Abstract
A rapid procedure for the identification of Paenibacillus larvae subsp. larvae, the causal agent of American foulbrood (AFB) disease of honeybees (Apis mellifera L.), based on PCR and restriction fragment analysis of the 16S rRNA genes (rDNA) is described. Eighty-six bacterial strains belonging to 39 species of the genera Paenibacillus, Bacillus, Brevibacillus, and Virgibacillus were characterized. Amplified rDNA was digested with seven restriction endonucleases. The combined data from restriction analysis enabled us to distinguish 35 profiles. Cluster analysis revealed that P. larvae subsp. larvae and Paenibacillus larvae subsp. pulvifaciens formed a group with about 90% similarity; however, the P. larvae subsp. larvae restriction fragment length polymorphism pattern produced by endonuclease HaeIII was found to be unique and distinguishable among other closely related bacteria. This pattern was associated with DNA extracted directly from honeybee brood samples showing positive AFB clinical signs that yielded the restriction profile characteristic of P. larvae subsp. larvae, while no amplification product was obtained from healthy larvae. The method described here is particularly useful because of the short time required to carry it out and because it allows the differentiation of P. larvae subsp. larvae-infected larvae from all other species found in apiarian sources.
American foulbrood (AFB) disease caused by the spore-forming bacterium Paenibacillus larvae subsp. larvae (15) (formerly Bacillus larvae) is a highly contagious, cosmopolitan disease of bacterial origin affecting the larval and pupal stages of honeybees (Apis mellifera L.). Infected individuals turn brown and then black, and the resultant mass becomes a hard scale of material deposited on the side of the cell. AFB is one of the few bee diseases capable of killing a colony, and it presents unique problems for prevention and control because the spores can remain viable for long periods and survive under adverse environmental conditions (17, 17a, 17b). The disease spreads when spores are carried on drifting bees, hive parts, beekeepers' clothes, and contaminated pollen or honey.
Govan et al. (13) and Dobbelaere et al. (8) reported the use of PCR for rapid identification of P. larvae subsp. larvae by using primers derived from gene regions encoding 16S rRNA (rDNA). Specific primers designed by Govan et al. (13) produced a single amplicon, whereas those designed by Dobbelaere et al. (8) produced four amplicons. The results of their analysis of a limited number of species from apiarian sources did not allow them to differentiate P. larvae subsp. larvae from Paenibacillus larvae subsp. pulvifaciens, the cause of powdery scale disease (15, 16), because both subspecies showed the same pattern. Dobbelaere et al. (8) concluded that the high degree of similarity between 16S rRNA genes of the two subspecies, about 99.44%, does not permit the design of specific primers for either of the two subspecies.
In addition, several Paenibacillus species and species of the genera Bacillus, Brevibacillus, and Virgibacillus were consistently reported as being isolated from apiarian sources (2, 9, 10, 11, 12). The complex microbial community of sporeformers includes Paenibacillus alvei, Brevibacillus laterosporus, and Paenibacillus apiarius, which are considered secondary bacterial invaders of larvae infected with European foulbrood (EFB), and also P. larvae subsp. pulvifaciens and Bacillus coagulans, which cause diseases of minor economical impact (1, 5, 16, 19, 20). Nevertheless, these bacteria can easily contaminate and overgrow plates of the slow-growing fastidious P. larvae subsp. larvae, making the correct diagnosis of AFB difficult unless selective media are used (1, 2).
The aim of this study was to assess the feasibility of using restriction fragment length polymorphism analysis (RFLP) of PCR-amplified 16S rDNAs for the differentiation of P. larvae subsp. larvae from other Paenibacillus organisms and from other spore-forming bacteria from apiarian sources and to assess its applicability to the direct and rapid diagnosis of AFB.
Strains and media.
Eighty-six bacterial strains from diverse origins used in this study are listed in Table 1. For the isolation of P. larvae subsp. larvae strains from brood combs affected by AFB and from honey samples, previously described techniques were employed (2, 3). Brevibacillus laterosporus BLA 168 was isolated from honeybee larvae exhibiting symptoms of EFB, and Argentinian strains of P. alvei, Bacillus cereus, Bacillus mycoides, and Bacillus megaterium were recovered from honey as reported before (2).
TABLE 1.
Restriction patterns of PCR-amplified 16S rDNA genes among Paenibacillus, Bacillus, Brevibacillus, and Virgibacillus species and origins of the strains used in this study
| Species, strain designation, and geographical originl | Pattern obtained with restriction enzyme:
|
||||||
|---|---|---|---|---|---|---|---|
| AluI | MspI | HaeIII | HinfI | CfoI | RsaI | TaqI | |
| Paenibacillus larvae subsp. larvae | |||||||
| ATCC 2574, United States | B | B | A | NRSm | A | A | A |
| PL113, PL225, Argentinaa | B | B | A | NRS | A | A | A |
| PL295, PL296, United Statesa | B | B | A | NRS | A | A | A |
| PL201, PL203, Italya | B | B | A | NRS | A | A | A |
| PL212, PL213, Canadaa | B | B | A | NRS | A | A | A |
| PL228, PL230, Francea | B | B | A | NRS | A | A | A |
| PL252, PL254, Spainb | B | B | A | NRS | A | A | A |
| PL284, PL286, Uruguayc | B | B | A | NRS | A | A | A |
| PL289, PL290, Japand | B | B | A | NRS | A | A | A |
| PL56, PL57, Swedene | B | B | A | NRS | A | A | A |
| PL29, PL31, New Zealandf | B | B | A | NRS | A | A | A |
| PL90, PL91, Germanyg | B | B | A | NRS | A | A | A |
| PL68, PL70, Polandh | B | B | A | NRS | A | A | A |
| PL75 (CCM4483), PL76 (CCM4485), Czech Republic | B | B | A | NRS | A | A | A |
| PL301, PL302, UKi | B | B | A | NRS | A | A | A |
| PL100, Tunisiaa | |||||||
| PL304, PL305, Belgiumj | |||||||
| Paenibacillus larvae subsp. pulvifaciens | |||||||
| CCM 38 (CCM), Czech Republic | B | B | B | B | A | A | A |
| NRRL B-3688, NRRL B-3670 | B | B | B | B | A | A | A |
| NRRL B-14154, NRRL B-14152 | B | B | B | B | A | A | A |
| ATCC 13537 | B | B | B | B | A | A | A |
| SAG 4689-3, SAG 4689-6, United Statesb | B | B | B | B | A | A | A |
| Paenibacillus lentimorbus NRRL B-2522 | F | D | NRS | F | A | A | A |
| Paenibacillus macquariensis NRRL B-14306 | B | D | D | D | A | C | A |
| Paenibacillus glucanolyticus NRRL B-14679 | B | E | G | E | A | E | D |
| Paenibacillus peoriae NRRL B-14750 | B | E | H | G | A | A | A |
| Paenibacillus curdlanolyticus NRRL B-23243 | J | E | NRS | K | E | D | B |
| Paenibacillus kobensis NRRL B-23299 | D | J | I | B | G | B | E |
| Paenibacillus dendritiformis NRRL B-666 | N | E | E | C | A | B | C |
| Paenibacillus lautus NRRL NRS-1000 | B | E | G | D | A | A | A |
| Paenibacillus validus NRRL NRS-1347 | N | E | E | C | A | B | C |
| Paenibacillus alginolyticus NRRL NRS-1351 | H | D | J | J | D | C | B |
| Paenibacillus chondroitinus NRRL NRS-1356 | B | D | D | H | A | B | A |
| Paenibacillus illinoisensis | B | D | H | D | A | A | A |
| Paenibacillus alvei | |||||||
| NRRL B-383 | I | E | NRS | H | A | A | A |
| m437a, m361, Argentinaa | I | E | NRS | H | A | A | A |
| Paenibacillus amylolyticus NRRL B142 | E | E | F | C | A | A | C |
| Paenibacillus apiarius ATCC 29575 | B | E | NRS | A | C | A | |
| Paenibacillus macerans NRRL NRS-924 | A | H | C | B | B | D | B |
| Paenibacillus pabuli NRRL B-510 | B | F | H | D | A | F | A |
| Paenibacillus polymyxa NRRL B_510 | A | F | D | B | A | D | B |
| Paenibacillus azotofixans NRRL B-142 | G | F | H | G | A | A | A |
| Paenibacillus chibensis ATCC 11377 | B | E | L | G | A | A | A |
| Paenibacillus thiaminolyticus | A | D | C | C | B | D | B |
| Paenibacillus popilliae ATCC 14706 | B | D | NRS | C | C | A | A |
| Paenibacillus borealis KK19, Finlandk | B | D | D | D | A | C | A |
| Bacillus azotoformans NRRL B-14310 | A | I | O | L | B | D | B |
| Bacillus circulans ATCC 4515 | C | C | C | C | A | B | B |
| Bacillus cereus | |||||||
| ATCC 11778 | D | E | E | C | A | B | C |
| m432, m436, Argentinaa | D | E | E | C | A | B | C |
| Bacillus coagulans ATCC 35670 | A | D | C | B | B | D | B |
| Bacillus licheniformis NRRL B-1001 | D | D | C | B | A | D | B |
| Bacillus megaterium | |||||||
| NRRL B-939 | A | E | C | C | A | B | C |
| m412, m440a, Argentinaa | A | E | C | C | A | B | C |
| Bacillus mycoides | |||||||
| ATCC 10206 | K | E | K | C | A | B | C |
| m425, m440b, Argentinaa | K | E | K | C | A | B | C |
| Bacillus thuringiensis ATCC 10792 | D | E | E | C | A | B | C |
| Bacillus pumilus ATCC 7061 | A | D | C | B | B | D | B |
| Bacillus subtilis ATCC 10783 | A | D | C | B | B | D | B |
| Bacillus sphaericus ATCC 245 | A | E | C | C | A | D | C |
| Bacillus firmus ATCC 8247 | G | F | D | C | A | A | C |
| Virgibacillus pantothenticus ATCC 14567 | L | E | M | B | F | G | C |
| Brevibacillus laterosporus | |||||||
| CCT 31 (CCT) | M | G | N | C | A | B | B |
| BLA 168, Argentinaa | M | G | N | C | A | B | B |
A. M. Alippi, Centro de Investigaciones de Fitopatología, Facultad de Ciencias Agrarias y Forestales, UNLP, La Plata, Argentina.
P. Avalos, Laboratorio y Estación Cuarentenaria Lo Aguirre, SAG, Chile.
C. Piccini, Laboratorio de Microbiología, Instituto de Investigaciones Biológicas Clemente Estable, Montevideo, Uruguay.
A. Kataoka, Research Institute for Animal Science in Biochemistry and Toxicology, Kanawaga, Japan.
I. Fries, Bee Division, Swedish University of Agricultural Sciences, Uppsala, Sweden.
M. R. Goodwin, Apicultural Research Unit, Ruakura Research Center, Hamilton, New Zealand.
U. Rdest, Biozentrum der Universität Würzburg, Lehrstuhl Mikrobiologie, Würzburg, Germany.
M. Jelinski, Instytut Weterynarii, Swarzdez, Poland.
B. Dancer, School of Pure and Applied Biology, University of Wales, Cardiff, United Kingdom.
W. Dobbelaere, Veterinary and Agrochemical Research Centre, Brussels, Belgium.
S. Elo, Department of Biosciences, Division of General Microbiology, University of Helsinki, Finland.
ATCC, American Type Culture Collection, Rockville, Md.; CCM, Czech Collection of Microorganisms, Brno, Czech Republic; CCT, Coleçao de Culturas Tropical, Fundaçao André Tosello, Campinas, SP, Brazil; NRRL, Northern Regional Research Laboratory, Peoria, Ill.
NRS, no recognition site.
P. larvae subsp. larvae, P. larvae subsp. pulvifaciens, P. amylolyticus, P. lautus, P. illinoisensis, and P. chibensis strains were grown on MYPGP agar (6) at 37°C for 48 h; the other Paenibacillus species were grown on MYPGP agar at 30°C for 24 to 48 h, except P. macquariensis, which was grown at 22°C, and P. dendritiformis, which was grown on Luria-Bertani agar at 37°C. Bacillus, Brevibacillus, and Virgibacillus species were grown on tryptic soy agar at 30°C for 24 h, with the exception of B. coagulans, which was incubated at 37°C. Purity was confirmed by colony morphology and microscopic examination of bacterial smears.
DNA preparation.
Bacterial cells for DNA extraction were grown at the appropriate temperature and medium under aerobic conditions for 24 to 48 h according to the species used. For bacterial DNA preparation, a rapid procedure using whole cells from plates was employed (3). After centrifugation to remove bacterial debris and resin, the supernatant was used as the DNA template.
PCR amplification and RFLP analysis of 16S rDNA.
Primers U1 and U2 described by Ash et al. were used for PCR amplification of 16S rRNA genes from Bacillus, Paenibacillus, Brevibacillus, and Virgibacillus species (4). These primers were derived from conserved regions and capable of amplifying about 1.1 kb of 16S rDNA from Bacillus species and closely related genera. The PCR mixtures, which contained 1.5 μl of deoxynucleotide mixture (2 mM each), 1.25 μl of a mixture of both primers (10 mM each), 1.5 μl of Promega (Buenos Aires, Argentina) reaction buffer, 1.0 μl of MgCl2 (25 μM), 5 μl of supernatant DNA, and sterile deionized water to bring the final volume to 25 μl, were pretreated at 94°C before 1 U of Taq polymerase (Promega Corp.) was added. PCR amplification was carried out according to the protocol of Ash et al. (4). PCR products were examined by using agarose (0.8%) gel electrophoresis and visualized by using ethidium bromide and UV light.
After amplification, subsamples of about 5 μl were incubated overnight with endonucleases RsaI, HaeIII, MspI, AluI, HinfI, TaqI, and CfoI (Promega) according to the manufacturer's specifications. RFLP analysis was performed by electrophoresis in a 2% agarose gel at 80 V for 2.30 h.
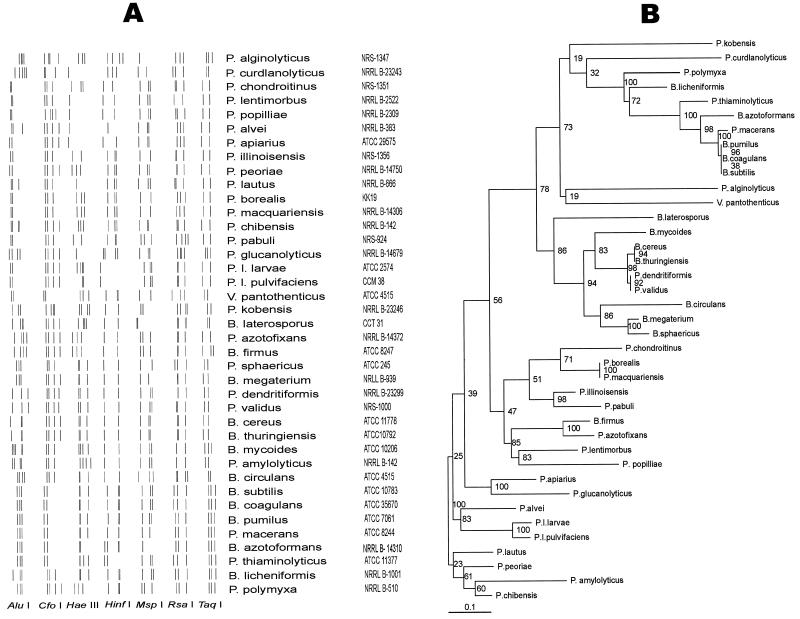
We found that species belonging to the genera Paenibacillus, Bacillus, Brevibacillus, and Virgibacillus consistently yielded a PCR amplification product of about 1,100 bp. In our analysis of the 16S rRNA gene, we assayed the variation at seven restriction sites that were thought to provide an RFLP pattern diagnostic of P. larvae subsp. larvae. It was found that analysis of 86 strains from different sources and classified as belonging to 39 species allowed us to place them in 35 composite profiles following digestion with RsaI, HaeIII, MspI, AluI, HinfI, TaqI, and CfoI by using the “combined gels” option of Gelcompare. The program FreeTree (14) was used for the construction of a phylogenetic tree (Fig. 1B) and for jackknife analysis by using a binary matrix based on RFLP characters (Nei-Li distances; neighbor-joining tree-construction method; 1,000 resampled data sets).
FIG. 1.
(A) Combined restriction patterns of PCR-amplified 16S rDNA from representative species of the genera Paenibacillus, Bacillus, Brevibacillus, and Virgibacillus obtained by using the endonucleases AluI, CfoI, HaeIII, HinfI, MspI, RsaI, and TaqI. (B) Phylogenetic tree constructed on the basis of RFLP data by the neighbor-joining method using FreeTree software. Jackknife values are indicated at the branching points (1,000 replicates).
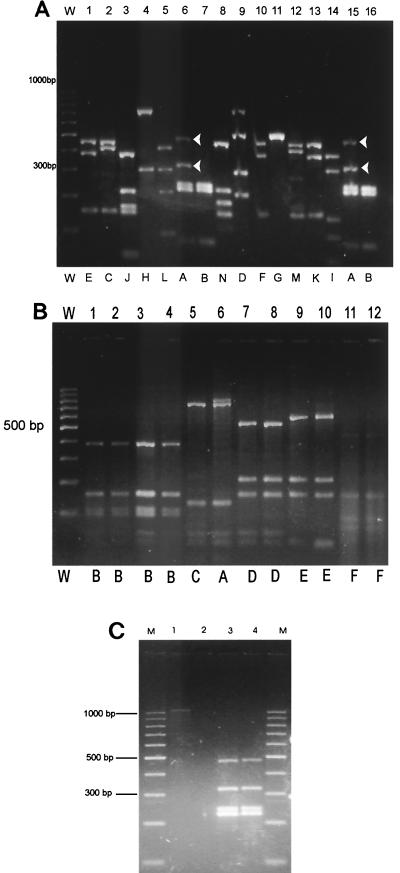
The result of the analysis shown in Fig. 1 revealed that P. larvae subsp. larvae and P. larvae subsp. pulvifaciens formed a group with about 90% similarity. However, the HaeIII restriction pattern of P. larvae subsp. larvae was found to be unique and allowed us to distinguish it from other closely related bacteria. Indeed, none of eight P. larvae subsp. pulvifaciens strains we examined showed the two HaeIII fragments of about 300 and 470 bp, respectively, which were characteristic of P. larvae subsp. larvae strains (n = 32) (Fig. 2A). Further evidence that supported subspecies differentiation was obtained, extending this analysis to 365 isolates of P. larvae subsp. larvae from different origins that showed the same HaeIII restriction pattern (data not shown); in addition, HinfI restriction analysis, unlike with P. larvae subsp. pulvifaciens, revealed no recognition site in P. larvae subsp. larvae strains (Table 1). On the other hand, profiles obtained with AluI, CfoI, RsaI, and TaqI were found to be identical in both subspecies. In addition, the MspI restriction patterns shown by P. larvae subsp. larvae and subsp. pulvifaciens were identical, whereas they differed from that of other species we examined (Fig. 2B). This relatedness between P. larvae subsp. larvae and P. larvae subsp. pulvifaciens is in agreement with previous evidence obtained by using a polyphasic approach (15). Differences between pairs of restriction patterns, as found here, could be simply explained in terms of gain or loss of only one or two restriction sites, which indicates indeed that these two subspecies are genetically closely related.
FIG. 2.
(A) Gel electrophoresis of a PCR-amplified 16S rDNA fragment of 1,100 bp digested with HaeIII. Similar restriction patterns were grouped and assigned the same letter (A to N). Lanes: W, molecular weight marker (100-bp ladder; Biodynamics, Buenos Aires, Argentina); 1, B. cereus ATCC 11778; 2, Paenibacillus macerans ATCC 8244; 3, Paenibacillus alginolyticus NRRL NRS-1347; 4, Paenibacillus peoriae NRRL B-14750; 5, Paenibacillus chibensis NRRL B-142; 6, P. larvae subsp. larvae ATCC 2574; 7, P. larvae subsp. pulvifaciens CCM 38; 8, Brevibacillus laterosporus CCT 31; 9, Paenibacillus borealis KK19; 10, Paenibacillus amylolyticus NRRL B 142; 11, Paenibacillus glucanolyticus NRRL B-14679; 12, Virgibacillus pantothenticus ATCC 14567; 13, B. mycoides (ATCC 10206); 14, Paenibacillus kobensis NRRL B-23246; 15, P. larvae subsp. larvae Pl 113; 16, P. larvae subsp. pulvifaciens ATCC 13537. Fragments of about 300 and 470 bp, respectively, that distinguish P. larvae subsp. larvae (lanes 6 and 15) from P. larvae subsp. pulvifaciens (lanes 7 and 16) are indicated by arrowheads. (B) Gel electrophoresis of a PCR-amplified 16S rDNA fragment of 1,100 bp digested with MspI. Similar restriction patterns were grouped and assigned the same letter (A to G). Lanes: W, molecular weight marker (100-bp ladder; Biodynamics); 1, P. larvae subsp. larvae ATCC 2574; 2, P. larvae subsp. larvae Pl 113; 3, P. larvae subsp. pulvifaciens CCM 38; 4, P. larvae subsp. pulvifaciens SAG; 5, Bacillus circulans ATCC 4515; 6, Bacillus firmus ATCC 8247; 7, Paenibacillus azotofixans NRRL B-14372; 8, Brevibacillus laterosporus CCT 31; 9, Bacillus subtilis ATCC 10783; 10, Paenibacillus macerans ATCC 8244; 11, P. alvei NRRL B-383; and 12, Bacillus thuringiensis ATCC 10792. (C) Agarose gel showing PCR-RFLP results from healthy and diseased larvae. Lanes: M, molecular weight marker (100-bp ladder; Biodynamics); 1, PCR of AFB-diseased larvae using primers U1/U2; 2, PCR of healthy larvae; 3, PCR-amplified 16S rDNA fragment of 1,100 bp from P. larvae subsp. larvae ATCC 2574 digested with HaeIII; 4, PCR-amplified 16S rDNA fragment from AFB-infected larvae digested with HaeIII.
In a few cases, pairs or groups (e.g., B. cereus and B. thuringiensis; B. subtilis, B. coagulans, and B. pumilus; and P. borealis and P. macquariensis) were not differentiated by the set of endonucleases that we used (Table 1; Fig. 1A). The use of other endonucleases or DNA sequencing may provide a basis for their differentiation.
We conclude that the 16S rRNA gene is polymorphic among the aerobic spore-forming bacterial species predominant in apiarian sources. However, intraspecies polymorphism was not detected among the 32 P. larvae subsp. larvae strains obtained from diverse geographic regions. More interesting, we found that restriction pattern analysis revealed a distinct genotype for P. larvae subsp. larvae which may be useful for its identification, since the use of the endonucleases MspI, HinfI, and HaeIII would result in the recognition of P. larvae subsp. larvae among apiarian bacteria.
Direct detection in honeybee larva samples.
Current procedures to detect AFB disease are based on direct field inspection of the hives and on the use of selective bacterial growth media combined with PCR methods (1, 2, 3, 13). Overall, they possess some limitations, since occasionally clinical symptoms are ambiguous and several days are required to reach a conclusive diagnosis. Therefore, in order to assess whether the 16S rDNA-RFLP analysis might be useful to reveal and confirm P. larvae subsp. larvae infection in hives, we carried out the following assays. Larvae exhibiting clinical symptoms of AFB were removed from the cells by using a toothpick and thoroughly mixed with 1 ml of sterile distilled water (two larval remains or scales per tube). One hundred microliters of this mixture was diluted in 900 μl of sterile distilled water, vortex mixed, and centrifuged at 3,200 × g for 5 min. Fifty microliters of the supernatant was heated at 95°C for 15 min (8) and centrifuged at 3,200 × g for 5 min. Subsamples of the supernatant were used as DNA templates in the PCR amplification as described above. Similar treatment was applied to healthy larvae 2, 5, and 19 days old and also to larval remains infected with chalkbrood caused by the fungus Ascosphaera apis (18) and EFB caused by the bacterium Melissococcus plutonius (formerly Melissococcus pluton) (5, 7), which were assessed as controls. AFB, EFB, and chalkbrood were confirmed by using standard microscopic and microbiological techniques (1, 5, 7, 18, 19). The results in Fig. 2C reveal that DNA extracted from larva samples associated with AFB symptoms consistently amplified the 1,100-bp fragment which, after incubation with endonuclease HaeIII, gave an RFLP pattern identical to that found to be characteristic of P. larvae subsp. larvae. No amplification was detected with extracts from healthy larvae, EFB-diseased larvae, or chalkbrood mummies (dried dead larvae affected by chalkbrood disease). Furthermore, by using larval samples carrying mixed spore-forming bacterial populations that had been described by Alippi (1), a unique HaeIII restriction pattern identical to that of P. larvae subsp. larvae was observed (data not shown). We assume that the high level of P. larvae subsp. larvae spores present in larva samples may indicate that DNA from P. larvae subsp. larvae outcompetes those from other bacteria as a template in the PCR.
Finally, DNA fingerprint analysis using the primers BOX, REP, and ERIC revealed four different genotypes within the P. larvae subsp. larvae collection we examined, which were demonstrated to be genetically diverse even though the 16S rDNA-RFLP pattern was identical (3; Alippi et al., unpublished data).
Our study provides a method that appears to be helpful in distinguishing P. larvae subsp. larvae from other Paenibacillus organisms, particularly those that are closely related, such as P. larvae subsp. pulvifaciens, and also from the spore-forming species which are commonly found in samples from apiarian environments. Since this procedure allows the identification of P. larvae subsp. larvae obtained either from culture or from larvae, we believe it can be applied to the reliable and rapid diagnosis of AFB (in about 4 h), in contrast to classical microbiological methods, which require at least 2 days.
Acknowledgments
This research was supported by a grant from ANPCyT, Argentina (BID 1201/OC-AR PICT 08-03857), and from the IFS, Sweden (A.M.A.). A.M.A. and O.M.A. are Career Investigators of CIC and CONICET, respectively, and A.C.L. is a recipient of a scholarship from ANPCyT, Argentina.
We thank the curator of the NRRL Collection, N. Nakamura, for supplying bacterial cultures, those (listed in Table 1) who provided AFB samples or bacterial isolates, and F. J. Reynaldi for collecting larval samples.
REFERENCES
- 1.Alippi, A. M. 1991. A comparison of laboratory techniques for the detection of significant bacteria of the honey bee, Apis mellifera, in Argentina. J. Apic. Res. 30:75-80. [Google Scholar]
- 2.Alippi, A. M. 1995. Detection of Bacillus larvae spores in Argentinian honeys by using a semi-selective medium. Microbiologia 11:343-350. [PubMed] [Google Scholar]
- 3.Alippi, A. M., and M. Aguilar. 1998. Characterization of isolates of Paenibacillus larvae subsp. larvae from diverse geographical origin by the polymerase chain reaction and BOX primers. J. Invertebr. Pathol. 72:21-27. [DOI] [PubMed] [Google Scholar]
- 4.Ash, C., F. G. Priest, and M. D. Collins. 1993. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Leeuwenhoek J. Microbiol. 64:253-260. [DOI] [PubMed] [Google Scholar]
- 5.Bailey, L. 1983. Melissococcus pluton, the cause of European foulbrood of honeybees (Apis spp.). J. Appl. Bacteriol. 55:65-69. [Google Scholar]
- 6.Dingman, D. W., and D. P. Stahly. 1983. Medium promoting sporulation of Bacillus larvae and metabolism of medium components. Appl. Environ. Microbiol. 46:860-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djordjevic, S. P., K. Noone, L. Smith, and M. Hornitzky. 1998. Development of a hemi-nested PCR assay for the specific detection of Melissococcus pluton. J. Apic. Res. 37:165-174. [Google Scholar]
- 8.Dobbelaere, W., D. C. De Graaf, J. E. Peeters, and F. J. Jacobs. 2001. Development of a fast and reliable method for American foulbrood disease (Paenibacillus larvae subsp. larvae) using a 16S rRNA gene based PCR. Apidologie 32:363-370. [Google Scholar]
- 9.Gilliam, M. 1978. Bacteria belonging to the genus Bacillus isolated from selected organs of queen honey bees, A. mellifera. J. Invertebr. Pathol. 31:389-391. [DOI] [PubMed] [Google Scholar]
- 10.Gilliam, M. 1979. Microbiology of pollen and bee bread: the genus Bacillus. Apidologie 10:269-274. [Google Scholar]
- 11.Gilliam, M., and D. B. Prest. 1987. Microbiology of feces of the larval honey bee, Apis mellifera. J. Invertebr. Pathol. 49:70-75.
- 12.Gilliam, M., and D. K. Valentine. 1976. Bacteria isolated from the intestinal contents of foraging worker honey bees, Apis mellifera: the genus Bacillus. J. Invertebr. Pathol. 28:275-276. [Google Scholar]
- 13.Govan, V. A., M. H. Allsopp, and S. Davidson. 1999. A PCR detection method for rapid identification of Paenibacillus larvae. Appl. Environ. Microbiol. 65:2243-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampl, V., A. Pavlicek, and J. Flegr. 2001. Construction and bootstrap analysis of DNA fingerprinting-based phylogenetic trees with the freeware program FreeTree; application to trichomonad parasites. Int. J. Syst. E vol. Microbiol. 51:731-735. [DOI] [PubMed] [Google Scholar]
- 15.Heyndrickx, M., K. Vandemeulebroecke, B. Hoste, P. Janssen, K. Kersters, P. De Vos, N. A. Logan, N. Ali, and R. C. W. Kerkeley. 1996. Reclassification of Paenibacillus (formerly Bacillus) pulvifaciens (Nakamura 1984) Ash et al., 1994, a later subjective synonym of Paenibacillus (formerly Bacillus) larvae (White 1906) Ash et al. 1994, as a subspecies of P. larvae, with emended descriptions of P. larvae as P. larvae subsp. larvae and P. larvae subsp. pulvifaciens. Int. J. Syst. Bacteriol. 46:270-279. [DOI] [PubMed] [Google Scholar]
- 16.Katznelson, H. 1950. Bacillus pulvifaciens (n. sp.), an organism associated with powdery scale of honey-bee larvae. J. Bacteriol. 59:153-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matheson, A., and M. Reid. 1992. Strategies for the prevention and control of American foulbrood. Part I. Am. Bee J. 132:399-402. [Google Scholar]
- 17a.Matheson, A., and M. Reid. 1992. Strategies for the prevention and control of American foulbrood. Part II. Am. Bee J. 133:471-475. [Google Scholar]
- 17b.Matheson, A., and M. Reid. 1992. Strategies for the prevention and control of American foulbrood. Part III. Am. Bee J. 143:534-547. [Google Scholar]
- 18.Rose, J. B., M. Christensen, and W. T. Wilson. 1984. Ascosphaera species inciting chalkbrood in North America and a taxonomic key. Mycotaxon 19:41-55. [Google Scholar]
- 19.Shimanuki, H., and D. A. Knox. 1991. Diagnosis of honey bee diseases. Agricultural handbook no. AH-690. U.S. Department of Agriculture, Washington, D.C.
- 20.Vandenberg, J. D., and H. Shimanuki. 1990. Isolation and characterization of Bacillus coagulans associated with half-moon disorder of honey bees. Apidologie 21:233-241. [Google Scholar]