Abstract
Fourteen bacterial strains capable of producing a trypsin-dependent antimicrobial substance active against Clostridium perfringens were isolated from human fecal samples of various origins (from healthy adults and children, as well as from adults with chronic pouchitis). Identification of these strains showed that they belonged to Ruminococcus gnavus, Clostridium nexile, and Ruminococcus hansenii species or to new operational taxonomic units, all from the Clostridium coccoides phylogenetic group. In hybridization experiments with a probe specific for the structural gene encoding the trypsin-dependent lantibiotic ruminococcin A (RumA) produced by R. gnavus, seven strains gave a positive response. All of them harbored three highly conserved copies of rumA-like genes. The deduced peptide sequence was identical to or showed one amino acid difference from the hypothetical precursor of RumA. Our results indicate that the rumA-like genes have been disseminated among R. gnavus and phylogenetically related strains that can make up a significant part of the human fecal microbiota.
Up to 1014 bacteria are present in the human intestinal tract. The microbiota that reside in the colon are essentially anaerobic: Bacteroides, Bifidobacterium, Eubacterium, Clostridium, Fusobacterium, Peptococcus, Peptostreptococcus, and Ruminococcus have been reported to be the predominant genera of the large bowel (34). The involvement of these microbiota in the salvage of energy from nondigested dietary compounds can lead to both toxification and detoxification of metabolic compounds (13). These microbes also affect the immune system of the host (7). Another role is the protection of the host against invasion by potentially pathogenic microorganisms (5), referred to as colonization resistance. Numerous interbacterial antagonisms taking place within the gut have been reported. However, very few have been elucidated thus far at the molecular level.
The production of toxic metabolites, such as bacteriocins, has been shown to be involved in the colonization process of complex ecosystems and in colonization resistance phenomena. Many lactic acid bacteria can produce such compounds (16, 19), and it is well established that they play a crucial protective role in the dairy industry (39). The rat urinary tract (2) or nasopharyngeal region (21) and the human oral cavity (12) are other ecosystems in which bacteriocins could protect against bacterial infections.
In the recent years, a large number of molecules belonging to the different families of bacteriocins have been well characterized genetically and biochemically. Numerous bacterial strains capable of producing bacteriocins were isolated from mammalian digestive ecosystems (1, 3, 10, 17, 18, 20, 33). Thus, it has been suggested that the role of bacteriocins in the gut could be similar to the one they play in other complex ecosystems (28, 29).
Bacteriocins are proteinaceous, ribosome synthesized, antibacterial compounds. Among bacteriocins, the lantibiotic family is characterized by the presence of posttranslationally modified residues such as lanthionines, β-methyllanthionines, and didehydrated residues. Their production requires a complex machinery responsible for biosynthesis, posttranslational modification, export, self-immunity, and in some cases regulation. The genes encoding these functions are generally grouped in clusters according to similar organizational schemes (15, 32).
Ruminococcin A (RumA), a lantibiotic produced by a Ruminococcus gnavus strain from a human fecal sample, was recently characterized both biochemically (3) and genetically (9). The genes responsible for RumA biosynthesis were shown to be organized into three transcriptional units (synthesis and export, immunity, and regulation). Their regulation was shown to be dependent on the proteolytic activity of trypsin and is probably mediated by a two-component signal transduction system (9). The aims of the present study were to determine (i) whether the structural gene encoding RumA is present among bacteria of the dominant human fecal microbiota and (ii) whether rumA-positive strains belong to the R. gnavus species. We show here that the structural genes encoding RumA have been distributed among strains of R. gnavus and other phylogenetically related bacteria isolated from healthy children and adults, as well as from adults with chronic pouchitis. The dissemination of these genes within bacteria of the dominant fecal microbiota reinforces the hypothesis that RumA could be involved in bacterium-bacterium interactions that take place within the human gut.
MATERIALS AND METHODS
Bacterial strains and media.
The following type strains were used in this study: R. gnavus ATCC 29149T (American Type Culture Collection, Manassas, Va.), Clostridium nexile DSMZ 1787T, and R. hansenii DSMZ 20583T (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany). R. gnavus strain E1 was been isolated from the predominant fecal microbiota of a healthy adult (27). The C. perfringens CpA strain (27) was used as the reference target strain. Other strains were isolated during this work (Table 1).
TABLE 1.
Identification of bacterial strains isolated from human fecal samples
| Straina | Originb | G+C content (%) | % DNA-DNA homology with R. gnavus | Max 16S rDNA identity (%)/reference strain | % DNA-DNA homology/reference strainc |
|---|---|---|---|---|---|
| LEMG25 | HC | 42 | 86 | ||
| LEMB04* | HC | 39 | 39 | 99.4/C. nexile | 81/C. nexile |
| LEMV95* | HC | 41 | 82 | ||
| LEMV98* | HC | 38 | 39 | 97.4/R. hansenii | 90/R. hansenii |
| LEMV99* | HC | 43 | 86 | ||
| LEMB86 | HC | 42 | 71 | ||
| LEMV61 | HA | 41 | 20 | 94/E. formicigeneransd | New OTU |
| E1** | HA | 43 | 80 | ||
| LEMV62** | HA | 42 | 87 | ||
| LEMV63** | HA | 41 | 25 | 95/R. torques | New OTU |
| LEMV78 | HA | 43 | 20 | 95/R. gnavus | New OTU |
| LEMB53 | CP | 42 | 85 | ||
| LEMV50 | CP | 41 | 81 | ||
| LEMV58 | CP | 40 | 86 | ||
| LEMV66 | CP | 41 | 85 |
Strains isolated from the same fecal sample are marked with one asterisk. Strains isolated from the same volunteer but in fecal samples obtained after at least 1- year intervals are marked with two asterisks.
Bacterial strains producing a trypsin-dependent anti-C. perfringens substance were isolated from human fecal samples of healthy children (HC), healthy adults (HA), or adults with chronic pouchitis (CP).
Strains exhibiting a 16S rDNA identity <97% with any sequence of a cultivable organism present in the data bank were considered as a new OTU.
Eubacterium formicigenerans.
All strains were grown in an anaerobic cabinet in brain heart infusion broth supplemented with yeast extract and hemin (BHI-YH; BHI [Difco Laboratories, Detroit, Mich.] supplemented with 5 g of yeast extract [Difco Laboratories] and 5 mg of hemin [Sigma-Aldricht Chimie, St. Quentin Fallavier, France]/liter ). When required, trypsin from bovine pancreas (type XIII; l-1-tosyl-amide phenylalanyl chloride treated; Sigma-Aldricht Chimie) was added to BHI broth or solid medium at a final concentration of 50 μg/ml.
Isolation of strains putatively producing a trypsin-dependent anti-C. perfringens substance.
Immediately after collection, 1-g aliquots of the fecal samples were introduced into an anaerobic cabinet, 10-fold serially diluted with LCY broth (11), plated onto BHI-YH agar plates, and incubated for 48 h at 37°C. One hundred clones from a 10−9 or 10−8 dilution were placed by using a toothpick onto BHI-YH plates supplemented with trypsin and allowed to grow for 48 h. Then, 100 μl of culture of the sensitive indicator strain C. perfringens CpA (37°C, 16 to 24 h) was added to 10 ml of BHI-YH agar medium and overlaid onto the plates prior to an additional 24 h of incubation at 37°C. Fecal clones were considered positive when they were surrounded by a clearing zone reflecting growth inhibition of the CpA strain.
DNA extraction and manipulation.
Bacterial chromosomal DNA was isolated by using a Nucleospin C+T kit (Machery-Nagel GmbH & Co., Düren, Germany). Restriction enzymes and T4 polynucleotide kinase were purchased from Gibco-BRL (Life Technologies SARL, Eragny, France) and MBI Fermentas (Vilnius, Lithuania) and used as recommended by the manufacturers. When necessary, DNA fragments were recovered from agarose gels by using a Nucleospin Extract kit (Machery-Nagel).
RAPD fingerprinting.
Randomly amplified polymorphic DNA (RAPD) profiles were obtained according to a previously described protocol (35) with three different primers—OlB06 (5′-TGCTCTGCCC-3′), OlB07 (5′-GGTGACGCAG-3′), or OlB08 (5′-GTCCACACGG-3′)—in independent reactions. Amplification reactions were carried out in a GeneAmp PCR system 2400 (Perkin-Elmer-Cetus, Norwalk, Conn.) by using 20 to 100 ng of the bacterial chromosomal DNA as a template. The reaction mixture included 1.5 mmol of MgCl2/liter, 2 μmol of primer/liter, 7.5 U of DNA polymerase (AmpliTaq; Appligène, Strasbourg, France), 200 μmol of each deoxynucleoside triphosphate/liter, and 10 mmol of Tris-HCl (pH 9.0)/liter in a 100-μl final volume. The PCR conditions used (30 cycles) included annealing at 36°C (2 min), polymerization at 72°C (2 min), and denaturation at 94°C (1 min).
Analysis and comparison of the RAPD profiles.
Portions (20 μl) of the PCR amplification products were separated by electrophoresis in 1% Seakem GTG agarose (Tebu, France) gels in Tris-borate-EDTA alongside the 123-bp ladder from Gibco-BRL (Life Technologies SARL, Eragny, France). Each gel contained 10 lanes of PCR products and 3 lanes of ladder located at both sides and in the center. Ethidium bromide-stained gels were photographed under UV light by using Polaroid film type 665, and negative pictures were digitized by using a Hewlett-Packard Scanjet IIcx/T linked to a computer. For each strain, the three RAPD patterns were merged for analysis and comparison by using the GelCompare Programme (Applied Maths, Sint-Martens-Latem, Belgium) (40). This program allows (i) normalization of electrophoresis patterns to compensate for minor differences in migration, (ii) calculation of a Pearson's coefficient of similarity between patterns, and (iii) clustering of the patterns by using the unweighed pair group method for arithmetic averages (UPGMA).
Bacterial identification by DNA-DNA hybridization.
The degree of DNA-DNA binding was determined quantitatively by spectrophotometry from renaturation rates according to a modification of the method of De Ley et al. (4). The temperature of renaturation was 25°C. DNA-DNA relatedness values were calculated after incubation for 21 and 24 min, after removal from the calculation of the first 3 min of renaturation. The G+C composition of the strains was determined as described by Marmur and Doty (24).
Amplification and cloning of 16S rDNA.
Amplification of 16S ribosomal DNA (rDNA) was performed with the oligonucleotide primers F515 [5′-GTGCCAGC(AC)GCCGCGG-3′], R930 [5′-G(CT)CCCCGTCAATTC(AC)T-3′], F915 [5′-A(GT)GAATTGACGGGG(GA)C-3′], and R1406 [5′-ACGGGCGGTGTGT(GA)C-3′], which correspond to conserved sequences of the bacterial 16S rRNA gene (from positions 515 to 530, 930 to 915, 915 to 930, and 1406 to 1392 on the Escherichia coli 16S rRNA, respectively) (23). PCR conditions used included annealing at 42°C (for 30 s) for fragment A (position 515 to position 930) or at 55°C (for 30 s) for fragment B (position 915 to position 1406), extension at 72°C (45 s), and denaturation at 94°C (2 min). Amplification reactions (30 cycles) were carried out in a GeneAmp PCR system 2400 (Perkin-Elmer-Cetus) with 75 ng of the E1 strain chromosomal DNA as a template. Fragments A and B were then cloned by using the LigATor kit (R&D Systems, Abingdon, United Kingdom) according to the manufacturer's recommendations.
rumA-like gene detection.
The presence of rumA-like genes in the genome of bacterial strains producing a tryspsin-dependent antimicrobial substance was explored by Southern blot hybridization according to standardized methods (30). The oligonucleotide ol30 (5′-AAAAACAATCAGCCACGAATGCAATATGAA-3′) was γ-32P labeled and used as a rumA-specific probe (9). Hybridization experiments were carried out under highly stringent conditions at 50°C.
rumA-like gene amplification.
The chromosomal region harboring the rumA-like genes was amplified by using the oligonucleotide OlK1 (5′-GGAGGAAAGATAAAAATTGATAGTGAACCTAATAG-3′) and OlA4 (5′-AGACCACAAAACTTCTTATGCATACTTACCTCCTG-3′) designated on the basis of the RumA locus sequence (accession number AJ276653 [9]). The PCR conditions included annealing at 50°C (for 30 s), extension at 72°C (for 3 min), and denaturation at 94°C (for 1 min). Amplification reactions (30 cycles) were carried out in a GeneAmp PCR system 2400 (Perkin-Elmer-Cetus) with 500 ng of the bacterial strains chromosomal DNA as a template.
DNA sequencing and analysis.
Nucleotide sequences were determined by the dideoxy chain terminator method (31) by using the Prism TM Ready Reaction d-Rhodamine Terminator sequencing kit (Applied Biosystems Division) in a ABI PRISM 310 Genetic Analyzer (Perkin-Elmer-Cetus). DNA or protein homology searches were carried out with the programs included in the Genetics Computer Group sequence analysis software package (University of Wisconsin).
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this study were deposited in the NCBI (Bethesda, Md.) GenBank Sequence Database. The accession numbers for partial 16S rDNA sequences are as follows: LEMV61, AF446129; LEMV63, AF446128; and LEMV78, AF446130. The accession numbers of the sequences of the chromosomal region harboring the rumA-like genes are as follows: LEMB04, AF439551; LEMB53, AF439554; LEMV58, AF439549; LEMV66, AF439548; LEMV95, AF439550; LEMV98, AF439552; and LEMV99, AF439553.
RESULTS
Isolation and RAPD analysis of strains producing a trypsin-dependent anti-C. perfringens substance.
Eighteen human fecal samples obtained from various ecological contexts, including five from healthy children between 6 and 10 months of age, nine from healthy adults, and four from adults with chronic pouchitis, were freshly collected. The total bacterial populations were estimated on BHI-YE medium to be between 5 × 1010 and 5 × 1011 CFU/g (wet weight) of feces. Randomly chosen bacterial isolates representing at least 1/100 of the culturable population (bacterial counts of between 5 × 108 and 5 × 109 CFU/g of feces) were screened for the production of a trypsin-dependent anti-C. perfringens substance. Two healthy-child and six healthy-adult samples were negative, but 1 to 4 positive clones, all of which were strict anaerobic gram-positive cocci, could be isolated from all of the other samples (three from healthy children, three from healthy adults, and four from adults with chronic pouchitis), providing us with 18 clones.
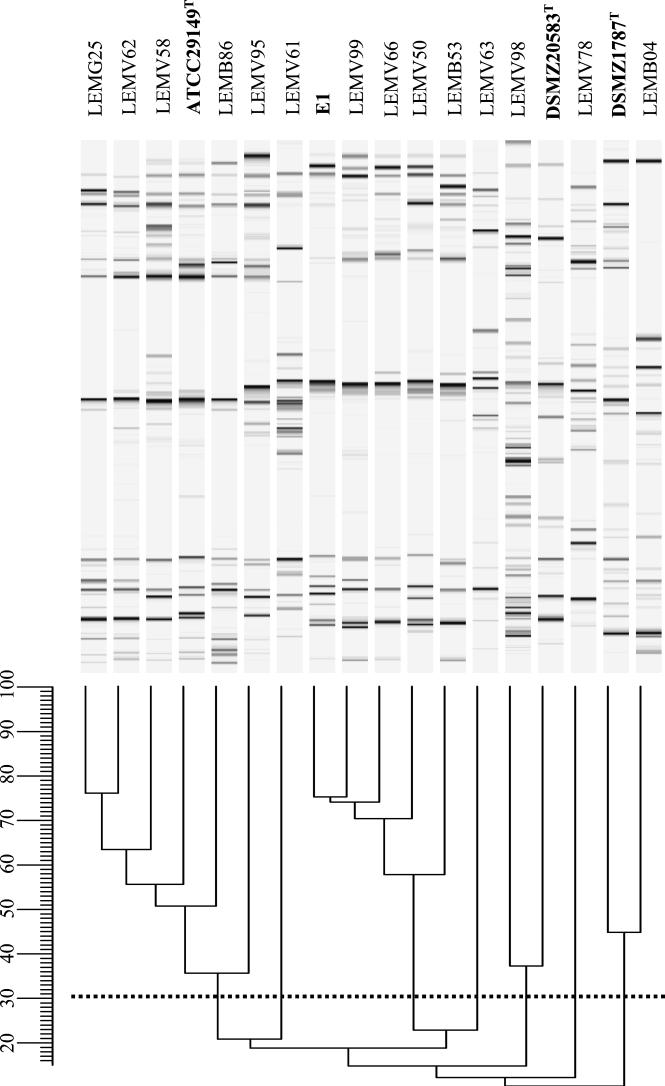
Since several bacterial isolates could be obtained from the same sample, RAPD patterns of positive clones were compared to eliminate redundant isolates. The R. gnavus ATCC 29149T reference strain and the previously characterized R. gnavus E1 strain, which is capable of producing the trypsin-dependent lantibiotic RumA, were also included in the analysis. RAPD profiles were obtained with three different primers, OlB06, OlB07, and OlB08, in separate reactions. Fourteen strains exhibited clearly differentiated profiles (Table 1 and Fig. 1); redundant clones were eliminated from further experiments.
FIG. 1.
Comparison of the RAPD patterns. RAPD profiles were obtained in three independent reactions with the oligonucleotide primers OlB6, OlB7, and OlB8 and then merged for analysis. Reference strains C. nexile DSMZ 1787T, R. hansenii DSMZ 20583T, and R. gnavus ATCC 29149T and the R. gnavus E1 strain are indicated in boldface letters. The horizontal dotted line indicates the value of 30 for the coefficient of similarity (Pearson's coefficient × 100).
At a cutoff level of 30 (Pearson's coefficient × 100), 5 of these 14 strains strains (i.e., LEMG25, LEMV62, LEMV58, LEMB86, and LEMV95) clustered with R. gnavus ATCC 29149T, and 4 strains (LEMV99, LEMV66, LEMV50, and LEMB53) clustered with R. gnavus E1 (Fig. 1). The RAPD patterns of five other strains (LEMV61, LEMV63,LEMV98, LEMV78, and LEMB04) took up separate positions in the analysis.
Identification of the producing strains.
Since similarity in the RAPD patterns suggested that the majority of the positive strains might be related to the R. gnavus species, DNA-DNA hybridization experiments were carried out with the R. gnavus ATCC 29149T reference strain. As expected, the nine strains (LEMG25, LEMV62, LEMV58, LEMB86, LEMV95, LEMV99, LEMV66, LEMV50, and LEMB53) related to R. gnavus according to their RAPD profiles exhibited more than 70% DNA-DNA homology with ATCC 29149T (Table 1), demonstrating that they belonged to R. gnavus. For the other five strains (LEMV61, LEMV63, LEMV98, LEMV78, and LEMB04), the level of DNA-DNA homology was <40%, indicating that they did not belong to this species.
The non-R. gnavus strains were identified by 16S rDNA sequencing. Two fragments, A and B, of the 16S rDNA were amplified, cloned, and sequenced, providing sequence information on an 861-bp rDNA fragment. The 16S rDNA sequence of LEMB04 strain showed 99.4% identity to C. nexile. DNA-DNA hybridization experiments demonstrated that LEMB04 strain had 81% homology with C. nexile DSMZ 1787T and thus confirmed that it belonged to this species (Table 1). Similarly, LEMV98 was shown to belong to the R. hansenii species since it shared 97.4% 16S rDNA identity and had 90% DNA-DNA homology with the type strain of this species (DSMZ 20583T) (Table 1).
For LEMV78, the16S rDNA sequence percentage of identity to known species was not >95% (R. gnavus, Table 1). For the LEMV61 and LEMV63 strains, the highest percentage of identity of their 16S rDNA sequence was observed with unculturable bacteria from human intestinal communities adhufec420 (34) and L37A (accession number AF253389), respectively. The percentage of identity to cultured bacterial strains was <95% (Eubacterium species, Table 1). Thus, these three strains should be considered new operational taxonomic units (OTUs) (8).
All of the strains isolated in here belonged to the C. coccoides phylogenetic group (RDP group 2.30.4.1) (22) that includes some of the predominant bacterial genera found in the human large bowel (34).
In a second series of experiments, RAPD profiles were obtained from all strains, including R. hansenii DSMZ 20583T and C. nexile DSMZ 1787T (Fig. 1). Previous results for the strains identified as R. gnavus were confirmed: they were grouped in two clusters containing R. gnavus ATCC 29149T or R. gnavus E1. LEMV98 and LEMB04 were grouped with R. hansenii DSMZ 20583T and C. nexile DSMZ 1787T, respectively (Fig. 1). RAPD patterns of LEMV61, LEMV63, and LEMV78, putative members of new OTUs, did not exhibit a significant homology with any other strain.
Detection of rumA-like genes.
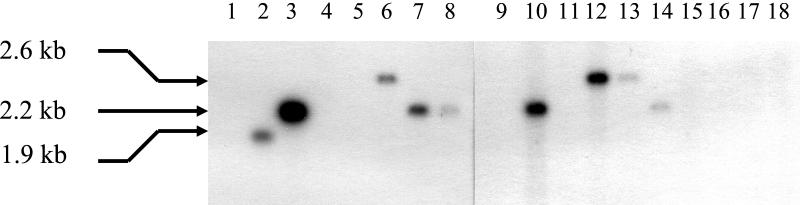
In order to determine whether the trypsin-dependent antimicrobial substance produced by the 14 newly identified strains could be related to RumA, the detection of rumA-like genes on their genome was undertaken. Chromosomal DNA was extracted, digested with EcoRI, and analyzed by Southern blot hybridization by using the specific-rumA oligonucleotide ol30 as a probe. R. gnavus E1 and ATCC 29149T strains were included as positive and negative controls, respectively. Apart from strain E1, 7 of the 14 strains gave a single positive signal (Fig. 2). Because of the highly stringent conditions used in this experiment, this result suggested that these strains probably harbored a rumA-like gene that is very similar to the one identified in strain E1 (9).
FIG. 2.
Detection of rumA-like genes by Southern blot hybridization. Lanes: 1 and 9, R. gnavus ATCC 29149T; 2, LEMB53; 3, LEMV58; 4, LEMV50; 5, LEMB86; 6, LEMV98; 7, LEMV95; 8 and 14, R. gnavus E1; 10, LEMV66; 11, LEMG25; 12, LEMB04; 13, LEMV99; 15, LEMV78; 16, LEMV63; 17, LEMV61; 18, LEMV62. The sizes of the EcoRI restriction fragments hybridizing with the ol30 rumA-specific probe are indicated on the left side of the figure.
DNA amplification and sequencing of the rumA-like genes.
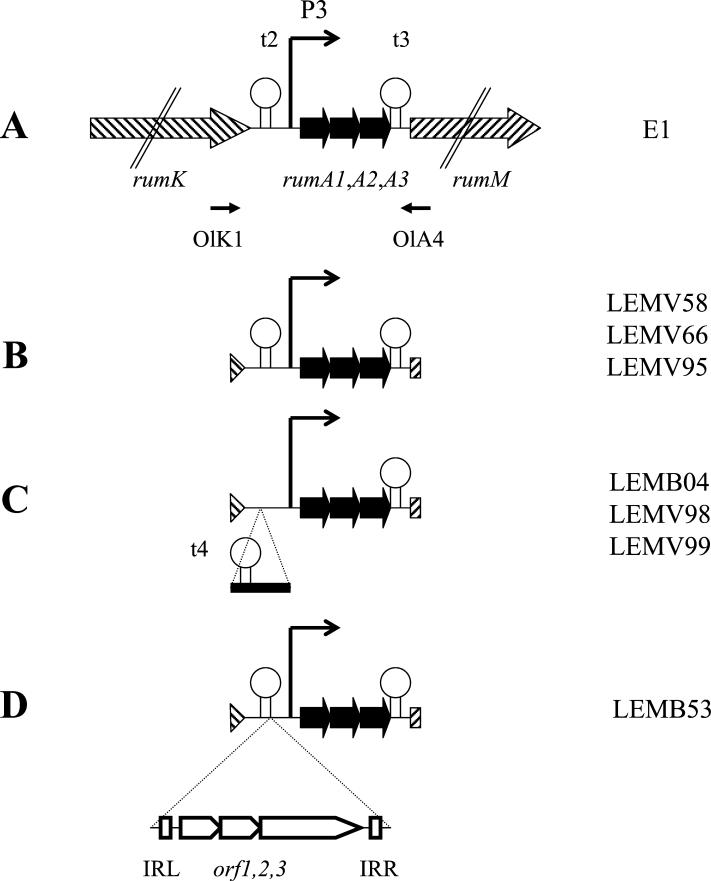
In strain E1, the structural gene encoding the RumA precursor is present in three copies located in between the rumK and rumM genes. These are proposed to encode a sensor histidine kinase and a modification enzyme supposed to catalyze dehydration and thioether bridge formation of lantibiotic precursors, respectively (9).
Two primers, OlK1 and OlA4, located at the 3′ terminus of rumK and at the 5′ terminus of rumM, respectively (Fig. 3A), were used to amplify total DNA extracted from the seven strains positively responding to rumA-specific probe ol30. For each strain, one main amplified fragment was obtained, with a size ranging from ca. 900 bp to 3.7 kbp. The fragments were extracted from agarose gels, purified, and sequenced.
FIG. 3.
Schematic representation of the chromosomal region harboring the rumA-like genes. The positions of rumA1, rumA2, and rumA3 are indicated with respect to rumK and rumM on the chromosome of strain E1. rumK and rumM genes are indicated as dashed arrows, rumA-like genes are indicated as black arrows, and orf1, orf2, and orf3 are iindicated as open arrows. Their orientation indicates the direction of transcription. OlK1 and OlA4 are primers used to amplify and sequence the corresponding DNA region. The strains corresponding to a given organizational scheme are indicated on the right side. t2, t3, and t4 are putative stem-loop structures that might act as rho-independent transcription terminators. P3 was previously suggested to act as the promoter of rumA genes in strain E1 (9). White boxes indicate the two imperfect 27-bp inverted repeats IRL and IRR bordering orf1, orf2, and orf3.
The analysis of the sequence data revealed that, as in strain E1, three copies of the structural rumA gene were systematically present on each amplified fragment (Fig. 3). The rumA1-, rumA2-, and rumA3-like genes were located on a 700-bp fragment exhibiting >90% identity to the corresponding strain E1 fragment. The G+C content of this fragment ranged from 32 to 34%, a level clearly different from the average values for the rest of the chromosome (38 to 43%, Table 1). For a given strain, the three copies of the rumA-like genes encoded the same putative peptide. Moreover, the peptide sequence deduced from the rumA-like genes of four strains (LEMB53, LEMV58, LEMV66, and LEMV95) were identical to the hypothetical RumA precursor previously characterized (9). In the three other strains (LEMB04, LEMV98, and LEMV99), one difference in the same putative residue (at position 15) was observed.
In strains LEMV58, LEMV66, and LEMV95, the amplified fragment was the same size as in strain E1 and showed >92% identity to the fragment of strain E1 (Fig. 3B).
In strains LEMB04, LEMV98, and LEMV99, the amplified fragments were 1,160 bp long and shared >99% identity. The supplementary 400-bp fragment, located between the 3′ terminus of rumK and the 5′ terminus of rumA1 (Fig. 3C), did not show any significant homology with the DNA sequence described above. This fragment harbored a 27-bp imperfect inverted repeat, which was predicted to form a stem-loop structure with a ΔG of −15.1 kcal/mol as shown by Tinoco et al. (38), that might act as a rho-independent transcription terminator. As mentioned above, in these strains, the peptide sequence deduced from the rumA-like genes exhibited one difference with the putative RumA precursor deduced from all other strains: one Asn residue located in position 15 of the hypothetical leader peptide replaced a Lys residue. Remarkably, LEMB04, LEMV98, and LEMV99 belonged to three different species (C. nexile, R. hansenii, and R. gnavus, respectively) and had been isolated from the same fecal sample together with the LEMV95 strain (Table 1).
The fragment amplified from LEMB53 strain was the largest. In addition to the rumA-like genes, three open reading frame genes—orf1, orf2, and orf3—were found (Fig. 3D) and predicted to code for hypothetical proteins consisting of 137, 114, and 538 amino acids, respectively. Orf1, Orf2, and Orf3 were similar to putative proteins associated with mobile genetic elements previously described in Yersinia pestis (26), Streptococcus pneumoniae TIGR4 (37), S. pyogenes (6), and E. coli (25), with some of them belonging to the IS66 family. The hypothetical genes orf1, orf2, and orf3 were located in between two imperfect 29-bp inverted repeats, suggesting that the complete structure could constitute an insertion sequence of the IS66 family. This putative mobile genetic element was located immediately downstream of the probable rho-independent transcription terminator t2 (Fig. 3D). No direct repeat could be detected close to this structure.
DISCUSSION
Fourteen bacterial strains capable of producing trypsin-dependent antimicrobial compounds active against C. perfringens were isolated from the dominant population of fecal samples collected in various ecological contexts from healthy adults and children and from adults with chronic pouchitis. They were identified as R. gnavus or closely related species belonging to the phylogenetically defined cluster C. coccoides. Three of them were new OTUs. The analysis of RAPD profiles showed that all of the R. gnavus strains clustered together with either the reference strain of this species, R. gnavus ATCC 29149T, or the R. gnavus E1 strain, which produced the trypsin-dependent lantibotic RumA, recently characterized by our group (3).
When the newly isolated strains were hybridized with a probe specific for the rumA structural gene, seven gave a positive response and seven were negative. Since the hybridization experiments were performed under highly stringent conditions, the trypsin-dependent anti-C. perfringens activity detected in the seven rumA-negative strains during the screening test may be due to the production of bacteriocins encoded by genes significantly different from those previously identified in the E1 strain. However, it may also be due to non-RumA-related antimicrobial compounds.
The seven positive strains were shown to harbor the structural gene encoding RumA (9). The peptide sequence deduced from these genes was nearly identical to the hypothetical RumA precursor. Only one variation in the amino acid composition of the leader peptide could be noticed in three strains isolated from the same sample. Variations in the amino acid composition of the leader peptide of a lantibiotic were previously demonstrated in M-type 49 group A Streptococcus strains, which harbor two tandem copies of the structural gene (14). As already described for strain E1 (9), all of the positive strains harbored three consecutive copies of the rumA gene, exhibiting a high percentage of nucleotide identity (>90%) and coding for the same probable peptide. This arrangement of genes was previously reported for strain E1 (9). However, the detection of a similar structure disseminated among several bacterial species of the dominant fecal microbiota was puzzling. The role, if any, of this particular structure on the regulation, the level of expression, or the structural DNA stability of this region remains to be evaluated.
In all of our strains, the rumA region exhibited a low G+C content (ca. 32 to 34%), which is clearly different from the rest of the chromosome of R. gnavus or phylogenetically related species (38 to 43%). This observation suggests that the rum genes were recently acquired by R. gnavus via horizontal genetic transfer from a bacterium with a lower G+C content.
Four rumA-positive strains were isolated from the same fecal sample collected from a healthy child. Three of these strains belonged to three different species (R. gnavus, R. hansenii, and C. nexile) and harbored an additional 400-bp fragment in between the rumK and rumA1 genes. These strains exhibited >95% identity in a 1.1-kb genome region spanning the rumA genes. These three strains were the only ones to harbor the same modification in the leader peptide sequence, suggesting that the genetic transfer of the rumA genes occurred within the digestive tract of the volunteer.
Initially, the proteinaceous nature of bacteriocins was evidenced by the loss of the antimicrobial activity after the action of various proteases, including trypsin (16, 18, 19). Therefore, the role of bacteriocins in the digestive tract, if any, has to be local as, for example, when there is a close relationship between the producer and the target strain; otherwise, bacteriocins would be destroyed by host or microbial proteases. In previous studies, it was shown that RumA seems adapted to the digestive ecosystem, since (i) trypsin, one of the main proteases of the gut, is necessary for RumA production; (ii) RumA is resistant to trypsin proteolytic activity; and (iii) RumA is active against two groups of targets: bacterial species belonging to the C. coccoides phylogenetic group and pathogenic Clostridium spp. (3, 9). Our new results suggest that the rumA-like genes may be located on a mobile genetic element transferable to different species of the C. coccoides cluster, leading to their dissemination in R. gnavus and related species that can reach dominant levels in the bacterial populations of the human gut.
In studies of bacteriocins produced by E. coli, Riley and Gordon concluded that “there might be a trade-off between the costs and the benefits of colicin production” (29). This argument is also true for lantibiotics such as RumA, the synthesis of which necessitates a complex genetic system (19). Thus, RumA might be considered as a powerful weapon allowing bacteria that produce it to compete efficiently for the same ecological niches with related species. It is remarkable that the rumA genes, initially identified in a bacterium isolated from the fecal microbiota of a healthy adult, were also present in fecal strains from a young child and in adults with chronic pouchitis. Thus, RumA could play a role in various ecological contexts, such as when the digestive microbiota is considered to have reached a steady state (as with healthy adults) and also when it undergoes variations due to increasing complexity (as with children) or profound modification of the ecosystem (as with chronic pouchitis).
Although several rumA-positive strains were isolated during the present study, the occurrence of RumA-producing bacteria among the fecal microbiota may still be underestimated. The detection of the rumA genes here was undertaken with bacterial strains representing at least 1% of the culturable fecal population. Previous studies have shown that only 21% of the total population detected microscopically through DAPI (4′,6′-diamidino-2-phenylindole) staining and 32 to 37.5% of the total population detected by oligonucleotide probe hybridization were able to grow anaerobically on a nonselective medium (34, 36). During our screening, no bacterial isolate producing a trypsin-dependent anti-C. perfringens substance could be obtained from two samples from healthy children and six samples from healthy adults. The assumption that positive strains simply did not exist in these samples cannot be rejected. However, such strains could be present but unculturable or at population levels lower than 5 × 108 CFU/g of feces.
Further studies are now needed to more precisely evaluate the impact of RumA production on the colonization capabilities of the producing strains and on the protection of the host against invasion by pathogenic Clostridium spp.
Acknowledgments
We thank E. Le Page for technical help and P. Tailliez for constructive discussion.
This work was supported by the grant “Actions Concertées Coordonnées Sciences du Vivant” from the French Ministry for Research and Technology and grant FAIR CT 95-0433 from the European Community. A.W. is grateful to the Fund for Scientific Research-Flanders for a position as Postdoctoral Research Fellow.
REFERENCES
- 1.Balla, E., L. M. Dicks, M. Du Toit, M. J. Van Der Merwe, and W. H. Holzapfel. 2000. Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecalis BFE 1071. Appl. Environ. Microbiol. 66:1298-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braude, A. I., and J. S. Siemienski. 1968. The influence of bacteriocins on resistance to infection by gram-negative bacteria. II. Colicin action, transfer of colicinogeny, and transfer of antibiotic resistance in urinary infections. J. Clin. Investig. 47:1763-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabard, J., C. Bridonneau, C. Phillipe, P. Anglade, D. Molle, M. Nardi, M. Ladire, H. Girardin, F. Marcille, A. Gomez, and M. Fons. 2001. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl. Environ. Microbiol. 67:4111-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Ley, J., H. Cattoir, and A. Reynaerts. 1970. The quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 5.Ducluzeau, R. 1988. Role of experimental microbial ecology in gastroenterology, p. 7-26. In E. Bergone-Berezin (ed.), Microbial ecology and intestinal infections. Springer-Verlag, Berlin, Germany.
- 6.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 8.Godon, J. J., E. Zumstein, P. Dabert, F. Habouzit, and R. Moletta. 1997. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 63:2802-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez, A., M. Ladiré, F. Marcille, and M. Fons. 2002. Trypsin mediates growth phase-dependent transcriptional regulation of genes involved in the biosynthesis of ruminococcin A, a lantibiotic produced by a Ruminococcus gnavus strain from a human intestinal microbiota. J. Bacteriol. 184:18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon, D. M., M. A. Riley, and T. Pinou. 1998. Temporal changes in the frequency of colicinogeny in Escherichia coli from house mice. Microbiology 144:2233-2240. [DOI] [PubMed] [Google Scholar]
- 11.Hautefort, I., B. Fléchon, J. Degrouard, and M. Fons. 2000. Adhesion to the digestive mucosa is not sufficient for durable persistence of different Lactobacillus fermentum strains in the digestive tract of mice. Microb. Ecol. Health Dis. 12:48-56. [Google Scholar]
- 12.Hillman, J. D., A. L. Dzuback, and S. W. Andrews. 1987. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J. Dent. Res. 66:1092-1094. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, R., and I. R. Rowland. 2000. Metabolic activities of the gut microflora in relation to cancer. Microb. Ecol. Health Dis. 12(Suppl. 2):179-185. [Google Scholar]
- 14.Hynes, W. L., V. L. Friend, and J. J. Ferretti. 1994. Duplication of the lantibiotic structural gene in M-type 49 group A Streptococcus strains producing streptococcin A-M49. Appl. Environ. Microbiol. 60:4207-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack, R., G. Bierbaum, C. Heidrich, and H. G. Sahl. 1995. The genetics of lantibiotic biosynthesis. Bioessays 17:793-802. [DOI] [PubMed] [Google Scholar]
- 16.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalmokoff, M. L., D. Lu, M. F. Whitford, and R. M. Teather. 1999. Evidence for production of a new lantibiotic (butyrivibriocin OR79A) by the ruminal anaerobe Butyrivibrio fibrisolvens OR79: characterization of the structural gene encoding butyrivibriocin OR79A. Appl. Environ. Microbiol. 65:2128-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalmokoff, M. L., and R. M. Teather. 1997. Isolation and characterization of a bacteriocin (Butyrivibriocin AR10) from the ruminal anaerobe Butyrivibrio fibrisolvens AR10: evidence in support of the widespread occurrence of bacteriocin-like activity among ruminal isolates of B. fibrisolvens. Appl. Environ. Microbiol. 63:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 20.Laukova, A., and M. Marekova. 1993. Antimicrobial spectrum of bacteriocin-like substances produced by rumen staphylococci. Folia Microbiol. 38:74-76. [DOI] [PubMed] [Google Scholar]
- 21.LiPuma, J. J., H. Richman, and T. L. Stull. 1990. Haemocin, the bacteriocin produced by Haemophilus influenzae: species distribution and role in colonization. Infect. Immun. 58:1600-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marmur, J., and P. Doty. 1962. Determination of base composition of deoxyribonucleic acid from its thermal denaturation temperature. J. Mol. Biol. 5:109-118. [DOI] [PubMed] [Google Scholar]
- 25.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 26.Perry, R. D., S. C. Straley, J. D. Fetherston, D. J. Rose, J. Gregor, and F. R. Blattner. 1998. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect. Immun. 66:4611-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramare, F., J. Nicoli, J. Dabard, T. Corring, M. Ladire, A. M. Gueugneau, and P. Raibaud. 1993. Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl. Environ. Microbiol. 59:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley, M. A. 1998. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 32:255-278. [DOI] [PubMed] [Google Scholar]
- 29.Riley, M. A., and D. M. Gordon. 1999. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 7:129-133. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siezen, R. J., O. P. Kuipers, and W. M. de Vos. 1996. Comparison of lantibiotic gene clusters and encoded proteins. Antonie Leeuwenhoek 69:171-184. [DOI] [PubMed] [Google Scholar]
- 33.Siragusa, G. R. 1992. Production of bacteriocin inhibitory to Listeria species by Enterococcus hirae. Appl. Environ. Microbiol. 58:3508-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suau, A., R. Bonnet, M. Sutren, J. J. Godon, G. R. Gibson, M. D. Collins, and J. Dore. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tailliez, P., J. Tremblay, S. D. Ehrlich, and A. Chopin. 1998. Molecular diversity and relationship within Lactococcus lactis, as revealed by randomly amplified polymorphic DNA (RAPD). Syst. Appl. Microbiol. 21:530-538. [DOI] [PubMed] [Google Scholar]
- 36.Tannock, G. W., K. Munro, H. J. Harmsen, G. W. Welling, J. Smart, and P. K. Gopal. 2000. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 66:2578-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 38.Tinoco, I., Jr., P. N. Borer, B. Dengler, M. D. Levin, O. C. Uhlenbeck, D. M. Crothers, and J. Bralla. 1973. Improved estimation of secondary structure in ribonucleic acids. Nat. New Biol. 246:40-41. [DOI] [PubMed] [Google Scholar]
- 39.Vandenbergh, P. A. 1993. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol. Rev. 12:221-238. [Google Scholar]
- 40.Vauterin, L., and P. Vauterin. 1992. Computer-aided objective comparison of electrophoresis patterns for grouping and identification of microorganisms. Eur. Microbiol. 1:37-41. [Google Scholar]