Abstract
Short- and long-term persistence of pathogenic (i.e., tumor forming) agrobacteria in soil was investigated in six nursery plots with a history of high crown gall incidence. No pathogenic Agrobacterium strains were isolated in soil samples taken in fall and winter in any plots, but such strains were isolated from both bulk soils and weed rhizospheres (over 0.5 × 105 pathogenic CFU/g of bulk soil or rhizosphere) in three out of six plots in spring and summer. PCR amplifications of a vir sequence from DNA extracted from soil confirmed the presence of Ti plasmids in summer and their absence in fall and winter. The results indicate that strains that harbor a Ti plasmid had an unforeseen positive fitness versus Ti plasmid-free strains in soil and rhizosphere in spring and summer in spite of the apparent absence of tumor, and hence of opines. The gain of fitness occurred during a bloom of all cultivable agrobacteria observed only in conducive soils. An evolution of the pathogenic population was recorded during a 4-year period in one particularly conducive soil. In 1990, the pathogenic population in this soil consisted of only biovar 1 strains harboring both octopine- and nopaline-type Ti plasmids. In 1994, it consisted of only nopaline-type Ti plasmids equally distributed among biovar 1 and 2 strains. These results suggest that nopaline-type Ti plasmids conferred a better survival ability than octopine-type Ti plasmids to biovar 2 agrobacteria under the present field conditions.
Agrobacterium spp. are a genus in the family Rhizobiaceae. Strains of Agrobacterium harboring a tumor-inducing (Ti) plasmid cause the plant disease crown gall, which is characterized by uncontrolled proliferation of plant tissues (e.g., tumors). In the course of the infection process, a region of the Ti plasmid, the T-DNA, is transferred to and integrated into the plant genome (13, 34). In the transformed plant cell, T-DNA genes encode enzymes responsible for the uncontrolled synthesis of the plant hormones auxin and cytokinin, which account for the appearance of plant tissue hyperplasia. Other T-DNA genes encode enzymes responsible for the biosynthesis of particular compounds by the transformed plant cells, called opines. Opines are utilized as energy sources by strains harboring a Ti plasmid. Some opines induce the conjugal transfer of the Ti plasmids. Opines are relatively specific niches for strains harboring Ti plasmids, and tumors that produce opines are favorable habitats for both the proliferation of agrobacteria and the conjugal spread of Ti plasmids (10).
Ti plasmids are large genetic elements, representing about 5% of the agrobacterial genome (1). As a consequence, they might negatively affect the fitness of Agrobacterium in the absence of opines. When population dynamics were monitored in the presence of opines, the closely related Ri plasmid was found to improve the growth and thus the fitness of a strain harboring this plasmid over those of a plasmid-free strain. Conversely, in the absence of opine, Ri and related plasmids hampered the bacterial growth and thus acted as a genetic load (15). After the removal of diseased plants from bulk soil, and hence after the disappearance of opine sources, pathogenic agrobacteria should be negatively selected and should decline more or less rapidly. Probably this has been the empirical rationale for leaving contaminated soils fallow to eliminate pathogenic agrobacteria. This assumption comes, however, in sharp contrast to regular outbursts of crown gall, which appears to be the main bacterial disease in stone-fruit tree nurseries in Mediterranean countries.
Almost all agrobacteria, including both pathogenic and nonpathogenic forms, are able to live as saprophytes in soil by consuming nutrients of soil or plant origin. However, the survival of pathogenic agrobacteria in soil and/or the rhizosphere is poorly documented in spite of interest in obtaining such information about the primary inoculum of a major soilborne disease. However, isolation of pathogenic strains from pasture soil that had never been tilled or cultivated (28) and from a contaminated soil left fallow for 6 years (2) demonstrates the potential for long-term survival of pathogenic agrobacteria in soil.
In the present study, we report data generated from the monitoring of pathogenic populations over 4 years in one plot and over several seasons in six plots that provide new insights into the ecology of Agrobacterium and Ti plasmids in soil.
MATERIALS AND METHODS
Sampling procedure.
Bulk soil and weed root samples were collected from six plots in different locations in Algeria (Table 1). The plot areas were in the size range of 3,000 to 5,000 m2. In each plot, eight sampling points regularly distributed were chosen along plot diagonals and pooled in one homogenized sample for analysis. By using this procedure, the eight individual sampling points of each plot could be resampled over time within a ca. 3-m2 zone, giving comparable homogenized samples over time. At each sampling point, about 50 g of bulk soil was collected in the top 15 to 20 cm of soil. A weed plant growing close to the sampling point was carefully removed from the soil with most of its root system and collected separately. All samples were collected in sterile black polyethylene bags. Pooled soil and pooled weed rhizospheres were processed within 24 h. Soil texture, pH, and information about agricultural history of field plots were recorded (Table 1). Samplings were performed March 1990 (2), 2 November 1993, 6 January 1994, 18 April 1994, and 23 June 1994 at Beni Tamou, the Mitidja flat, Blida's wilaya, in a fallow plot. This plot was a former stone-fruit nursery from which diseased materials were completely removed in 1984 and which has since tilled annually in the fall. All five of the other plots were known to have a history of high crown gall incidence 1 year ago. They were located in nurseries that produced stone-fruit scions, in the Tlemcen's wilaya, at an elevation of 600 m or more. Diseased materials were removed during the winter of 1996 to 1997, and samplings were performed 18 to 22 October 1997, 6 to 10 February 1998, 12 to16 April 1998, and 3 to 7 July 1998. Pathogenic strains were isolated from rhizospheres of the following dominant weeds: Lavatera trimestris and Chrysanthemum segetum in Beni Tamou; Solanum nigrum and various grasses, including Bromus sp., in Boughrara; and Raphanus raphanistrum and Sinapis arvensis in Ouled Mimoun.
TABLE 1.
Location, previous crops, and soil characteristics of the plots studied
| Location | Previous and current crops | % of soil texture
|
pH | ||
|---|---|---|---|---|---|
| Clay | Loam | Sand | |||
| Beni Tamou | Peach until 1984, then left fallow | 28 | 67 | 5 | 8.4 |
| Boughrara | Peach rootstock then peach grafted on almond | 51 | 39 | 10 | 7.8 |
| Ouled Mimoun | Peach, almond then almond grafted on almond | 60 | 33 | 7 | 7.8 |
| Sidi Abdli 1 | Peach, almond then peach grafted on almond | 34 | 47 | 19 | 7.8 |
| Sidi Abdli 2 | Grapevine then almond grafted on almond | 34 | 47 | 19 | 7.8 |
| Saf Saf | Almond rootstock then peach grafted on almond | 47 | 26 | 27 | 8.2 |
Isolation and cultivation of agrobacteria.
Soil samples were processed as follows. One gram of soil was pounded in a sterile mortar in the presence of 10 ml of sterile distilled water. The resulting suspension was left for 30 min at room temperature and then serially diluted 10-fold. One hundred microliters of each suspension was spread onto plates containing Agrobacterium selective media 1A and 2E (3) and onto nonselective MG medium (17). MG medium was useful in complementing the other two media to determine the total number of Agrobacterium-like colonies, because it enables the simultaneous growth of the two biovars and allows the possible isolation of peculiar strains not recovered with selective media. Plates were incubated for 1 week at 27°C before counting. Root samples were treated as follows. After the removal of the weakly adherent soil particles by gentle manual shaking, 1 g of the root system with tightly adherent soil particles was crushed in a mortar in the presence of 10 ml of sterile distilled water. The resulting suspensions were processed as indicated above. Agrobacterium-like colonies were selected from each medium and purified onto potato dextrose agar (Difco Laboratories) and MG medium according to the description of Moore et al. (21). Purified agrobacterial colonies were stored in sterile distilled water at 4°C for further analysis.
Pathogenicity determination.
The tumor-forming ability of each isolate was determined by the method of Moore et al. (21), by inoculating three wounded stems of 4-week-old tomato seedlings (cv. Marmande) and three wounded leaves of Kalanchoe daigremontiana cultivated in the greenhouse with dense suspensions of 48-h-old bacterial cultures. Tumor formation was assessed by visual inspection 3 weeks after inoculation. The presence of a Ti plasmid in pathogenic strains and its absence in nonpathogenic strains were verified by DNA hybridizations with Ti plasmid probes as indicated below.
Biovar affiliation.
Biovars were determined according to biochemical and physiological tests (21), which included Gram staining, oxidation of lactose to 3-ketolactose, growth on Simmon's citrate sodium medium, growth on l-tyrosine, tolerance to 2% sodium chloride, growth and pigmentation on ferric ammonium citrate medium, acid production from erythritol, and growth at 35°C on solid NGA (21). Results were scored after 7 days at 28°C. The biovar affiliation was determined for all pathogenic strains and for 180 nonpathogenic isolates arbitrarily chosen whatever the sampling season from Beni Tamou, Ouled Mimoun, and Boughrara plots (30 from soil and 30 from rhizosphere in each plot).
Sensitivity to agrocin 84.
Strains were tested for agrocin sensitivity on MG agar plates by the method of Stonier (31) as modified by Cooksey and Moore (5). Mannitol glutamate plates were inoculated by spreading a loopful of strain K84, which had recently been cultivated on MG medium, at the center of each plate (over a circular zone about 3 mm in diameter). Plates were incubated for 48 h at room temperature. Resuspensions of the isolates in water for assay (ca. 108 CFU/ml) were sprayed as a fine mist onto the surface of the medium containing K84. Growth inhibition of the assayed strain was recorded after 72 h at 28°C. The agrocin 84-sensitive strain C58 and the agrocin 84-resistant strain B6 were used as positive and negative controls, respectively.
Opine synthesis and catabolism.
The presence of opines in tumors was investigated after extraction of opines from plant tissues and concentration and separation by high-voltage paper electrophoresis at pH 1.9 as previously described (9). Opine utilization was assayed by inoculating individual strains into 200 μl of a degradation cocktail, which consisted of AT minimal medium (25) supplemented with ammonium sulfate (1.0 g/liter); yeast extract (100 mg/liter); and the opines octopine (5 mM), nopaline (5 mM), cucumopine (4 mM), agropine (2.5 mM), mannopine (2.5 mM), and mannopinic acid (5 mM). The inoculated cocktail was incubated at 28°C for 7 days. Utilization of opines was assessed by investigating their disappearance from the degradation cocktail by high-voltage paper electrophoresis (9).
Extraction of DNA from soil.
Extraction of DNA from soil was done as described by Frostegård et al. (12). Extraction and purification of DNA from soil were performed immediately after soil samplings. The soil samples were sieved (2 by 2 mm), and 4× 250-mg soil aliquots were resuspended in 0.5 ml of TENP buffer (50 mM Tris, 20 mM EDTA disodium salt [pH 9.0], 100 mM NaCl, 1% [wt/vol] polyvinyl polypyrrolidone [Sigma Chemical Co. St. Louis, Mo]). The soil suspension was vortexed for 1 min and homogenized in a rotary shaker for 2 h at room temperature. The suspensions were centrifuged for 10 min at ca. 8,000 × g at 4°C. The DNA dissolved in the supernatant was precipitated with 3 M sodium acetate and isopropanol. DNA extracted from 4× 250 mg of soil was pooled and resuspended in 100 μl of TE8 buffer (50 mM Tris HCl, 20 mM EDTA [pH 8.0]) and then was further purified once on a Sephacryl S200 microtube purification cartridge (Pharmacia Biotech, Upsalla, Sweden) and twice on an Elutip d column (Schleicher & Schuell, Dassel, Germany), both as recommended by the manufacturers. The purified DNA was precipitated according to standard procedures with ethanol, and the pellet was resuspended in 10 μl of ultrapure water.
PCR detection and quantification of Ti plasmids.
The technique used for PCR detection of Ti plasmid sequences in soil microflora DNA was adapted from the method described by Picard et al. (26). A 246-bp conserved region of the vir region of the Ti plasmid was amplified with primers F14 (FGPvirG15′) (GAA CGT GTT TCA ACG GTT CA) and F44 (FGPvirB11 + 21) (TGC CGC ATG GCG CGT TGT AG), which are highly efficient for specific detection of Ti plasmids (6, 24). The concentration of the target sequence was estimated by serially diluting the template DNAs before PCR as previously described (24). For this purpose, soil DNA extracts were diluted 10-fold up to 10−6. PCRs were performed in 0.5-ml microtubes in a final volume of 25 μl containing reaction buffer (10 mM Tris-HCl [pH 8.3], 50 mM MgCl2, 0.01% gelatin), the four deoxynucleoside triphosphates (dNTPs; 20 μM each), the two primers (1 μM each), 2 U of Taq DNA polymerase (Gibco-BRL), and 1 μl of the diluted template DNA. Cyclings were performed in a dry-block thermocycler (Perkin-Elmer) with an initial denaturation step of 5 min at 95°C followed by 40 cycles of denaturation for 1 min at 95°C, annealing for 1 min at 55°C, and extension for 1 min at 72°C. PCR products were separated by electrophoresis in 2% standard agarose gels and stained with ethidium bromide.
Colony hybridization.
Isolates were tested for the presence of a Ti plasmid by hybridization of colonies patched onto a nylon membrane, with nonradioactive probes obtained by PCR amplification of Ti plasmid regions of C58 by the procedure described by Mougel et al. (22). The occurrence of a Ti plasmid was probed with a fragment of the common tmr gene obtained with primers F49 (=FGPtmr530) (CCA TGT TGT TTG CTA GCC AG) and F50 (=FGPtmr701′) (CCT TCG AAT CCG TCG AAA GC), while nopaline-type Ti plasmids were specifically probed with a fragment of the nos gene of C58 obtained with primers F139 (=FGPnos975) (GGC AAT TAC CTT ATC CGC AA) and F140 (=FGPnos1236′) (CAC CAT CTC GTC CTT ATT GA). Patched colonies of C58 (pTiC58, nopaline-type Ti plasmid) and B6 (pTiB6, octopine-type Ti plasmid) and the Ti plasmid-free derivative strains C58C1 were used as positive and negative controls, respectively. The presence and characterization of a Ti plasmid were verified for all pathogenic strains and for the 180 arbitrarily chosen nonpathogenic isolates described above.
Statistical analyses.
Statistical analyses were done as described by Dagnelie (8) for testing the independence of proportions. The confidence interval of proportions, determined by a table obtained from Hald (16), was used to determine the confidence intervals of percentages of pathogenic strains over the total agrobacterial populations and, in turn, their densities in samples. The chi-square test was used to compare the relative distributions of pathogenic and nonpathogenic strains in different samples.
RESULTS
Temporal variations in the pathogenic agrobacterial population at Beni Tamou.
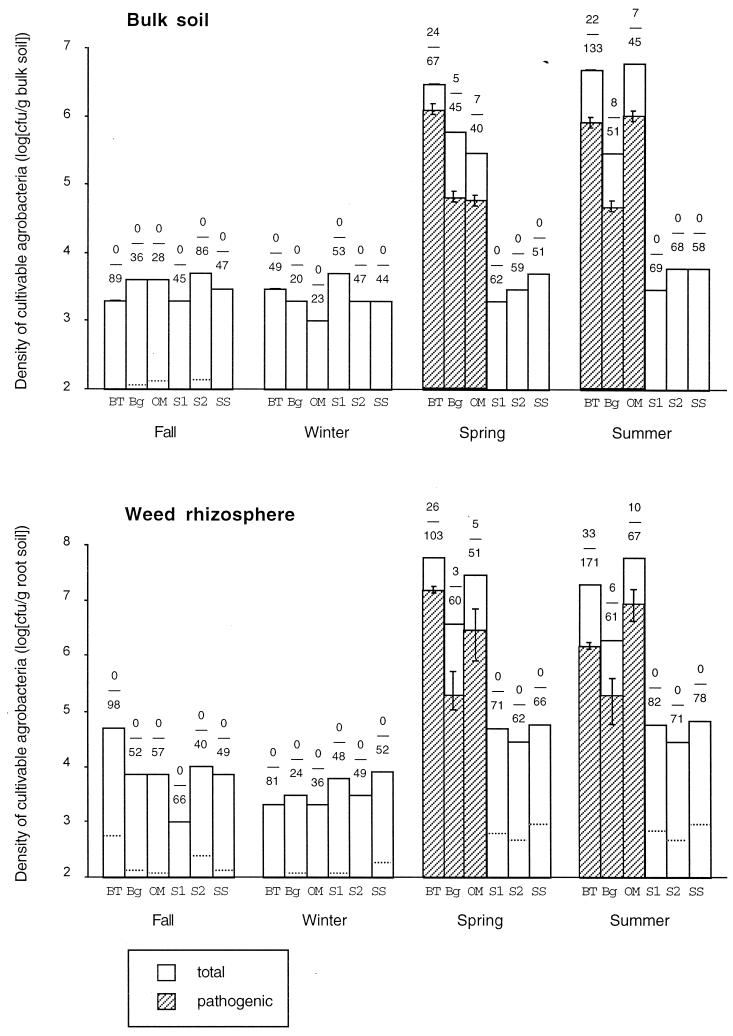
Our previous work from March 1990 revealed that 33% of the total Agrobacterium-like isolates recovered directly from the fallow field at Beni Tamou were pathogenic (2). To monitor the possible evolution of the pathogenic population, new samples were taken 3 years later at the same plot. The samples taken in November 1993 and January 1994 did not produce any pathogenic strains among the Agrobacterium-like colonies isolated directly from the bulk soil and the weed rhizosphere (Fig. 1). The differences between the number of pathogenic versus nonpathogenic strains in the 1990 sampling (24 versus 22) and the 1993-1994 samplings (0 versus 89 and 0 versus 49, respectively) performed in the bulk soil were highly significant (chi-square result = 56.5, df = 1, P < 10−4; and chi-square result = 34.2, df = 1, P < 10−4, respectively). These results indicate a drop in the pathogenic Agrobacterium population below the threshold of detection in the test plot. Nevertheless, samples taken in April 1994 and June 1994 allowed the isolation of pathogenic along with nonpathogenic isolates, whether these came from bulk soil or weed rhizosphere. From March 1990 to June 1994, significant fluctuations in the ratio of pathogenic versus total agrobacteria were thus observed in this test plot. Considering that the same sampling and identification procedures were performed by the same person (Z.K.), the source of variation was assumed to be the sampling dates. The observation that pathogenic populations fluctuated over time and season was unexpected. Therefore, investigations were undertaken in other test plots to determine whether the phenomenon is reproduced elsewhere.
FIG. 1.
Seasonal density fluctuations of total and pathogenic Agrobacterium-like colonies isolated directly from bulk soil and weed rhizosphere in six different plots: BT, Beni Tamou, 1993-1994; Bg, Boughrara, 1997-1998; OM, Ouled Mimoun, 1997-1998; S1, Sidi Abdli 1, 1997-1998; S2, Sidi Abdli 2, 1997-1998; SS, Saf Saf, 1997-1998. Ratios indicate the numbers of pathogenic over total agrobacteria recovered per sample. Dotted lines indicate the maximum absolute number of pathogens (generally below 2 log CFU/g). Vertical bars indicate a 0.95 confidence interval determined by the binomial distribution.
Seasonal fluctuations in total, cultivable agrobacterial populations in different plots.
Agrobacteria were recovered from bulk soils and/or weed rhizospheres in five other plots located in Western Algeria, far from Beni Tamou. In a first step, fluctuations in the numbers of total, cultivable agrobacteria were monitored over several seasons. Estimates of the total number of cultivable agrobacterial populations were determined by MG plate counting, but similar counts were obtained with media 1A and 2E, indicating that Agrobacterium-like colonies counted with MG were predominantly a mixture of biovars 1 and 2. Agrobacterium-like colonies were arbitrarily picked in similar amounts from the three media for further analyses. Morpho-biochemical tests performed on all 165 pathogenic and 180 arbitrarily selected nonpathogenic isolates recovered from the three media confirmed that Agrobacterium-like colonies consisted of bona fide agrobacteria in all instances.
The density of total, cultivable agrobacteria was found to fluctuate over the season at Boughrara, Ouled Mimoun, and as at Beni Tamou (Fig. 1). The average Agrobacterium densities in these three plots were 3.3 × 103, 2.0 × 103, 1.3 × 106, and 3.8 × 106 CFU/g of bulk soil for fall, winter, spring, and summer samples, respectively. As an indication, in March 1990 at Beni Tamou, the total number of Agrobacterium-like colonies was 3 × 107 CFU/g of bulk soil. Conversely, in the plots located at Sidi Abdli 1 and 2 and Saf Saf, where no pathogenic isolates were recovered, the densities of cultivable agrobacteria were found to be low during all seasons: 3.3 × 103, 3.0 × 103, 3.3 × 103, and 5 × 103 CFU/g of bulk soil in fall, winter, spring, and summer samples, respectively.
Similar results were obtained with the weed rhizosphere samples. The average Agrobacterium densities in Boughrara, Ouled Mimoun, and Beni Tamou plots were 2.1 × 104, 2.3 × 103, 3.1 × 107, and 2.7 × 107 CFU/g of root for fall, winter, spring, and summer samples, respectively. At Sidi Abdli 1 and 2 and Saf Saf, there were 6 × 103, 5.3 × 103, 4.7 × 104, and 5.3 × 104 CFU/g of root for fall, winter, spring, and summer samples, respectively.
Seasonal fluctuations of pathogenic agrobacterial population at various plots.
The newly investigated plots were stone-fruit nurseries reported to have a history of high crown gall incidences. Pathogenic agrobacteria were isolated in only two out of five plots (Fig. 1). In these two plots (Boughrara and Ouled Mimoun), the number of pathogenic isolates was also found to fluctuate over time. In both cases, pathogenic agrobacteria were not isolated in fall or winter (October 1997 and February 1998), but only in spring and summer (April and July 1998). Statistical analyses showed a highly significant effect of the season of the relative number of pathogenic versus nonpathogenic strains at each site for soil isolates (Beni Tamou, chi-square result = 50.8, df = 3, P < 10−4; Boughrara, chi-square result = 26.8, df = 3, P < 10−4; Ouled Mimoun, chi-square result = 28.0, df = 3, P < 10−4) as well as for rhizosphere isolates (Beni Tamou, chi-square result = 41.9, df = 3, P < 10−4; Boughrara, chi-square result = 27.6, df = 3, P < 10−4; Ouled Mimoun, chi-square result = 36.9, df = 3, P < 10−4). Our hypothesis of a fluctuation of pathogenic agrobacteria in soil according to the season was therefore verified at several plots.
Pathogenic strains could be isolated only when densities of Agrobacterium-like isolates were over 105 CFU/g in bulk soil and in the rhizosphere (Fig. 1). When pathogens were recovered, their estimated densities varied from 5 × 104 to 106 CFU/g of bulk soil and from 2 × 105 to 1.5 × 107 CFU/g of rhizosphere. When no pathogens were isolated, since from less than 1 of 20 up to less than 1 of 98 agrobacterial strains were pathogenic, the absolute numbers of pathogenic strains were at a maximum 140 and 900 CFU/g of bulk soil and rhizosphere, respectively (dotted lines in Fig. 1).
PCR-based quantification of pathogenic agrobacteria in soil DNAs.
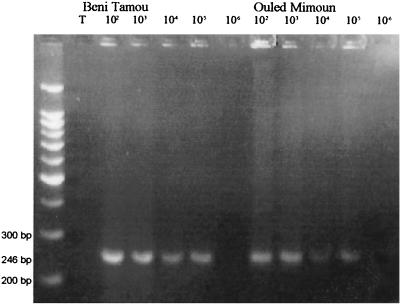
Since the results provided above were based on counting of cultivable agrobacteria, seasonal fluctuations of pathogens might be due to fluctuations in their cultivability instead of fluctuations in their actual population densities. To avoid the potential bias caused by the isolation techniques, densities of pathogenic populations in soils were estimated by direct PCR detection of Ti plasmid (i.e., vir) sequences in the whole DNA extracted from soil. DNA was extracted from soil samples obtained at Beni Tamou and Ouled Mimoun in fall (October 1999), winter (January 2000), and summer (July 2000). No amplifications were observed with fall or winter samples, while PCRs revealed the presence of sequences of the expected size with DNA templates obtained from soils collected in summer in both Beni Tamou and Ouled Mimoun (Fig. 2). The density of the target sequence was determined by a robust template dilution technique. The results indicated that the densities of vir sequences per gram of soil were below 103 copies of vir per g of soil in fall or winter and about 106 copies of vir per g of soil in summer in both Beni Tamou and Ouled Mimoun. Assuming that vir copies are likely individual Ti plasmids, the densities of Ti plasmid determined by direct PCR in summer 2000 (about 106/g in the two plots) and the densities of cultivable pathogenic agrobacteria determined in the summers of 1994 and 1998 (0.8 × 106 and 0.5 × 105 per g, for Beni Tamou and Ouled Mimoun, respectively) were equivalent. Similarly, in the absence of pathogens, both methods led to comparable results: less than 103 Ti plasmids per g or less than 140 CFU/g of soil. Fluctuations of the density of cultivable pathogenic agrobacteria over different seasons therefore appear to follow fluctuations of all physically present, pathogenic agrobacteria within the soil microflora.
FIG. 2.
PCR amplifications of vir sequences in the soil microflora DNA from summer samples from two plots. The quantities of DNA in PCR microtubes for dilutions 10−2, 10−3, 10−4, 10−5, and 10−6 correspond to the total DNA extracted from 1 mg, 0.1 mg, 0.01 mg, 1 μg, and 0.1 μg of bulk soil, respectively. The molecular size marker is a 100-bp ladder. Lane T, negative control with no DNA.
Biovar and Ti plasmid diversity of the pathogenic populations.
Strains were characterized at both biovar and Ti plasmid levels. Biovar analysis yielded unambiguous results, since all strains analyzed fell into either biovar 1 or 2. Pathogenic agrobacteria isolated from Beni Tamou (1994), Boughrara, and Ouled Mimoun were equally distributed in biovars 1 and 2 in both bulk soils and rhizospheres and in both spring and summer samples (Table 2). Opine catabolism, opine content of tumors, K84 sensitivity, and tmr and nos hybridization analyses also yielded unambiguous results, since all strains analyzed fell into either nopaline-type or octopine-type Ti plasmid groups (data not shown). A strong predominance of nopaline-type Ti plasmids was observed in the three plots, but some octopine-type Ti plasmids were also recovered in the rhizospheres of Boughrara and Ouled Mimoun. On the other hand, 179 out of the 180 arbitrarily selected, nonpathogenic isolates from Beni Tamou, Boughrara, and Ouled Mimoun were biovar 2, and 1 isolate was biovar 1, indicating a strong predominance of biovar 2 among nonpathogenic agrobacteria in both bulk soil and rhizosphere. The lack of Ti plasmid in the 180 nonpathogenic isolates was verified by tmr and nos hybridizations (data not shown).
TABLE 2.
Biovar and Ti plasmid type of pathogenic isolates
| Plot | Sampling date | No. of isolatesa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Nopaline type
|
Octopine type
|
||||||||
| Biovar 1
|
Biovar 2
|
Biovar 1
|
Biovar 2
|
||||||
| Soil | Root | Soil | Root | Soil | Root | Soil | Root | ||
| Beni Tamou | March 1990 | 16 | NAb | 0 | NA | 8 | NA | 0 | NA |
| April 18 1994 | 11 | 16 | 13 | 10 | 0 | 0 | 0 | 0 | |
| June 23 1994 | 3 | 28 | 19 | 5 | 0 | 0 | 0 | 0 | |
| Boughrara | April 12 1998 | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 |
| July 6 1998 | 2 | 2 | 6 | 1 | 0 | 3 | 0 | 0 | |
| Ouled Mimoun | April 15 1998 | 2 | 6 | 3 | 1 | 0 | 0 | 0 | 0 |
| July 7 1998 | 2 | 5 | 5 | 3 | 0 | 2 | 0 | 0 | |
The presence of nopaline-type Ti plasmids was determined by utilization of nopaline, synthesis of nopaline in incited tumors, sensitivity to K84, and hybridization with an nos probe. The presence of octopine-type Ti plasmids was determined by utilization of octopine, agropine, and mannopinic acid; synthesis of octopine, agropine, and mannopine in incited tumors; lack of sensitivity to K84; and lack of hybridization with an nos probe. Soil, bulk soil; Root, roots and soil particles tightly adhering to roots of weeds.
NA, not applicable.
Evolution of the pathogenic populations of Beni Tamou between 1990 and 1994.
In 1990, the 24 pathogenic strains recovered in Beni Tamou were biovar 1 strains harboring nopaline-type (16 isolates) or octopine-type (8 isolates) Ti plasmids (Table 2). In 1994, both biovar 1 and 2 strains were recovered, but only nopaline-type Ti plasmids were found, as determined by biochemical tests, sensitivity to K84, and hybridization with a nos probe (data not shown). The disappearance of octopine-type Ti plasmid between 1990 and 1994 was significant (chi-square result = 10.67, df = 1, P < 10−3), as were the lack of biovar 2 strains in 1990 and their presence in 1994 (chi-square result = 16.01, df = 1, P < 10−4). These results show a concomitant decrease in Ti plasmid diversity and an increase in host strain diversity of pathogenic populations during a 4-year period in the Beni Tamou plot.
DISCUSSION
Moore and Coocksey (20) pointed out the controversy over whether pathogenic agrobacteria are short- or long-term survivors in soil. The work initiated in 1990 by Bouzar et al. (2) substantiated the observation that pathogenic agrobacteria are long-term survivors. In a plot apparently bearing only symptomless weeds, pathogenic agrobacteria could be detected in the bulk soil 16 years after the removal of diseased plants. The present study showed, however, that the survivor status varied according to the soils. All of the plots investigated here are known to have been heavily crown galled, and pathogenic strains were probably released in large quantities in these soils. However, 1 year after uprooting of the diseased material in five plots, pathogenic strains could be recovered at only two plots. This 1-year period following removal of diseased material was sufficient for a tentative discrimination of the soils as being conducive to or suppressive for pathogenic agrobacteria. As evidenced at Beni Tamou, but likely as well for all other conducive soils, the current agronomic practice of leaving a field fallow may therefore be ineffective for curing a contaminated soil, thus risking infection during further planting of susceptible hosts.
Conducive soils are probably those that are the most favorable for Agrobacterium spp. either harboring or not harboring a Ti plasmid. In the present study, pathogenic agrobacteria were isolated in the soils that exhibited the highest densities of cultivable agrobacteria in both the bulk soil and the rhizosphere. However, the presence of bacteria hosting a Ti plasmid is not sufficient to allow the total population of Agrobacterium to reach these levels, since nonpathogenic strains—harboring no Ti plasmid—were isolated as well as pathogenic strains—frequently in higher numbers in favorable instances (Fig. 1). Members of the genus Agrobacterium are common in soil, but precise information on their density and seasonal fluctuations are not available, especially when compared to the wealth of information about the survival of Rhizobium inoculant in soils. Edaphic factors such as pH and texture have been reported to affect the population density of plant-associated microbes (23), including members of Rhizobiaceae such as Rhizobium (27). In our study, conducive soils did not show marked pH or texture differences from the suppressive ones (Table 1). A lower percentage of sand found in the conducive soils could perhaps provide them with a better capacity for water retention than suppressive soils. Indeed, soil moisture content and the variation of this parameter are key determinants of the composition of the microflora (7), but such data were not available for the studied soils.
The total number of cultivable agrobacteria fluctuated according to the season (Fig. 1), and the relatively low agrobacterial densities reached in fall and winter in conducive soils were similar to the densities recorded all during the year in suppressive soils. The bloom of total agrobacteria in spring is probably a response to a flush of nutrients in the weed rhizosphere, and this flush should be sufficient to have also caused a bloom of agrobacteria in the distant bulk soil. While rhizodeposition is known to be cyclical and to depend upon active plant growth (19), such a bloom, for unknown reasons, was not observed in the rhizospheres of suppressive plots. Interestingly, in a field study over three growing seasons, the populations of Rhizobium trifolii were found to vary from ca. 103 to 106 g of soil−1 according to soil and seasons (29). These numbers are comparable to those found in the present study. Moreover, in unlimed soils, the rhizobial populations fluctuated with season, with the smallest numbers following the summer-fall months, but where the soil was limed, the survival of rhizobia was less seasonally dependent. Whether soil plots in the present study were limed or not is not known. Our results, however, showed that seasonal fluctuation of the soil bacterium densities is a feature shared by several species of Rhizobiaceae.
A major result generated by the present study is the characterization of seasonal fluctuations in the ratio of pathogenic populations of Agrobacterium in soils and rhizospheres. This unforeseen finding was observed in several independent plots and, to the best of our knowledge, has not been reported previously for particular (i.e., pathogenic) strains of Agrobacterium. Seasonal shifts in the abundance and composition of the whole bacterial communities in rhizospheres have recently been reported (14, 30), but these results did not deal with relative fluctuations of particular strains within a single taxon. A seasonal pattern of ribotype reoccurrence was observed within the pseudomonad populations associated with sugar beet leaves (11), showing that seasonal fluctuation in the relative ratio of finely typed strains over the total population should also occur in other taxa.
No pathogenic strains were isolated in fall and winter in Boughrara, Ouled Mimoun, Sidi Abdli, and Saf Saf in spite of a probable high population density of pathogenic strains in all of those plots resulting from the previous outburst of crown gall reported 1 year ago (Fig. 1). This was not caused by fluctuations of the cultivability of pathogenic strains as verified by direct PCR detection and quantification of Ti plasmids (i.e., vir) sequences in the DNA extracted from soils (Fig. 2). Ti plasmids were not detected by PCR during winter 1999 in Beni Tamou and Ouled Mimoun in spite of their previous detection in pathogenic strains during summer 1994 in Beni Tamou and summer 1998 in Ouled Mimoun and later by PCR in summer 2000 in both plots. This indicates an annual periodicity in seasonal fluctuation of Ti plasmid populations. The disappearance—or at least their decrease below a detectable level—of pathogenic strains in fall and winter could simply fit the seasonal fluctuation of the total cultivable agrobacteria described above. Some of our data suggest, however, that the presence of a Ti plasmid in the host Agrobacterium decreased the fitness of the bacteria in soil and in symptomless rhizosphere. This occurred at least in Beni Tamou between March 1990 (2) and November 1993 (Fig. 1), when the ratio of pathogenic populations over total cultivable agrobacteria decreased significantly between the two sampling dates. The decrease in the pathogenic strain ratio in fall and winter experienced in Beni Tamou and suspected in other plots could be related to a low fitness in the bulk soil in the absence of an opine niche. This is substantiated by Guyon et al. (15) and by our own work performed with soil microcosms showing a faster decline of the population density of strain C58 compared to that of its plasmid-free derivatives (unpublished results). It is suspected, but remains to be experimentally confirmed, that in the suppressive soils, pathogenic invaders were less fit than Ti plasmid-free strains and thus decreased rapidly below the detection limit.
A troubling fact was the increase in the ratio of pathogenic over total agrobacteria between winter and spring samplings (Fig. 1), suggesting that pathogenic strains are more fit than nonpathogenic strains during the growing season of plants. A similar increase in plasmid incidence in the rhizosphere and phytosphere over time has been reported in pseudomonad populations by Lilley and Bailey (18). A study performed with a marked strain introduced as a seed dressing to sugar beets focused on acquisition of various indigenous plasmids that confer mercury resistance. Under those experimental conditions, Lilley and Bailey demonstrated that the fluctuation in plasmid-carrying strain numbers was related to an increase in the number of transconjugants of the marked strain, which could be collected only within a narrow temporal window coincident with the midseason maturation of crop. While the results are similar in the two studies, there is no evidence that the relative increase in pathogenic agrobacteria observed in the present study results directly from seasonal transconjugant events, even if Ti plasmid conjugations probably have a greater chance to occur during the growing season of plants. In our field survey of indigenous populations, the Ti plasmid-free strains are not totally the same strains as the plasmid-containing pathogenic strains, since the majority of the former belonged to biovar 2, while the latter were equally distributed into biovars 1 and 2. The difference in fitness survival between those different populations could thus be at least partly due to a host (chromosomal) difference(s) and not only to the presence or acquisition of a Ti plasmid. Actually, this phenomenon could also result from the occurrence of associations between hosts and Ti plasmids that fit particularly well. The recovery of a significantly higher proportion of pathogenic agrobacteria in spring than in winter suggested that those strains were a better fit than the Ti plasmid-free strains in three different plots. It cannot be excluded, however, that the same close association between Ti plasmid and bacterial host was dispatched by human activity in the various stone-fruit nurseries. Nevertheless, in the conducive soils, the pathogenic invaders that exhibited poor fitness during fall and winter recovered better fitness in spring, allowing them to become soil inhabitant. The improved fitness of strains with a Ti plasmid during spring was observed in bulk soil and/or symptomless weed rhizospheres, probably in the absence of the favorable opine niche. Unknown factors are able to specifically improve the growth of strains harboring Ti plasmid in the bulk soil and the rhizosphere of symptomless plants. These unknown factors could in part be opines secreted by plants with asymptomatic lesions or cryptic tumors. The discovery of the opine deoxy-fructosyl-glutamine (4, 33), which is a naturally occurring compound in wounded plant material (10), supports this hypothesis. On the other hand, other compounds of plant origin might be responsible for the selection of particular bacterial cells harboring or not harboring Ti plasmids. As a matter of fact, the related bacterium Rhizobium meliloti is selected at the root system of nonhost, nonlegume plants, such as hedge bindweed or belladona, because they efficiently degrade some alkaloid compounds synthesized by these plants (32).
The pathogenic population of Beni Tamou evolved between 1990 and 1994. Both octopine- and nopaline-type Ti plasmids were found in 1990, while only nopaline-type Ti plasmids were recovered in 1994, suggesting that the nopaline-type Ti plasmid itself provided better survivability to pathogenic agrobacteria in that soil. However, a correlative association was found between chromosomes and Ti plasmids in 1990 (2). Thus, the survival capacity of pathogenic strains could be determined by the associated chromosome as well. The particular host strain that harbored an octopine-type Ti plasmid in 1990 disappeared or dropped below the limit of detection in 1994, while those harboring a nopaline-type Ti plasmid survived better in that plot. The correlative association was, however, disrupted later, since only biovar 1 was found in 1990, while the prevalent nopaline-type Ti plasmid was found to be equally distributed in biovars 1 and 2 in 1994. An explanation could be that pathogenic biovar 2 was present with Ti plasmid in 1990, but in low numbers and was therefore not detected. For some reason, biovar 2 became more competitive and was then detected in 1994. Alternatively, nopaline-type Ti plasmids could have spread from biovar 1 to biovar 2. The spread of a Ti plasmid among various strains or species is thought to result from conjugal events. However, the conjugation of Ti plasmids is strongly repressed in the absence of the so-called conjugal opines (10). Here again, the evolution of the pathogenic population suggests that an effect normally mediated by opines could occur in a plot apparently bearing only symptomless plants and no opines. Whatever its cause, the shift of pathogenic populations from biovar 1 to biovar 2 suggests a selection in favor of biovar 2. Considering this fact, it is relevant to note that the Ti plasmid-free agrobacteria from that plot are predominantly biovar 2. Assuming that the Ti plasmid-free strains are the former indigenous agrobacteria of that soil, it is thus likely that the pathogenic population showed a shift toward the most adapted biovar of that soil, through a process of “naturalization” of a Ti plasmid in indigenous agrobacterial populations of the conducive soil.
Overall, the survival of Ti plasmid-harboring strains appeared to be the result of an equilibrium between the genetic load on the one hand and the fitness improvement determined by the Ti plasmid and/or the favorable association between Ti plasmid and particular chromosome on the other, this equilibrium being finely tuned by environmental conditions.
Acknowledgments
We thank M. A. Poirier for technical assistance and T. Vogel for discussion and manuscript revision.
This research was supported by funds from EU contract ERBIC18CT970198 “Integrated control of crown gall in Mediterranean countries.”
REFERENCES
- 1.Allardet-Servent, A., S. Michaux-Charachon, E. Jumas-Bilak, L. Karayan, and M. Ramuz. 1993. Presence of one linear and one circular chromosome in the Agrobacterium tumefaciens C58 genome. J. Bacteriol. 175:7869-7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouzar, H., D. Ouadah, Z. Krimi, J. B. Jones, M. Trovato, A. Petit, and Y. Dessaux. 1993. Correlative association between resident plasmids and the host chromosome in a diverse Agrobacterium soil population. Appl. Environ. Microbiol. 59:1310-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brisbane, P. G., and A. Kerr. 1983. Selective media for three biovars of Agrobacterium. J. Appl. Bacteriol. 54:425-431. [Google Scholar]
- 4.Chilton, W. S., A. M. Stomp, V. Béringue, H. Bouzar, V. Vaudequin-Dransart, A. Petit, and Y. Dessaux. 1995. The chrysopine family of amadori-type crown gall opines. Phytochemistry 40:619-628. [Google Scholar]
- 5.Cooksey, D. A., and L.W. Moore. 1980. Biological control of crown gall with fungal and bacterial antagonists. Phytopathology 70:506-509. [Google Scholar]
- 6.Cubero, J., M. C. Martinez, P. Llop, and M. M. Lopez. 1999. A simple and efficient PCR method for the detection of Agrobacterium tumefaciens in plant tumor. J. Appl. Microbiol. 86:591-602. [DOI] [PubMed] [Google Scholar]
- 7.Curl, E. A., and B. Truelove. 1986. The rhizosphere. Springer-Verlag, Berlin, Germany.
- 8.Dagnelie, P. 1975. Théorie et méthodes statistiques, vol. 2, p. 81-98. Les Presses Agronomiques de Gembloux, Gembloux, Belgium.
- 9.Dessaux, Y., A. Petit, and J. Tempé. 1992. Opines in Agrobacterium biology, p. 109-136. In D. P. S. Verma (ed.), Molecular signals in plant-microbe communications. CRC Press, Boca Raton, Fla.
- 10.Dessaux, Y., A. Petit, S. K. Farrand, and P. M. Murphy. 1998. Opines and opine-like molecules in plant-Rhizobiaceae interactions, p. 173-197. In A. Kondorosi and H. Spaink (ed.), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 11.Ellis, R. J., I. P. Thompson, and M. J. Bailey. 1999. Temporal fluctuations in the pseudomonad population associated with sugar beet leaves. FEMS Microbiol. Ecol. 28:345-356. [Google Scholar]
- 12.Frostegård, A., S. Courtois, V. Ramisse, S. Clerc, D. Bernillon, F. Le Gall, P. Jeannin, X. Nesme, and P. Simonet. 1999. Quantification of bias related to the extraction of DNA directly from soils. Appl. Environ. Microbiol. 65:5409-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelvin, S. B. 1992. Chemical signaling between Agrobacterium and its plant host, p. 137-167. In D. P. S. Verma (ed.), Molecular signals in plant-microbe communications. CRC Press, Boca Raton, Fla.
- 14.Gomes, N. C. M., H. Heuer, J. Schönfeld, R. Costa, L. Hagler-Mendonca, and K. Smalla. 2001. Bacterial diversity of the rhizosphere of maize (Zea mays) grown in tropical soil studied by temperature gradient gel electrophoresis. Plant Soil 232:167-180. [Google Scholar]
- 15.Guyon, P., A. Petit, J. Tempé, and Y. Dessaux. 1993. Transformed plants producing opines specifically promote growth of opine-degrading agrobacteria. Mol. Plant-Microbe Interact. 6:92-98. [Google Scholar]
- 16.Hald, A. 1952. Statistical tables and formulas. Wiley, New York, N.Y.
- 17.Keane, P. J., A. Kerr, and P. B. New. 1970.Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust. J. Biol. Sci. 23:585-595. [Google Scholar]
- 18.Lilley, A. K., and M. J. Bailey. 1997. The acquisition of indigenous plasmids by a genetically marked pseudomonad population colonizing the sugar beet phytosphere is related to local environmental conditions. Appl. Environ. Microbiol. 63:1577-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch, J. M., and J. M. Whipps. 1991. Substrate flow in the rhizosphere, p. 15-24. In D. L. Keister and P. B. Gregan (ed.), The rhizosphere and plant growth. Kluwer Academic Publichers, Dordrecht, The Netherlands.
- 20.Moore, L. W., and D. A. Cooksey. 1981. Biology of Agrobacterium tumefaciens: plant interactions. Int. Rev. Cytol. 13(Suppl.):15-46. [Google Scholar]
- 21.Moore, L. W., C. I. Kado, and H. Bouzar. 1988. Agrobacterium, p. 16-36. In N. W. Shaad (ed.), Laboratory guide for identification of plant pathogenic bacteria, 2nd ed. APS Press, Saint Paul, Minn.
- 22.Mougel, C., B. Cournoyer, and X. Nesme. 2001. Novel tellurite-amended media and specific chromosomal Ti plasmid probes for direct analysis of soil populations of Agrobacterium biovars 1 and 2. Appl. Environ. Microbiol. 67:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naseby, D. C., and J. M. Lynch. 1999. Effects of Pseudomonas fluorescens F113 on ecological functions in the pea rhizosphere are dependent on pH. Microb. Ecol. 37:248-256. [DOI] [PubMed] [Google Scholar]
- 24.Nesme, X., C. Picard, and P. Simonet. 1995. Specific DNA sequences for detection of soil bacteria, p. 111-139. In J. T. Trevors and J. D. van Elsas (ed.), Nucleic acids in the environment. Methods and applications. Springer-Verlag, Berlin, Germany.
- 25.Petit, A., J. Tempé, A. Kerr, M. Holsters, M. van Montagu, and J. Shell. 1978. Substrate induction of conjugative activity of Agrobacterium tumefaciens Ti plasmids. Nature 271:570-572. [Google Scholar]
- 26.Picard, C., C. Ponsonnet, E. Paget, X. Nesme, and P. Simonet. 1992. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl. Environ. Microbiol. 58:2717-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postama, J. C., H. Hok-A-Hin, and J. H. Oude Voshaar. 1990. Influence of the inoculum density on the growth and survival of Rhizobium leguminosarum biovar trifolii introduced into sterile and non-sterile loamy sand and silt loam. FEMS Microbiol. Ecol. 73:49-58. [Google Scholar]
- 28.Schroth, M. N., A. R. Weinhold, A. H. McCain, D. C. Hildebrand, and N. Ross. 1971. Biology and control of Agrobacterium tumefaciens. Hilgardia 40:537-552. [Google Scholar]
- 29.Slattery, J. F., and D. R. Coventry. 1993. Variation of soil populations of Rhizobium leguminosarum bv trifolii and the occurrence of inoculant rhizobia in nodules of subterranean clover after pasture renovation in North-Eastern Victoria. Soil Biol. Biochem. 25:1725-1730. [Google Scholar]
- 30.Smalla, K., G. Wieland, A. Büchner, A. Zock, J. Parzy, S. Kaiser, N. Roskot, H. Heuer, and G. Berg. 2001. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 67:4742-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stonier, T. 1960. Agrobacterium tumefaciens Conn. II. Production of an antibiotic substance. J. Bacteriol. 79:889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tepfer, D., A. Goldmann, N. Pamboukdjian, M. Maille, A. Lepingle, D. Chevalier, J. Dénarié, and C. Rosenberg. 1988. A plasmid of Rhizobium meliloti 41 encodes catabolism of two compounds from root exudate of Calystegium sepium. J. Bacteriol. 170:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaudequin-Dransart, V., A. Petit, C. Poncet, C. Ponsonnet, X. Nesme, J. B. Jones, H. Bouzar, W. S. Chilton, and Y. Dessaux. 1995. Novel Ti plasmids in Agrobacterium strains isolated from fig tree and chrysanthemum tumors and their opinelike molecules. Mol. Plant-Microbe Interact. 8:311-321. [DOI] [PubMed] [Google Scholar]
- 34.Zhu, J., P. M. Oger, B. Schrammeijer, P. J. J. Hooykaas, S. K. Farrand, and S. C. Winans. 2000. The bases of crown gall tumorigenesis. J. Bacteriol. 182:3885-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]