Abstract
The basidiomycete Collybia dryophila K209, which colonizes forest soil, was found to decompose a natural humic acid isolated from pine-forest litter (LHA) and a synthetic 14C-labeled humic acid (14C-HA) prepared from [U-14C]catechol in liquid culture. Degradation resulted in the formation of polar, lower-molecular-mass fulvic acid (FA) and carbon dioxide. HA decomposition was considerably enhanced in the presence of Mn2+ (200 μM), leading to 75% conversion of LHA and 50% mineralization of 14C-HA (compared to 60% and 20%, respectively, in the absence of Mn2+). There was a strong indication that manganese peroxidase (MnP), the production of which was noticeably increased in Mn2+-supplemented cultures, was responsible for this effect. The enzyme was produced as a single protein with a pI of 4.7 and a molecular mass of 44 kDa. During solid-state cultivation, C. dryophila released substantial amounts of water-soluble FA (predominantly of 0.9 kDa molecular mass) from insoluble litter material. The results indicate that basidiomycetes such as C. dryophila which colonize forest litter and soil are involved in humus turnover by their recycling of high-molecular-mass humic substances. Extracellular MnP seems to be a key enzyme in the conversion process.
Humic substances are the most widespread and ubiquitous natural nonliving organic materials in terrestrial and aquatic environments and represent the major fraction of soil organic matter (12, 33). In addition, they make up a substantial part of the fossil organic carbon incorporated into peat and low-rank coals (e.g., lignite and brown coal) (11). Humic substances comprise a physically and chemically heterogeneous mixture of biogenic, relatively high-molecular-mass compounds with mixed aliphatic and aromatic natures (35). Based on solubility in acids and alkalis, they can be divided into three main fractions: humic acid (HA), which is soluble in alkali and insoluble in acid; fulvic acid (FA), which is soluble in alkali and acid; and humin, which is insoluble in both alkali and acid (33). Humic substances are formed by secondary synthesis reactions (humification) during the decay process and transformation of biomolecules originating from dead organisms and microbial activity. Lignin and its transformation products, as well as polyphenols and derived polymers from lower plants and microorganisms, are important starting materials in this process and provide aromatic building blocks of high physicochemical stability (35).
Microbial degradation of humic substances—particularly of high-molecular-mass, aromatic moieties in HA and humin—is an important part of humus turnover and is therefore essential for maintaining the global carbon cycle (14). Despite this fact, little is known yet about the microorganisms which decompose and recycle humic matter. Since lignin, the complex aromatic polymer providing strength and rigidity to the cell walls and tissues of vascular plants, is a major parent material in the formation of humic substances (33), several authors studied the decomposition of natural and synthetic HA by white-rot fungi (2, 4, 6, 19, 23, 38, 41), which are the most effective lignin degraders in nature (16). Some of these basidiomycetous fungi which colonize wood (e.g., Phanerochaete chrysosporium, Trametes versicolor, and Nematoloma frowardii) were shown to disintegrate high-molecular-mass HA by forming lower-molecular-mass FA and carbon dioxide (CO2). Correlation was observed between the activity of extracellular peroxidases and HA degradation, and isolated manganese peroxidase (MnP) was even found to depolymerize and mineralize different HAs in vitro (5, 20, 40). However, since most white-rot fungi grow preferentially in compact wood (trunks, logs, branches, and stumps) and cannot compete in soil for a prolonged time (7, 28), it is doubtful whether they are involved to a large extent in humus decomposition under natural conditions (25). In the present study, therefore, we have focused on HA degradation by a true soil-colonizing basidiomycete, Collybia dryophila, which is a very common species in European and North American woodlands and grows in different layers of forest litter from both coniferous and deciduous trees (17, 24, 30).
MATERIALS AND METHODS
Soil-litter and litter HA (LHA).
Soil-litter samples (wet weight, 3 × 500 g) from a mixed pine-spruce forest (Pinus sylvestris and Picea abies) near Lahti (Finland) were collected at a depth of 5 cm. Samples were combined and sieved, using a <1-cm-pore-diameter sieve, to remove larger twigs, cones, stones, etc. The combined litter sample had a pH of 3.9 and a water content of 60% and was stored at 4°C.
LHA was extracted from litter (wet weight, 750 g) with 0.5 N NaOH (4 liters) by continuous agitation for 2 h (1). After sedimentation of the alkaline suspension, the dark-brown supernatant was filtered through Miracloth (Calbiochem, La Jolla, Calif.), centrifuged (8,500 × g), and filtered again (glass fiber filter GF52; Schleicher & Schuell, Dassel, Germany). LHA was precipitated by adjusting the pH level to 1.5 with 6 N HCl, and the suspension was left overnight at 4°C. After centrifugation, the LHA precipitate was washed with water and dried at 40°C.
Synthetic 14C-HA.
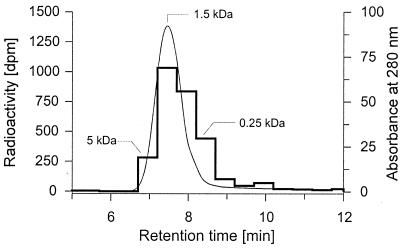
14C-labeled humic acid (14C-HA) was synthesized by spontaneous oxidative polymerization of [U-14C]catechol (specific radioactivity, 3.4 mCi mmol−1 = 125.8 MBq mmol−1; Sigma, Saint Louis, Mo.) in an alkaline solution (13, 40). 14C-catechol (1.38 × 107 dpm) and 11 mg of unlabeled catechol were dissolved in 1 ml of NaOH (0.1 N) which also contained Mn2+ (final concentration, 2 mM) to enhance the polymerization process (20). The reaction mixture was incubated with vigorous stirring at 25°C for 3 days. 14C-HA was precipitated by addition of 50 μl of 6 N HCl, followed by centrifugation. The 14C-HA pellet was washed twice with water and resuspended in 1 ml of 0.1 N NaOH, forming a dark-brown solution with a radioactivity of 4.75 × 103 dpm μl−1; i.e., about 35% of the original 14C-labeled catechol was incorporated in this preparation. The distribution of molecular mass and radioactivity of this preparation are shown in Fig. 1. A structural model of a similar humic substance, based on 13C-CP-TOSS nuclear magnetic resonance spectroscopic data, has been described recently (25).
FIG. 1.
HPSEC elution profile of a synthetic HA prepared from catechol, showing the distribution of molecular masses (thin line, spline curve) and radioactivity (bold line, step plot). Injection volume, 10 μl of the tenfold diluted stock solution (about 5 × 103 dpm).
Organism and culture conditions.
C. dryophila (Bull.:Fr.) P. Kumm. (russet tough-shank) strain K209, which has been grouped into the litter-decomposing fungi according to the classification system of Dix and Webster (7), was isolated from basidiocarps collected in the geographical area described above. They grew in soil litter consisting of needles, twigs, dead moss material, and humus-rich soil particles. Isolates were recovered by placing small pieces of fruit body plectenchyma aseptically on 2% malt extract agar (MEA) plates containing chloramphenicol (50 mg liter−1) and benomyl (50 mg liter−1) to prevent growth of bacteria and molds, respectively. Stock cultures of C. dryophila were maintained on MEA slants at 4°C and are deposited at the fungal culture collection of the Department of Applied Chemistry and Microbiology at University of Helsinki (Finland).
Precultures were grown on MEA plates, and agar plugs (10 mm in diameter) were used to inoculate liquid- and solid-state cultures. The basal liquid medium consisted of 10 g of glucose liter−1, 2 g of KH2PO4 liter−1, 0.5 g of MgSO4 · 7 H2O liter−1, 0.1 g of CaCl2 liter−1, 0.5 g of ammonium tartrate liter−1, 2.2 g of 2,2-dimethylsuccinate liter−1, and 0.1 g of yeast extract liter−1 (pH was adjusted to 5.0). Stationary culture cultivation was carried out in 1-liter Erlenmeyer flasks containing 150 ml of medium. In addition, certain cultures were supplemented with MnCl2 (200 μM) and/or LHA (250 mg liter−1 dissolved in 0.1 NaOH). Flasks were inoculated with 10 agar plugs (10 mm in diameter) and incubated at 20°C in the dark for 6 weeks. Every 3 to 7 days, samples (1.5 ml) were taken to determine enzyme activities and the absorbance at 450 nm (the wavelength of the brown color of LHA) (38). After 6 weeks of incubation, LHA-containing flasks were mixed with 1.5 ml of 10 N NaOH and shaken for 30 min (150 rpm). Aliquots of 1 ml were centrifuged, and 6 N HCl (20 μl) was added to the supernatant to precipitate LHA and separate it from FA. The FA was present in the yellowish acidic supernatant after further centrifugation, and the LHA pellet was resuspended and dissolved in 0.1 N NaOH. Both fractions were used for high-performance size exclusion chromatography (HPSEC) analyses. The dry mass of the fungal cultures was determined after filtration through glass-fiber filters and drying to constant weight at 70°C.
Production of MnP for further enzyme purification occurred in 1-liter tissue culture flasks containing 200 ml of basal medium plus 200 μM MnCl2 (34). Flasks were cultured at 24°C and harvested after 5 weeks.
Solid-state cultures consisted of sterilized litter (15 g; water content, 80%) in 100-ml Erlenmeyer flasks which were inoculated with 3 agar plugs (10 mm in diameter) of pregrown mycelium (11 ± 2 mg mycelial dry weight per plug). They were incubated at 20°C for 46 days and then extracted with 40 ml of distilled water by sonication (3 min) and shaking (30 min on a rotary shaker at 150 rpm). After centrifugation, samples were used for HPSEC, high-performance liquid chromatography (HPLC), and enzymatic measurements.
Mineralization experiments with radioactive HA.
Mineralization studies using 14C-HA were carried out in liquid culture containing the basal medium mentioned above either in the presence of Mn2+ (200 μM) or in its absence. 14C-HA (2.5 × 105 dpm) dissolved in 0.1 NaOH was added to 15-ml liquid cultures pregrown for 3 days (final 14C-HA concentration, 12.8 mg liter−1). Inoculated flasks (3 used in parallel; see Material and Methods) and uninoculated controls were sealed with rubber septa and aluminum caps. Incubation occurred at 24°C in the dark. 14C-labeled volatile compounds and 14CO2 were flushed out once a week with pure oxygen for 15 min and trapped by bubbling any gas released through two sequential flasks containing Opti-Fluor and Carbosorb/Opti-Fluor (Packard Instruments, Groningen, The Netherlands). After 6 weeks of incubation, residual 14C-HA was dissolved by addition of 15 ml 0.1 NaOH followed by sonication (3 min) and vigorous shaking (5 min). After filtration and centrifugation, aliquots of 100 μl were used to determine soluble radioactivity. The respective filters and the included fungal biomass were burned in a combustion chamber (Junitek, Turku, Finland) to confirm the identity of the bound 14C as evolving 14CO2. A liquid scintillation counter (model no. 1411; Wallac, Turku, Finland) was used for all radioactivity measurements.
HPSEC and HPLC.
An HPSEC method described previously (19, 22) was used for the determination of the molecular mass distribution of humic substances. Sodium polystyrene sulfonates (1.3 to 168 kDa) and biphenyl dicarboxylic acid (0.246 kDa) served as molecular mass standards.
Elution profiles of aqueous litter extracts were recorded by HPLC using an Aqua-C18 column (4.6 by 250 mm; Phenomenex, St. Torrance, Calif.) and an HP 1090 Liquid Chromatograph (Hewlett Packard, Waldbronn, Germany). Separations were run at a constant temperature of 40°C by using a stepwise gradient of 0 to 100% acetonitrile (0 and 15 min, 0%; 40 min, 50%; 45 min and 47 min, 100%; 55 min, 0%) in 0.05% phosphoric acid over 55 min with a constant flow rate of 0.75 ml min−1. Eluted substances were detected at 254 nm.
Enzyme assays.
The activity of MnP was measured at 270 nm by monitoring the formation of Mn3+-malonate complexes (37) or at 420 nm by monitoring the oxidation of ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate); ɛ420, 36 mM−1 cm−1] in the presence of HA, as described previously (21). Laccase activity was also determined by the oxidation of ABTS at 420 nm, but in the absence of H2O2 and Mn2+ (8). Lignin peroxidase activity was measured at 310 nm using the veratryl alcohol method (26). Enzyme activities were expressed in units (U), i.e., micromoles of product formed per minute.
Purification and characterization of MnP.
Culture fluid from tissue culture flasks (1.5 liter) supplemented with 200 μM Mn2+ was harvested after 5 weeks of incubation and separated from the mycelium by filtration through a glass fiber filter (GF52; Schleicher & Schuell). The filtrate was concentrated at 4°C by two steps of ultrafiltration-diafiltration (Filtron Omega cassette, 8-kDa cutoff: Filtron Technology Corp., Northboro, Mass.; Amicon chamber, YM10 membrane, 10-kDa cutoff: Millipore, Bedford, Mass.). Extracellular proteins were separated by anion exchange chromatography on a MonoQ column (Amersham Pharmacia Biotech, Uppsala, Sweden) with a 0.02 to 1 M NaAc (pH 5.5) gradient. The elution of absorbing material from the column was simultaneously monitored at 280 and 405 nm to record total protein and heme, respectively. Fractions of 0.5 ml each were collected at a flow rate of 0.5 ml min−1, and those containing MnP were pooled, concentrated, and diafiltrated (Filtron Microsep, 10-kDa cutoff) with double the amount of distilled water.
The molecular weight (MW) of C. dryophila MnP was determined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described previously (27), using a 4% stacking gel, a 12% resolving gel, and the Bio-Rad Mini Protean II PAGE chamber (Bio-Rad, Hercules, Calif.). Samples were boiled in the presence of SDS and β-mercaptoethanol for 3 min. After electrophoresis, gels were stained and protein bands were visualized with Coomassie brilliant blue R-250. For the determination of MW, a low-MW protein calibration kit was used (Amersham Pharmacia).
Analytical isoelectric focusing (IEF) was carried out with a Multiphor II electrophoresis system (Amersham Pharmacia). The IEF gel (7.5%) was prepared using ampholines of isoelectric points (pIs) 2.5 to 5.0 and 3.5 to 10.0 (Pharmacia). The pH gradient of the gel was measured with a surface electrode (Sentek, Braintree, United Kingdom) and a pH meter (Orion, Beverly, Mass.), and in addition, a protein standard kit (Amersham Pharmacia) was used. Gels were stained with phenol red according to the method of Pease et al. (29).
RESULTS
LHA decomposition and enzyme activities.
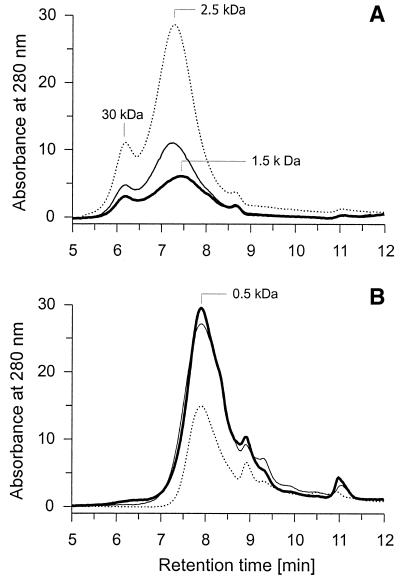
C. dryophila grew relatively slowly in liquid media, and after only 3 weeks, the whole surface was covered with a white mycelium layer. In the presence of both Mn2+ and LHA, biomass production was noticeably stimulated (2.18 ± 0.07 g liter−1) compared with that of nonsupplemented cultures (1.01 ± 0.09 g liter−1) and cultures containing either Mn2+ (1.09 ± 0.08 g liter−1) or LHA (1.25 ± 0.05 g liter−1). HPSEC elution profiles of LHA recovered from fungal cultures show that about 75% of the material was converted in Mn2+-containing cultures, whereas only 60% was converted in the absence of Mn2+ (Fig. 2). Molecular mass distribution of residual LHA was changed towards lower masses only in the presence of Mn2+ (from 2.5 to 1.5 kDa for the predominant molecular mass) (Fig. 2A). Part of LHA was transformed into lower-molecular mass FA (∼0.5 kDa) which was, in contrast to the original LHA, not precipitable with HCl (Fig. 2B). The largest amount of FA was found in cultures supplemented with Mn2+, though the difference from Mn2+-free cultures was slight. In both cases, the FA concentration was about twice as high as in the uninoculated controls.
FIG. 2.
HPSEC elution profiles of LHA recovered from liquid cultures of C. dryophila grown in the presence or absence of Mn2+ (A) and of the respective FA formed (B). Bold lines, fungal cultures supplemented with 200 μM MnCl2; thin lines, fungal cultures without Mn2+; dotted lines, controls without fungus.
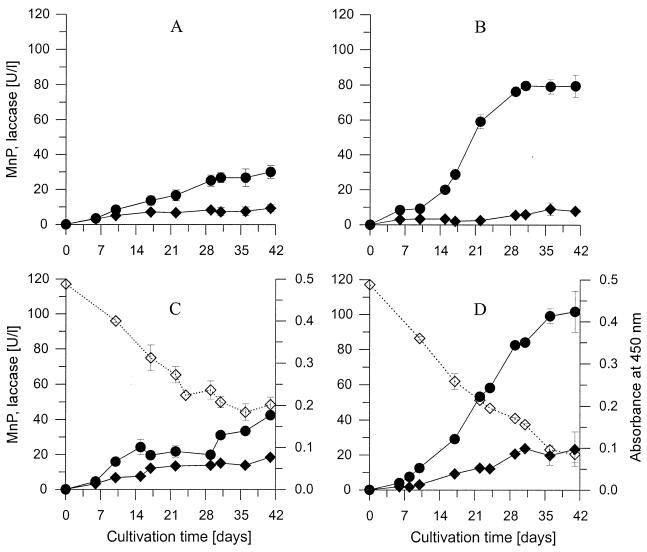
Analysis of extracellular enzymatic activities revealed that C. dryophila produced MnP and laccase (Fig. 3). In the absence of Mn2+ and LHA, the fungus secreted only small amounts of both enzymes (a maximum of 9 U of laccase liter−1 and 30 U of MnP liter−1) (Fig. 3A). Mn2+ stimulated the production of MnP considerably (a maximum of 80 U liter−1), whereas that of laccase was not influenced (Fig. 3B). The presence of LHA in the medium resulted in an increase of both laccase (a maximum of 18 U liter−1) and MnP (a maximum of 43 U liter−1) activities (Fig. 3C), although the latter was only half as much as that for Mn2+-supplemented cultures. The highest levels of activity of MnP and laccase were observed in cultures containing both Mn2+ and LHA, reaching maximum levels of 102 and 23 U liter−1, respectively. The decolorization (bleaching) of LHA medium was most prominent in the presence of Mn2+ and agreed with the results of the HPSEC analysis (Fig. 3C and D).
FIG. 3.
Production of MnP (closed circles) and laccase (closed diamonds) by C. dryophila in liquid culture and decolorization of LHA (open diamonds). (A) Medium without Mn2+ and LHA. (B) Medium with 200 μM Mn2+ (MnCl2) but without LHA. (C) Medium with 250 mg of LHA liter−1 but without Mn2+. (D) Medium with 200 μM Mn2+ and 250 mg of LHA liter−1. Decolorization of LHA (panels C and D) was monitored by the decrease in absorbance at 450 nm. Data points represent means of three parallels with standard deviations.
Mineralization of 14C-HA.
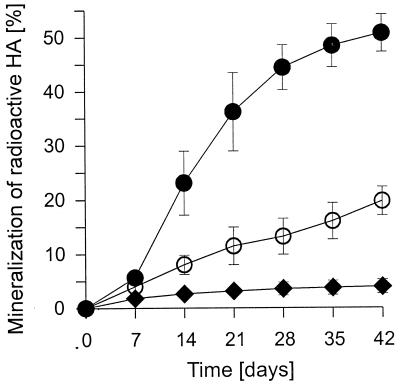
Figure 4 shows the time courses of 14CO2 evolution from 14C-HA by liquid cultures of C. dryophila. In the absence of Mn2+, the fungus mineralized about 20% of the 14C-HA, while 35% was incorporated into the biomass or closely bound to the cell surface (Table 1). The presence of Mn2+ in the medium caused a noticeable stimulation of 14CO2 production (an increase of about 50%) and reduced the amount of 14C associated with the fungal mycelium. Substantial 14CO2 formation started after 1 week of incubation and reached an average rate of approximately 2.2% of 14CO2 per day during the following 2 weeks. Interestingly, at the same time MnP production had begun to increase in the LHA experiment (compare Fig. 3D). After 3 weeks, 14C-HA decomposition declined, thus reaching a mineralization rate of only 0.3% 14CO2 per day during the last week of incubation. In contrast, 14CO2 release by Mn2+-free cultures was almost linear throughout the experiment.
FIG. 4.
Mineralization of 14C-HA by C. dryophila in liquid culture. Closed circles, fungus in Mn2+-supplemented medium; open circles, fungus in Mn2+-free medium; closed diamonds, control without fungus. Data points represent means of three parallels with standard deviations.
TABLE 1.
Balance of radioactive carbon from a 14C-labeled synthetic humic acid (14C-HA; 2.5 × 105 dpm), added to stationary liquid cultures of Collybia dryophila K209, after 42 days growtha
| Sample | % of 14C in:
|
||||
|---|---|---|---|---|---|
| 14CO2 | 14C-labeled volatile compounds | 14C-labeled NaOH-soluble material | Residual 14C after combustion | Total 14C | |
| C. dryophila | 18.9 ± 3.1 | 0.8 ± 0.3 | 47.1 ± 6.3 | 35.3 ± 2.8 | 102.1 |
| C. dryophila + Mn2+b | 49.4 ± 2.8 | 1.4 ± 1.1 | 34.2 ± 4.3 | 12.9 ± 2.2 | 97.9 |
| Control without fungus | 3.7 ± 0.4 | <0.1 | 97.8 ± 0.9 | 1.9 ± 0.3 | 103.6 |
Values given are the means ± standard deviations of three experiments.
Concentration, 200 μM MnCl2.
In both the presence and absence of Mn2+, only negligible amounts of 14C-labeled volatile organic compounds were formed (Table 1). A small part of 14C-HA (approximately 3.5%) was also converted to 14CO2 by the controls, particularly in the beginning of the experiment, indicating spontaneous oxidation reactions.
Purification of MnP.
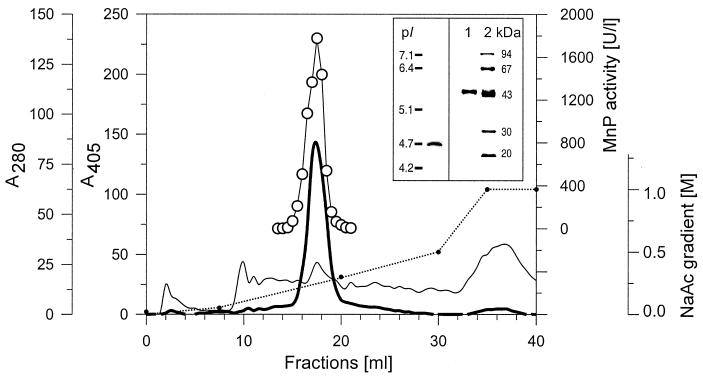
To produce larger amounts of MnP for subsequent purification, C. dryophila was cultured in 1-liter tissue culture flasks, which ensured a favorable oxygen supply. Using the Mn2+-supplemented medium, the fungus produced considerably more MnP (269 U liter−1) under these conditions than in the Erlenmeyer flasks. After two steps of ultrafiltration-diafiltration, the concentrated culture fluid (988 μg of protein ml−1) was fractionated by fast protein liquid chromatography (FPLC) (Fig. 5). The elution profile showed one large heme peak (405 nm), with high MnP activity corresponding to a smaller protein peak (280 nm; 41 μg of protein ml−1). The pooled FPLC peak of MnP was analyzed by IEF and denaturing SDS-PAGE. IEF showed one enzyme band with a pI of 4.7 (stained with phenol red). SDS-PAGE followed by Coomassie staining also gave a single band with a molecular mass of 44 kDa (see insets in Fig. 5).
FIG. 5.
FPLC protein profile of 5-week-old concentrated culture filtrate from C. dryophila grown in the presence of Mn2+ (200 μM). The sodium acetate (NaAc) gradient is represented by a dotted line. Absorbance levels at 405 nm (bold line) and 280 nm (thin line) represent the elution of heme-containing protein and total protein, respectively, along with the MnP activity levels (open circles). Inset: IEF (left) and SDS-PAGE (right) analyses of the pooled MnP peak (lane 1) and molecular mass markers (lane 2).
Effect of C. dryophila on solid litter.
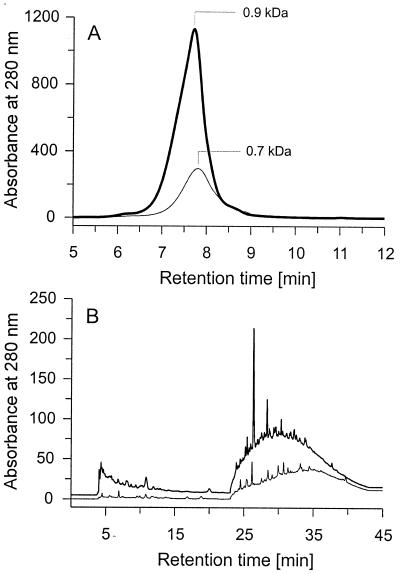
C. dryophila grew well on sterilized soil litter, and after one week, the whole surface of the solid material was covered with white mycelium. At the end of incubation on day 46, the material was thoroughly grown through by the fungus and showed a noticeably lighter color than the uninoculated controls. The already low pH of the original litter samples dropped further during the growth of C. dryophila (from 3.9 to 3.1). HPSEC elution profiles of aqueous litter extracts revealed that, in comparison to that of the uninoculated litter, the amount of water-soluble material was considerably increased as the result of fungal activity (Fig. 6A). The major part of these yellowish compounds in the extract showed physicochemical characteristics of FAs, and only a small fraction (<5%), representing polar HA, could be precipitated with HCl. The predominant molecular mass of FA was around 0.9 kDa, which was slightly higher than that of FA in the uninoculated litter (0.7 kDa). HPLC analysis using a reversed-phase C18 column gave similar results. Considerably more water-soluble material which absorbed at 280 nm, including highly polar fragments which were eluted with pure water from the column, was detected in litter extracts overgrown by the fungus (Fig. 6B). Enzymatic activities detected in the aqueous litter extracts were relatively low, probably due to the interference of soluble litter products with the assays (MnP: maximum level, 12 U liter−1 of litter extract; laccase: maximum level, 29 U liter−1 of litter extract).
FIG. 6.
HPSEC (A) and reversed phase C18-HPLC (B) elution profiles of aqueous litter extracts. Thin lines represent controls without fungus; bold lines represent litter samples which were inoculated with C. dryophila for 46 days.
DISCUSSION
The litter-decomposing C. dryophila was found to be capable of decomposing both natural HA from soil litter and synthetic 14C-HA prepared from catechol in liquid culture. The conversion process resulted in the formation of lower-molecular-mass FA and carbon dioxide. HA decomposition was considerably enhanced in the presence of Mn2+, which stimulated the production of MnP. C. dryophila grew well on sterilized solid litter and released substantial amounts of FA from the water-insoluble material during growth.
Decolorization (bleaching) of different high-molecular-mass HAs, indicating their conversion into low-molecular-mass FA, has been reported for a number of white-rot fungi under cometabolic conditions (i.e., in the presence of an assimilable carbon source such as glucose). Among 20 species of basidiomycetes tested for their ability to decolorize an HA from podzol soil, four white-rot fungi (Trametes versicolor, T. suaveolens, T. rubescens, and Hypholoma fasciculare) were found to attack the dissolved humic material and bleach it completely (23). In another screening test using HA from podzol, black peat, and brown coal, the white-rot fungus Bjerkandera (Gloeoporus) adusta turned out to be particularly active (41). The fungus not only bleached HAs but also changed their physicochemical properties towards higher polarity. In connection with the investigation of brown coal bioconversion, further HA-attacking white-rot fungi were discovered, e.g., Phanerochaete chrysosporium, Trametes versicolor, and two unidentified species isolated from coal mining areas (31, 38). Indications were found that lignin peroxidase, laccase, and above all, MnP were involved in the bleaching and depolymerization process (10, 32, 38, 39). Our findings show that C. dryophila also produced at least two ligninolytic enzymes, MnP and laccase (with MnP predominant), during LHA conversion.
The first experiments for monitoring the cometabolic decomposition of 14C-HA by white-rot fungi were performed with P. chrysosporium. The fungus mineralized about 35% of a 14C-HA extracted from a U-14C-wheat straw compost in liquid culture within 18 days (15). The same fungal species was later used to degrade a synthetic 14C-melanoidine prepared from 14C-glucose and glycine by the Maillard reaction method (2). Within an incubation period of 15 days, P. chrysosporium released ca. 17% of the 14C-labeled material as 14CO2. The mineralization process was stimulated in the presence of veratryl alcohol (23.5% 14CO2), which was proposed to enhance LiP activity and thereby melanoidine degradation. In the same study, the author also reported the decolorization and depolymerization of natural HA isolated from forest soil by P. chrysosporium (2). Later, T. versicolor was found to mineralize 14C-melanoidine more effectively than P. chrysosporium, and in addition to that of LiP, high levels of MnP were detected in HA- and melanoidine-containing culture filtrates (4). Culture filtrate and purified MnP of T. versicolor was shown to produce 14CO2 from 14C-melanoidine in a cell-free reaction system (5). This remarkable observation was later confirmed using a more recalcitrant 14C-HA, prepared as in the present study from 14C-catechol, and MnP from the agaric white-rot fungus N. frowardii (20). Our finding that Mn2+ in the medium stimulated the MnP level and the 14CO2 production from 14C-HA indicates that this enzymatic system is also involved in the mineralization process in C. dryophila. The molecular mass value (44 kDa) and pI (4.7) of the purified C. dryophila MnP are in the typical range for MnPs from white-rot fungi and other litter decomposers (16, 34). Unlike most other MnPs, which produce sets of isoforms that differ, that of C. dryophila showed only a single peak in the FPLC elution profile and one sharp band in the IEF analysis.
The finding that the pH of litter samples inoculated with C. dryophila decreased during the fungal growth is in concordance with those of earlier studies, which showed the secretion of oxalic and other organic acids by various basidiomycetous fungi (17, 18, 36). Organic acids not only support MnP activity but also have an effect of their own in the mobilization of humic material that might subsequently undergo enhanced depolymerization (3, 9). Thus, FA and other polar fragments which form from litter as a consequence of C. dryophila treatment might be the result of concerted action by enzymatic and nonenzymatic agents. In this context, it cannot be ruled out that fungal metabolites also account for the variety of water-soluble products found in the litter extracts, although such compounds were not detected in the respective liquid cultures.
C. dryophila, representing a cosmopolitan species that belongs to the litter-decomposing basidiomycetes, possesses a ligninolytic enzyme system, based on MnP, which is similar to those of certain wood-degrading white-rot fungi (e.g., Ceriporiopsis subvermispora, Dichomitus squalens, and Phanerochaete sordida) (16) and which enables the fungus to disintegrate high-molecular-mass humic substances. In conclusion, we propose that litter-decomposing basidiomycetes play an important role in the recycling of organic carbon bound in the soil organic matter.
Acknowledgments
This work was supported financially by the Finnish Graduate School in Environmental Science and Technology (EnSTe) and the Academy of Finland (Finnish Biodiversity Research Program), Biodiversity and Humus in Forest and Lake Ecosystems project grant 39590, the Academy of Finland project grant 39906 (to A.H.), and Bioconversion of Recalcitrant Soil Organic Matter by Litter-Decomposing Basidiomycetous Fungi (Academy of Finland) project grant 52063 (to M.H.).
REFERENCES
- 1.Almendros, G., and J. Dorado. 1999. Molecular characteristics related to the biodegradability of humic acid preparations. Eur. J. Soil Sci. 50:227-236. [Google Scholar]
- 2.Blondeau, R. 1989. Biodegradation of natural and synthetic humic acids by the white-rot fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 55:1282-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, M. S., K. A. Feldman, C. S. Brown, and E. T. Grey. 1990. Isolation and identification of the coal-solubilizing agent produced by Trametes versicolor. Appl. Environ. Microbiol. 56:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehorter, B., and R. Blondeau. 1992. Extracellular enzyme activities during humic acid degradation by the white rot fungi Phanerochaete chrysosporium and Trametes versicolor. FEMS Microbiol. Lett. 94:209-216. [Google Scholar]
- 5.Dehorter, B., and R. Blondeau. 1993. Isolation of an extracellular Mn-dependent enzyme mineralizing melanoidins from the white-rot fungus Trametes versicolor. FEMS Microbiol. Lett. 109:117-123. [Google Scholar]
- 6.Dehorter, B., C. Y. Kontchou, and R. Blondeau. 1992. 13C-NMR spectroscopic analysis of soil humic acids recovered after incubation with some white-rot fungi and actinomycetes. Soil Biol. Biochem. 24:667-673. [Google Scholar]
- 7.Dix, N. J., and J. Webster. 1995. Fungal ecology. Chapman & Hall, London, United Kingdom.
- 8.Eggert, C., U. Temp, and K. E. L. Eriksson. 1996. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 391:144-148. [DOI] [PubMed] [Google Scholar]
- 9.Fakoussa, R. M. 1994. The influence of different chelators on the solubilization/liquefaction of different pretreated and natural lignites. Fuel Process. Technol. 40:183-192. [Google Scholar]
- 10.Fakoussa, R. M., and P. J. Frost. 1999. In vivo-coal decolorization of coal-derived humic acids by laccase-excreting fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 52:60-65. [Google Scholar]
- 11.Fakoussa, R. M., and M. Hofrichter. 1999. Minireview: microbiology and biotechnology of coal degradation. Appl. Microbiol. Biotechnol. 52:25-40. [DOI] [PubMed] [Google Scholar]
- 12.Frimmel, F. H. 2001. Aquatic humic substances, p. 301-324. In M. Hofrichter and A. Steinbüchel (ed.), Biopolymers. Lignin, humic substances and coal, vol. 1. Wiley-VCH, Weinheim, Germany.
- 13.Grieser, K., and W. Ziechmann. 1988. Wechselwirkungen zwischen Huminstoffen und Peroxidase. Mitt. Dtsch. Bodenkundl. Ges. 56:153-159. [Google Scholar]
- 14.Haider, K. 1998. Physical and chemical stabilisation mechanisms of RSOM. Mittl. Dtsch. Bodenkundl. Ges. 87:119-132. [Google Scholar]
- 15.Haider, K., and J. P. Martin. 1988. Mineralization of 14C-labelled humic acids and humic-acid bound 14C-xenobiotics by Phanerochaete chrysosporium. Soil Biol. Biochem. 20:425-429. [Google Scholar]
- 16.Hatakka, A. 2001. Biodegradation of lignin, p. 129-180. In M. Hofrichter and A. Steinbüchel (ed.), Biopolymers. Lignin, humic substances and coal, vol. 1. Wiley-VCH, Weinheim, Germany.
- 17.Hintikka, V. 1970. Studies on white-rot humus formed by higher fungi in forest soils. Comm. Inst. For. Fenn. 67:1-68. [Google Scholar]
- 18.Hintikka, V., K. Korhonen, and O. Näykki. 1979. Occurrence of calcium oxalate in relation to the activity of fungi in forest litter and humus. Karstenia 19:58-64. [Google Scholar]
- 19.Hofrichter, M., and W. Fritsche. 1997. Depolymerization of low-rank coal by extracellular fungal enzyme systems. II. The ligninolytic system of the coal-humic-acid-degrading fungus Nematoloma frowardii b19. Appl. Microbiol. Biotechnol. 47:419-424. [Google Scholar]
- 20.Hofrichter, M., K. Scheibner, I. Schneegaβ, D. Ziegenhagen, and W. Fritsche. 1998. Mineralization of synthetic humic substances by manganese peroxidase from the white-rot fungus Nematoloma frowardii. Appl. Microbiol. Biotechnol. 49:584-588. [Google Scholar]
- 21.Hofrichter, M., D. Ziegenhagen, T. Vares, M. Friedrich, M. G. Jäger, W. Fritsche, and A. Hatakka. 1998. Oxidative decomposition of malonic acid as basis for the action of manganese peroxidase in the absence of hydrogen peroxide. FEBS Lett. 434:362-366. [DOI] [PubMed] [Google Scholar]
- 22.Hofrichter, M., T. Lundell, and A. Hatakka. 2001. Conversion of milled pine wood by manganese peroxidase from Phlebia radiata. Appl. Environ. Microbiol. 67:4588-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst, H. M., A. Burges, and P. Latter. 1963. Some aspects of the biochemistry of humic acid decomposition by fungi. Phytochemistry 1:227-231. [Google Scholar]
- 24.Jordan, M. 1995. Encyclopedia of fungi of Britain and Europe. David & Charles, Newton Abbot, Devonshire, United Kingdom.
- 25.Kästner, M., and M. Hofrichter. 2001. Biodegradation of humic substances, p. 349-378. In M. Hofrichter and A. Steinbüchel (ed.), Biopolymers. Lignin, humic substances and coal, vol. 1. Wiley-VCH, Weinheim, Germany.
- 26.Kirk, T. K., S. Croan, M. Tien, K. E. Murtagh, and R. L. Farrell. 1986. Production of multiple ligninases by Phanerochaete chrysosporium: effect of selected growth conditions and use of a mutant strain. Enzyme Microb. Technol. 8:27-32. [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature (London) 227:680-688. [DOI] [PubMed] [Google Scholar]
- 28.Martens, R., and F. Zadrazil. 1992. Screening of white rot fungi for their ability to mineralize polycyclic aromatic hydrocarbons in soil, p. 505-510. In J. Klein (ed.), Preprints of the International Symposium on Soil Decontamination using Biological Processes. DECHEMA e.M., Frankfurt, Germany. [DOI] [PubMed]
- 29.Pease, E. A., S. D. Aust, and M. Tien. 1991. Heterologous expression of active manganese peroxidase from Phanerochaete chrysosporium using the Baculovirus expression system. Biochem. Biophys. Res. Commun. 179:897-903. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, R. 1991. Mushrooms of North America. Little, Brown & Company Inc., New York, N.Y.
- 31.Ralph, J. P., and D. E. A. Catcheside. 1994. Decolourisation and depolymerisation of solubilised low-rank coal by the white-rot basidiomycete Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 42:536-542. [Google Scholar]
- 32.Ralph, J. P., and D. E. A. Catcheside. 1999. Transformation of macromolecules from brown coal by lignin peroxidase. Appl. Microbiol. Biotechnol. 52:70-77. [DOI] [PubMed] [Google Scholar]
- 33.Senesi, N., and E. Loffredo. 2001. Soil humic substances, p. 247-299. In M. Hofrichter and A. Steinbüchel (ed.) Biopolymers. Lignin, humic substances and coal, vol 1. Wiley-VCH, Weinheim, Germany.
- 34.Steffen, K. T., M. Hofrichter, and A. Hatakka. 2002. Purification and characterization of manganese peroxidases from the litter-decomposing basidiomycetes Agrocybe praecox and Stropharia coronilla. Enzyme Microb. Technol. 30:550-555. [Google Scholar]
- 35.Stevenson, F. J. 1994. Humus chemistry. Genesis, composition, reactions, 2nd ed. John Wiley & Sons, New York, N.Y.
- 36.Takao, S. 1965. Organic acid production by basidiomycetes. I. Screening of acid-producing strains. Eur. J. Appl. Microbiol. 13:732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wariishi, H., K. Valli, and M. H. Gold. 1992. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelator. J. Biol. Chem. 267:23688-23695. [PubMed] [Google Scholar]
- 38.Willmann, G., and R. M. Fakoussa. 1997. Biological bleaching of water-soluble coal macromolecules by a basidiomycete strain. Appl. Microbiol. Biotechnol. 47:95-101. [Google Scholar]
- 39.Willmann, G., and R. M. Fakoussa. 1997. Extracellular oxidative enzymes of coal-attacking fungi. Fuel Process. Technol. 52:27-41. [Google Scholar]
- 40.Wunderwald, U., G. Kreisel, M. Braun, M. Schulz, C. Jäger, and M. Hofrichter. 2000. Formation and degradation of a synthetic humic acid derived from 3-fluoro-catechol. Appl. Microbiol. Biotechnol. 53:441-446. [DOI] [PubMed] [Google Scholar]
- 41.Ziechmann, W. 1980. Huminstoffe. BI-Wissenschaftsverlag, Weinheim, Germany.