Abstract
The epiphytic fitness of Salmonella enterica was assessed on cilantro plants by using a strain of S. enterica serovar Thompson that was linked to an outbreak resulting from cilantro. Salmonella serovar Thompson had the ability to colonize the surface of cilantro leaves, where it was detected by confocal laser scanning microscopy (CLSM) at high densities on the veins and in natural lesions. The population sizes of two common colonizers of plant surfaces, Pantoea agglomerans and Pseudomonas chlororaphis, were 10-fold higher than that of the human pathogen on cilantro incubated at 22°C. However, Salmonella serovar Thompson achieved significantly higher population levels and accounted for a higher proportion of the total culturable bacterial flora on cilantro leaves when the plants were incubated at warm temperatures, such as 30°C, after inoculation, indicating that the higher growth rates exhibited by Salmonella serovar Thompson at warm temperatures may increase the competitiveness of this organism in the phyllosphere. The tolerance of Salmonella serovar Thompson to dry conditions on plants at 60% relative humidity was at least equal to that of P. agglomerans and P. chlororaphis. Moreover, after exposure to low humidity on cilantro, Salmonella serovar Thompson recovered under high humidity to achieve its maximum population size in the cilantro phyllosphere. Visualization by CLSM of green fluorescent protein-tagged Salmonella serovar Thompson and dsRed-tagged P. agglomerans inoculated onto cilantro revealed that the human pathogen and the bacterial epiphyte formed large heterogeneous aggregates on the leaf surface. Our studies support the hypothesis that preharvest contamination of crops by S. enterica plays a role in outbreaks linked to fresh fruits and vegetables.
The incidence of human pathogens on fresh fruits and vegetables has been a growing concern in industrialized countries in the last decade. Although generally associated with consumption of meat products, outbreaks of salmonellosis are increasingly linked to contaminated fruits and vegetables in the United States, as well as in many other countries (6). In addition to large international epidemics of Salmonella infections originating from sprouts grown from contaminated seeds (13), outbreaks of salmonellosis have been linked to lettuce and shelled peanuts (Infect. Dis. News Brief, August and October 2001, respectively; http://www.hc-sc.gc.ca/pphb-dgspsp/bid-bmi/dsd-dsm/nb-ab/index.html), cilantro (1), tomatoes (5), cantaloupe (18), and unpasteurized orange juice (2). These outbreaks have raised concerns over the possible preharvest colonization of crops by Salmonella enterica. A survey of domestic produce in the United States revealed that 2.6, 1.6, and 1.8% of the cantaloupe, cilantro, and lettuce samples, respectively, were contaminated with Salmonella spp. (http://www.cfsan.fda.gov/). A complementary survey of imported produce showed that 3.5% of the samples that were analyzed were contaminated with Salmonella spp., with cilantro accounting for 1.6% of the overall contamination (http://www.cfsan.fda.gov/).
The interaction of S. enterica with its vertebrate hosts has been the subject of intense studies. In contrast, our knowledge of the fitness of this enteric pathogen on plant surfaces is greatly lacking. Most studies of the growth of human pathogens in the plant environment have been performed with cut plants or plant tissue to simulate the infection of produce during postharvest handling and food processing. We have shown previously that a strain of S. enterica serovar Thompson that was linked epidemiologically to an outbreak of salmonellosis associated with the consumption of cilantro grew rapidly and to high concentrations on chopped cilantro leaves and in salsa made from contaminated cilantro leaves (1). Presumably, S. enterica benefited from the moist and nutrient-rich environment provided by the damaged plant tissue. In contrast, the leaf surface of an intact plant, which can harbor a bacterial population of up to 106 to 107 cells per g of leaf, is a much harsher environment, in which there are large fluctuations in nutrient and water availability, temperature, and UV radiation (7).
The objective of this study was to investigate the ability of S. enterica to grow and survive on the surfaces of leaves of live plants. We used cilantro as a plant model in combination with a strain of S. enterica serovar Thompson that was epidemiologically linked to an outbreak originating from contaminated cilantro in California (1) to demonstrate that S. enterica colonized the cilantro phyllosphere and that its population reached significant levels under warm and humid conditions.
MATERIALS AND METHODS
Strains, plasmids, and growth media.
S. enterica serovar Thompson strain RM1987 was isolated from a patient in a salmonellosis outbreak that was linked to cilantro in California in 1999 (1). S. enterica serovar Newport strain RM1655, S. enterica serovar Derby strain RM1313, and S. enterica serovar Enteritidis strain RM1250 were isolated from alfalfa seeds associated with an outbreak, from ground turkey, and from mayonnaise, respectively. Pantoea agglomerans strain FC1 and Pseudomonas chlororaphis strain CIL12 were isolated from cilantro plants grown in California in a field and in a greenhouse, respectively; both strains were identified by fatty acid analysis (Microbial ID, Newark, Del.). Spontaneous rifampin-resistant mutants of Salmonella serovar Thompson strain RM1987, P. agglomerans strain FC1, and P. chlororaphis strain CIL12 were isolated from Luria-Bertani agar plates containing 100 μg of rifampin per ml that were streaked with the wild-type strains and incubated at 37 or 20°C, as appropriate. The rifampin-resistant mutants were designated Salmonella serovar Thompson strain RM1987R, P. agglomerans strain FC1R, and P. chlororaphis strain CIL12R, respectively.
Salmonella serovar Thompson strain RM1987 was labeled intrinsically by transformation with plasmid pWM1007, which carries the gene encoding the green fluorescent protein (GFP) transcribed from a Campylobacter jejuni consensus promoter (16). P. agglomerans FC1R was transformed with plasmid pWM1013, which contains the gene encoding the fluorescent protein DsRed (BD Biosciences Clontech, Palo Alto, Calif.) on the same backbone as pWM1007 (16). These strains were used for observations with a confocal laser scanning microscope (CLSM). All strains were routinely grown at 28°C in Luria-Bertani broth (Becton Dickinson, Sparks, Md.) amended with kanamycin (50 μg/ml) or rifampin (100 μg/ml) when appropriate.
Plant inoculation.
Ten microliters of cells from stationary-phase cultures grown in Luria-Bertani broth at 28°C were added to 1 liter of potassium phosphate buffer (0.5 mM, pH 7.0) to obtain an inoculum suspension containing ca. 2 × 10 4 cells per ml. This resulted in a 10 5-fold dilution of spent cultured medium in the inoculation buffer. Cell suspensions in spent culture medium were used for inoculation instead of washed cells in order to avoid potential artifacts caused by inoculation of bacterial aggregates (instead of single cells) that are generated by repeated centrifugation during washing steps. The results of comparative experiments revealed that cultured cells of Salmonella serovar Thompson that were centrifuged and either resuspended in their supernatant or washed to remove culture medium grew at the same rate and achieved the same population levels following inoculation onto cilantro plants and incubation under high humidity and at 26°C.
Cilantro plants (Coriandrum sativum cv. Leisure) that were grown in a greenhouse to the four- to six-true-leaf stage were inoculated by immersion of the upper part of each plant in the bacterial suspension for 2 s. The control treatment consisted of immersing the plants in buffer without inoculum. A minimum of four replicate pots containing eight plants each were used per treatment. Immediately after inoculation, at least three leaves were removed at random from each pot for each treatment and assessed to determine the initial inoculum population as described below. The plants were then incubated at 24°C under fluorescent lights in a humid chamber that allowed the presence of visible free water on the leaves. When the effect of temperature on the growth of Salmonella serovar Thompson on plants was tested, the pots were individually bagged after inoculation and placed in plant growth chambers under fluorescent and incandescent lights at the appropriate temperatures. For exposure of the bacteria to water stress on plants, the plants were incubated first for 18 h at 26°C in a humid chamber and then in a growth chamber at 50 or 60% relative humidity at 26°C for 24 h to trigger low water availability on the leaf surface. The plants were then placed in a humid chamber again to allow the bacteria to recover from water stress under conditions where free water was present on the leaves. In all the experiments, the pots were randomized within the humid chamber and the growth chambers. Each experiment was repeated twice independently with different bacterial cultures and different sets of plants.
Plants that were used for observation of Salmonella serovar Thompson on leaves with the CLSM were inoculated as described above, except that the inoculum concentration was 2 × 10 5 cells per ml. The plants were incubated in a humid chamber at 24°C immediately after inoculation.
Estimation of bacterial populations.
At each sampling time, a minimum of three leaves were removed at random from the cilantro plants in each replicate pot (resulting in a minimum sample size of 12 leaves per treatment) and placed in 10 ml of potassium phosphate buffer (10 mM, pH 7.0) in a conical centrifuge tube. The leaves were then sonicated in a sonicator bath for 75 s and immediately vortexed vigorously for 30 s to dislodge the bacterial cells from the leaf surface and break up bacterial aggregates. Homogenization of the leaves in buffer prior to plating did not result in significantly higher numbers of cells recovered from the cilantro leaves than the numbers recovered by this method. The suspensions obtained from the leaf washing procedures were plated by hand when populations were expected to be low, or they were dilution plated with an automated plater (Autoplate 4000; Spiral Biotech Inc., Norwood, Mass.) onto appropriate culture media for estimation of bacterial populations by hand or by automated counting (QCount; Spiral Biotech Inc.).
Suspensions of cells recovered from leaves were plated onto Salmonella-Shigella agar (Becton Dickinson), Luria-Bertani agar with rifampin, and King's medium B agar (9) for enumeration of cells of Salmonella serovar Thompson, P. agglomerans FC1R or P. chlororaphis CIL12R, and indigenous bacteria, respectively. Salmonella serovar Thompson strain RM1987R was used for osmotolerance studies; cells of this strain were enumerated on Luria-Bertani agar with rifampin.
CLSM observations.
The localization of Salmonella serovar Thompson(pWM1007) and P. agglomerans(pWM1013) on inoculated cilantro was determined by examining sampled leaves or leaf disks that were mounted in glycerol-phosphate-buffered saline (50:50) with a Leica TCS-NT confocal microscope equipped with argon (488 nm), krypton (568 nm), and He/Ne (633 nm) lasers (Leica Microsystems, Wetzlar, Germany). The bacterial cells were visualized on leaves by using a 40×/0.70 PL FLUOTAR or 63×/1.2 W PL APO objective. The dye SYTO 62 (Molecular Probes, Eugene, Oreg.) was used to observe the total bacterial microflora in the phyllosphere. GFP fluorescence, DsRed fluorescence, and SYTO 62 fluorescence were detected with emission filter sets BP525/50, BP600/30, and LP645, respectively. Autofluorescence of the chlorophyll of leaves was detected with emission filter set LP590 when only GFP-labeled cells were observed and with emission filter set LP645 when a combination of DsRed- and GFP-labeled cells was examined or when SYTO 69-stained cells were examined.
Statistical methods.
Statistical calculations were performed with the program Instat, version 3.0 (GraphPad Software, Inc., San Diego, Calif.).
RESULTS
Colonization of cilantro.
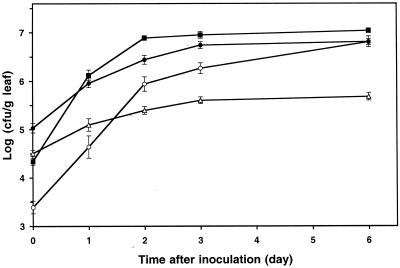
Comparative studies were undertaken to test the ability of Salmonella enterica serovar Thompson to grow and colonize plant surfaces relative to that of two common epiphytic bacteria, P. chlororaphis and P. agglomerans. Population studies revealed that Salmonella serovar Thompson grew 15-fold on leaves of cilantro plants incubated at room temperature (22°C) under humid conditions (Fig. 1). The bacterial epiphytes, including the indigenous bacterial flora on the control plants, grew at higher rates and reached maximum population sizes that were 17-fold higher than those of Salmonella serovar Thompson (Fig. 1). Additionally, Salmonella serovar Thompson accounted for 80% of the microbial carrying capacity of cilantro leaves 3 days after inoculation, whereas P. agglomerans FC1R and P. chlororaphis CIL12R represented nearly the total culturable leaf bacterial flora (data not shown).
FIG. 1.
Population dynamics of P. chlororaphis CIL12R (▪), P. agglomerans FC1R (•), and Salmonella serovar Thompson (▵) after inoculation of each strain individually onto cilantro plants and incubation of the plants at 22°C. The growth of indigenous bacteria on control plants (○) is also shown. Each data point indicates the mean of the log-transformed bacterial population size for 12 leaves. Each error bar indicates ±1 standard error of the mean.
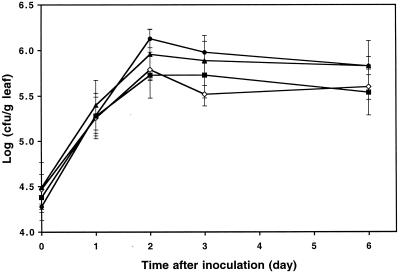
In order to determine if the epiphytic fitness of Salmonella serovar Thompson on cilantro is representative of that of the species S. enterica, cilantro plants were inoculated separately with four different serovars of S. enterica from various animal and nonanimal food sources. All four serovars, namely, S. enterica serovar Derby, S. enterica serovar Newport, S. enterica serovar Enteritidis, and S. enterica serovar Thompson, which were isolated from a turkey, alfalfa sprouts that were associated with an outbreak, mayonnaise, and a human patient affected with salmonellosis from an outbreak linked to cilantro, respectively, displayed very similar colonization trends (Fig. 2). The population size of each serovar on cilantro leaves increased by ca. 25- to 100-fold within 2 days after inoculation, and the population sizes were not significantly different (F = 1.93, P < 0.14). The survival data for the serovars were very similar after 6 days of incubation of the inoculated cilantro plants. It is noteworthy that the overall population sizes of S. enterica on cilantro were larger in the experiments described above, which were performed at 24°C, than in the experiments in which the fitness of this organism was compared to that of epiphytes (Fig. 1), which were carried out at 22°C. In a separate experiment, S. enterica serovar Typhimurium appeared to have growth patterns also resembling those of Salmonella serovar Thompson on cilantro plants (data not shown).
FIG. 2.
Colonization of cilantro leaves by strains of S. enterica serovar Derby (•), S. enterica serovar Newport (▴), S. enterica serovar Thompson (⋄), and S. enterica serovar Enteritidis (▪) that were linked to outbreaks from turkey, alfalfa sprouts, cilantro, and mayonnaise, respectively. Each data point indicates the mean of the log-transformed population size for a serovar inoculated onto cilantro plants incubated at 24°C. Each error bar indicates ±1 standard error of the mean of the log-transformed bacterial population size for 12 leaves.
Visualization of Salmonella serovar Thompson on leaves by CLSM.
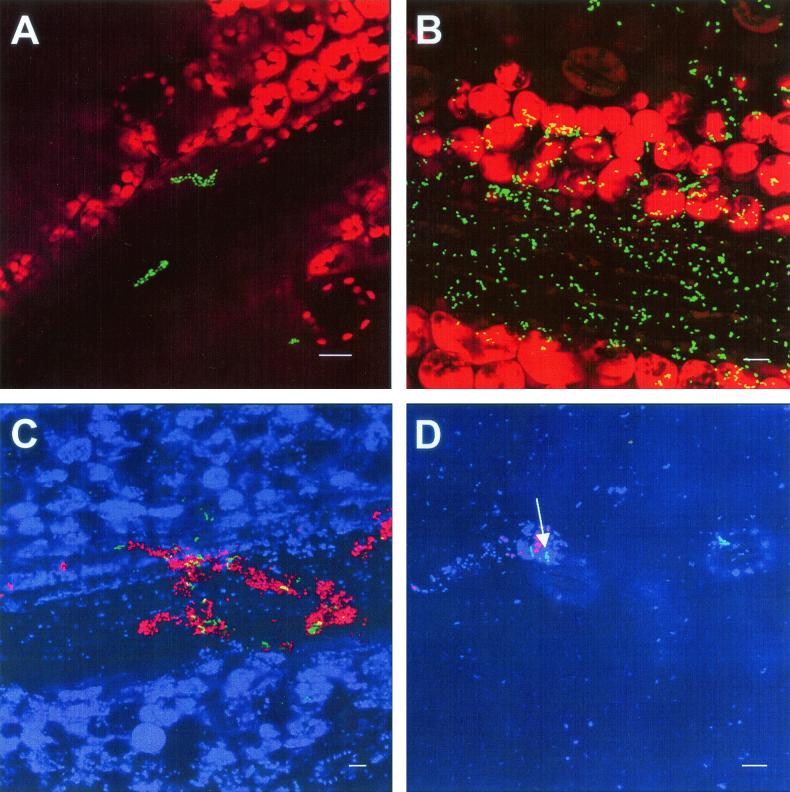
In order to observe the distribution of Salmonella serovar Thompson cells on the surfaces of leaves, we inoculated cilantro plants with low concentrations of Salmonella serovar Thompson(pWM1007) marked with a constitutively expressed GFP and visualized Salmonella serovar Thompson cells at different sampling times by CLSM. Plasmid pWM1007 was maintained in 93% of strain RM1987 cells over 40 cell generations and thus was considered to be stable in this strain. Two days after inoculation, microcolonies of Salmonella serovar Thompson were apparent mainly on the surfaces of major leaf veins and areas adjacent to the veins (Fig. 3A), as well as on the longitudinal edges of petioles. Large colonies and aggregates of Salmonella serovar Thompson cells were observed infrequently above plant epidermal cells and their junctions in regions between veins. Salmonella serovar Thompson was also detected infrequently in stomatal openings. Senescent leaves, which appeared 9 days after inoculation, harbored significant populations of Salmonella serovar Thompson that were located primarily on veins and neighboring areas, particularly those at the base of the leaf where the leaf blade joins the petiole (Fig. 3B). Coinoculation of GFP-labeled Salmonella serovar Thompson and DsRed-labeled P. agglomerans revealed that Salmonella serovar Thompson was often found associated with this enteric species on the cilantro leaf surface and that both organisms were detected in large and mixed aggregates (Fig. 3C). Salmonella serovar Thompson also formed mixed colonies with the indigenous bacterial flora, as evidenced by the bacterial cells that were stained with SYTO 62 but not marked with GFP or DsRed and were adjacent to Salmonella serovar Thompson cells (Fig. 3D).
FIG. 3.
Visualization by CLSM projected z series of the localization of Salmonella serovar Thompson(pWM1007) in the phyllosphere of inoculated cilantro plants. (A) Microcolonies of Salmonella serovar Thompson (GFP) above a vein of a cilantro leaf 2 days after inoculation. (B) High density of Salmonella serovar Thompson (GFP) cells in the vein area of a senescent cilantro leaf 9 days after inoculation. (C) Large aggregate of Salmonella serovar Thompson (GFP) and P. agglomerans (DsRed) cells on the vein of a cilantro leaf 7 days after inoculation. (D) Heterogeneous bacterial aggregate comprised of Salmonella serovar Thompson (GFP), P. agglomerans (DsRed), and natural microflora (visualized with the stain SYTO 62 and assigned the pseudocolor blue) (arrow) on the cilantro phylloplane. Bars, 10 μm.
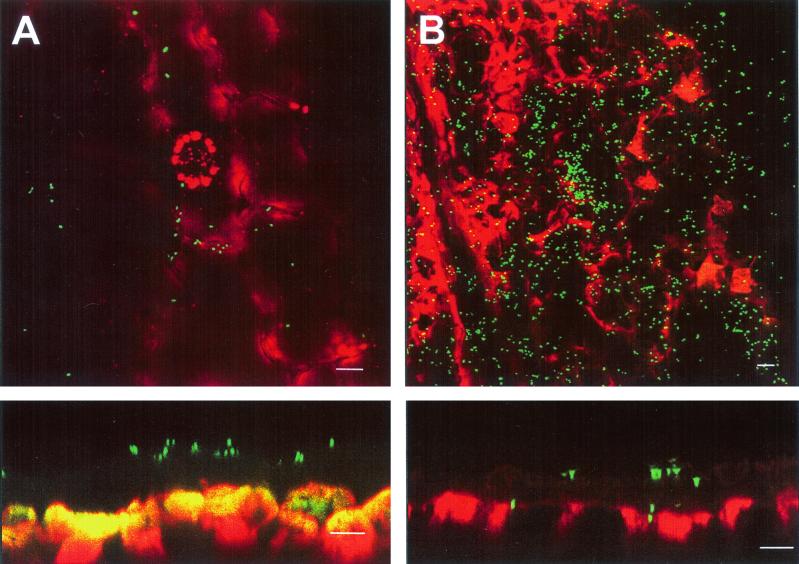
The localization of Salmonella serovar Thompson was investigated on cilantro plants that developed lesions 9 days postinoculation due to infection by a component of the natural leaf microflora. Although Salmonella serovar Thompson was not detected in every lesion of a particular leaf, it was present at extremely high densities in some lesions, as demonstrated in Fig. 4. The CLSM image of a leaf lesion shown in Fig. 4B (top panel) revealed that Salmonella serovar Thompson colonized the damaged tissue and reached high population densities in that environment compared to the population detected on healthy tissue (Fig. 4A, top panel). Optical cross sections of the same areas showed that whereas Salmonella serovar Thompson was present on the surface of the cuticle of healthy leaf tissue (Fig. 4A, lower panel), it was found within the plant tissue after gaining access through the disrupted cuticle of the damaged region of the leaf (Fig. 4B, lower panel).
FIG. 4.
Visualization by CLSM of Salmonella serovar Thompson (GFP) on the cuticle of a healthy leaf (A) and within the damaged tissue of a naturally diseased leaf (B) after inoculation onto cilantro plants. The top panels show the localization of Salmonella serovar Thompson in a projected z series of xy optical sections. The lower panels show corresponding cross sections which reveal the localization of Salmonella serovar Thompson above (A) and within (B) the plant tissue by a projected series of xz optical sections and by a single xz optical section, respectively. Note the amplification of the red and green signals above saturation which aids in detection of the very dimly fluorescent cuticle layer of the leaf surface. Bars, 10 μm.
Effect of temperature on colonization.
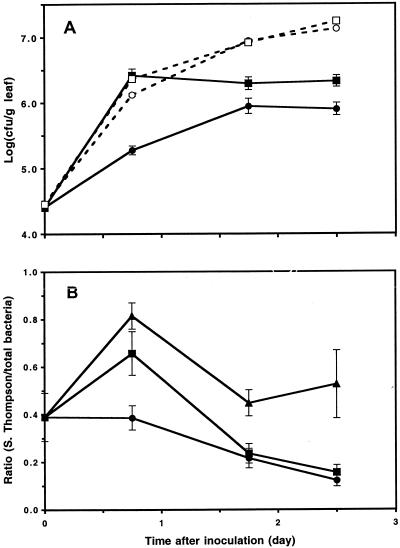
Temperature had a significant effect on the rate of colonization of cilantro leaves by Salmonella serovar Thompson. Within 18 h after inoculation of Salmonella serovar Thompson onto cilantro, the population size of this organism was 14-fold higher on leaves of plants incubated at 30°C than on leaves of plants incubated at 24°C (Fig. 5A). Moreover, the growth rate of Salmonella serovar Thompson on inoculated plants and the growth rate of the total culturable bacteria on uninoculated plants (control plants) were nearly identical at 30°C during this initial growth period. In contrast, the growth rate of Salmonella serovar Thompson was ca. sevenfold lower than that of the total culturable bacteria on control plants at 24°C (Fig. 5A). It is noteworthy that despite identical growth rates in the initial phase of colonization of the leaf surface at 30°C, the population size of Salmonella serovar Thompson on cilantro did not increase significantly after 18 h postinoculation, whereas that of the total culturable bacteria on control leaves continued to increase to at least eightfold-higher levels at 60 h after inoculation (Fig. 5A).
FIG. 5.
Effect of temperature on the colonization of cilantro plants by Salmonella serovar Thompson and by the natural bacterial flora. (A) Population dynamics of Salmonella serovar Thompson (solid symbols) on leaves of inoculated plants and population dynamics of the natural bacterial flora (open symbols) on leaves of control plants. Cilantro plants were incubated at 24°C (circles) or at 30°C (squares). (B) Ratio of the population size of Salmonella serovar Thompson to the population size of the total bacteria on individual leaves of inoculated plants incubated at 24°C (•), 30°C (▪), and 37°C (▴). Each error bar indicates ±1 standard error of the mean of the log-transformed bacterial population size (A) or population size ratio (B) for 15 leaves.
Analysis of the ratio of the population size of Salmonella serovar Thompson to the population size of the total culturable bacteria measured on the same leaves revealed that the mean ratios were higher at 30 and 37°C than at 24°C (Tukey-Kramer multiple comparison test on square root-transformed ratios; q = 4.16 and 6.67; P < 0.05 and 0.001, respectively) 18 h after inoculation (Fig. 5B). During this period, the average ratio of the size of the human pathogen population to the size of the total bacterial flora population did not change at 24°C, but it increased at 30 and 37°C. After this initial colonization phase, the mean ratios at all temperatures decreased, reflecting continued growth of the indigenous bacterial population while the Salmonella serovar Thompson population approached or had reached its maximum level (Fig. 5).
Tolerance to water stress in the phyllosphere.
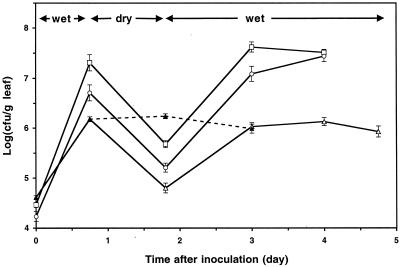
The tolerance of Salmonella serovar Thompson to water stress on plant surfaces was assessed by exposing Salmonella serovar Thompson to alternating wet and dry conditions on cilantro plants. These studies showed that the size of the population of Salmonella serovar Thompson viable cells declined at least 24-fold during dry conditions in the phyllosphere, which were simulated by incubating the plants at 60% relative humidity (Fig. 6). This decrease was smaller than the decreases observed for the sizes of the P. agglomerans and P. chlororaphis populations, which declined 43- and 32-fold, respectively, during the same period (Fig. 6). Moreover, the population size attained by Salmonella serovar Thompson after recovery from water stress during incubation of the plants under high-humidity conditions was similar to the maximum population size reached by Salmonella serovar Thompson on a subset of plants that were maintained under wet conditions during the entire experiment (Fig. 6). P. agglomerans and P. chlororaphis recovered from dry conditions, and their population sizes reached the high levels attained on plants by these species. When Salmonella serovar Thompson was subjected to greater water stress by incubating the plants at 50% relative humidity, the population size decreased 36-fold, but again it recovered to nearly the maximum level observed under wet conditions on cilantro leaves (data not shown).
FIG. 6.
Tolerance of Salmonella serovar Thompson (▵), P. agglomerans (○), and P. chlororaphis (□) to alternating wet and dry conditions in the cilantro phyllosphere at 26°C. The data points indicate the means of the log-transformed population size of each strain on cilantro leaves during a period of wet conditions, followed by exposure to 60% relative humidity and a recovery period under wet conditions. The dotted line shows the population size of Salmonella serovar Thompson on plants that were incubated under wet conditions throughout the experiment (▴). Each error bar indicates ±1 standard error of the mean of the log-transformed bacterial population size for 14 leaves.
DISCUSSION
The possibility that preharvest contamination of fruits and vegetables by S. enterica may result in epidemics of food-borne illness has prompted speculation that this human pathogen can grow and persist in the plant environment. The notion that human pathogens can colonize plants in the field challenges the concept that the pathogens are fit solely in what has been thought to be their natural environment, which is the intestinal tracts of animals and, sporadically, of humans. Our results demonstrate clearly that Salmonella serovar Thompson achieved significant population levels in the cilantro phyllosphere after inoculation at low concentrations onto cilantro plants. This was further shown by the distinct microcolonies and the large aggregates that Salmonella serovar Thompson formed primarily in the vein regions of cilantro leaves 3 and 9 days after inoculation, respectively, as observed by CLSM. The common presence of epiphytic bacteria on the veins of leaves has been reported previously (10). Indeed, both Salmonella serovar Thompson and P. agglomerans, a common epiphyte, were found to be parts of large heterogeneous aggregates on the leaf veins when they were coinoculated onto cilantro. At the time of inoculation, microscopy revealed that only individual cells of Salmonella serovar Thompson were present and that they were scattered at distant locations on the leaf surface. However, cells of Salmonella serovar Thompson also were observed at a higher frequency on the veins than in other areas of the leaves. These results suggest that there is a correlation between the location of Salmonella serovar Thompson inoculum cells and the location of Salmonella serovar Thompson aggregates observed after plant incubation. This may be explained by the high hydrophobicity of the waxy and trichome-free leaf surface of cilantro and the tendency of surface water to accumulate in the depressions of the leaf veins. Leben (10) demonstrated that areas above the veins of cucumber leaves had enhanced wettability and harbored a high proportion of the bacterial flora of the leaves. The presence of water may increase the leaching of nutrients onto the plant surface (24), as well as the solubility of substrates. High consumption of fructose and/or sucrose by epiphytic bacteria was detected by CLSM near the vein cells of bean leaves with the use of a bacterial reporter for these carbohydrates (11). The increase in the density of Salmonella serovar Thompson in the vein areas as early as 2 days after inoculation indicates that the external physicochemical environment of the veins provided sufficient free water and nutrient resources for Salmonella serovar Thompson to multiply. This observation may have significant implications for leafy crops that are sprinkler irrigated with water contaminated with human pathogens.
Although we used a strain of Salmonella serovar Thompson that was implicated in an epidemic linked to cilantro, there is so far no evidence of an association between a particular serotype of S. enterica and any plant species to which an outbreak has been linked. In our study, the lack of a difference in the abilities of various Salmonella serovars associated with epidemics caused by various animal or plant foods to colonize cilantro indicates that the serotypes do not confer differential fitness to S. enterica on plants. In addition, the comparative fitness studies of Salmonella serovar Thompson and two successful colonizers of aerial plant surfaces, P. agglomerans and P. chororaphis (8), revealed that the bacterial epiphytes consistently reached higher population levels than Salmonella serovar Thompson on cilantro. These initial results suggest that S. enterica is not as well adapted as common bacterial epiphytes to exploit the cilantro phyllosphere as a habitat. However, it is interesting that in a previous study, a strain of Salmonella serotype Typhimurium achieved under humid conditions at 24°C the same high population levels on bean and corn plants as pathogenic and nonpathogenic Pseudomonas syringae strains (20). Thus, it is likely that our studies performed with the cilantro microcosm do not reflect the entire range of colonization of plants by S. enterica. The maximum population size that S. enterica can achieve in the phyllosphere depends not only on the genetic potential of the organism to exploit this habitat but also on the different microenvironments provided by various plant species exposed to different growth conditions (24).
Temperature also may have a major effect on the colonization of plants by Salmonella serovar Thompson. Since Salmonella serovar Thompson and other human pathogens grow optimally at 37°C (8), temperature may greatly affect their fate once they land on a plant surface. Bacterial population studies performed at 22 to 24°C do not fully reflect the potential for these pathogens to colonize plants under temperature conditions that are optimum for growth of the pathogens and still relevant to field conditions. Our temperature studies indicated that higher growth rates of Salmonella serovar Thompson at 30°C increased the colonization level on cilantro leaves within the first 18 h after inoculation. During this period, Salmonella serovar Thompson colonized the phyllosphere at the same rate as the aerobic bacteria of noninoculated cilantro plants and accounted for a higher proportion of the total aerobic bacterial flora of inoculated plants at 30 and 37°C than at 24°C. Although higher temperatures may have induced higher rates of nutrient leaching from the plant cells to the leaf surface (24), the resulting enhanced availability of nutrients to the bacteria cannot fully account for the large increase in the population size of Salmonella serovar Thompson in the first 18 h after inoculation, since the indigenous bacterial population on the control plants was only slightly larger at 30°C than at 24°C. It is likely that higher growth rates at warm temperatures enabled Salmonella serovar Thompson to utilize a larger share of the limited nutrients available on the plant surface, thus increasing the competitiveness of this organism against indigenous bacteria in the early colonization phase.
The failure of Salmonella serovar Thompson to achieve final population levels as high as those of the indigenous bacteria independent of temperature may be explained by the limited ability of Salmonella serovar Thompson to utilize a wide range of nutrient sources on the leaf surface. It is likely that S. enterica is better adapted evolutionarily to metabolize the carbon and nitrogen sources found in the animal and human gut than those found on plant surfaces. Previous studies have indicated that the level of carbon-containing nutrients limits the growth of bacteria on leaves (15, 27, 28). Glucose and fructose are among the most abundant simple sugars present on leaves (15, 19). Therefore, it is possible that Salmonella serovar Thompson utilizes simple carbon sources, such as these sugars, on plants, resulting in an initial burst of growth, but then is unable to assimilate many of the more complex nutrients found on the phylloplane. Optimal growth rates would enable Salmonella serovar Thompson to use a higher proportion of the nutrients that it is capable of metabolizing and thus to develop larger populations on leaves. A comparison of the carbon and nitrogen compounds present on leaves to the carbon and nitrogen compounds that S. enterica can metabolize should provide important insight into the competitiveness of this human pathogen in the phyllosphere. Nevertheless, the ability of Salmonella serovar Thompson to reach high densities rapidly on plants under warm and humid conditions is significant considering the direct correlation between infectious dose and severity of illness that has been described for many serovars of S. enterica, including serovar Thompson (4). These data are relevant also to the potential role of postharvest temperature abuse of produce in human illness.
Also relevant to preharvest contamination of produce is the tolerance of S. enterica to dry conditions on plant surfaces. Bacterial cells on leaves experience frequent periods of water stress, which may result in osmotic and/or matric stress, as well as in low nutrient availability. Previous studies have shown that the sizes of epiphytic bacterial populations in the field decrease after prolonged periods of dry weather and increase when plants are wet, such as following rain and irrigation (7). Our results indicate that Salmonella serovar Thompson has a tolerance to low water availability on cilantro leaves under dry conditions at 60% relative humidity for 24 h that is similar to that of P. agglomerans and P. chlororaphis. It should be noted that these experiments were conducted in a growth chamber with heavy airflow. Therefore, it is unlikely that Salmonella serovar Thompson cells were protected in the humid environment of the laminar layer above the leaf surface. The death of Salmonella serovar Thompson that occurred under low relative humidity conditions and the subsequent growth during recovery under humid conditions were comparable to results reported for similar experiments with epiphytic bacteria on bean plants (12, 14). We also observed that the population size of Salmonella serovar Thompson did not increase after the organism was inoculated onto cilantro plants that were kept under conditions of 40 to 50% relative humidity for 4 days (data not shown). This result is similar to that reported by O'Brien and Lindow (20), who showed that Salmonella serovar Typhimurium failed to grow at a low relative humidity on bean and corn plants (20). Conversely, it is possible that S. enterica cells entered a viable but nonculturable state under desiccation conditions, resulting in underestimation of the viable population size on dry leaves by direct plating on selective medium. Pedersen and Leser (21) reported that as little as 0.1% of the viable population of Enterobacter cloacae, a species closely related to S. enterica, was culturable 14 days after inoculation onto bean plants in the field (21). The possible presence of viable but nonculturable cells of S. enterica in the phyllosphere has important implications for pathogen surveillance on produce and for risk assessment studies, and this area is an important area for future studies. It is noteworthy that the size of the population of Salmonella serovar Thompson on cilantro remained low for 4 days at 40 to 50% relative humidity and then fully recovered to the initial level during subsequent incubation of the cilantro plants under humid conditions (data not shown). Thus, our studies indicate that Salmonella serovar Thompson can persist on plant surfaces under dry conditions for extended periods of time. Other studies have shown that S. enterica is capable of surviving for more than 1 year in disinfected poultry houses (3) and for at least 280 days in a dry cow manure-soil mixture (17). The ability of Salmonella serovar Thompson to tolerate desiccation and to recover efficiently from water stress in the phyllosphere suggests that it could be present at low levels in the field under dry conditions but develop significant populations during subsequent high-humidity conditions resulting from dew, rain, or irrigation water.
Our observations of GFP-labeled Salmonella serovar Thompson on cilantro leaves suggest that Salmonella serovar Thompson colonizes mainly the leaf surface and may not be able to evade environmental stresses by hiding in the substomatal cavities, as was hypothesized for pathogenic strains of P. syringae on bean leaves (26). Conversely, leaf lesions, by which Salmonella serovar Thompson gains access to the internal plant tissue, may provide shelter from various stresses and abundant nutrients. A survey of the marketplace revealed higher incidences of Salmonella species on fruits and vegetables affected by soft rot than on undamaged produce (25). The high densities of Salmonella serovar Thompson detected in natural lesions of cilantro inoculated in the laboratory suggest that pre- and postharvest plant injury may be an important factor in the occurrence of Salmonella outbreaks due to produce. Studies to investigate the behavior of S. enterica in plant lesions are under way in our laboratory.
Our study demonstrates that Salmonella serovar Thompson has the ability to grow and survive on plant surfaces but is less fit than two bacterial epiphytes under the same conditions. These results challenge the notion that a human pathogen such as S. enterica is solely fit to colonize the enteric tissues of its vertebrate hosts. The interactions between human and animal pathogens and their hosts have been redefined by the demonstration of common virulence factors in Pseudomonas aeruginosa involved in pathogenicity in lettuce and Arabidopsis thaliana and in animals (22, 23). The large population sizes that Salmonella serovar Thompson reached rapidly on cilantro plants under warm conditions further support the hypothesis that human pathogens may find a particular ecological niche on plant surfaces. Our study provides evidence that salmonellosis outbreaks can originate from preharvest contamination of fruits and vegetables by S. enterica.
Acknowledgments
We thank William Miller for the gift of plasmids pWM1007 and pWM1013 and Denyse Goff for technical assistance.
This work was funded by U.S. Department of Agriculture Agricultural Research Service CRIS project 201-5325-210-40.
REFERENCES
- 1.Campbell, V. J., J. Mohle-Boetani, R. Reporter, S. Abbott, J. Farrar, M. T. Brandl, R. E. Mandrell, and S. B. Werner. 2001. An outbreak of Salmonella serotype Thompson associated with fresh cilantro. J. Infect. Dis. 183:984-987. [DOI] [PubMed] [Google Scholar]
- 2.Cook, K. A., T. E. Dobbs, W. G. Hlady, et al. 1998. Outbreak of Salmonella serotype Hartford infections associated with pasteurized orange juice. JAMA 280:1504-1509. [DOI] [PubMed] [Google Scholar]
- 3.Davies, R. H., and C. Wray. 1995. Observations of disinfection regimens used on Salmonella enteritidis infected poultry units. Poultry Sci. 74:638-647. [DOI] [PubMed] [Google Scholar]
- 4.Glynn, J. R., and D. J. Bradley. 1992. The relationship between infecting dose and severity of disease in reported outbreaks of Salmonella infections. Epidemiol. Infect. 109:371-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedberg, C. W., F. J. Angulo, K. E. White, C. W. Langkop, W. L. Schell, M. G. Stobierski, A. Schuchat, J. M. Besser, S. Dietrich, L. Helsel, P. M. Griffin, J. W. McFarland, and M. T. Osterholm. 1999. Outbreaks of salmonellosis associated with eating uncooked tomatoes: implications for public health. Epidemiol. Infect. 122:385-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedberg, C. W., K. L. MacDonald, and M. T. Osterholm. 1994. Changing epidemiology of food-borne disease: a Minnesota perspective. Clin. Infect. Dis. 18:671-682. [DOI] [PubMed] [Google Scholar]
- 7.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams (ed.). 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams and Wilkins, Baltimore, Md.
- 9.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 10.Leben, C. 1988. Relative humidity and the survival of epiphytic bacteria with buds and leaves of cucumber plants. Phytopathology 78:179-185. [Google Scholar]
- 11.Leveau, J. H. J., and S. E. Lindow. 2001. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 98:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindow, S. E., G. Andersen, and G. A. Beattie. 1993. Characteristics of insertional mutants of Pseudomonas syringae with reduced epiphytic fitness. Appl. Environ. Microbiol. 59:1593-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahon, B. E., A. Ponka, W. N. Hall, K. Komatsu, S. E. Dietrich, A. Siitonen, G. Cage, P. S. Hayes, M. A. Lambert-Fair, N. H. Bean, P. M. Griffin, and L. Slutsker. 1997. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. J. Infect. Dis. 175:876-882. [DOI] [PubMed] [Google Scholar]
- 14.Manulis, S., A. Haviv-Chesner, M. T. Brandl, S. E. Lindow, and I. Barash. 1998. Differential involvement of indole-3-acetic acid biosynthetic pathways in pathogenicity and epiphytic fitness of Erwinia herbicola pv. gypsophilae. Mol. Plant-Microbe Interact. 11:634-642. [DOI] [PubMed] [Google Scholar]
- 15.Mercier, J., and S. E. Lindow. 2000. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 66:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, W. G., A. H. Bates, S. T. Horn, M. T. Brandl, M. R. Wachtel, and R. E. Mandrell. 2000. Detection on surfaces and in Caco-2 cells of Campylobacter jejuni cells transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 66:5426-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitscherlich, E., and E. H. Marth. 1984. Microbial survival in the environment. Springer Verlag, Berlin, Germany.
- 18.Mohle-Boetani, J., R. Reporter, and S. B. Werner. 1999. An outbreak of Salmonella serogroup Saphra due to cantaloupes from Mexico. J. Infect. Dis. 180:1361-1364. [DOI] [PubMed] [Google Scholar]
- 19.Morgan, J. V., and H. B. Tukey. 1964. Characterization of leachate from plant foliage. Plant Physiol. 39:590-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien, R. D., and S. E. Lindow. 1989. Effect of plant species and environmental conditions on epiphytic population sizes of Pseudomonas syringae and other bacteria. Phytopathology 79:619-627. [Google Scholar]
- 21.Pedersen, J. C., and T. D. Leser. 1992. Survival of Enterobacter cloacae on leaves and in soil detected by immunofluorescence microscopy in comparison with selective plating. Microb. Releases 1:95-102. [Google Scholar]
- 22.Rahme, L. G., E. G. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and in animals. Science 268:1899-1902. [DOI] [PubMed] [Google Scholar]
- 23.Rahme, L. G., M. W. Tan, L. Le, S. M. Wong, R. G. Tompkins, S. B. Calderwood, and F. M. Ausubel. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 94:13245-13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tukey, H. B. 1970. The leaching of substances from plants. Annu. Rev. Plant Physiol. 21:305-324. [Google Scholar]
- 25.Wells, J. M., and J. E. Butterfield. 1997. Salmonella contamination associated with bacterial soft-rot of fresh fruits and vegetables in the marketplace. Plant Dis. 81:867-872. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, M., S. S. Hirano, and S. E. Lindow. 1999. Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl. Environ. Microbiol. 65:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson, M., and S. E. Lindow. 1994. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning. Appl. Environ. Microbiol. 60:4468-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson, M., and S. E. Lindow. 1994. Ecological similarity and coexistence of epiphytic ice-nucleating (Ice+) Pseudomonas syringae strains and a non-ice-nucleating (Ice−) biological control agent. Appl. Environ. Microbiol. 60:3128-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]