Abstract
To monitor the ability of the food-borne opportunistic pathogen Bacillus cereus to survive during minimal processing of food products, we determined its heat-adaptive response. During pre-exposure to 42°C, B. cereus ATCC 14579 adapts to heat exposure at the lethal temperature of 50°C (maximum protection occurs after 15 min to 1 h of pre-exposure to 42°C). For this heat-adaptive response, de novo protein synthesis is required. By using two-dimensional gel electrophoresis, we observed 31 heat-induced proteins, and we determined the N-terminal sequences of a subset of these proteins. This revealed induction of stress proteins (CspB, CspE, and SodA), proteins involved in sporulation (SpoVG and AldA), metabolic enzymes (FolD and Dra), identified heat-induced proteins in related organisms (DnaK, GroEL, ClpP, RsbV, HSP16.4, YflT, PpiB, and TrxA), and other proteins (MreB, YloH, and YbbT). The upregulation of several stress proteins was confirmed by using antibodies specific for well-characterized heat shock proteins (HSPs) of B. subtilis. These observations indicate that heat adaptation of B. cereus involves proteins that function in a variety of cellular processes. Notably, a 30-min pre-exposure to 4% ethanol, pH 5, or 2.5% NaCl also results in increased thermotolerance. Also, for these adaptation processes, protein synthesis is required, and indeed, some HSPs are induced under these conditions. Collectively, these data show that during mild processing, cross-protection from heating occurs in pathogenic B. cereus, which may result in increased survival in foods.
Bacillus cereus is one of the major food-borne pathogenic bacteria and a common contaminant of food and dairy products. It is a gram-positive, facultatively anaerobic, spore-forming bacterium that is the causative agent of two types of food poisoning, the emetic and diarrheal forms. The mechanism of the pathogenesis of B. cereus has not yet been clarified fully. The vegetative bacterial cells can produce a number of virulence factors, including enterotoxins, of which at least three have been characterized (13, 21). One of the reasons that B. cereus is an important cause of food poisoning in industrialized parts of the world (13) might be the increasing demand of consumers for fresher and more natural food products, which results in a reduction in the intrinsic preservation of foods (12). The microbial safety and stability of most minimally processed foods are based on the application of combined preservative factors, of which (mild) heating is the most common preservation technique used (22).
Bacteria have evolved adaptive networks to face the challenges of a changing environment and to survive under conditions of stress (1). An initial nonlethal heat dose can induce transient resistance to subsequent heat treatment, a phenomenon termed thermotolerance. Heat-induced thermotolerance has been studied in several food pathogens, such as Listeria monocytogenes (28) and B. cereus (23). For both psychrotrophic and mesophilic B. cereus variants, increased thermotolerance at 50°C is observed after incubation under mild heat conditions (37 or 40°C for several hours). Several proteins were induced during heat pre-exposure, but these were not identified (23). The mechanisms of heat adaptation and the production of heat-induced proteins have not been studied in B. cereus.
In a wide variety of bacteria, the heat shock response includes increased synthesis of a set of conserved heat shock proteins (HSPs) (41). The molecular genetics of the heat shock response has been most extensively studied in Escherichia coli and B. subtilis (14, 41). Classical HSPs are the molecular chaperones (e.g., DnaK, GroEL, and their cohorts) or ATP-dependent proteases (e.g., ClpP). These proteins play roles in protein folding, assembly, and repair and prevention of aggregation under stress and nonstress conditions. The chaperones and proteases act together to maintain quality control of cellular proteins (11). The B. subtilis heat-inducible genes are divided into four different classes based on their regulatory mechanisms (8, 14). Class I genes encode classical chaperones such as DnaK, DnaJ, GroES, and GroEL, the expression of which involves a highly conserved CIRCE (controlling inverted repeat of chaperone expression) operator sequence, which is the binding site for the HrcA repressor. Class II genes are induced by both heat and other stresses, such as salt or ethanol. Expression of these genes is regulated by the alternative sigma factor σB. Class III genes form part of the CtsR stress response regulon and include genes encoding the ClpP protease and two ATPases, ClpC and ClpE. Class IV genes are devoid of regulation by the CIRCE operator sequence and σB, as well as CtsR, and the regulation of these genes remains to be elucidated. This group includes, among others, the genes encoding the ClpX ATPase and FtsH (8, 14). Recent DNA microarray experiments with B. subtilis revealed that more than 100 genes are heat induced (15). In addition, array experiments on σB-regulated genes indicated that approximately 100 genes belong to this regulon and that these genes are involved in a variety of cellular processes, including protective processes, (post)transcriptional regulation, solute influx and efflux, and carbon metabolism (29, 30).
For several bacteria, it has been observed that stress exposures other than heat, such as exposure to ethanol, acid, or oxidative stress or during macrophage survival, might result in increased thermotolerance. Several HSPs are also induced under these conditions (1, 2, 3, 41). Common regulatory pathways might be responsible for the production of HSPs under different stress conditions, and in B. subtilis and Staphylococcus aureus, for example, a central role in these processes is thought to be played by σB (7, 14). Understanding cross-adaptation to different stresses and the involvement of stress proteins during these exposures might be instrumental in optimizing processing conditions to guarantee the microbial safety of food products (5). Another important applicative aspect of the heat shock response is the observation that B. subtilis cells pre-exposed to mild heat produce spores that are more heat stable. (26). Understanding the heat-adaptive response of vegetative cells might also shed light on the mechanism of heat resistance of spores.
In this report, we provide evidence for a heat-protective response in B. cereus ATCC 14579. By using a proteomics approach, we observed the production of heat shock proteins. A reference map of the protein components of B. cereus was generated, and a group of 31 HSPs was identified. The role of these proteins in heat adaptation is discussed.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
The mesophilic strain B. cereus ATCC 14579 was used throughout this study. Cells were grown at 30°C in brain heart infusion (BHI) medium (Difco, Le Pont de Claix, France) with shaking at 130 rpm. Growth of B. cereus was monitored at 30, 35, 40, and 45°C by plate counting and measurement of optical density at 600 nm.
Viability and thermotolerance of B. cereus exposed to different stresses.
B. cereus cells, cultured at 30°C, were pre-exposed to mild heat treatment at 42°C for 0, 7.5, 15, 30, 60, 120, or 240 min, after which their thermotolerance at 50°C was determined. Cultures in the mid-exponential growth phase (optical density at 600 nm, 0.5), grown at 30°C, were harvested by centrifugation (3,000 × g, 10 min), resuspended in preheated BHI medium, and kept at 42°C for the above-mentioned time periods. Consecutively, cells were exposed to 50°C and viable counts were measured after 0, 5, 10, 15, 20, and 25 min of exposure. For all heat exposures, three independent experiments were performed and samples were plated in duplicate for each time point. The heat tolerance of cells pre-exposed to the following other stresses was also assessed: low temperature (7°C for 2 h), salt upshock (additional 2.5% [wt/vol] NaCl at 30°C for 30 min), low pH (pH 5 adjusted with lactic acid at 30°C for 30 min), and the presence of ethanol (4% [vol/vol] ethanol at 30°C for 30 min). The heat sensitivity of cells pre-exposed to these stresses was analyzed in BHI medium as described above. The pre-exposures to heat or other stresses were also performed in the presence of chloramphenicol (100 μg ml−1) to inhibit de novo protein synthesis.
Protein extraction from B. cereus.
Total cellular protein extractions were performed essentially as described by Wouters et al. (38). For each sample, 10-ml cultures were concentrated and, consecutively, cells were disrupted by bead beating with an MSK cell homogenizer (B. Braun Biotech International, Melsungen, Germany) and zirconium beads (0.1-mm diameter; Biospec Products, Bartlesville, Okla.) six times for 1 min (with cooling on ice between treatments). Subsequently, proteins in the homogenate were analyzed by Western blotting and two-dimensional gel electrophoresis (2D-E). The protein concentration in cell extracts was determined by using the bicinchoninic acid assay (Sigma Chemical Co., St. Louis, Mo.).
Western blotting.
Protein extracts (40 μg) were separated by using sodium dodecyl sulfate-12.5% polyacrylamide gels in a Criterion II vertical electrophoresis system (Bio-Rad, Richmond, Calif.) with a molecular size standard containing proteins of 94, 67, 43, 30, 20, and 14 kDa. After electrophoresis, proteins were electroblotted at 100 V on nitrocellulose membranes (Bio-Rad) and blocked with 0.1% sodium caseinate. Blots were then incubated with either GroEL, GroES, DnaK, DnaJ, ClpC, ClpP, ClpX, or FtsH rabbit antibodies raised against these proteins of B. subtilis. The antibodies were generous gifts of W. Schumann, University of Bayreuth, Bayreuth, Germany (GroEL, DnaK, DnaJ, and FtsH); K. Turgay, Albert-Ludwigs-Universität, Freiburg, Germany (ClpC); and U. Gerth, Ernst-Moritz-Arndt-Universität, Greifswald, Germany (ClpP and ClpX). Immunocomplexes were incubated with goat anti-rabbit peroxidase and visualized with 3,3′-diaminobenzidine tetrahydrochloride.
Protein analysis by 2D-E.
Protein analysis was performed with a Multiphor 2D-E system (Pharmacia Biotech, Uppsala, Sweden) as described previously (38). Equal amounts of protein (50 μg) were separated on isoelectric-point gels at pI 4 to pI 7 and subsequently on homogeneous sodium dodecyl sulfate-12 to 14% polyacrylamide gels (Pharmacia Biotech). The gels were silver stained as described by Blum et al. (4). The experiments were performed in duplicate or triplicate, and representative gels are shown. The gels were analyzed, integrated, and normalized by using PD-Quest software (Bio-Rad). Induction factors for each heat-induced protein were calculated as the ratio of the normalized spot values in a stress gel to those in the control gel.
Determination of N-terminal amino acid sequences.
For determination of the N-terminal amino acid sequences of specific spots, protein samples (1.5 mg) were separated on the 2D-E gels under conditions identical to those used for the running of analytical gels. The proteins were blotted on a polyvinylidene difluoride membrane optimized for protein transfer (Amersham Life Science, Buckinghamshire, England) with a Trans-Blot unit in accordance with the instructions of the manufacturer (Bio-Rad) and stained with Coomassie brilliant blue. Protein spots were cut from the blot and subjected to consecutive Edman degradation and subsequent analysis with the model 476A Protein Sequencing System (Applied Biosystems, Foster City, Calif.) at the Sequence Center, University Utrecht (Utrecht, The Netherlands). By using BlastP and the B. cereus ATCC 14579 genome sequence database (Integrated Genomics, Chicago, Ill.; www.integratedgenomics.com), the derived N termini were screened for sequence similarities.
RESULTS
Heat sensitivity and thermotolerance of B. cereus pre-exposed to mild-heat stresses.
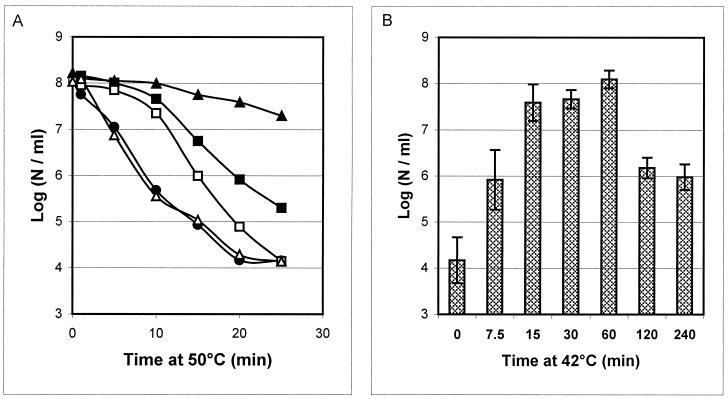
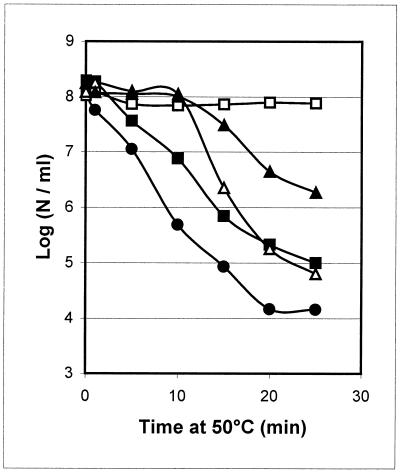
In order to select optimal temperatures for the heat exposure experiments, the growth rates of B. cereus ATCC 14579 at different temperatures in BHI medium were defined. The maximum growth rates determined were 0.8, 1.1, 1.0, and 0.3 h−1 at 30, 35, 40, and 45°C, respectively. No growth was observed at 50°C within 24 h after inoculation. A temperature of 30°C was chosen as the standard growth temperature in order to expose B. cereus cells to a significant temperature upshift (from 30 to 42°C) to study their thermotolerance at the lethal temperature of 50°C. Upon exposure of mid-exponential-phase cells grown at 30 to 50°C, a 4-log reduction in viable counts was observed after 20 min (Fig. 1A). Cells pre-exposed to 42°C for 15 min showed a less-than-0.5-log reduction in viable cells after 20 min at 50°C (Fig. 1A). Thus, pre-exposed cells showed strongly enhanced thermotolerance at 50°C compared to control cells. An increase in heat survival was also observed after pre-exposure at 42°C for 7.5 min, but to a lesser extent (approximately 100-fold greater survival). Longer pre-exposures of 30 min and 1 h at 42°C also produced a major increase in B. cereus thermotolerance compared to control cells (3- to 4-log increased survival after 20 min of heat treatment; Fig. 1B). After 2 and 4 h of pre-exposure, the thermotolerance decreased and was similar to that observed after 7.5 min of pre-exposure at 42°C (Fig. 1B). Interestingly, in the presence of chloramphenicol (an inhibitor of de novo protein synthesis) during pre-exposure at 42°C for 15 min, no increase in thermotolerance was observed compared to that of control cells. Exposure to heat in the presence of chloramphenicol for 7.5 min only partly blocked the development of thermotolerance, which is possibly explained by the time required to obtain a complete block of protein synthesis (Fig. 1A).
FIG. 1.
Effect of mild heat treatment on the survival of B. cereus ATCC 14579 at 50°C. (A) Survival (log number of cells [N] per milliliter) of exponential-phase control cells grown at 30°C (circles) and that of cells pretreated for 7.5 (squares) and 15 (triangles) min at 42°C in the absence and presence of chloramphenicol (closed and open symbols, respectively). (B) Survival, after 20 min at 50°C, of mid-exponential-phase B. cereus ATCC 14579 cells pretreated at 42°C for 0, 7.5, 15, 30, 60, 120, and 240 min.
Production of HSPs in B. cereus exposed to mild heat conditions.
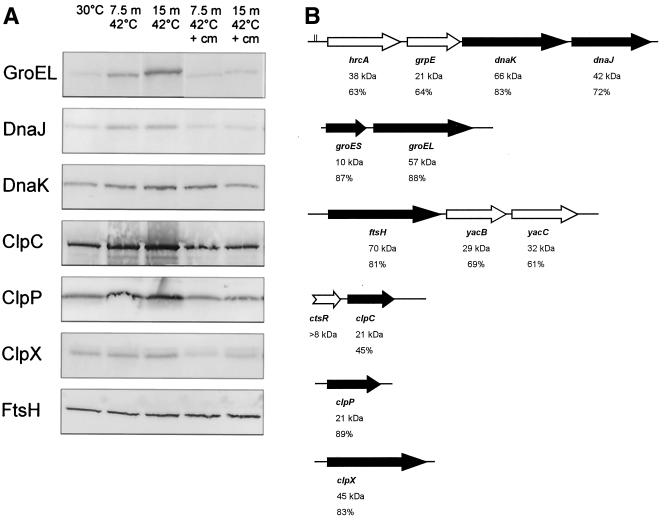
By using Western blotting with antibodies against the well-characterized B. subtilis HSPs GroEL, GroES, DnaK, DnaJ, ClpC, ClpP, ClpX, and FtsH, we analyzed the heat-induced production of these proteins in B. cereus. Except for GroES, all B. subtilis HSP antisera cross-reacted to their B. cereus counterparts (Fig. 2A). We observed a significant (greater than threefold) induction of all HSPs after exposure of B. cereus to 42°C for 7.5 and 15 min. The only exception was FtsH, which was not heat induced. In the presence of chloramphenicol, no increased HSP levels were found (except for DnaK, which was slightly induced after 7.5 min), which indicates that the increased HSP levels are dependent on de novo protein synthesis. The genes encoding all of the above-described HSPs could be identified in the B. cereus genome sequence, including the groES gene. The gene clusters encoding these HSPs show orientations identical to those observed for their homologs in B. subtilis. Upstream of the gene encoding HrcA, a CIRCE element is found that exactly matches the conserved CIRCE sequence of B. subtilis (TTAGCACTC-N9-GAGTCGTAA) (14; Fig. 2B). Because the upstream region of the groESL gene cluster is lacking in the B. cereus genome sequence, no CIRCE element could be identified upstream this operon. No other perfect CIRCE sequences were observed in the B. cereus genome sequence.
FIG. 2.
(A) Western blot analysis of HSP (GroEL, DnaK, DnaJ, ClpC, ClpP, ClpX, and FtsH) production in B. cereus. Cells were grown to mid-exponential phase at 30°C (control) and exposed to mild heat treatment (42°C) for 7.5 and 15 min (m) in the absence and presence of chloramphenicol (cm). (B) Schematic representation of the gene clusters encoding the analyzed HSPs in B. cereus (black arrows) and proteins homologous to heat-induced proteins in B. subtilis (white arrows; HrcA, GrpE, the transcriptional regulator YacB, the redox regulated chaperone YacC, and CtsR), which were not found to be heat induced in B. cereus in our study. For each gene, the percent identity to the B. subtilis homologs and the molecular mass of the encoded protein are indicated. The CIRCE sequence preceding the gene encoding HrcA is indicated by two vertical lines.
2D-E of extracts of B. cereus cells exposed to mild heat stress conditions.
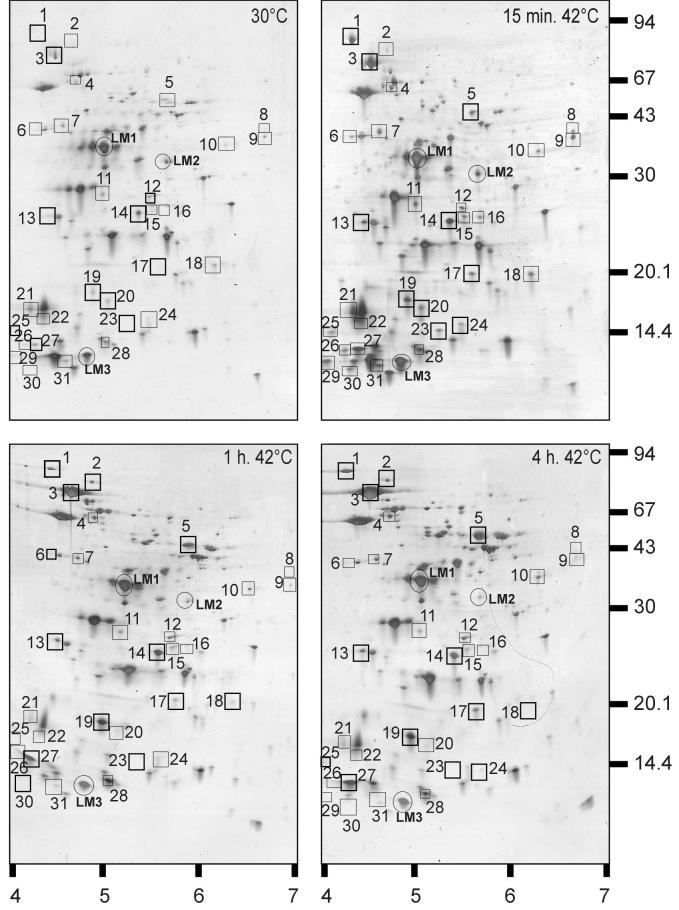
2D-E was used to gain an overview of the proteins induced by heat shock. On gels containing extracts of control and heat-shocked B. cereus cells, a total of approximately 250 proteins could be identified. Detailed analysis of the 2D-E gels revealed that 31 proteins were induced twofold or more in B. cereus by 15 min, 1 h, or 4 h of incubation at 42°C (Fig. 3; Table 1). The HSPs could be grouped on the basis of the time points of maximal production. For the first group (proteins 2, 3, 4, 5, 10, and 17), the induction factors increased upon longer exposure to 42°C and were significantly higher after 4 h than after 15 min of incubation at 42°C. The majority of HSPs (proteins 6, 7, 8, 9, 11, 15, 16, 18, 20, 21, 22, 23, 24, 25, 29, 30, and 31) fall into a second category of proteins that were maximally produced after 15 min at 42°C but whose levels decreased again upon longer exposure, returning to (near) prestimulus levels. A third group (proteins 1, 12, 13, 14, 19, 26, 27, and 28) were induced after 15 min at 42°C and stayed approximately at this level during longer heat exposure (up to 4 h; Table 1). The N-terminal sequences of a subset of HSPs were determined (Table 2). Firstly, the identified proteins include HSPs reported for other organisms, such as DnaK, GroEL, ClpP, SodA, Hsp16.4, PpiB, RsbV, SpoVG, and TrxA. Next, a set of proteins were also identified that are induced in response to stresses other than heat in several bacteria but have been shown to be heat induced in B. cereus: YflT, CspB, and CspE. In addition, some B. cereus HSPs have not been reported to be heat induced before, such as YbbT, AldA, MreB, FolD, Dra, and YloH. The genes corresponding to the identified proteins were determined by using the B. cereus genome sequence, and percentages of homology to related proteins were calculated (Table 2).
FIG. 3.
2D-E of extracts of exponential-phase B. cereus cells grown at 30°C and after mild heat treatment (42°C) for 15 min, 1 h, and 4 h. Molecular masses (in kilodaltons) of marker bands (right side) and pI ranges (bottom) are indicated. Heat-induced proteins are boxed and numbered (see also Tables 1 and 2).
TABLE 1.
Induction factorsa of heat shock proteins of B. cereus ATCC 14579
| Spot no. | Proteinb | Groupc | Heated (42°C) for:
|
Stressed by exposure to:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| 15 mind | 1 h | 4 h | 7°C | NaCl | Ethanol | pH 5 | |||
| 1 | DnaK | I | >20 | >20 | >20 | 4.2 | >20 | 3 | 2.5 |
| 2 | ND | I | 7.6 | 7.4 | 8.9 | 20 | 14.9 | 13.7 | |
| 3 | GroEL | I | 3.5 | 5.5 | 7.5 | 2.1 | |||
| 4 | YbbT | I | 3.8 | 4.6 | 2.1 | 2.4 | 2.5 | 2.6 | |
| 5 | AldA | I | 10.9 | 12.9 | 2.2 | ||||
| 6 | ND | II | 2.6 | 4.8 | 8.6 | 3.7 | |||
| 7 | MreB | II | 2.3 | 2.5 | 2 | ||||
| 8 | ND | II | 4 | 2.8 | 3.7 | 3.1 | |||
| 9 | ND | II | 2.9 | 3 | 3.1 | ||||
| 10 | FolD | I | 2.8 | 6.3 | 3.1 | ||||
| 11 | Dra | II | 2 | 4.5 | |||||
| 12 | SodA | III | 2 | 2 | 2 | 2.3 | 4.8 | ||
| 13 | ND | III | 2 | 2 | 2.1 | ||||
| 14 | ClpP | III | 3.5 | 3.9 | 4.1 | 3.6 | |||
| 15 | ND | II | 2.2 | 2.4 | 2.4 | ||||
| 16 | ND | II | 2 | ||||||
| 17 | HSP16.4 | I | 8 | 4.4 | 16.2 | ||||
| 18 | PpiB | II | 2 | 3 | 2.3 | ||||
| 19 | YflT | III | 4.1 | 6.1 | 5.3 | ||||
| 20 | ND | II | 2.4 | 2.9 | |||||
| 21 | ND | II | 3.9 | 2.9 | |||||
| 22 | ND | II | 7.1 | ||||||
| 23 | RsbV | II | >20 | 3.7 | 4 | ||||
| 24 | SpoVG | II | 2.5 | 3.8 | |||||
| 25 | TrxA | II | >20 | >20 | |||||
| 26 | ND | III | 4.9 | 2 | 2 | 5.2 | 3.5 | ||
| 27 | YloH | III | 8 | 7 | 10.4 | ||||
| 28 | ND | III | 2.3 | 2.2 | 2 | 2.9 | |||
| 29 | ND | II | 17.6 | 9.9 | 2.2 | ||||
| 30 | CspE | II | 4.2 | 3.5 | |||||
| 31 | CspB | II | 2.9 | 2.2 | |||||
Normalized value in stress gel/normalized value in control gel.
Identified heat-induced protein. ND, N-terminal amino acid sequence not determined.
Grouping is based on time point of induction (see text for details).
Less-than-twofold induction.
TABLE 2.
List of identified heat shock proteins of B. cereus ATCC 14579
| HSP no. | Designation | N-terminal sequencea | Molecular massb (kDa) | Description | % Identity (closest hit) |
|---|---|---|---|---|---|
| 1c | DnaK | SKIIGIID | 72 | Heat shock chaperone | 83 (B. subtilis) |
| |||||||| | |||||
| SKIIGIID | |||||
| 3 | GroEL | MAKDIKFSEEA | 60 | 60-kDa chaperonin | 89 (B. subtilis) |
| ||||||||||| | |||||
| MAKDIKFSEEA | |||||
| 4 | YbbT | XXKYFGTD | 48 | Phosphoglucosamine mutase | 78 (B. subtilis) |
| |||||| | |||||
| MGKYFGTD | |||||
| 5 | AldA | MRXGIPXE | 40 | Alanine dehydrogenase | 73 (B. sphaericus) |
| |||||| | |||||
| MRIGIPTE | |||||
| 7 | MreB | MFGFXGF | 36 | Rod shape-determining protein | 84 (B. subtilis) |
| |||||| | 36 | ||||
| MFGFGGF | |||||
| 10 | FolD | XVAVIIKG | 31 | Methylene tetrahydrofolate dehydrogenase | 68 (B. subtilis) |
| ||||||| | |||||
| MVAVIIKG | |||||
| 11 | Dra | MNAIKLI | 24 | Deoxyribose phosphate aldolase | 76 (B. subtilis) |
| ||||||| | |||||
| MNIAKLI | |||||
| 12 | SodA | XAKHELPN | 23 | Superoxide dismutase | 79 (B. stearothermophilus) |
| ||||||| | |||||
| MAKHELPN | |||||
| 14 | ClpP | MNLIPTVIEQ | 21 | ATP-dependent protease | 90 (B. subtilis) |
| |||||||||| | |||||
| MNLIPTVIEQ | |||||
| 17 | HSP16.4 | MRNLFPE | 16 | Heat shock chaperone | 35 (S. thermophilus) |
| ||||||| | |||||
| MRNLFPE | |||||
| 18 | PpiB | MKTLGYI | 16 | Peptidyl-prolyl cis-trans isomerase | 74 (B. halodurans) |
| ||||||| | |||||
| MKTLGYI | |||||
| 19 | YflT | METXYRK | 17 | General stress protein 17M | 47 (B. subtilis) |
| |||||| | |||||
| METKYRK | |||||
| 23 | RsbV | MNLAIN | 12 | Anti-sigma factor B antagonist | 95 (B. anthracis) |
| |||||| | |||||
| MNLAIN | |||||
| 24 | SpoVG | MEVTDVR | 10 | Stage V sporulation protein G | 85 (B. subtilis) |
| ||||||| | |||||
| MEVTDVR | |||||
| 25 | TrxA | XAIVNAND | 11 | Thioredoxin | 72 (B. halodurans) |
| ||||||| | |||||
| MAIVNAND | |||||
| 27 | YloH | MLXP | 7 | RNA polymerase omega subunit | 67 (B. subtilis) |
| ||| | |||||
| MLNP | |||||
| 30 | CspE | MQGKVK | 7 | Cold shock protein E | 92 (B. cereus) |
| |||||| | |||||
| MQGKVK | |||||
| 31 | CspB | MTLTGKV | 71 | Cold shock protein B | 97 (B. cereus) |
| ||||||| | |||||
| MTLTGKV |
The upper sequence is the derived N-terminal sequence, and the lower sequence represents the N-terminal sequence of the respective protein in the B. cereus ATCC 14579 genome. The letter X indicates an unknown residue.
Calculated molecular mass based on the encoded gene sequence.
The N-terminal sequences of proteins 2, 6, 8, 9, 13, 15, 16, 20, 21, 22, 26, 28, and 29 have not been determined.
Thermotolerance of B. cereus pre-exposed to different stress conditions.
To verify whether other stresses affect heat survival, B. cereus cells were exposed to 7°C, 2.5% NaCl, 4% ethanol, or pH 5 prior to exposure to heat treatment at 50°C. All of these pretreatments resulted in increased thermotolerance, although to different levels (Fig. 4). The greatest cross-protection was provided by salt stress; no cell number reduction was observed after 25 min of incubation at 50°C, compared to a 4-log reduction in control cells. No reduction in cell counts was noted during the first 10 min upon exposure to 50°C after ethanol or low-pH pretreatment. However, after 25 min at 50°C, 2- and 3.3-log reductions were observed after the low-pH and ethanol exposures, respectively. Pre-exposure to a low temperature had a marginal effect (a 10-fold increase) on B. cereus thermotolerance (Fig. 4). In the presence of chloramphenicol during the stress pre-exposures, some thermotolerance was acquired compared to that of control cells (a maximal 20-fold increase in salt-, pH-, and ethanol-stressed cells and no increase in low-temperature-stressed cells after 25 min at 50°C; data not shown). This indicates that the increase in thermotolerance obtained during these processes is not dependent solely on de novo protein synthesis.
FIG. 4.
Effect of pre-exposure to stress on survival of B. cereus cells at 50°C. Survival (log number of cells [N] per milliliter) of exponential-phase control cells grown at 30°C (circles) and that of cells pretreated for 30 min at 30°C with 2.5% NaCl (open squares), 4% ethanol (open triangles), and pH 5 (closed triangles) and cells preincubated at 7°C for 2 h (closed squares).
Production of HSPs in B. cereus exposed to different stress conditions.
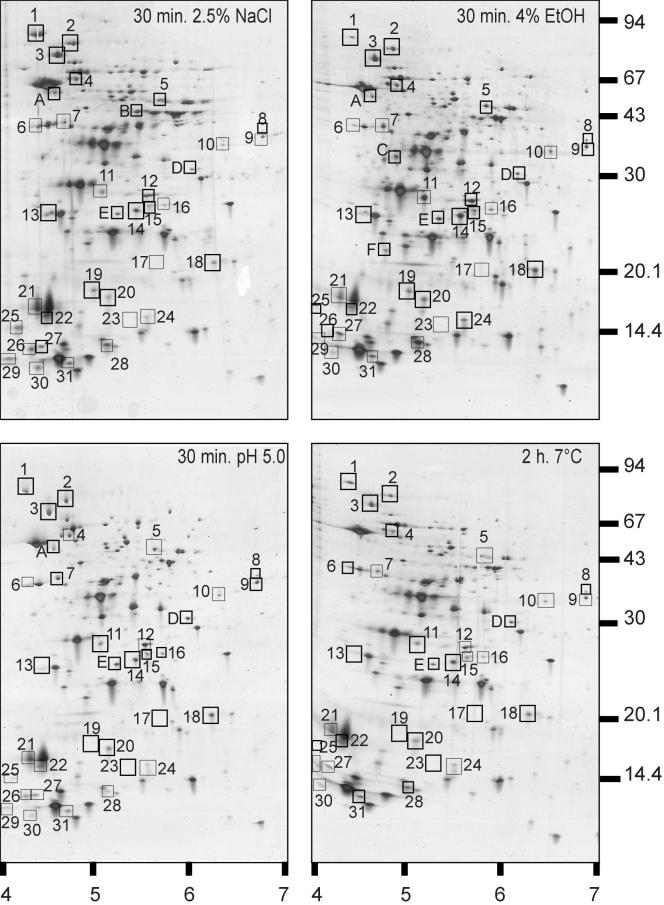
Next, we analyzed whether the identified B. cereus HSPs are induced by stresses other than heat by using Western blotting or 2D-E. By using Western blotting, we observed that the production of B. cereus HSPs GroEL, DnaK, DnaJ, ClpC, ClpP, ClpX, and FtsH increased after exposure to 4% ethanol. Upon exposure to NaCl, the production of DnaJ, DnaK, ClpC, and ClpX increased. Under low-pH and low-temperature conditions, a weak induction of only DnaK and DnaJ was found (Fig. 5). Next, comparison of the 2D-E gels showed that 23 of the 31 HSPs identified were also induced during one of the stress exposures tested (Fig. 6; Table 2). Enhanced (greater than twofold) production levels were observed for 10, 16, 10, and 7 proteins after exposure to salt, ethanol, a low pH, and a low temperature, respectively. Strikingly, three HSPs (DnaK, protein 2, and YbbT) were upregulated under all of the stress conditions tested. The production of four proteins (GroEL, protein 21, TrxA, and CspE) increased only during the heat and salt stresses, while the enhanced production of seven proteins (AldA, FolD, Dra, ClpP, SpoVG, protein 28, and CspB) overlapped during heat and ethanol exposures (Table 2). Finally, we also observed increased production of non-heat-induced proteins after exposure to the different stress conditions (Fig. 6).
FIG. 5.
Western blot analysis of HSP (GroEL, DnaK, DnaJ, ClpC, ClpP, ClpX, and FtsH) production in B. cereus. Cells were grown to mid-exponential phase at 30°C (column 1) and exposed to mild heat (42°C, 15 min) (column 2), a low temperature (7°C, 2 h) (column 3), 2.5% NaCl (30°C, 30 min) (column 4), 4% ethanol (30°C, 30 min) (column 5), or pH 5 (30°C, 30 min) (column 6).
FIG. 6.
2D-E of extracts of B. cereus cells exposed to 2.5% NaCl, 4% ethanol, or pH 5 for 30 min at 30°C or to a low temperature (7°C) for 2 h. Molecular masses (in kilodaltons) of marker bands (right side) and pI ranges (bottom) are indicated. HSPs are boxed and numbered. Proteins typically induced by exposure to a stress other than heat are boxed and lettered in each panel for the respective stress conditions.
DISCUSSION
In the present study, we analyzed the heat stress response of B. cereus ATCC 14579. During a shift from 30 to 42°C, B. cereus cells develop an increased tolerance to lethal heat exposure at 50°C. This phenomenon of increased thermotolerance after mild pre-exposure has been shown in other bacteria (2, 3, 23, 28, 41) and is now also known in B. cereus ATCC 14579. Furthermore, it was shown that protein synthesis is required to obtain this increase in thermotolerance. By using specific antibodies against the HSPs DnaK, DnaJ, GroEL, ClpC, ClpP, and ClpX, we showed that the production of these proteins increases during mild heat exposure and that this increase is absent in the presence of chloramphenicol. 2D-E revealed that 31 B. cereus proteins were induced during heat shock. Determination of the N-terminal sequences of a series of these proteins revealed that these proteins are involved in a variety of cellular processes. The majority of the proteins identified are homologous to stress proteins in other microorganisms but also to proteins involved in sporulation and in the biosynthesis of cellular compounds.
Several of the proteins identified belong to the group of chaperones (DnaK and GroEL) and proteases (ClpP) that have been described in great detail in other microorganisms (for reviews, see references 11 and 41). The induction of proteins of this type forms a highly conserved response to heat stress, and their induction by heat in B. cereus confirms their universal importance in stress adaptation. Other chaperones that are heat induced in B. cereus are HSP16.4, PpiB, YloH, CspB, and CspE. HSP16.4 is a member of the small heat shock protein (Hsp20) family and shows the greatest homology to a family of this type of proteins in the lactic acid bacterium Streptococcus thermophilus (34). PpiB is a peptidyl-prolyl cis-trans isomerase that can catalyze the refolding of proteins in B. subtilis. Although the disruption of ppiB in B. subtilis did not increase the sensitivity of the cells to heat stress, it is upregulated during heat shock (10). YloH is the omega subunit of RNA polymerase, and it has recently been shown to enhance the in vitro reconstitution of E. coli core RNA polymerase (9). YloH might act as a core RNA polymerase-specific chaperone that counters the core enzyme destabilization caused by elevated temperatures. To our knowledge, this is the first example of stress induction of the omega subunit of RNA polymerase, possibly suggesting a role for YloH in stress adaptation of the transcription machinery. Finally, in this chaperone family, two cold shock proteins, CspB and CspE, were induced upon heat stress. CspB and CspE have been described in Bacillus weihenstephanensis WSBC10201 and were only mildly induced upon cold shock (25), which is in agreement with our observations. Cold shock proteins are believed to act as RNA chaperones under low-temperature and other stress conditions (39). Here we show that CspB and CspE of B. cereus are induced during heat shock and osmotic or ethanol shock, respectively.
Within the group of B. cereus HSPs, we found several homologs to stress proteins. A protein with a possible role in protection of the cell against oxidative oxygen species is the thioredoxin TrxA. Thioredoxins are small, heat-stable proteins, and in B. subtilis, thioredoxin is induced under several stress conditions. Thioredoxin has multiple functions: it can serve as a hydrogen donor, it has been implicated in the formation of disulfide bonds in proteins, and it may be involved in the defense against oxidative stress (17, 32). Interestingly, the important regulatory protein RsbV is also induced by heat shock. RsbV is the anti-sigma factor antagonist of the alternative sigma factor σB, and its upregulation suggests the activation of σB under these conditions in B. cereus. Next, SodA is a superoxide dismutase that is highly homologous to superoxide dismutases from other members of the family Bacillaceae. This enzyme not only plays a role in protecting vegetative cells from reactive oxygen species but is also important in sporulation, as it is involved in the assembly of the insoluble matrix of the spore (16, 18). Finally, the role of the general stress protein YflT is unclear. It is also upregulated under heat shock conditions in B. subtilis (37).
Apart from SodA, two other HSPs of B. cereus have a potential role in sporulation. Firstly, AldA is homologous to the Ald protein in B. subtilis, which catalyzes the deamination of alanine to pyruvate and ammonia and is required for normal sporulation. The generation of pyruvate by this mechanism may be a source of energy during the sporulation process (33). SpoVG is involved in sporulation as a negative regulator of the pathway leading to asymmetric septation. SpoVG is upregulated upon heat shock in B. subtilis, and this may function to block the sporulation process and favor the vegetative growth of cells (24).
The production of several proteins presumably involved in metabolic processes also increased at 42°C in B. cereus. FolD is a methylene-tetrahydrofolate dehydrogenase that is involved in the biosynthesis of essential cellular compounds such as purines, methionine, and histidine. Dra is the last enzyme in the cascade of the catabolism of deoxyribonucleosides and is thought to furnish the cells with an extra source of energy (31, 35). YbbT is homologous to a phosphoglucose mutase in B. subtilis that can be involved in the conversion of glucose 1-phosphate into glucose 6-phosphate in glycolysis. YbbT is also closely related (68% identity) to GlmM, a phosphoglucosamine mutase from S. aureus that is involved in peptidoglycan production and methicillin resistance (19, 40). Finally, the production of rod shape-determining protein MreB increased in B. cereus. MreB was identified in B. cereus ATCC 10987 by Narahara et al. (27) and was recently characterized as the bacterial homologue of actin (20, 36). In B. subtilis, this protein is essential as its deletion causes inflated morphology and, ultimately, cell lysis (20). The upregulation of MreB in B. cereus may be required for retention of the rod-shaped cell form under stress conditions.
On the basis of the time after which heat-activated production occurred, the B. cereus HSPs can be divided into three groups: I, increasing induction within the exposure time (up to 4 h); II, maximum induction after 15 min with a decrease to basic levels after 1 or 4 h; III, constant overproduction during heat exposure (15 min to 4 h). By analogy to B. subtilis, the grouping of the B. cereus HSPs might correlate to common regulatory features. Transient induction of σB-regulated genes and proteins in B. subtilis upon heat exposure has been observed (14, 15), which correlates to that of the B. cereus group II HSPs. Indeed, group II contains a regulator of σB, RsbV, which might point to σB-dependent expression of the genes in this group. In addition, two genes that are σB dependent in B. subtilis (trxA and ppiB; 14, 32) are found in this group. However, group III also contains some genes that are σB regulated in B. subtilis (yflT and sodA; 14, 29). Continuous production of the classical heat shock chaperones and proteases, such as GroEL and DnaK, upon heat exposure has been observed (correlating to groups I and III of the B. cereus HSPs). This might relate to regulation by HrcA via CIRCE elements. Indeed, close to the transcriptional start of the hrcA-grpE-dnaK-dnaJ operon, which encodes, among other proteins, DnaK (group III), a CIRCE element is present. GroEL, which is HrcA regulated in B. subtilis, also falls into the cluster of proteins the production of which increases during heat exposure (group I).
In relation to these regulatory phenomena, it is important to note that B. cereus ATCC 14579 developed cross-protection from heat after exposure to salt, a low pH, and ethanol, stresses in which common regulators may be involved. We also observed that the increase in the thermotolerance of B. cereus cells exposed to stresses other than heat is not solely protein synthesis dependent. An alternative or complementary mechanism can be the accumulation of compatible solutes or osmoprotectants that might function as thermoprotectants, as has been shown, for example, for glycine betaine in E. coli (6).
The increased use of mild heat preservation treatments and hurdle technology makes food products more susceptible to bacterial contamination than heavily processed foods. This development may have contributed to the reported rise in B. cereus-related food poisoning outbreaks in developed countries (21). Within the concept of hurdle technology, it is important to keep in mind that microorganisms can be more resistant to adverse conditions after a previous stress exposure and thus survive normally lethal conditions that occur during food processing. Here, we analyzed the initial responses of B. cereus to heat and other stresses. This will contribute to the further understanding of B. cereus adaptive mechanisms that may be applicable to food processing and cause increased survival of B. cereus during food processing. Even when more severe heat treatments or other preservation strategies are used, the understanding of the stress response of vegetative cells is of vital importance, as this process may lead to the generation of more resistant spores (26). In the development of cross-protection, key regulators, such as σB, are thought to play a central role. In future studies, we will characterize σB of B. cereus and study genes controlled by this sigma factor.
Acknowledgments
P. M. Periago thanks the Spanish Ministerio de Educación y Ciencia for a research fellowship.
We gratefully acknowledge Wolfgang Schumman, Kuersad Turgay, and Uwe Gerth for the generous gift of antibodies. Integrated Genomics (Chicago, Ill.) is acknowledged for the use of the B. cereus genome sequence database.
REFERENCES
- 1.Abee, T., and J. A. Wouters. 1999. Microbial stress response in minimal processing. Int. J. Food Microbiol. 50:65-91. [DOI] [PubMed] [Google Scholar]
- 2.Arsene, F., T. Tomoyasu, and B. Bukau. 2000. The heat response of Escherichia coli. Int. J. Food Microbiol. 55:3-9. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt, J., U. Völker, A. Völker, H. Antelmann, R. Schmid, H. Mach, and M. Hecker. 1997. Specific and general stress proteins in Bacillus subtilis—a two dimensional protein electrophoresis study. Microbiology 143:999-1017. [DOI] [PubMed] [Google Scholar]
- 4.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 5.Bower, C. K., and M. A. Daeschel. 1999. Resistance responses of microorganisms in food environments. Int. J. Food Microbiol. 50:33-44. [DOI] [PubMed] [Google Scholar]
- 6.Caldas, T., N. Demont-Caulet, A. Ghazi, and G. Richarme. 1999. Thermoprotection by glycine betaine and choline. Microbiology 145:2543-2548. [DOI] [PubMed] [Google Scholar]
- 7.Chan, P. F., S. J. Foster, E. Ingham, and M. O. Clements. 1998. The Staphylococcus aureus alternative sigma factor σB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J. Bacteriol. 180:6082-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derré, I., G. Rapoport, and T. Msadek. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in Gram-positive bacteria. Mol. Microbiol. 31:117-131. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh, P., A. Ishihama, and D. Chatterji. 2001. Escherichia coli RNA polymerase subunit omega and its N-terminal domain bind full-length beta' to facilitate incorporation into the alpha(2)beta subassembly. Eur. J. Biochem. 268:4621-4627. [DOI] [PubMed] [Google Scholar]
- 10.Gothel, S. F., C. Scholz, F. X. Schmid, and M. A. Marahiel. 1998. Cyclophilin and trigger factor from Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions. Biochemistry 37:13392-13399. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman, S., S. Wickner, and M. R. Maurizi. 1997. Protein quality control: triage by chaperones and proteases. Genes Dev. 11:815-823. [DOI] [PubMed] [Google Scholar]
- 12.Gould, G. W., T. Abee, P. E. Granum, and M. V. Jones. 1995. Physiology of food poisoning microorganisms and the major problems in food poisoning control. Int. J. Food Microbiol. 28:121-128. [DOI] [PubMed] [Google Scholar]
- 13.Granum, P. E. 2001. Bacillus cereus, p. 373-381. In M. Doyle, L. Beuchat, and T. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, D.C.
- 14.Hecker, M., W. Schumann, and U. Völker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 15.Helmann, J. D., M. F. W. Wu, P. A. Kobel, F.-J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriques, A. O., L. R. Melsen, and C. P. Moran, Jr. 1998. Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J. Bacteriol. 180:2285-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren, A. 1989. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 264:13963-13966. [PubMed] [Google Scholar]
- 18.Inaoka, T., Y. Matsumura, and T. Tsuchido. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 181:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolly, L., S. Wu, J. van Heijenoort, H. de Lencastre, D. Mengin-Lecreulx, and A. Tomasz. 1997. The femR315 gene from Staphylococcus aureus, the interruption of which results in reduced methicillin resistance, encodes a phosphoglucosamine mutase. J. Bacteriol. 179:5321-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, L. J., R. Carballido-Lopez, and J. Errington. 2001. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104:913-922. [DOI] [PubMed] [Google Scholar]
- 21.Kotiranta, A., K. Lounatmaa, and M. Haapasalo. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189-198. [DOI] [PubMed] [Google Scholar]
- 22.Leistner, L. 2000. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 55:181-186. [DOI] [PubMed] [Google Scholar]
- 23.Mahakarnchanakul, W., and L. R. Beuchat. 1999. Effect of shift in growth temperature on tolerance of psychrotrophic and mesophilic strains of Bacillus cereus to heat and sodium chloride. J. Food Prot. 62:57-64. [DOI] [PubMed] [Google Scholar]
- 24.Matsuno, K., and A. L. Sonenshein. 1999. Role of SpoVG in asymmetric septation in Bacillus subtilis. J. Bacteriol. 181:3392-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayr, B., T. Kaplan, S. Lechner, and S. Scherer. 1996. Identification and purification of a family of dimeric major cold shock protein homologs from the psychrotrophic Bacillus cereus WSBC 10201. J. Bacteriol. 178:2916-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Movahedi, S., and W. Waites. 2000. A two-dimensional protein gel electrophoresis study of the heat stress response of Bacillus subtilis cells during sporulation. J. Bacteriol. 182:4758-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narahara, A., K. Naterstad, T. Kristensen, R. Lopez, P. Bork, and A. B. Kolsto. 1992. Cloning of a gene from Bacillus cereus with homology to the mreB gene from Escherichia coli. Gene 122:181-185. [DOI] [PubMed] [Google Scholar]
- 28.Pagán, R., S. Condón, and F. J. Sala. 1997. Effects of several factors on the heat-shock-induced thermotolerance of Listeria monocytogenes. Appl. Environ. Microbiol. 63:3225-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price, C. W., P. Fawcett, H. Cérémonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome wide analysis of the general stress response of Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 31.Saxild, H. H., L. N. Andersen, and K. Hammer. 1996. dra-nupC-pdp operon of Bacillus subtilis: nucleotide sequence, induction by deoxyribonucleosides, and transcriptional regulation by the deoR-encoded DeoR repressor protein. J. Bacteriol. 178:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharf, C., S. Riethdorf, H. Ernst, S. Engelmann., U. Völker, and M. Hecker. 1998. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J. Bacteriol. 180:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siranosian, K. J., K. Ireton, and A. D. Grossman. 1993. Alanine dehydrogenase (ald) is required for normal sporulation in Bacillus subtilis. J. Bacteriol. 175:6789-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solow, B. T., and G. A. Somkuti. 2000. Comparison of low-molecular-weight heat stress proteins encoded on plasmids in different strains of Streptococcus thermophilus. Curr. Microbiol. 41:177-181. [DOI] [PubMed] [Google Scholar]
- 35.Tozzi, M. G., F. Sgarrella, D. Barsacchi, and P. L. Ipata. 1984. Induction of deoxyribose-5-phosphate aldolase of Bacillus cereus by deoxyribonucleosides. Biochem. Int. 9:319-325. [PubMed] [Google Scholar]
- 36.Van den Ent, F., L. A. Amos, and J. Lowe. 2001. Prokaryotic origin of the actin cytoskeleton. Nature 413:39-44. [DOI] [PubMed] [Google Scholar]
- 37.Völker, U., S. Engelmann, B. Maul, S. Riethdorf, A. Völker, R. Schmid, H. Mach, and M. Hecker. 1994. Analysis of the induction of stress proteins of Bacillus subtilis. Microbiology 140:741-752. [DOI] [PubMed] [Google Scholar]
- 38.Wouters, J. A., B. Jeynov, F. M. Rombouts, W. M. de Vos, O. P. Kuipers, and T. Abee. 1999. Analysis of the role of 7 kDa cold-shock proteins of Lactococcus lactis MG1363 in cryoprotection. Microbiology 145:3185-3194. [DOI] [PubMed] [Google Scholar]
- 39.Wouters, J. A., F. M. Rombouts, O. P. Kuipers, W. M. de Vos, and T. Abee. 2000. The role of cold-shock proteins in low-temperature adaptation of food-related bacteria. Syst. Appl. Microbiol. 23:165-173. [DOI] [PubMed] [Google Scholar]
- 40.Wu, S., H. de Lencastre, A. Sali, and A. Tomasz. 1996. A phosphoglucomutase-like gene essential for the optimal expression of methicillin resistance in Staphylococcus aureus: molecular cloning and DNA sequencing. Microb. Drug Resist. 2:277-286. [DOI] [PubMed] [Google Scholar]
- 41.Yura, T., M. Kanemori, and M. Y. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.