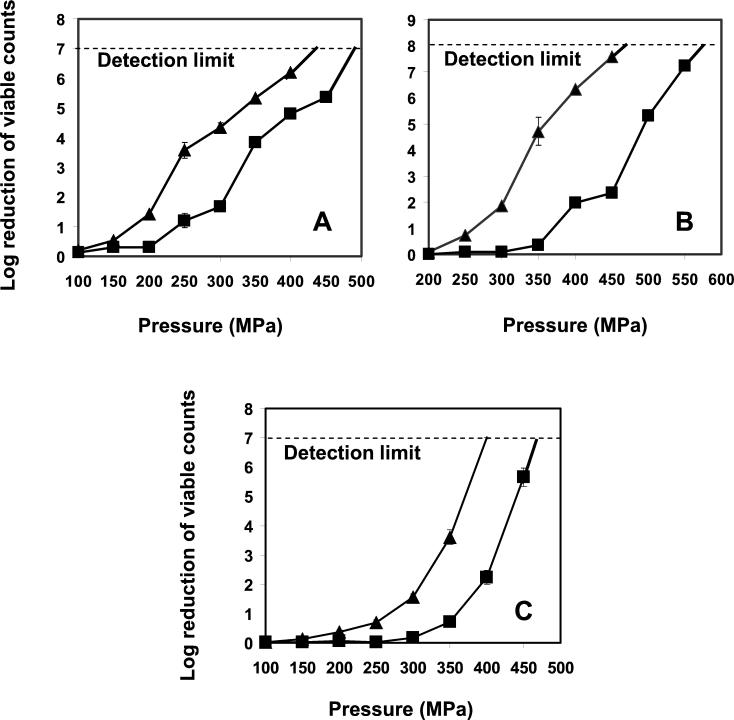
The HHP resistance of mid-exponential-phase cultures of the piezotolerant L. monocytogenes AK01 (grown at 30°C) was higher than that of the wt strain over the range of pressures tested (150, 200, 250, 300, 350, 400, 450, and 500 MPa), showing maximally a 2.5-log-unit difference (Fig. 3A). Notably, more than a 6-log-unit reduction in viable counts was achieved, for both the wt and AK01, with pressures greater than 400 MPa. A similar difference in resistance to HHP between the wt and strain AK01 was observed for cells grown at 8°C and harvested at mid-exponential phase. Overall, cells grown at 8°C were maximally 1 log unit more resistant to the whole range of pressures tested than those grown at 30°C (data not shown).
FIG. 3.
Reductions in viable numbers of wt L. monocytogenes (▴) and AK01 (▪) after exposure to different pressures for 20 min at 20°C. Cells were grown in BHI broth at 30°C with shaking (160 rpm). (A) Cells were harvested in mid-exponential phase and resuspended in ACES buffer before treatment. The detection limit was a 7-log-unit reduction of the viable counts, which was exceeded by the wt at 450 MPa and by AK01 at 500 MPa. (B) Cells were harvested in stationary phase and resuspended in ACES buffer before treatment. The detection limit was an 8-log-cycle reduction of the viable counts, which was exceeded by the wt at 500 MPa and by AK01 at 600 MPa. (C) Cells were harvested in mid-exponential phase and resuspended in semiskim milk before treatment. The detection limit was a 7-log-cycle reduction of the viable counts, which was exceeded by the wt at 400 MPa, and by AK01 at 500 MPa. Experiments were performed in duplicate and error bars represent standard deviations.
The growth phase of cells affected the HHP resistance of both the wt and strain AK01. When cultures reached stationary phase, their HHP resistance increased strongly, but also in this case, strain AK01 had a piezotolerance that was higher (maximally 5.2 log units) than that of the wt (Fig. 3B). While pressure inactivation of more than 6 log units was achieved at 450 MPa for stationary-phase cells of the wt, pressures close to 600 MPa were required for cells of AK01.
The reductions of the viable numbers of mid-exponential-phase cells from strain AK01 and the wt (grown at 30°C) were overall 2 to 3 log units lower in milk than in ACES buffer over the whole range of pressures (150 to 500 MPa). Again, AK01 was more resistant to HHP than the wt (Fig. 3C).
Stability of HHP resistance.
Exponential-phase cultures of AK01 had the same HHP resistance after ∼30 and ∼70 generations. Their HHP resistance was tested at 250 and 300 MPa, resulting in 1.2- and 1.6-log-unit reductions of the viable counts, respectively, independent the number of generations, versus 3.4- and 4.4-log-unit reductions for the wt control cultures (data not shown). This suggests that AK01 retained its HHP-resistant character, indicating a stable phenotype.
Phase transition temperature.
The in situ membrane fluidity and the melting curve of L. monocytogenes AK01 were identical to those of the wt strain as determined by Fourier transform infrared spectroscopy (data not shown). The Tm was centered between 10 and 11°C, with the beginning of melting around −3°C and the end of the melting around 23°C.
Heat treatment.
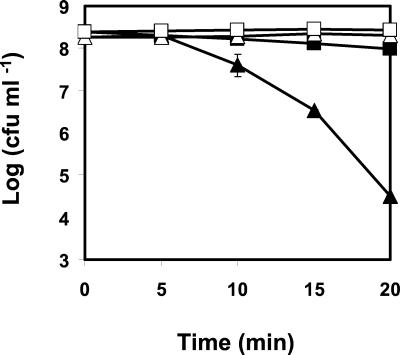
Exponential-phase cultures of AK01 grown at 22°C were more resistant to heat treatment than the wt control cells. After 20 min at 55°C, only a 0.4-log-unit reduction in viable numbers was observed for the piezotolerant strain, compared to a 3.7-log-unit reduction of viable numbers for the wt (Fig. 4). Similarly, subjecting mid-exponential-phase cells of AK01 grown at 30°C to heat treatment (55°C for 20 min) resulted in no reduction of the viable counts, while a 2.3-log-unit reduction in viable numbers was observed for the wt (data not shown).
FIG. 4.
Effect of heat treatment at 55°C on viable numbers of wt L. monocytogenes (▴) and AK01 (▪). The viability of both cultures in absence of heat treatment is also represented for the wt (▵) and AK01 (□). Cells were cultivated aerobically at 22°C in BHI broth (pH 7), harvested, and resuspended in ACES buffer (50 mM, pH 7.0) before treatment. Values are means of triplicate measurements from a representative experiment. Bars represent standard deviations (n = 3)
Acid treatment.
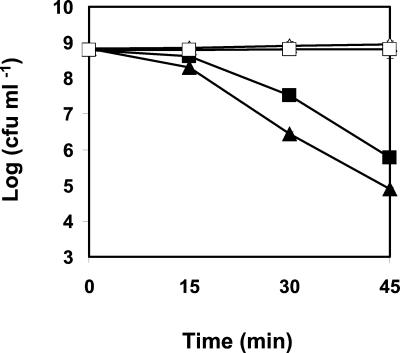
Exponential-phase cells of the wt L. monocytogenes grown at 30°C showed an approximate 1-log-higher reduction of their viable counts during exposure to pH 2.5 for 45 min than those of strain AK01 grown at the same temperature (Fig. 5).
FIG. 5.
Effect of exposure to pH 2.5 on viable numbers of wt L. monocytogenes (▴) and the piezotolerant strain AK01 (▪). The viability of both cultures in absence of HCl at pH 7 is also represented for the wt (▵) and the piezotolerant strain (□). Cells were cultivated aerobically at 30°C in BHI broth (pH 7). Experiments were performed in BHI broth adjusted to pH 2.5 with HCl. Values are means of triplicate measurements from a representative experiment. Bars represent standard deviations (n = 3)
H2O2 treatment.
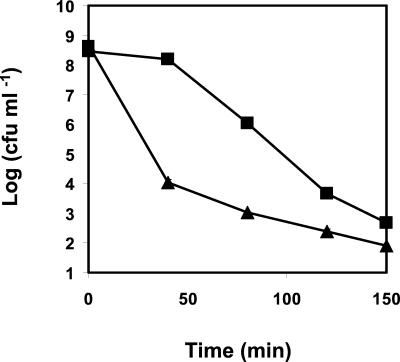
Exposure of both L. monocytogenes strains to 0.2% (wt/vol) hydrogen peroxide showed that AK01 had a higher resistance to H2O2 than the wt. During the first 40 min of exposure, the population of AK01 showed a reduction of only ∼0.5 log unit in the viable numbers, compared to a ∼4.5-log-unit reduction in viable numbers for the wt population. After 60 min, the viable counts of AK01 started to decrease, but they remained at least 1 log unit higher than those of the wt after 150 min (Fig. 6).
FIG. 6.
Effect of exposure to 0.2% H2O2 on viable numbers of wt L. monocytogenes (▴) and the piezotolerant strain AK01 (▪). Experiments were performed in BHI broth, at 30°C, with shaking, in the absence of light. Values are means of triplicate measurements from a representative experiment. Bars represent standard deviations (n = 3).
Maximum NaCl concentration for growth.
The growth of wt and AK01 L. monocytogenes was negatively affected at increasing NaCl concentrations. We did not observe marked differences between the wt and strain AK01, and the maximum concentration of NaCl at which growth occurred was 12.5% (wt/vol) for both strains throughout 50 h.
DISCUSSION
In this study, we characterized the piezotolerant strain AK01 of L. monocytogenes Scott A, which was isolated from a wt population after exposure to HHP treatment of 400 MPa for 20 min. The survival of exponential- and stationary-phase cells of strain AK01 upon pressurization was more than 2 log units higher than that of wt L. monocytogenes over a broad range of pressures tested. The piezotolerance of AK01 was retained for at least 40 generations in rich medium. These results suggest a stable phenotype, likely resulting from altered cellular properties, rather than a short-lived adaptation. The HHP phenotype was accompanied by a slightly lower μmax than that of the wt and by altered morphological characteristics, namely, the absence of flagella and elongation of cells.
To date, only a limited number of studies have demonstrated the occurrence of piezotolerant strains derived from microorganisms that can be present in foods. To our knowledge, this is the first report describing an L. monocytogenes piezotolerant strain. In a study performed by Iwahashi et al. (8), piezotolerant mutants of Saccharomyces cerevisiae were obtained upon treatment of cells with mutagenic substances such as N-methyl-N′-nitro-N-nitrosoguanidine. Furthermore, Hauben et al. (5) reported the isolation of HHP mutants of E. coli after numerous subsequent selective HHP cycles. By contrast, L. monocytogenes AK01 was selected upon a single HHP cycle that reduced the initial wt population of ∼109 CFU ml−1 to such an extent that the only colony formed on the agar was AK01.
We investigated whether the difference in HHP tolerance between the piezotolerant strain and the wt strain could be attributed to altered membrane properties, since there is evidence that an increased membrane fluidity of natural and artificial membranes gives rise to an increased high-pressure resistance (15). Importantly, strain AK01 and the wt had identical in situ membrane fluidities, indicating that the piezotolerant phenotype was not originating from a higher membrane fluidity of strain AK01 than of the wt. Similar findings have been reported for piezotolerant E. coli mutants, which showed no significant differences in fatty acid composition and outer membrane properties compared with the wt strain (5). While the difference in the piezotolerances of strain AK01 and the wt could not be linked to their membrane fluidities, wt and AK01 cells grown at 8°C had similarly increased HHP resistances compared with cells grown at 30°C upon pressurization at 20°C. This can be explained by a higher membrane fluidity of L. monocytogenes cells cultured at low temperatures compared with 30°C (24).
The piezotolerant strain L. monocytogenes AK01 had increased resistance to heat, acid, and H2O2 compared with the wt. An increased heat resistance of piezotolerant strains of E. coli compared to the wt has also been described by Hauben et al. (5), who demonstrated that E coli piezotolerant mutants were thermotolerant at 58 and 60°C but not at higher temperatures. In addition, Iwahashi et al. (8) reported increased thermotolerance of a piezotolerant mutant of S. cerevisiae compared to the wt. It is possible that a number of similar cellular properties underlie pressure and heat resistance, since both high pressure and heat destabilize the quaternary structure of proteins (9). This is supported by the findings that E. coli wt cells showed an induced expression of 55 proteins upon exposure to a pressure upshift to 55 MPa, many of which are also induced by heat shock (26). A correlation between pressure resistance and resistance to organic acids has previously been established for a variety of strains from different species (2), while increased oxygen tolerance was observed for a piezotolerant mutant of S. cerevisiae (8). Although it is not clear what mechanisms underlie the increased resistance of the piezotolerant strain to the different stresses, it could possibly be attributed to altered expression levels of proteins involved in the (general) stress response. Furthermore, the piezotolerance of strain AK01 and the wt was increased in the stationary phase compared with the exponential phase of growth, which might be related to the increased expression of genes involved in stationary-phase stress survival (13).
Important in relation to food processing is our observation that reductions in viable numbers of the wt and the AK01 strain were lower upon pressurization in milk than in buffer. A lower sensitivity of cells to HHP treatment in milk and other food matrices has previously been observed (18, 23, 17) and has been attributed to the protective effect of food components like sugars, free amino acids, and vitamins (17). On the other hand, it has been shown that HHP treatment combined with other preservation factors has a synergistic effect on the inactivation of microorganisms. We recently demonstrated that combined treatment with HHP and the essential oil compound carvacrol had a strong synergistic effects (10), while other authors demonstrated an effective control of pressure-resistant food-borne microbes by combined treatment with HHP and acid (2).
The piezotolerance observed for AK01 is quite significant considering that pressures in the range of 300 to 600 MPa are selected for pasteurization purposes (17) and that this resistance could increase in certain cases due to protective effects of some food constituents (17, 23). The application and optimization of combined processing in food systems therefore seems to be required to ensure effective inactivation of pressure-resistant strains in foods. Furthermore, the occurrence of piezotolerant isolates urges further investigation of the mechanisms underlying HHP resistance of microorganisms. Using protein two-dimensional gel electrophoresis, we have been able to identify proteins that are differentially expressed in the wt strain and the piezotolerant strain AK01. We recently obtained evidence that a single mutation in a key regulator of stress proteins is responsible for the observed phenotype of strain AK01. Such insights may resolve problems caused by phenomena like tailing and contribute to the design of safe, accurate, and feasible HHP treatments.
Acknowledgments
We thank Jan Dijksterhuis, Felix Thiel, and Adrian van Aelst for help with electron microscopy; F. Hoekstra and Marc Alberda for performing Fourier transform infrared spectroscopy and analysis of the results; and Vasilis Valdramidis for technical assistance.
This research was supported by the Wageningen Center of Food Sciences.
REFERENCES
- 1.Alpas, H., N. Kalchayanand, F. Bozoglu, A. Sikes, C. P. Dunne, and B. Ray. 1999. Variation in resistance to hydrostatic pressure among strains of food-borne pathogens. Appl. Environ. Microbiol. 65:4248-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpas, H., N. Kalchayanand, F. Bozoglu, and B. Ray. 2000. Interactions of high hydrostatic pressure, pressurisation temperature and pH on death and injury of pressure-resistant and pressure-sensitive strains of foodborne pathogens. Int. J. Food Microbiol. 60:33-42. [DOI] [PubMed] [Google Scholar]
- 3.Cheftel, J. C. 1995. High pressure, microbial inactivation and food preservation. Food Sci. Technol. Int. 1:75-90. [Google Scholar]
- 4.Cox, J. L. 1989. A perspective on listeriosis. Food Technol. 43:52-59. [Google Scholar]
- 5.Hauben, K. J. A., D. H. Bartlett, C. C. F. Soontjens, K. Cornelis, E. Y. Wuytack, and C. W. Michiels. 1997. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl. Environ. Microbiol. 63:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hite, B. H. 1899. The effect of pressure in the preservation of milk. Bull. W.Va. Univ. Agric. Exp. Station Morgantown 58:15-35. [Google Scholar]
- 7.Hoekstra, F. A., J. H. Crowe, and L. M. Crowe. 1991. Effect of sucrose on phase behaviour of membranes in intact pollen of Typha latifolia L., as measured with Fourier transform infrared spectroscopy. Plant Physiol. 97:1073-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwahashi, H., S. Fujii, K. Obuchi, S. C. Kaul, A. Sato, and Y. Komatsu. 1993. Hydrostatic pressure is like high temperature and oxidative stress in the damage it causes to yeast. FEMS Microbiol. Lett. 108:53-58. [DOI] [PubMed] [Google Scholar]
- 9.Jaenicke, R. 1991. Protein stability and molecular adaptation to extreme conditions. Eur. J. Biochem 202:715-728. [DOI] [PubMed] [Google Scholar]
- 10.Karatzas, A. K., E. P. W. Kets, E. J. Smid, and M. H. J. Bennik. 2001. The combined action of carvacrol and high hydrostatic pressure on Listeria monocytogenes Scott A. J. Appl. Microbiol. 90:463-469. [DOI] [PubMed] [Google Scholar]
- 11.Knorr, D., V. Heinz, D.-U. Lee, O. Schlüter, and M. Zenker. 1998. High pressure processing of foods: introduction, p. 9-20. In K. Autio (ed.), Proceedings of VTT symposium “Fresh novel foods by high pressure.” Technical Research Centre of Finland, Espoo, Finland.
- 12.Linton, R. H., J. B. Webster, M. D. Pierson, J. R. Bishop, and C. R. Hackney. 1992. The effect of sublethal heat shock and growth atmosphere on the heat resistance of Listeria monocytogenes Scott A. J. Food Prot. 55:284-287. [DOI] [PubMed] [Google Scholar]
- 13.Lou, Y., and A. E. Yousef. 1996. Resistance of Listeria monocytogenes to heat after adaptation to environmental stresses. J. Food Prot. 59:5465-5471. [DOI] [PubMed] [Google Scholar]
- 14.Lovett, J. 1989. Listeria monocytogenes, p. 283-310. In M. P. Doyle (ed.), Foodborne bacterial pathogens. Marcel Dekker, New York, N.Y.
- 15.MacDonald, A. G. 1992. Effects of high hydrostatic pressure on natural and artificial membranes, p. 67-74. In C. Balny, R. Hayashi, K. Heremans, and P. Masson (ed.), High pressure and bio/technology. INSERM, Paris, France.
- 16.Metrick, C., D. G. Hoover, and D. F. Farkas. 1989. Effects of high hydrostatic pressure on heat-resistant and heat-sensitive strains of Salmonella. J. Food Sci 54:1547-1549. [Google Scholar]
- 17.Palou, E., A. López-Malo, G. V. Barbosa-Cánovas, and B. G. Swanson. 1999. High-pressure treatment in food preservation, p. 533-576. In M. S. Rahman (ed.), Handbook of food preservation. Marcel Dekker, New York, N.Y.
- 18.Patterson, M. F., M. Quinn, R. Simpson, and A. Gilmour. 1995. The sensitivity of vegetative pathogens to high hydrostatic pressure in phosphate buffered saline and foods. J. Food Prot. 58:542-549. [DOI] [PubMed] [Google Scholar]
- 19.Schlegel, G. H. 1992. General microbiology, 7th ed. Cambridge University Press, Cambridge, United Kingdom.
- 20.Smelt, J. P. P. M. 1998. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 9:152-158. [Google Scholar]
- 21.Smelt, J. P. P. M., and C. Hellemons. 1998. High pressure treatment in relation to quantitative risk assessment, p. 27-38. In K. Autio (ed.), Proceedings of VTT symposium “Fresh novel foods by high pressure.” Technical Research Centre of Finland, Espoo, Finland.
- 22.Somero, G. N. 1992. Adaptations to high hydrostatic pressure. Annu. Rev. Physiol. 54:557-577. [DOI] [PubMed] [Google Scholar]
- 23.Styles, M. F., D. G. Hoover, and D. F. Farkas. 1991. Response of Listeria monocytogenes and Vibrio parahaemolyticus to high pressure. J. Food Sci. 56:1404-1407. [Google Scholar]
- 24.Verheul, A., N. J. Russell, R. van't Hof, F. M. Rombouts, and T. Abee. 1997. Modifications of membrane phospholipid composition in nisin-resistant Listeria monocytogenes Scott A. Appl. Environ. Microbiol. 63:3451-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker, S. J., P. Archer, and J. G. Banks. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157-162. [DOI] [PubMed] [Google Scholar]
- 26.Welch, T. J., A. Farewell, F. C. Neidhardt, and D. H. Bartlett. 1993. Stress response of Escherichia coli to elevated hydrostatic pressure. J. Bacteriol. 175:7170-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yayanos, A. A., and E. C. Pollard. 1969. A study of he effects of hydrostatic pressure on macromolecular synthesis in Escherichia coli. Biophys. J. 9:1464-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yayanos, A. A. 1995. Microbiology to 10,500 metres in the deep sea. Annu. Rev. Microbiol. 49:777-805. [DOI] [PubMed] [Google Scholar]