Abstract
Human cytomegalovirus (HCMV) is the leading cause of congenital viral infection and mental retardation. HCMV infection, while causing asymptomatic infections in most immunocompetent subjects, can be transmitted during pregnancy from the mother with primary (and also recurrent) infection to the fetus. Hence, careful diagnosis of primary infection is required in the pregnant woman based on the most sensitive serologic assays (immunoglobulin M [IgM] and IgG avidity assays) and conventional virologic and molecular procedures for virus detection in blood. Maternal prognostic markers of fetal infection are still under investigation. If primary infection is diagnosed in a timely manner, prenatal diagnosis can be offered, including the search for virus and virus components in fetal blood and amniotic fluid, with fetal prognostic markers of HCMV disease still to be defined. However, the final step for definite diagnosis of congenital HCMV infection is detection of virus in the blood or urine in the first 1 to 2 weeks of life. To date, treatment of congenital infection with antiviral drugs is only palliative both prior to and after birth, whereas the only efficacious preventive measure seems to be the development of a safe and immunogenic vaccine, including recombinant, subunit, DNA, and peptide-based vaccines now under investigation. The following controversial issues are discussed in the light of the most recent advances in the field: the actual perception of the problem; universal serologic screening before pregnancy; the impact of correct counseling on decision making by the couple involved; the role of prenatal diagnosis in ascertaining transmission of virus to the fetus; the impact of preconceptional and periconceptional infections on the prevalence of congenital infection; and the prevalence of congenitally infected babies born to mothers who were immune prior to pregnancy compared to the number born to mothers undergoing primary infection during pregnancy.
INTRODUCTION
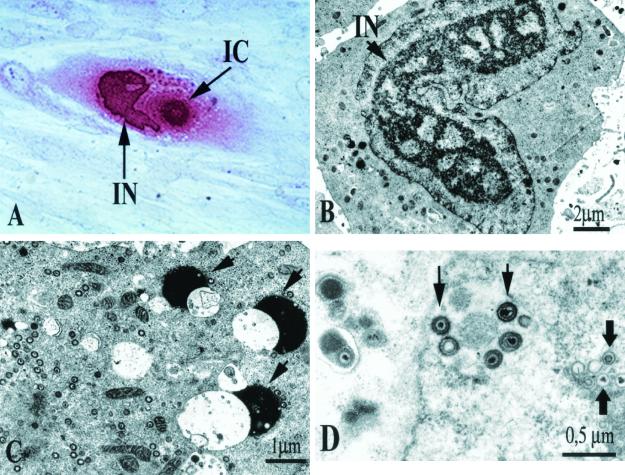
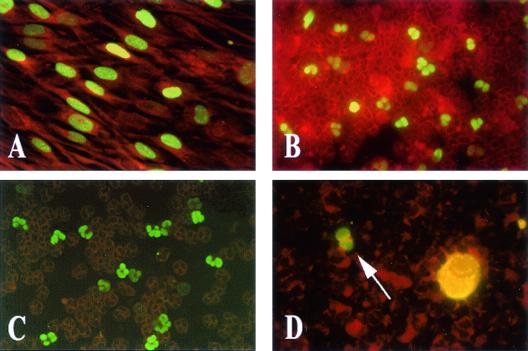
Human cytomegalovirus (HCMV) is the vernacular name of human herpesvirus 5, a highly host-specific virus of the Herpesviridae family. HCMV is the largest virus in the family and is morphologically indistinguishable from other human herpesviruses. HCMV, like all herpesviruses, undergoes latency and reactivation in the host. Although HCMV has been shown to infect a broad spectrum of cells in vivo (246), the only cells that are fully permissive for HCMV replication in vitro are human fibroblasts. In these cells, virus replication results in the formation of intranuclear and intracytoplasmic inclusion bodies (Fig. 1A), with the former full of nucleocapsids (Fig. 1B) and the latter containing several dense bodies (Fig. 1C). Nucleocapsids acquire the envelope from the nuclear membrane or cytoplasmic vacuoles (Fig. 1D).
FIG. 1.
HCMV replication in human embryonic lung fibroblast cell cultures. (A) HCMV-infected human fibroblast 120 h postinfection (following immunoperoxidase staining with human antibodies), showing intranuclear (IN) and intracytoplasmic (IC) inclusion bodies. (B to D) Electron microscopy of HCMV-infected human fibroblasts. (B) Horseshoe-shaped intranuclear inclusion (IN). (C) Dense bodies (arrows). (D) Maturing virus particles at the level of the nuclear membrane.
HCMV is a virus of paradoxes. It can be a potential killer or a lifelong silent companion. These two aspects are confirmed in an exemplary manner by the circumstances, vividly reviewed by Thomas H Weller (287), surrounding the isolation of the first HCMV strains. In 1956, Margaret G. Smith recovered the first HCMV isolate from the submaxillary salivary gland tissue of a dead infant and the second isolate from the kidney tissue of a baby dying of cytomegalic inclusion disease (250). The same year, Rowe and coworkers, who recovered adenoviruses by observing cytopathic changes in uninoculated cultures of human adenoids, noted unique focal lesions and intranuclear inclusions primarily in the fibroblast component of cultures of adenoidal tissues from three asymptomatic children (228). The cytopathic effect of the new virus strain (AD169) very closely resembled that of the Davis strain that was observed 1 year later by Weller and colleagues in human embryonic skin muscle tissue cultures inoculated with a liver biopsy taken from a 3-month-old infant with microcephaly, jaundice, hepatosplenomegaly, chorioretinitis, and cerebral calcifications (288). The same group of researchers isolated two additional HCMV strains: the Kerr strain from the urine of a newborn with petechiae, hepatosplenomegaly, and jaundice, and the Esp. strain from the urine of an infant with hepatosplenomegaly, periventricular calcification, and chorioretinitis (288).
In the following years, HCMV also showed its pathogenic properties in organ transplant recipients, patients with AIDS, and cancer patients, while it gained the leading position among infectious agents responsible for mental retardation, intellectual impairment, and deafness.
Presently, HCMV infection is mostly controlled in immunocompromised patients by available antiviral drugs, yet it continues to maintain its role as the most dangerous infectious agent for the unborn infant. Thus, HCMV infection is still a major health problem, warranting strong preventive measures.
The major scope of this review will be to analyze and update the diagnostic and prognostic implications of primary HCMV infections in pregnancy in the mother, fetus, and newborn. Special emphasis will be given to less-investigated issues, such as detection of virus and viral products in the blood of the mother during primary HCMV infection, the presence of clinical signs and symptoms in the mother, prenatal diagnosis of congenital infection in amniotic fluid and fetal blood, maternal and fetal prognostic markers of HCMV infection and disease, and the impact of counseling. Measures of treatment and prevention of congenital HCMV infection will be mentioned briefly. The last part of the review will deal with the most controversial issues, in particular, how the problem of HCMV infections in pregnancy is perceived by the scientific community and public health authorities. Is preconception serologic screening justified, and should HCMV-seronegative women be prospectively monitored? What are the limits of prenatal diagnosis (false-positive and false-negative results)? What is the role of preconceptional and periconceptional infections in HCMV transmission to the fetus? Are reactivated infections significant in transmission of virus to the fetus?
Finally, one additional goal is to focus the attention of the scientific community on the problem of congenital HCMV infection and to appeal for international collaboration. We truly need to develop and implement consensus strategies for prevention of congenital HCMV infection, ideally through a vaccine.
EPIDEMIOLOGY OF VERTICAL HCMV TRANSMISSION
The term vertical transmission is used here to indicate HCMV transmission from mother to fetus during pregnancy, thus excluding virus transmission from mother to newborn infant. Due to latency following primary infection and periodic reactivation of HCMV replication causing recurrent infections, in utero transmission of HCMV may follow either primary or recurrent infections (4, 75, 237, 261, 264). It is commonly recognized that primary HCMV infections are transmitted more frequently to the fetus and are more likely to cause fetal damage than recurrent infections (75). In addition, it seems that primary infection occurring at an earlier gestational age is related to a worse outcome (50, 264).
Initially, the role of recurrent maternal infections in causing congenital infections was supported by three independent reports describing congenital infections in consecutive pregnancies (62, 145, 260). In all three reports, the first newborn was severely affected and the second one was subclinically infected. Molecular epidemiological studies indicated that in each of three pairs of congenitally infected siblings, the viruses were identical to each other when examined by restriction fragment length polymorphism analysis (265). However, the first convincing evidence of the possible transmission of HCMV from immune mothers to the fetus came from a prospective study showing that 10 congenitally infected infants were born to immune mothers within a group of 541 infants of women who were seropositive before pregnancy (261), with a prevalence of 1.9%. Subsequently, similar findings were observed in a geographic area where nearly the entire population was immune to HCMV during childhood, and the prevalence of congenital infection was found to be 1.4% (237).
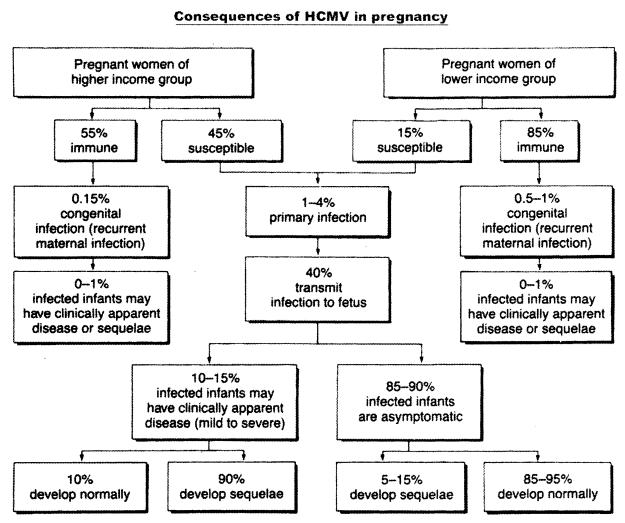
In 1985, Stagno and Whitley (259) estimated the maternal risk of acquiring either primary or recurrent HCMV infection in pregnancy as well as the risk of intrauterine transmission to the offspring in two groups of women of low or high socioeconomic status. Their estimates showed that the risk of primary maternal infection was about three times higher among the higher-income susceptible women (45%), compared to 15% in the lower-income group (Fig. 2). In both groups, transmission to the fetus occurred in about 40% of cases, with delivery of about 10 to 15% symptomatic and 85 to 90% asymptomatic congenitally infected newborns. Among the asymptomatic newborns, about 10% developed sequelae, while about 90% of infants that were asymptomatic at birth developed normally. On the other hand, the rate of congenital infections from recurrent maternal infection was 0.15% in the higher-income group of pregnant women, who were 55% immune, and 0.5 to 1% in the lower-income group, which was 85% immune, i.e., 3 to 7 times higher. However, the rate of clinically apparent disease was low and similar (0 to 1%) in both groups.
FIG. 2.
Characteristics of HCMV infection in pregnancy. (From S. Stagno and R. J. Whitley [259], used with permission.)
It is currently accepted that congenital HCMV infection may be the consequence of either a primary or recurrent maternal infection (263). Recurrent infections may consist of either reactivation of the virus strain causing primary infection or reinfection by a new virus strain. Recently, the incidence of symptomatic congenital HCMV infections in immune mothers has been shown to be similar in primary and recurrent maternal infections (28). In addition, symptomatic congenital infections appear to be mostly caused by reinfection of immune mothers during pregnancy by a new HCMV strain (30). This conclusion was based on demonstration of the appearance of antibodies directed against new epitopes of glycoprotein H of HCMV not present in the blood prior to the current pregnancy. Sequencing of the glycoprotein H gene has confirmed the presence of a new virus strain in the reported cases (30). On the other hand, congenital infections following reactivated maternal infection are mostly asymptomatic (265).
In conclusion, the true frequency and clinical importance of congenital HCMV infections from recurrent maternal infections remain to be determined in long-term prospective studies. However, primary HCMV infection continues to be the major viral cause of congenital infections, with significant morbidity. Recent findings related to the potential role of recurrent maternal infection in symptomatic congenital infection complicate but will not interfere with efforts aimed at developing a safe and efficacious vaccine.
PATHOGENESIS OF CONGENITAL INFECTION
In the case of primary maternal infection, the antiviral immune response begins proximate to virus transmission to the fetus, whereas in the case of recurrent infection, virus transmission occurs in the presence of both humoral and cell-mediated immune responses. As a result, viremia occurs as a rule only in primary infections (216), whereas it is either absent or undetectable in recurrent infections of the immunocompetent host (216) and common in recurrent infections of immunocompromised patients (67, 137, 147, 179). Since, following primary HCMV infection, intrauterine transmission occurs in only 30 to 40% of cases, an innate barrier seems to partially prevent vertical transmission (4, 50, 110, 264). In addition, a similar event seems to occur among infected newborns, less than 15% of whom show clinically apparent infection, in the great majority of cases resulting from primary maternal infection (4, 50, 75, 264, 293). Finally, in reactivated maternal infections, the risk of symptomatic congenital infection is even markedly lower (4, 110, 264), as shown by the few symptomatic infants reported in the past to have been born to mothers who were immune before pregnancy. In fact, although existing immunity does not prevent transmission of the virus to the fetus, reactivated infections are less likely to cause damage to the offspring than primary infections (75).
Multiple mechanisms of immune evasion for HCMV could relate to the pathogenic role of the virus. Recently, expression of immune evasion genes US3, US6, and US11 of HCMV in the blood of solid organ transplant recipients has been investigated, showing that, after clinical recovery, transcripts of these genes remain detectable, indicating that persistent low viral activity may have implications for long-term control of HCMV infection (106).
Little is still known about the mechanisms of HCMV transmission to the fetus. It has been reported that about 15% of women undergoing primary infection during the first months of pregnancy abort spontaneously, showing placental but not fetal infection (110, 124). Subsequently in the course of pregnancy, placental infection has been shown to be consistently associated with fetal infection (177).
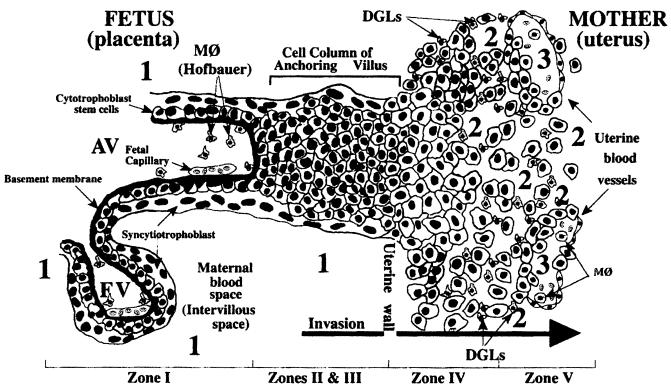
Understanding the mechanisms of HCMV transmission to the fetus implies elucidation of some major steps in placental development (44, 48). The development of the placenta requires differentiation of specialized epithelial stem cells, referred to as cytotrophoblasts, in both floating villi, where they fuse into multinucleate syncytiotrophoblasts covering the villous surface, and anchoring villi, where they aggregate into columns of single cells invading the endometrium and the first third of the myometrium (interstitial invasion). While the syncytiotrophoblast is in direct contact with maternal blood, mediating transport of multiple substances to and from the fetus, the cytotrophoblast columns also invade maternal arterioles (endovascular invasion) by replacing endothelial and smooth muscle cells and thus generating a hybrid cell population of fetal and maternal cells inside uterine vessels (Fig. 3).
FIG. 3.
Diagram of a longitudinal section that includes a floating and an anchoring chorionic villus at the fetal-maternal interface near the end of the first trimester of human pregnancy. The anchoring villus (AV) functions as a bridge between the fetal and maternal compartments, whereas the floating villus (FV), containing macrophages (M℘, Hofbauer cells) and fetal blood vessels, is bathed by maternal blood. Cytotrophoblasts in the anchoring villus (zone I) form cell columns that attach to the uterine wall (zones II and III). Cytotrophoblasts then invade the uterine interstitium (decidua and first third of the myometrium; zone IV) and maternal vasculature (zone V), thereby anchoring the fetus to the mother and accessing the maternal circulation. Zone designations mark areas in which cytotrophoblasts have distinct patterns of stage-specific antigen expression, including integrin and HLA-G. Decidual granular leukocytes (DGLs) and macrophages (M℘) in maternal blood and fetal capillaries in villous cores are indicated. Areas proposed as sites of natural HCMV transmission to the placenta in utero are numbered 1, 2, and 3. (From Fisher et al. [74], used with permission.)
Syncytiotrophoblasts upregulate expression of the neonatal immunoglobulin G (IgG) Fc receptor, involved in transport of maternal IgG to the fetus (160, 244). In parallel, invading cytotrophoblasts initiate expression of adhesion molecules, such as integrin α1β1, and proteinases, which are required for invasion, besides molecules inducing maternal immune tolerance, such as HLA-G (143, 174) and interleukin-10 (226, 227). Additionally, in the process called pseudovasculogenesis, invading cells modify the phenotype of their adhesion molecules, mimicking that of endothelial cells by expressing αvβ3 integrin, a marker of angiogenic endothelium, and vascular endothelial cadherin, a marker of cell polarization (48, 295).
That placenta behaves as a reservoir in which HCMV replicates prior to being transmitted to the fetus has been experimentally shown in the guinea pig, which, as in humans, has a hemomonochorial placenta with a single trophoblast layer separating fetal from maternal circulation (162). In experimental infection of the guinea pig with species-specific CMV, the virus disseminates hematogenously to the placenta, from which it is transmitted to the fetus in about 25% of cases. The guinea pig CMV also persists in placental tissues long after virus has been cleared from blood (108). Recently, a greater understanding of the human placenta has been achieved by using two in vitro models for the study of trophoblast populations lying at the maternal-fetal interface, villous explants and isolated cytotrophoblasts (72-74). These data, coupled with immunohistochemical studies of in vivo HCMV-infected placentas (177, 247) and recent findings on HCMV latency (121, 252), have led to new hypotheses for routes of transmission of HCMV to the fetus in primary and reactivated maternal HCMV infection.
During primary infection of the mother, leukocytes carrying infectious virus (79, 81, 211, 216) may transmit HCMV infection to uterine microvascular endothelial cells (E. Maidji, E. Percivalle, G. Gerna, S. Fisher, and L. Pereira, Abstr. 8th International Cytomegalovirus Workshop, abstr. p. 31, 2001). These cells are in direct contact with cytotrophoblasts of anchoring villi invading maternal arterioles and forming hybrids of maternal-fetal cells (Fig. 3). Infected cytotrophoblasts may in turn transmit the infection to underlying tissues of villous cores, including fibroblasts and fetal endothelial cells (247), thus spreading to the fetus. An alternative model of transmission, in the case of primary maternal infection, is spreading of infection to the villous stroma by infected maternal leukocytes through breaches of the syncytiotrophoblast layer (126, 134). A further hypothesis has been raised suggesting possible transportation of the virus as antibody-coated HCMV virions by a process of transcytosis through intact syncytiotrophoblasts similar to that advocated for transport of maternal IgG to the fetus (74). Finally, syncytiotrophoblasts may be directly infected, but the infection proceeds slowly and remains predominantly cell associated (126) until infected cells are eliminated during the physiological turnover (251). This hypothesis therefore excludes transmission through virus replication in syncytiotrophoblasts.
In the case of congenital HCMV infection following recurrent maternal infection, it must be considered that the placenta is a hemiallograft inducing local immunosuppression in the uterus (74, 227). This may cause reactivation of latent virus in macrophages of the uterine wall, with HCMV transmission to the invading cytotrophoblasts. Then, virus could spread in a retrograde manner to anchoring villi and subsequently to the fetus (177). In this regard, HCMV establishes a true latent infection in CD14+ monocytes, which can be reactivated upon allogeneic stimulation of monocyte-derived macrophages from healthy blood donors (252). Reactivation of latent HCMV is dependent on the production of gamma interferon in the differentiation process (253). These data await confirmation by other laboratories.
As a consequence of placental infection, HCMV impairs cytotrophoblast differentiation and invasiveness, as shown in vitro (74). This could explain early abortion occurring in women with primary infection. In addition, HCMV infection impairs cytotrophoblast expression of HLA-G, thus activating the maternal immune response against the cytotrophoblast subpopulation expressing this molecule (74).
DIAGNOSIS OF PRIMARY INFECTION DURING PREGNANCY
By far the major role in transmitting HCMV infection to the fetus is played by primary infections of the mother during pregnancy. In fact, the rate of vertical transmission was found to be 0.2 to 2.2% in previously seropositive mothers undergoing recurrent infection during pregnancy (28, 258) and 20 to 40% in pregnant women with primary infection (258, 259). Thus, the ratio of transmitting to nontransmitting mothers is on the order of 1:100 between those with recurrent and those with primary infection. In this respect, diagnosis of primary infection during pregnancy is a major task of the diagnostic virology laboratory. It may be achieved in the majority of cases through concurrent analysis of the following factors: serum antibodies, virus detection in blood, and clinical signs and symptoms.
Serology
Seroconversion.
The diagnosis of primary HCMV infection is ascertained when seroconversion is documented, i.e., the de novo appearance of virus-specific IgG in the serum of a pregnant woman who was previously seronegative. However, such an approach is feasible only when a screening program is adopted and seronegative women are identified and prospectively monitored. In this respect, screening programs are not approved by public health authorities of the great majority of developed countries, as reported elsewhere (see Universal Serology Screening). Thus, detection of HCMV-specific antibodies or IgG in the blood of a pregnant woman in the absence of prepregnancy antibody determination does not lead to suspicion of primary infection. HCMV-specific IgM antibody must be determined for this purpose. Although detection of specific IgM is not sufficient per se to diagnose primary HCMV infection (IgM can also be detected during reactivations), primary infection is consistently associated with the presence of a virus-specific IgM antibody response.
IgM assays.
Several serologic assays have been used in the past to detect HCMV-specific IgM antibodies both in whole serum and serum fractions obtained by sucrose density gradient centrifugation or column chromatography. These include complement fixation, anticomplement immunofluorescence, indirect hemagglutination, and radioimmunoassay (220). More recently, enzyme-linked immunosorbent assays (ELISAs) have been more widely used in both the indirect ELISA (146, 234) and the capture ELISA format with either labeled antigen or antibody (235, 279). The indirect ELISA shows the following potential sources of error when performed on whole serum: (i) competitive inhibition due to the presence of specific IgG; (ii) interference due to rheumatoid factor of the IgM class (IgM-RF) or to IgM-RF reactive only with autologous complexed IgG; and (iii) interference due to IgM antibody reactive with cellular antigens (38). All these interfering factors could be readily eliminated by mixing serum samples with anti-human gamma chain serum (38).
However, following the development of ELISA technology, most initial IgM indirect ELISAs were replaced by IgM capture assays based on selective binding of IgM antibody to the solid phase. In capture ELISAs, while IgG does not interfere, IgM-RF may cause false results by competing with viral IgM for anti-IgM binding sites on the solid phase, complexing with specific IgG, which in turn binds viral antigens, reacting directly with the labeled viral antibody, and mutual interference with antinuclear antibody. More precisely, in capture ELISAs, the presence of the sole IgM-RF (or IgG-RF) does not cause false-positive results, which have been observed to occur in serum samples containing both IgM-RF and IgG-antinuclear antibody (180). Initially, capture ELISAs with enzyme-labeled antigen appeared to be the most promising assays (235, 279). However, after a few years, it was recommended, for specificity control of test results, that human serum samples be tested in parallel with viral and cell control labeled antigens (185). In addition, false-positive results due to the presence of both RF and antinuclear antibody, as reported above, could be avoided in capture ELISAs employing labeled F(ab′)2 fragments of specific antibody instead of the IgG fraction (180).
In order to avoid false-positive results, we developed a capture ELISA IgM assay (213) with a mixture of viral antigen and mouse monoclonal antibody to the nonstructural HCMV major DNA-binding proteins (pp52 or ppUL44) as a detector system. This phosphoprotein is prominent in HCMV-infected cells (77, 97) and is known to be recognized primarily by human IgM during the convalescent phase of a primary HCMV infection (150). According to this approach, antinuclear antibody of the IgM class bound to the solid phase will not give false reactions because only IgM antibody reactive to pp52 are recognized by the specific monoclonal antibody.
Different levels of specificity were determined with this assay. General specificity, determined on a series of unselected IgM-negative serum samples from an adult population, was 100%. Stringent specificity, evaluated on a series of potentially interfering serum samples from patients who had Epstein-Barr virus-related infectious mononucleosis, autoimmune diseases, or rheumatoid factor or who had been treated with radioimmunotherapy based on the use of mouse monoclonal antibody, was 96.3%. Finally, clinical specificity, determined on a series of IgM-negative serum samples drawn prior to onset of primary HCMV infection, was 100%. Thus, the overall specificity was 98.9% (363 of 367 IgM-negative serum samples tested). The sensitivity, assayed on 277 IgM-positive serum samples, was 100%. Comparison of the results obtained by this assay with those given by enzyme-labeled antigen showed that the HCMV p52-specific IgM antibody response paralleled that obtained by using enzyme-labeled antigen, thus representing a major component of it, i.e., a major part of the antibody response within the IgM class. In addition, this study showed that, while HCMV-specific IgM drops sharply in titer in normal subjects within 2 to 3 months after onset of infection and is virtually undetectable within 12 months, in immunocompromised patients such a response persists much longer.
Thus, in pregnant women, detection of HCMV IgM antibody may be related to a primary infection occurring during pregnancy when the IgM titer falls sharply in sequential blood samples. The presence of low, slowly decreasing levels of IgM may indicate a primary infection initiated some months earlier and possibly prior to pregnancy. These findings are basically in agreement with previous reports describing a broad HCMV IgM antibody response (111, 188).
An additional risk of HCMV IgM ELISA is a false-positive result due to primary Epstein-Barr virus infection acting as a potent B-cell stimulator and resulting in the production of HCMV IgM antibody in HCMV-immune individuals (53). Dual HCMV and Epstein-Barr virus infection has also been reported (59).
Recombinant IgM assays.
Besides the lack of standards for HCMV IgM serology, the high level of discordance among commercial assays for detection of HCMV-specific IgM (156) has been attributed to the lack of standardization of the viral preparations used. More recently, in an attempt to improve the specificity of conventional ELISAs and to overcome the discordant results given by commercial kits based on use of crude viral preparations, HCMV IgM immunoassays have been developed based on recombinant HCMV proteins or peptides. The HCMV-coded proteins reactive with IgM antibody are both structural and nonstructural (39, 144, 151, 213, 223, 224, 280). Major structural proteins include pp150 (UL32), pp65 (UL83), and pp38 (UL80a), while nonstructural proteins include pp52 (UL44) and p130 (UL57). Vornhagen et al. (281) developed a recombinant HCMV IgM ELISA for Biotest (Biotest AG, Dreieich, Germany) with only peptides derived from nonstructural proteins pp52 (amino acids 297 to 433) and p130 (amino acids 545 to 601). In particular, it was found that the indicated portion of the UL57 gene product is a dominant IgM antigen which may be superior in both sensitivity and specificity to fragments from other HCMV proteins for detection of IgM antibodies during primary HCMV infection.
Recombinant proteins and their fragments have been studied in a Western blot or immunoblot assay for their reactivity to IgM-positive serum samples prior to being included in an ELISA. The group of M. P. Landini, in close association with Abbott Laboratories (Abbott Park, Ill.), developed two versions of the HCMV IgM immunoblot assay with both recombinant proteins or peptides and viral proteins from purified virus preparations (152, 155, 158). In the new version of the assay (152), the viral section of a slot blot contains the entire viral proteins pp150 (UL32), pp82, pp65 (UL83), and pp28 (UL99) purified by gel electrophoresis, while the recombinant section contains only portions of pp150 (amino acids 595 to 614 and 1006 to 1048), p130 (amino acids 545 to 601 and 1144 to 1233), pp52 (amino acids 202 to 434), and pp38 (amino acids 117 to 383).
A preliminary evaluation of the new immunoblot assay indicated that 13 of 80 (16%) IgG- and IgM-negative serum samples and as many as 38 of 200 (19%) IgG-positive, IgM-negative serum samples did react with one or more of the viral or recombinant proteins, while 126 of 126 (100%) IgM-positive serum samples reacted variably. In order to render these highly nonspecific results interpretable, an algorithm for reading of test results had to be introduced. Thus, only serum samples reactive with at least one viral and one recombinant protein or serum samples reactive with at least three recombinant protein bands were considered positive for IgM. By using this approach, a sensitivity of 100% and specificity of 98.6% were reached with respect to the consensus of two of the most used commercial ELISAs (Behring AG, Marburg, Germany, and DiaSorin, Saluggia, Italy).
This assay was used as a reference test for development of the Abbott AxSYM CMV IgM microparticle enzyme immunoassay, with microparticles coated with the indicated portions of three structural (pp150, amino acids 595 to 614 and 1006 to 1048; pp65, amino acids 297 to 510; and pp38, amino acids 117 to 373) and one nonstructural (p52, amino acids 202 to 434) protein. This assay, when compared to a consensus given by three commercial HCMV IgM immunoassays (discordant results were resolved by immunoblot), showed a relative sensitivity, specificity, and agreement of greater than 95%. In addition, the assay was able to detect seroconversion very early and displayed a higher positive reactivity rate than the commercial assays tested on pregnant women (168). The level of cross-reactivity was 3.3%. The diagnostic utility of the AxSYM IgM assay in detecting low levels of IgM antibody (not detected by other commercial assays) in some serum samples is stressed by the finding that some of these serum samples contain low-avidity IgG (154), a marker of primary HCMV infection (see IgG Avidity Assay).
At least one additional approach has been reported, with a combination of two HCMV peptides derived from pp150 (UL32, amino acids 1011 to 1048) and pp52 (UL44, amino acids 266 to 293) for IgM detection and a combination of peptides from pp150 (amino acids 1011 to 1048), pp28 (amino acids 130 to 160), and gB (amino acids 60 to 81) for optimal IgG detection (107). Sensitivity was 96.4% for the IgM assay with respect to a viral lysate-based ELISA.
Although the development of immunoassays based on use of recombinant viral proteins or peptide epitopes represents major progress towards standardization of serological assays, these assays do not appear to be reliable from the diagnostic standpoint due to exceedingly high sensitivity and somewhat low specificity. In a recent study, 10 of 42 (23.8%) potentially cross-reactive or interfering serum samples were scored IgM-positive with a commercial ELISA based on use of recombinant HCMV antigens, whereas two commercial ELISAs based on use of viral lysates detected zero and one positive sample, respectively, in the same panel of problematic serum samples (46). Indeed, false-positive results still represent the major pitfall of HCMV IgM serology. In this respect, in a recent retrospective review of 325 consecutive pregnant women referred to our laboratory over a 2-year period because of a positive IgM result and a suspicion of primary HCMV infection, as many as 188 (57.8%) were found to be IgM negative by two different in-house-developed capture ELISAs in the absence of primary infection (207).
Interpretation of positive IgM results.
Once the specificity of a positive IgM result has been verified, the interpretation of the clinical significance of IgM antibody present in the serum of a pregnant woman begins. We must recall that the IgM antibody response, which is currently detected in primary HCMV infections of both immunocompetent and immunocompromised patients, may also be detected during recurrent infections of the immunocompromised person, but generally not in the immunocompetent host. Thus, IgM detection in the serum of a pregnant woman is likely to be a reliable marker of a primary HCMV infection. However, IgM can reveal different clinical situations which can be related to the acute phase of a primary HCMV infection, the convalescent phase of a primary HCMV infection, or the persistence of IgM antibody.
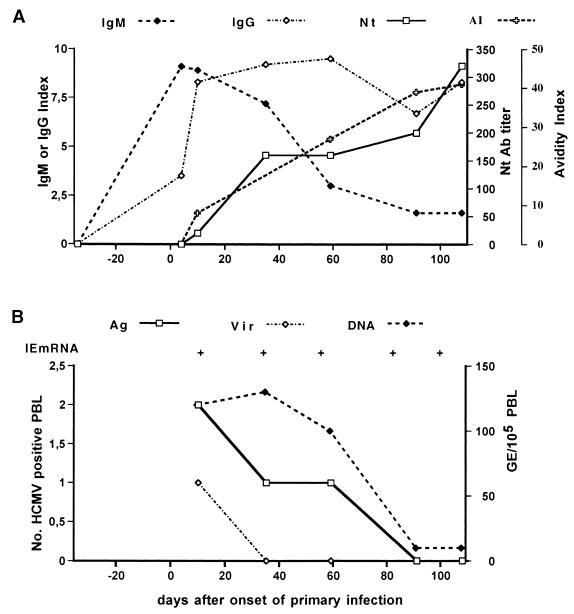
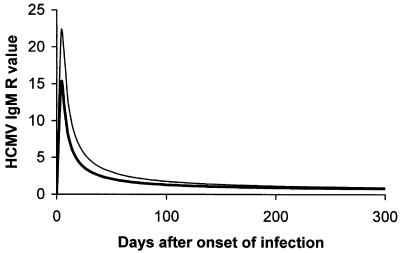
The kinetics of the HCMV-specific IgM antibody response during primary infection may vary greatly among individuals and depends substantially on the test or commercial kit used for testing. However, in general, high to medium levels of IgM antibody (peak titers) can be detected during the first 1 to 3 months after the onset of infection (acute or recent phase), after which the titer starts declining (convalescent or late phase) (Fig. 4; M. G. Revello and G. Gerna, unpublished data). By using two capture ELISAs, it was shown that of nine immunocompetent individuals, four became negative for IgM within 6 months, three within 12 months, while two remained IgM positive for more than a year after the onset of primary infection (213). A recent study compared the sensitivities of the same two in-house-developed IgM capture assays based on use of viral lysates (213) and a commercially available recombinant IgM assay (168). The kinetics of the IgM antibody response as determined on 213 sequential serum samples from 76 pregnant women with primary HCMV infection was grossly overlapping (Fig. 5), showing a low-level IgM antibody response persisting for several months (M. G. Revello, G. Gorini, M. Parea, and G. Gerna, unpublished data).
FIG. 4.
(A) Kinetics of IgG, IgM, and neutralizing (Nt) antibody (Ab) response as well as IgG avidity index (AI) in a pregnant woman with primary HCMV infection. (B) Kinetics of infectious virus and different virus products in the blood of the same pregnant woman as in A during the convalescent phase of a primary HCMV infection. Ag, antigenemia; Vir, viremia; DNA, DNAemia; IE mRNA, immediate-early mRNA; +, positive; −, negative; GE, genome equivalents; PBL, peripheral blood leukocytes. (M. G. Revello and G. Gerna, unpublished data.)
FIG. 5.
Kinetics of IgM antibody response in 76 pregnant women with primnary HCMV infection as determined in 213 sequential serum samples by using two in-house-developed capture assays in parallel. IgM assays were based on the use of (thin line) virus lysate (213) and (thick line) a commercial recombinant IgM assay (168). (M. G. Revello, G. Gorini, M. Parea, and G. Gerna, unpublished data.)
We define persistent IgM antibody response as the detection of stable levels of HCMV-specific IgM antibody for longer than 3 months. Although varying among different individuals, levels of persistent IgM antibody are mostly low, perhaps representing the sustained tail of an IgM response following a primary infection in some subjects (207). In a recent survey of 137 pregnant women confirmed to be positive for HCMV-specific IgM, only 60 (43.8%) were diagnosed as having primary HCMV infection acquired during pregnancy, whereas 39 (28.5%) had persistent IgM. In 38 (27.8%) of the 137 women, the IgM kinetics could not be determined due to the availability of only a single serum sample (207).
IgG avidity assay.
When the presence of HCMV-specific IgM antibody in the serum of a pregnant woman cannot be directly related to a primary infection during pregnancy, an IgG avidity assay can help distinguish primary from nonprimary HCMV infection. This assay is based on the observation that virus-specific IgG of low avidity is produced during the first months after onset of infection, whereas subsequently a maturation process occurs by which IgG antibody of increasingly higher avidity is generated. Only IgG antibody of high avidity is detected in subjects with remote or recurrent HCMV infection. Avidity levels are reported as the avidity index, expressing the percentage of IgG bound to the antigen following treatment with denaturing agents, such as 6 M urea. The utility of the assay in diagnosing a primary infection has been reported for a variety of viruses (7, 17, 102, 123, 125, 142, 283). Measurement of IgG avidity is also of value in determining the duration of primary HCMV infection (20, 23, 101, 156, 207).
We have shown that mean avidity index values relevant to serum samples collected less than 3 months after onset of primary infection were 21% ± 13%, whereas mean avidity index values for serum samples from subjects with remote HCMV infection were 78% ± 10% (207). Thus, the presence of high IgM levels and a low avidity index are highly suggestive of a recent (less than 3 months) primary HCMV infection. In a recent study, an avidity index above 65% during the first trimester of pregnancy could reasonably be considered a good indicator of past HCMV infection, whereas in all women with a low avidity index (≤50%), there was a risk of congenital HCMV infection. The risk increased with the gestational age at the time of testing (20). That is, only 2 of 12 (16.7%) women with a low avidity index during the first trimester of pregnancy transmitted the infection to the fetus, whereas in utero infection of the fetus was found in 6 of 15 (40.0%) women with a low avidity index detected during the second or third trimester of pregnancy (20), approaching the transmission rate reported by several groups (110, 131, 264, 293). A negative predictive value of 100% was found when the avidity index was determined to be high or moderate before 18 weeks of gestation (157, 169). On the other hand, when the avidity index was calculated at 21 to 23 weeks of gestation, it failed to identify some women who transmitted the virus, with a negative predictive value of 90.9% (169).
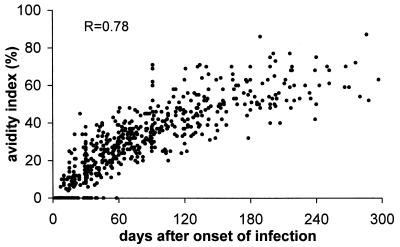
Figure 6 shows the maturation of HCMV-specific IgG avidity in 560 sequential serum samples from 176 immunocompetent individuals with primary HCMV infection (M. G. Revello and G. Gerna, unpublished data). It can be observed that in the interval between 4 and 6 months after the onset of infection, while most avidity index values are intermediate, a minor portion are either low (<30%) or high (>50%). This implies that in some pregnant women examined during the first trimester of pregnancy, a low avidity index may be related to a primary infection acquired prior to conception (false-positive result with respect to primary infection during pregnancy), while a high avidity index observed in the second trimester of pregnancy does not necessarily exclude a primary infection acquired during pregnancy. Recently, the ability of three IgG avidity assays to detect a primary HCMV infection was found to approximate 100%, whereas the ability to exclude a recent infection was shown to range from 96% to 32%. These data indicate that standardization of the assay is urgently needed (22).
FIG. 6.
Kinetics of IgG avidity index (maturation of HCMV-specific IgG) in 560 serum samples from 176 pregnant women with primary HCMV infection. (M. G. Revello, and G. Gerna, unpublished data.)
Neutralizing antibody.
It has also been reported recently that determination of HCMV neutralizing antibody may be an additional useful parameter for identification and timing of primary HCMV infection via a single serum sample (60). A neutralizing antibody response was not detected for 15 weeks (range, 14 to 17 weeks) after onset of primary infection. On this basis, it was concluded that the absence of neutralizing antibody during the convalescent phase of a primary HCMV infection is a reliable marker of primary infection, whereas the presence of neutralizing antibody rules out a primary infection in the previous 15 weeks. However, although it is well known that the neutralizing antibody response is the last to be mounted after a primary HCMV infection (256, 267), the reported 15-week delay appears too extended, at least for immunocompetent subjects.
When we tested 89 serum samples from 22 pregnant women with primary HCMV infection with the same neutralizing assay, we found neutralizing antibodies in 9 of 20 (45%) serum samples collected within 30 days, 20 of 23 (87%) serum samples collected within 30 to 60 days, and in all 46 (100%) serum samples collected >60 days after onset (Fig. 4) (M. G. Revello and G. Gerna, unpublished data). Thus, the absence of neutralizing antibody in a serum sample from a pregnant woman containing HCMV IgG and IgM may indeed provide additional evidence of recent primary infection. In contrast, the presence of neutralizing antibody is of no help in interpreting a positive IgM result.
Conclusions.
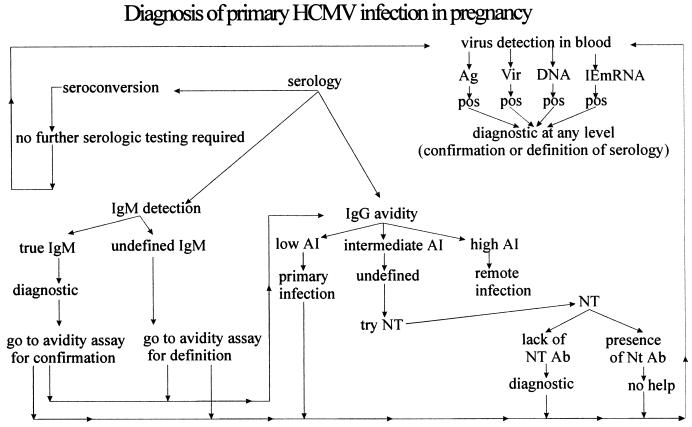
The most definitive diagnosis of primary HCMV infection in a pregnant woman is by detection of seroconversion, i.e., the appearance of HCMV-specific IgG antibody during pregnancy in a previously seronegative woman (Fig. 7). When this result cannot be achieved, detection of IgM antibody during pregnancy as well as during follow-up (whenever possible) can be used to determine clinically significant primary HCMV infection. Further testing by the IgG avidity test may be of great help in both confirming and clarifying the clinical significance of IgM antibody. When, at the end of the diagnostic algorithm, a primary HCMV infection is either diagnosed or suspected, prenatal diagnosis should be offered to a pregnant woman to verify whether the infection has been transmitted to the fetus. However, prior to performance of prenatal diagnostic procedures, the diagnosis of primary infection may be further confirmed or substantially supported by performing assays for detection of virus or virus products in the blood of the mother (Fig. 7).
FIG. 7.
Schematic of diagnosis of primary HCMV infection in pregnancy, including both serologic and virologic approaches. AI, avidity index; Ag, antigenemia; Vir, viremia; DNA, DNAemia; IE mRNA, immediate-early mRNA; NT, neutralization test; Ab, antibody; pos, positive.
Detection of Virus and Viral Products in Maternal Blood
Following primary infection, HCMV can be recovered from multiple body fluids such as saliva, urine, and vaginal secretions for a variable period of time. However, virus shedding from the same body sites may occur during reactivations and reinfections as well. Thus, the recovery of HCMV from these biological materials does not allow differentiation between primary and nonprimary infections in either immunocompetent or immunocompromised individuals. In the last decade, it has been clearly shown that only detection and quantitation of HCMV in blood has a high predictive value for HCMV disease in immunocompromised patients with either primary or recurrent HCMV infections (25, 78, 95, 116, 132, 166, 225, 243, 277). In addition, virus detection in blood has been reported to be diagnostic of primary HCMV infection in immunocompetent individuals (216), whereas in immunocompromised patients it is indicative of both primary and nonprimary infections.
During the last decade, several methods have been developed to detect and quantify HCMV in blood. The most widely used assays include determination of viremia, i.e., infectious HCMV in blood; determination of antigenemia, i.e., number of pp65-positive peripheral blood leukocytes; quantification of HCMV DNA in whole blood (DNAemia), leukocytes (leuko-DNAemia), or plasma; determination of immediate-early and late mRNA (RNAemia); and search for the presence of circulating cytomegalic endothelial cells (CEC) in blood. An extended review of the methodological aspects and clinical applications of different assays for quantitation of HCMV has been published recently (24).
Viremia.
Conventional methods for determination and quantitation of viremia are time-consuming because they are based on the appearance of cytopathic effect and include determination of 50% tissue culture infectious doses and plaque assays. These methods have been replaced by the “shell vial” assay, which provides results within 24 h. Following its introduction in the early 1980s (98), the assay was later rendered quantitative based on the assumption that each p72-positive fibroblast in a human fibroblast monolayer is infected by a single leukocyte carrying infectious virus (93). The shell vial monolayer is stained with either the immunofluorescence or the immunoperoxidase technique and a monoclonal antibody reactive with the HCMV major immediate-early protein (93). Then, the number of positive nuclei is counted (Fig. 8A). Since it was shown that a single monoclonal antibody may not identify virus strains with mutations in the relevant epitope of the major immediate-early protein, virus identification in our laboratory is performed with a pool of monoclonal antibodies reactive to different epitopes of p72 (G. Gerna, E. Percivalle, and M. G. Revello, unpublished data).
FIG. 8.
(A) Viremia, indicating the presence in a shell vial monolayer of HCMV p72-positive fibroblast nuclei following cocultivation with peripheral blood leukocytes carrying infectious virus and immunostaining by fluorescein-conjugated p72-specific monoclonal antibody. (B) Antigenemia ex vivo, showing immunofluorescent staining with a pool of monoclonal antibodies of pp65-positive peripheral blood polymorphonuclear leukocytes from a patient with AIDS and disseminated HCMV infection. (C) Antigenemia in vitro, showing pp65-positive polymorphonuclear leukocytes from a healthy blood donor following cocultivation with HCMV-infected human umbilical vein endothelial cells and immunofluorescent staining with the same pool of pp65-specific monoclonal antibodies used in B. (D) Circulating cytomegalic endothelial cell with a pp65-positive leukocyte (arrow). Immunofluorescent staining was done with a pool of pp65-specific monoclonal antibodies.
In immunocompromised patients, the presence of HCMV viremia is commonly associated with a high risk of developing HCMV disease (91, 116). Thus, its determination represents a useful parameter for initiation of antiviral treatment (78), monitoring of the efficacy of antiviral treatment (82), and detection of treatment failure due to emergence of a drug-resistant HCMV strain (88). However, major disadvantages of the viremia assay are its low sensitivity, the toxicity of peripheral blood leukocyte suspension for fibroblast monolayers, and the loss of HCMV viability in stored clinical samples (24).
Antigenemia.
The antigenemia assay (Fig. 8B) detects and quantifies peripheral blood leukocytes, mostly polymorphonuclear leukocytes and, to a much lesser extent, monocytes/macrophages, which are positive for the HCMV lower matrix phosphoprotein pp65 (105, 209). This HCMV protein, which was initially believed to be the major immediate-early protein p72 (214, 278), is transferred to polymorphonuclear leukocytes from infected permissive cells via transitory microfusion events between two adhering cells (81, 211). The antigenemia assay has been optimized (92) and standardized (83) by using in vitro-infected leukocytes (Fig. 8C). The methodological aspects of this assay have been reviewed recently (24).
Experience obtained with transplant recipients has shown that antigenemia becomes positive earlier than viremia but later than DNAemia at the onset of infection, and it becomes negative later than viremia but earlier than DNAemia in the advanced stage of a systemic infection (89); high antigenemia levels are often associated with HCMV disease; the assay is widely used for monitoring of HCMV infections and antiviral treatment (25, 78, 116, 166, 172, 277); and during ganciclovir treatment of primary HCMV infections, antigenemia levels may increase for up to 2 to 3 weeks despite the efficacy of treatment as shown by the disappearance of viremia, prompting clinicians to erroneously change antiviral drugs (26, 94, 117, 183). A major advantage of the antigenemia assay is rapidity in providing results in a few hours, while major disadvantages are the limited number of samples processed per test run and the subjective component in slide reading (24).
DNAemia.
Detection and quantification of HCMV DNA in blood has become a major diagnostic tool for transplant recipients. To this purpose, two major approaches have been used, PCR and hybridization techniques. For PCR, two main types of competitors have been used in the quantitative-competitive PCR: homologous competitors containing small deletions or insertions with respect to the target sequence (27, 76), and heterologous competitors having the target sequence for primers as the target nucleic acid but differing in the intervening sequence (84, 90, 95). In addition to in-house-developed methods, a commercially available method has been developed by Roche (Cobas Amplicor CMV monitor test; Roche Molecular Systems, Branchburg, N.J.) for both detection and quantification of HCMV DNA (55, 128). Finally, a new and interesting approach to the quantification of viral DNA is the detection and measurement of PCR products as they accumulate, thus overcoming the limited linear dynamic range of the traditional quantitative PCR. This technique, referred to as real-time PCR, is now being tested (Perkin Elmer, Applied Biosystems, Foster City, Calif.) and is based on the release of fluorescent dye molecules at each PCR cycle, the intensity of which is proportional to the amount of DNA in the sample (127).
Among the hybridization techniques amplifying the signal generated rather than the viral DNA itself, two have become commercially available for quantification of HCMV DNA: the Digene hybrid capture system CMV DNA assay (version 2.0; Abbott Laboratories, Abbott Park, Ill.) and the branched DNA assay (Bayer, Chiron Corporation, Emeryville, Calif.). The hybrid capture system is based on the formation of a DNA-RNA hybrid which is captured by a monoclonal antibody specific for the hybrid and is then reacted with the same monoclonal antibody labeled with alkaline phosphatase. The hybrid is finally detected with a chemiluminescent substrate, whose emission is proportional to the amount of target DNA present in the sample (173). The second-generation hybrid capture system assay has been reported to have increased sensitivity (24) and, thus could be considered for detection of viral DNA in the blood of immunocompetent hosts (see below). The branched DNA assay is based on the use of branched DNA amplifiers (branched probes) containing multiple binding sites for an enzyme-labeled probe. The target DNA sequence binds to the branched DNA molecule, and the complex is revealed by a chemiluminescent substrate whose light emission is directly proportional to the target DNA present in the sample (40, 141).
In immunocompromised patients, HCMV DNA quantification has been shown to be useful for follow-up of disseminated infections and evaluation of the efficacy of antiviral treatment. In addition, it is useful for the diagnosis and local evaluation of the effect of antiviral treatment at special body sites, such as the eye and nervous system (84, 210). Finally a special application concerns its use for prenatal diagnosis of HCMV infection and for quantification of viral DNA in amniotic fluid samples (see below).
RNAemia.
Detection of HCMV transcripts in blood is currently considered a marker of HCMV replication in vivo and late viral transcripts in particular are considered to better reflect active HCMV replication and dissemination (100, 148). With reverse transcription-PCR, false-positive results may result from the difficulty in differentiating between RNA- and DNA-derived PCR products in the case of unspliced transcripts (85). Unlike reverse transcription-PCR, detection of mRNAs by the recently introduced nucleic acid sequence-based amplification (NASBA) method, which allows specific amplification of unspliced RNA in a DNA background (42), appears very useful for different populations of transplant recipients (8, 19).
Recently, two retrospective studies, in which preemptive therapy of both solid organ and hematopoietic stem cell transplant recipients was antigenemia guided, monitoring of HCMV pp67 mRNA (a late viral transcript) by NASBA appeared to be a promising tool for initiation and termination of preemptive therapy for solid organ transplant recipients with reactivated HCMV infection (87), whereas monitoring of immediate-early mRNA expression appeared to be a useful parameter for initiation of preemptive therapy in hematopoietic stem cell transplant recipients (86). At this time, prospective studies with NASBA assays are ongoing in transplant recipients, whereas preliminary data on the kinetics of immediate-early mRNA in immunocompetent individuals with primary HCMV infection are already available (208).
Endotheliemia.
The term endotheliemia was introduced to indicate HCMV-infected CEC in the peripheral blood of immunocompromised patients. CEC were first described in 1993 by two independent groups (104, 196) and were shown to be endothelial in origin and fully permissive for HCMV replication. CEC are derived from infected endothelial cells of small blood vessels, which progressively enlarge until they detach from the vessel wall and enter the bloodstream. More recently, CEC have been studied in hematopoietic stem cell transplant recipients (232) and in AIDS patients with disseminated HCMV infection (96). In recent years, the introduction of highly active antiretroviral therapy for AIDS patients and the adoption of prophylactic and preemptive therapy approaches for transplant recipients have nearly eliminated CEC from blood of these patient groups. However, CEC may still be found in the blood of fetuses (Fig. 8D) and newborns with symptomatic congenital HCMV infection (M. G. Revello, E. Percivalle, and G. Gerna, unpublished data).
Virus and viral products in blood of immunocompetent persons as an aid for diagnosis of primary infection.
Although there is an extensive amount of data obtained from studies with immunocompromised patients (78, 166, 274), very few data are available on the presence of HCMV in the blood of immunocompetent individuals with primary infection (33, 138, 140, 221, 222). In particular, little has been done to assess the diagnostic value of virus detection in the blood of nonimmunocompromised patients. Recently, an investigation was conducted on the peripheral blood leukocytes of 52 immunocompetent individuals (40 pregnant women) with primary HCMV infection by quantitation of pp65 antigenemia, viremia, and leukoDNAemia (216). pp65 antigenemia was detected in 12 of 21 (57.1%), 4 of 16 (25%), and 0 of 10 patients examined 1, 2, and 3 months after onset, respectively. Viremia was detected in 5 of 19 (26.3%) patients during the first month only. Finally, leukoDNAemia was detected in 20 of 20, 17 of 19 (89.5%), and 9 of 19 (47.3%) patients tested 1, 2, and 3 months after onset, respectively. Four (26.6%) of 15 patients were still DNAemia positive at 4 to 6 months, whereas none were positive at >6 months. No assay was positive in any of 20 subjects with remote infection or of 9 subjects with recurrent infection. In addition, virus levels were low by all assays. The conclusion of the study was that primary HCMV infection can be rapidly and specifically diagnosed whenever any of the studied virologic markers is detected in blood. On this basis, dating of the onset of infection can also be attempted (216).
Viremia, i.e., virus recovery from blood, allows diagnosis of primary infection in about 25% of cases during the first month after onset. In fact, HCMV could not be recovered from the blood of 86 blood donors (249) and in only one study was HCMV isolation from the blood of 2 of 35 normal donors reported (56). However, in this case, the interpretation of positive HCMV recovery from the blood of healthy people may hypothetically be referred to the convalescent phase of an unknown asymptomatic primary infection. Antigenemia may allow diagnosis of primary HCMV infection in 50% of patients in the first month and in 25% of patients in the second month after onset of infection. Again, positive antigenemia was never reported in immune healthy subjects or in patients prior to transplantation. Finally, DNAemia and, in particular, leukoDNAemia allow diagnosis of primary HCMV infection in 100% of subjects examined within 1 month after onset of infection and in 98% of those tested within 2 months.
The presence of viral DNA in the leukocytes of healthy people is a more controversial issue. While in three reports viral DNA was found in virtually all seropositive healthy adult volunteers and in most seronegative persons when monocytes (268, 273) or peripheral blood leukocytes (14) were examined, other investigators failed to detect viral DNA by PCR in monocytes (16) or peripheral blood leukocytes (78, 135, 229, 240) or reported low (4 to 6%) positivity rates (34, 249). Recent studies support the concept that viral DNA is not detected in the peripheral blood leukocytes of HCMV-seropositive immunocompetent individuals (216, 294), emphasizing the utility of viral DNA detection in blood as a parameter for diagnosing primary HCMV infection.
More recently, a new virologic parameter has been found to be useful for diagnosis of primary HCMV infection, detection of immediate-early mRNA by the NASBA technology. The clinical specificity of this parameter was first assessed in healthy individuals with remote or recurrent HCMV infection, showing its consistent negativity. Then, immediate-early mRNA was detected in the blood of all subjects with primary HCMV infection in the first month after onset of infection, whereas the proportion of positive subjects declined over time and became negative ≥6 months after onset of infection (208). Thus, immediate-early mRNA kinetics appears to be comparable to that already reported for viral DNA, which suggests that detection of immediate-early mRNA in the blood of immunocompetent individuals can be considered an additional marker of recent primary HCMV infection. However, if immediate-early mRNA detection appears to be slightly more sensitive than DNA detection in diagnosing the early phase of primary HCMV infection (86), it appears to be slightly less sensitive in detecting the late phase of primary HCMV infection (208).
Clinical Signs and Symptoms
The great majority of primary HCMV infections in the immunocompetent host are clinically silent (190). In addition, less than 5% of pregnant women with primary infection are reported to be symptomatic, and an even smaller percentage suffer from a mononucleosis syndrome (192). Thus, a primary HCMV infection cannot generally be diagnosed on clinical grounds alone. However, careful collection of the clinical history may be extremely useful for detecting minor clinical symptoms and dating the onset of infection. Whenever a primary HCMV infection is diagnosed in a pregnant woman, an interview is mandatory. Apart from major clinical findings observed in HCMV mononucleosis (such as fever, cervical adenopathy, sore throat, splenomegaly, hepatomegaly, and rash) which are not commonly detectable, if a pregnant woman is carefully questioned by experienced personnel, minor symptoms typical of HCMV mononucleosis, such as malaise, fatigue, headache, and myalgia, can be recalled, allowing quite precise dating of the onset of infection in the majority of cases (207). In addition, a slight increase in serum levels of liver enzymes (alanine transaminase, aspartate transaminase) may help in dating the onset of infection.
In a survey conducted on 60 pregnant women with primary HCMV infection, mild clinical symptoms and/or liver function abnormalities were detected in as many as 38 (60%) (207). In a more recent survey (M. G. Revello, and G. Gerna, unpublished data) conducted on 244 pregnant women with primary HCMV infection, clinical symptoms were present in 166 (68.1%), fever (60.2%), fatigue (48.8%), and headache (26.5%) being the most frequent symptoms (Table 1). In addition, 70 (42.1%) women reported three or more symptoms. The high rate of symptomatic primary HCMV infections in pregnancy may be explained by the careful medical interview. Whether the pregnancy-associated immunosuppression might play a critical role remains to be determined.
TABLE 1.
Clinical and laboratory findings in 244 pregnant women with primary HCMV infectiona
| Clinical symptoms or abnormallaboratory findings | No. (%) of women |
|---|---|
| Absent | 78 (32.0) |
| Present | 166 (68.0) |
| Fever | 100 (60.2) |
| Fatigue | 81 (48.8) |
| Headache | 44 (26.6) |
| Arthralgiae/Myalgiae | 25 (15.1) |
| Rhinitis | 25 (15.1) |
| Pharyngitis | 23 (13.9) |
| Cough | 16 (9.6) |
| Elevated liver enzymes | 60 (36.1) |
| Lymphocytosis | 20 (12.0) |
| Cumulative no. of symptoms/abnormalities per subject | |
| One | 49 (29.5) |
| Two | 48 (28.9) |
| Three or more | 70 (42.2) |
M. G. Revello and G. Gerna, unpublished data.
Dating of primary HCMV infections in pregnancy is crucial for at least three reasons. The first refers to prognosis, in the sense that primary infection acquired just before conception is generally assumed to represent a lower risk than primary infection acquired during pregnancy. The second refers to prenatal diagnosis. It seems to be important to delay prenatal diagnosis as long as possible with respect to the onset of infection in order to minimize the rate of false-negative results (178, 184). In fact, false-negative results have been reported despite the use of the most sensitive techniques available (206, 215). Finally, primary infection early in pregnancy implies greater likelihood of congenital disease.
Diagnosis of Recurrent Infection in the Mother
It is generally accepted that transplacental transmission of HCMV infection represents a major risk during primary infection. However, over the past 20 years, data have accumulated that recurrent HCMV infections during pregnancy may also cause congenital infections. Thus, there is a rough correlation between the rate of maternal seropositivity and the rate of congenital infections (258). The estimated risk of intrauterine HCMV transmission during both primary and recurrent HCMV infections of pregnant women from low-income and high-income backgrounds in the United States is reported in Fig. 2.
Diagnosis of recurrent infection can be accomplished by virus isolation or viral antigen or viral DNA detection in clinical samples, such as samples from the genital tract or cervix or urine, other than blood in the absence of concomitant serologic and virologic markers of primary HCMV infection, i.e., HCMV-specific IgM, low-avidity IgG, absence of neutralizing antibodies, and absence of virus and viral markers in blood. In this respect, it is very important to emphasize that, in order to ascertain the diagnosis of a congenital HCMV infection following a recurrent infection of the mother, at least the following requirements must be satisfied: the mother must be defined as immune to HCMV at least 1 year prior to pregnancy; a prepericonceptional HCMV infection (see below) must be excluded; and HCMV should be recovered from the genital tract. Thus, only prospective well-designed and extended epidemiological studies will be able to define the true impact of recurrent maternal infections (either reactivations or reinfections) in determining congenital HCMV infections in the future.
Maternal Prognostic Markers of Fetal Infection
Thus far, no reliable prognostic markers of transmission of HCMV infection to the fetus have been identified in the mother. Recently, no correlation has been found between virus load in blood and the clinical course of HCMV infection in primary infections of immunocompetent subjects (216), in contrast to immunocompromised patients (78, 82). In addition, no correlation has been found between virus load in blood and intrauterine transmission of the infection (216). Similarly, human immunodeficiency virus type 1 has been reported to be transmitted from mother to fetus within a wide range of maternal plasma human immunodeficiency virus type 1 RNA levels (257). Furthermore, no correlation was found either between persistence of viral DNA in blood for 3 months or >3 months and the risk of fetal infection or between gestational age and the risk of intrauterine transmission (216). With respect to the last issue, it was found, in agreement with a previous study (264), that HCMV transmission occurred in 50%, 40%, and 71% of fetuses after maternal infection in the first, second, and third trimester of pregnancy, respectively (216).
In addition, virus recovery during pregnancy from the cervical tract or urine in both primary and recurrent infections is a poor indicator of risk of intrauterine transmission (258). The neutralizing antibody response has also been investigated as a potential prognostic marker of intrauterine transmission. Lower neutralizing antibody titers were detected in transmitting mothers with primary HCMV infection compared to nontransmitters, suggesting an association of neutralizing activity and intrauterine transmission (29). In the same study, a significant correlation was also observed between neutralizing activity and antibody avidity, thus suggesting that a maturation of antibody avidity is necessary for production of high levels of neutralizing antibodies, while a defect or delay in avidity maturation may play a role in intrauterine HCMV transmission (29).
Symptomatic congenital HCMV infections have been noted in infants born to mothers with prepregnancy anti-HCMV immunity (28). Moreover, intrauterine transmission of HCMV from immune mothers to their infants has been related to reinfection with a different virus strain capable of causing symptomatic infections, as measured by the acquisition of new antibody specificities against epitopes of the glycoprotein H of the reinfecting HCMV strain (30). However, only prospective studies will be able to define the frequency of reinfection in immune pregnant women and its clinical impact on congenital infections.
Finally, a lymphoproliferation assay against HCMV has been reported to provide an early marker of fetal infection after primary HCMV infection in pregnancy (269). In that study, all eight women with positive lymphoproliferative response gave birth to uninfected babies, whereas four of six women with negative responses delivered congenitally infected babies. Those findings suggested that depression of cell-mediated immunity in pregnant women after primary infection may represent a marker of fetal infection.
COUNSELING
Once a diagnosis of primary HCMV infection has been achieved, the woman should receive sufficient information to make informed choices about further testing and options. This step is generally indicated with the term counseling. The term itself is vague and, in a way, misleading. Indeed, it is well recognized that the counselor is not supposed to give suggestions, opinions, or advice; rather, his or her role is that of facilitating informed choice by giving information and helping people to make decisions that reflect their value systems. Similarly, many terms such as informed decision, effective decision, and evidence-based choices are used to encompass informed choice.
There is a growing tendency to consider informed choice as being “based on relevant knowledge, consistent with the decision-maker's values and behaviorally implemented” (187). According to this definition, an informed choice to undergo a test, such as prenatal diagnosis, occurs when the woman has relevant knowledge about the test, has a positive attitude towards undergoing a test, and undergoes it. An informed choice to decline a test occurs when the woman has a negative attitude towards undergoing a test, has relevant knowledge about the test, and does not undergo it. As a consequence, whenever the woman does not have relevant information or her attitudes are not reflected in her behavior, her choice should be considered uninformed. With this classification, very recently a model has been developed to provide a measure of informed choice capable of assessing both knowledge and values in relation to Down's syndrome testing in pregnancy (171). It would be very interesting to prove the validity of this approach for HCMV specifically. One of the major benefits would be to determine whether decisions are informed and, if not, the types of interventions required to increase rates of informed choice.
Since no study has so far specifically addressed the issue of counseling of pregnant women for HCMV, data are not available concerning the number of health professionals actually providing the counseling, be it specialists in infectious diseases, virology, microbiology, psychology, obstetricians, or midwives. Similarly, nothing is known about how counseling is structured and performed or about the outcome, i.e., effect of counseling on informed decision making. Finally, it must be stressed that, at least in Italy, very few health professionals have received specific training in counseling, and in most instances, including our own, the counselor is a self-taught health professional with specific knowledge and wide experience. In less fortunate (from the woman's standpoint) but not infrequent cases, the health professional providing the counseling has neither specific knowledge nor experience, which often has disastrous consequences.
In our experience, counseling is a complex process that proceeds step by step and is tailored to each individual. The first, most crucial step is the diagnosis of the mother. From a practical point of view, we do not provide extensive information on the possible clinical outcome until a diagnosis is firmly established. In particular, whenever a woman is referred to us because of IgM positivity detected in other laboratories during routine screening, we do explain what HCMV is and how one becomes infected. However, we focus primarily on the possible meaning of the laboratory results and multiple diagnostic options (false-positive result, persistent IgM, cross-reactive IgM due to herpesvirus infections other than HCMV, HCMV-specific IgM to be related to a preconceptional infection or to a primary infection in pregnancy). We do anticipate that only the last alternative may carry some risks to the fetus, and we explain to the woman that extensive information will be given only when the final diagnosis is reached. In this way, sufficient information is given to justify additional blood samplings and the time required for definite diagnosis without overly upsetting the woman.
Once an acute or recent primary HCMV infection is diagnosed with certainty or high probability, the woman is given complete information about the risks of transmission, possible clinical outcome for the child, therapeutic possibilities in the case of symptomatic disease at birth, as well as prenatal diagnosis (if gestation time allows this option). All information is given within a framework that is as neutral as possible and in an unhurried fashion. Evidence (research)-based information is tailored to single cases, according to timing of maternal infection, certainty of diagnosis, and time of gestation. Possibilities and limitations of prenatal diagnosis, including the event of a false-negative result, are discussed in detail. If the mother has an acute or recent infection and is still viremic, the possibility of iatrogenic transmission is also discussed. The woman is also informed about the possibility of terminating the pregnancy, but she is referred to her obstetrician for specific counseling.
Finally, if the woman undergoes prenatal testing and the fetus is found to be infected, results of prenatal diagnosis are discussed during an additional counseling session in order to provide the woman with the most accurate picture of fetal conditions based on biochemical/hematological, virological, and ultrasound findings. The woman (or the couple) then makes the final decision about continuation or termination of the pregnancy.
DIAGNOSIS OF CONGENITAL INFECTION IN THE FETUS
After more than a decade, there are still those who do not favor prenatal diagnosis and those who consider prenatal diagnosis a major achievement in monitoring pregnancy. The main reasons of those who are against prenatal diagnosis are that the predictive value of a negative result is not yet quantified and because there is no specific antiviral treatment during pregnancy, the only clinical decision which can be made following prenatal diagnosis is whether or not to terminate the pregnancy; also, because only 35 to 40% of primary maternal infections are transmitted to the fetus (265) and the great majority of congenital infections are asymptomatic (75), most pregnant women may prefer not to pursue prenatal diagnosis or termination of pregnancy (189). Reasons supporting prenatal diagnosis are to study of the natural history of congenital HCMV infection; to better prepare the family to face the health problems of the infant or young child; and to allow identification of prognostic markers of HCMV disease. Prenatal diagnosis may also represent the step preceding the potential introduction of antiviral therapy in the future (113). Finally, it can assist in decisions about continuing or terminating the pregnancy.
Clinical samples currently used for prenatal diagnosis are fetal blood drawn by cordocentesis and amniotic fluid obtained by amniocentesis. Cordocentesis was introduced by Daffos et al. (45) in the early 1980s and allows fetal blood sampling via the umbilical cord. It is usually performed after 17 weeks of gestation and is completed in a few minutes. Complications of cordocentesis, which occur at a low rate, may include transient bleeding, transient fetal bradycardia (7 to 9%), premature delivery (<2.0 to 5.0%), and fetal loss (1.7 to 1.9%) (45, 285). Amniocentesis was first introduced by Bevis (15) for diagnosis of immune hemolytic anemia and by Davis (49) for diagnosis of congenital HCMV infection. Although rare, complications of amniocentesis may include fetal loss (<1%), leakage of amniotic fluid, and vaginal bleeding (114). By 1992, 20 cases of congenital HCMV infection diagnosed by amniocentesis were reported, as reviewed by Grose et al. (115). In subsequent years, the number of reports of congenital HCMV infection diagnosed prenatally increased progressively, with a major contribution provided by European investigators (58, 65, 118, 153, 159, 165, 178, 184, 206, 215, 294).
Major clinical indications for prenatal diagnosis are documented primary HCMV infection in the mother, diagnosed according to the criteria reported above, and ultrasonographic abnormalities, known to be found frequently in fetal HCMV infection (such as intrauterine growth retardation, hydrops or ascites, and central nervous system abnormalities).
Fetal Blood
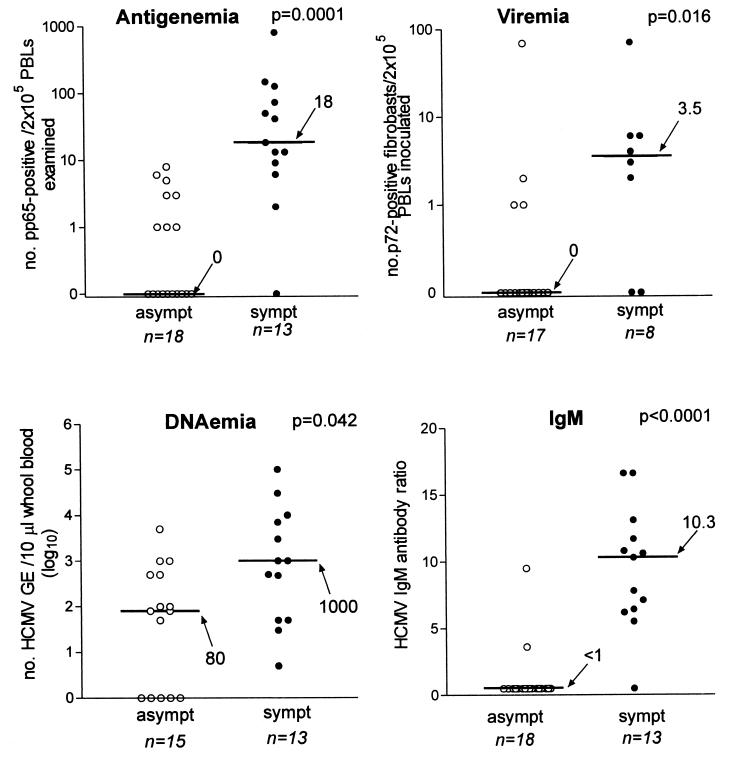
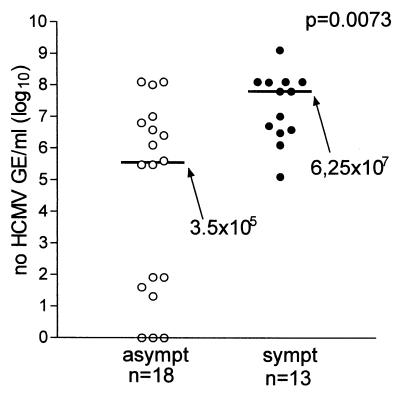
Fetal blood and amniotic fluid samples are often drawn in parallel during procedures for prenatal diagnosis. Fetal blood can be used for both determination of HCMV-specific IgM antibody and quantification of viral load (Fig. 9). However, the utility of IgM determination in fetal blood remains to be fully assessed (58, 149, 167). In addition, while several studies have established the diagnostic and prognostic value of the determination of viral load in the blood of immunocompromised patients, the clinical significance of the presence of virus and viral components in the blood of fetuses exposed to HCMV has never been fully investigated. Fetal blood may allow assessment of biochemical and hematological parameters, such as hemoglobin and platelet counts, and measurement of liver enzymes (γ-glutamyl transferase, alanine aminotransferase, and aspartate aminotransferase). These nonspecific tests, although per se not very useful as prognostic markers of fetal disease, could help as complementary assays (164). Among the HCMV-specific assays, IgM antibody, which can be determined after 20 weeks of gestation, may be more helpful, even though this assay is known to possess a limited diagnostic value due to its low (20% to 75%) sensitivity (58, 149, 167).
FIG. 9.
Median levels (horizontal lines with values beside arrows) of HCMV antigenemia, viremia, and DNAemia and IgM ratio in fetal blood of symptomatic (sympt) and asymptomatic (asympt) congenitally infected fetuses and newborns. All parameters evaluated were significantly higher in symptomatic than in asymptomatic subjects. Fetuses were considered symptomatic if they had ultrasonographic abnormalities, and newborns were considered symptomatic if they were born with clinical symptoms. PBLs, peripheral blood leukocytes; GE, genome equivalents. (M. G. Revello and G. Gerna, unpublished data.)
In two subsequent studies by the same group, the sensitivities of HCMV-specific IgM determination in fetal blood were 55.5% and 57.9%, while the specificity was 100% in both studies (215, 217). While looking for prognostic markers of symptomatic infection, the authors observed that both frequency and levels of virus-specific IgM antibody were significantly higher in congenitally infected fetuses with ultrasound or biochemical/hematological abnormalities than in fetuses with normal ultrasound and biochemical findings (217). This finding was shown not to be related to the time of maternal infection, to the interval between onset of maternal infection and time of prenatal diagnosis, or to gestational age at amniocentesis. The finding was interpreted as potentially due to the fact that IgM-negative fetuses were sampled early during the infection on the basis of the following observations: four of seven IgM-negative fetuses had virus-specific IgM at birth, and an additional infant developed IgM antibody 65 days after birth, and the great majority of IgM-negative fetuses had a low viral load in blood and normal biochemical and hematological values. It is reasonable to assume that IgM-negative fetuses at 20 to 23 weeks of gestation could become positive in the advanced stages of pregnancy and thus not differ from fetuses with high-level IgM antibody. However, IgM levels were consistently higher in fetuses with HCMV disease, implying that this parameter represents a true prognostic marker of congenital HCMV disease.
The same study addressed the issue of the diagnostic and prognostic value of viral load in fetal blood. While in the past, viremia was found to be negative in all eight HCMV-infected fetuses examined (131), in the above-mentioned study, evaluation of the prenatal diagnostic value of different tests for diagnosis of congenital infection on fetal blood showed that the sensitivity of antigenemia was 57.9%; of viremia, 55.5%; and of leukoDNAemia, 82.3%, while the specificity was 100% for all assays (217). Although only antigenemia reached levels of significance, higher levels of all virologic parameters determined were found in the groups of fetuses with ultrasound or abnormal laboratory findings compared to apparently normal congenitally infected fetuses. The following major conclusions were drawn from the study: no assay for detection of virus or virus components in fetal blood was sensitive enough to significantly improve prenatal diagnosis of intrauterine transmission of the virus; however, tests performed on fetal blood are confirmatory of results achieved on amniotic fluid (see below); fetuses with normal biochemical, hematological, and ultrasound findings, low or absent HCMV load in blood, and undetectable IgM antibody at 20 to 24 weeks of gestation may have a more favorable outcome; and taken together, virologic, laboratory, and ultrasound findings may contribute to a better prognostic definition of fetal infection (217).
In a recent prospective study of 237 pregnancies at risk, in which prenatal diagnosis of congenital HCMV infection was achieved or excluded by amniocentesis with or without cordocentesis, the sensitivity of the IgM assay was comparable (51%) to that in previous studies (215, 217), whereas PCR, which was positive in 17 of 41 cases (sensitivity 41%), and culture, which was positive in 2 of 27 cases (sensitivity 7%), were by far less sensitive (164). In this study, IgM antibody was unexpectedly detected in one fetus proven uninfected at birth, whereas all the other positive fetal blood samples were in total agreement with positive amniocentesis results.
In unpublished studies on 86 pregnant women undergoing prenatal diagnosis for 88 fetuses (Table 2), specificities and positive predictive values of all assays on fetal blood were 100%, whereas sensitivities and negative predictive values of both DNAemia and immediate-early mRNA were around 85% (M. G. Revello and G. Gerna, unpublished data). Thus, when fetal blood was used for prenatal diagnosis, all uninfected fetuses were correctly detected by all assays, whereas about 15% of them were missed by the most sensitive assays.
TABLE 2.
Diagnostic value of different assays for prenatal diagnosis of congenital infection in 88 fetuses of 86 mothers with primary HCMV infection in pregnancya
| Clinical specimen | HCMV assay | Test result | No. of fetuses
|
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|
| Infected | Uninfected | |||||||
| Fetal blood | Antigenemia | Neg | 13 | 35 | 63.9 | 100 | 100 | 72.9 |
| Pos | 23 | 0 | ||||||
| Viremia | Neg | 17 | 31 | 41.4 | 100 | 100 | 64.6 | |
| Pos | 12 | 0 | ||||||
| DNAemia | Neg | 5 | 27 | 84.8 | 100 | 100 | 84.4 | |
| Pos | 28 | 0 | ||||||
| Immediate-early mRNA | Neg | 2 | 11 | 84.6 | 100 | 100 | 84.6 | |
| Pos | 11 | 0 | ||||||
| pp67 mRNA | Neg | 4 | 11 | 63.6 | 100 | 100 | 73.3 | |
| Pos | 7 | 0 | ||||||
| IgM antibody | Neg | 15 | 36 | 57.1 | 100 | 100 | 70.6 | |
| Pos | 20 | 0 | ||||||
| Amniotic fluid | Virus isolation | Neg | 8 | 44 | 81.8 | 100 | 100 | 84.6 |
| Pos | 36 | 0 | ||||||
| DNA | Neg | 3 | 38 | 92.7 | 100 | 100 | 92.7 | |
| Pos | 38 | 0 | ||||||
| Immediate-early mRNA | Neg | 2 | 32 | 92.6 | 97 | 96 | 94.1 | |
| Pos | 25 | 1 | ||||||
| pp67 mRNA | Neg | 2 | 33 | 92.0 | 100 | 100 | 94.3 | |
| Pos | 23 | 0 | ||||||
neg, negative; pos, positive; PPV, positive predictive value; NPV, negative predictive value. Source: M. G. Revello and G. Gerna, unpublished data.
Finally, there are two anecdotal observations demonstrating circulating CEC in the peripheral blood of two fetuses presenting with high viral load and ultrasound abnormalities (E. Percivalle and M. G. Revello, unpublished data). Circulating CEC have been detected in immunocompromised patients with disseminated HCMV infection (Fig. 8D), in association with very high levels of antigenemia and viremia and the presence of end organ disease (96, 104, 196, 232). The finding of these cells in congenitally infected fetuses indicates a disseminated infection comparable to those reported in immunocompromised patients (207).
Amniotic Fluid
Due to its high sensitivity and absolute specificity (100%), HCMV isolation from amniotic fluid has been recognized as the reference method for prenatal diagnosis (115, 130, 131, 178). At the beginning of the 1990s, the striking increase in the number of reported cases of prenatal diagnosis of congenital HCMV infection by virus isolation after amniocentesis was partly due to improvement of the tissue culture technology (115). In fact, the availability of monoclonal antibodies to HCMV major immediate-early protein and shell vial cell cultures allowed diagnosis to be performed within 16 to 24 h after sample collection (93, 98). However, following the initial enthusiasm generated by findings showing that all cases of congenital infection could be diagnosed by virus isolation from amniotic fluid samples (130, 131, 165), several studies began documenting false-negative results of amniotic fluid cultures (57, 58, 178, 184).
With the advent of PCR, the question arose of whether the sensitivity of culture could be increased by using the PCR technique for HCMV DNA detection in amniotic fluid samples. In a retrospective study, the sensitivity of PCR for prenatal diagnosis was found to be only slightly superior (76.9%, or 10 of 13 cases detected) to virus culture (69.2%, or 9 of 13 cases detected) by either single-step or nested PCR (215). In other words, PCR could not avoid of three false-negative results out of 13 intrauterine infections diagnosed at birth.
Subsequently, a substantial increase in the sensitivity of the PCR assay for prenatal diagnosis was obtained with a modified protocol for nested PCR (206). In the new assay, multiple instead of single aliquots and 100 μl instead of 20 μl of amniotic fluid were amplified and tested. By this approach, low DNA levels (1 to 10 genome equivalents) could be detected in a variable number of replicates of six amniotic fluid samples from four fetuses that previously had false-negative results. The specificity of the new assay was 100%, as demonstrated by negative results on 29 amniotic fluid samples from 22 pregnant women with primary HCMV infection who did not transmit the infection. However, the new assay failed to result in a positive prenatal diagnosis in the first amniotic fluid sample from a retrospective case that required two subsequent procedures for final diagnosis and did not rule out an additional false-negative diagnosis 8 weeks after maternal infection when used prospectively. Therefore, although the use of a very sensitive technique such as PCR can increase the sensitivity of prenatal diagnosis of HCMV congenital infection, it is reasonable to assume that a delay in intrauterine transmission of the infection may represent a major obstacle to achieving 100% sensitivity (206).
These results were confirmed by other reports showing that even the combination of the most sensitive assays available, such as viral culture and PCR on amniotic fluid samples, may reach a sensitivity of about 70% to 80% (21, 58). In a recent prospective study, global sensitivity, specificity, and positive and negative predictive values of prenatal diagnosis for HCMV detection in amniotic fluid and fetal blood (taken together) were 80%, 99%, 98%, and 93%, respectively, while the percentages were nearly overlapping if prenatal diagnosis was based on PCR of amniotic fluid alone (164).
In a series of recent reports from a single group, the sensitivity of viral culture by the shell vial assay was found to be 50% to 62.5% and the specificity 100%, whereas the sensitivity of PCR was 100%, but surprisingly, the specificity was 67.3% to 83.3%, with a positive predictive value of 48% to 48.5% (118, 153, 169). This means that congenital HCMV infection was documented in only 12 of 27 fetuses or newborns found to be positive in amniotic fluid by PCR (153) or in 16 of 33 fetuses or newborns reported subsequently (118). Recently, two false-positive PCR results in amniotic fluid in a series of 96 uninfected newborns (specificity, 97.9%) have been reported (65). On the other hand, false-positive PCR results in amniotic fluid have never been reported by other groups (58, 164, 165, 215, 230). In fact, when virus was detected by PCR (or culture) in amniotic fluid samples, it was consistently recovered from fetal tissues or excreted by newborns in all reported cases (164, 207). Therefore, virus detection in amniotic fluid must be considered a marker of fetal and congenital infection (164). Also, in our experience, the detection of even small amounts of viral DNA (<100 genome equivalents/ml of amniotic fluid) has always correlated with congenital infection at birth (207).
In our recent, as yet unpublished study (Table 2), when prenatal diagnosis was based on amniotic fluid, the specificities and positive predictive value of all assays were again 100% or close to 100%, while the sensitivities and negative predictive value of the most sensitive assays for detection of viral DNA and mRNA were ≥92% (M. G. Revello and G. Gerna, unpublished data). This means that, while nearly all uninfected fetuses were identified by each assay, about 7 to 8% of infected fetuses were missed by molecular assays and thus scored as false negative.
Lazzarotto et al. (153) suggested that the high sensitivity of PCR could detect small amounts of virus which could be cleared by the defenses of the mother or fetus. In addition, the authors suggested that the term “rate of intrauterine transmission of CMV” should be applied to indicate the percentage of amniotic fluid-positive samples rather than the percentage of HCMV-infected newborns or fetuses. In this respect, extreme caution must be used in evaluating these thus far unconfirmed results, based on the following considerations: prenatal diagnosis is a very delicate task and, as a rule, irrevocable decisions must be taken on the basis of test results, and PCR assays and the related containment measures must be extensively validated before being used for diagnostic purposes.
The risk of HCMV transmission during antenatal diagnostic procedures performed in the presence of maternal DNAemia does not seem to be major, although it cannot be excluded (216). This conclusion seems to be supported by the observation that transmission rates were not different between women with a single prenatal sampling and women with multiple samplings and were not higher after initiation of prenatal diagnostic procedures compared to historical controls without prenatal intervention (164).
Apart from the most sensitive techniques used, the sensitivity of prenatal diagnosis may be increased by repeated sampling; increasing gestational age at time of amniocentesis; increasing the time between onset of maternal infection and time of amniocentesis; and repeating ultrasonographic examinations. With reference to the first point concerning multiple sampling, it was found that for all undiagnosed infected fetuses except one, only one prenatal sample was collected. On the other hand, of 24 infected fetuses with multiple samples taken at different times during pregnancy, prenatal diagnosis was positive in 23 (96%). Of 44 pregnancies with transmission of virus to the fetus, 12 infected fetuses were diagnosed upon the second sampling (164). The correlation of gestational age and virus transmission was documented by the finding that prenatal diagnosis showed a sensitivity of 30% (6 of 20) if the first amniotic fluid sample was taken before 21 weeks of gestation, whereas of 35 women tested for the first time after 21 weeks of pregnancy, 26 (74%) were diagnosed as transmitters, 25 (71%) with tests on amniotic fluid and 17 of 28 (61%) with tests on fetal blood (164).
The difference in sensitivity between amniocentesis before and after 21 weeks of gestation was found to be statistically significant. The same data were obtained by other groups (57, 165). In addition, a correlation was found between time elapsed after onset of maternal infection and time of positive amniocentesis (178). All infected fetuses were detected when a mean interval of 7 weeks between maternal symptoms and amniocentesis had elapsed (21, 165). Other authors recommended an interval of at least 4 weeks to avoid false-negative results (230). However, with these recommendations, a rate of 23% false-negative results would have been obtained, while with a time lapse of 7 weeks, positive antenatal diagnoses could be achieved in all cases (164). Repeated ultrasonographic examinations may help in only a small minority of fetuses with severe disseminated infection at autopsy (164). However, frequent ultrasonographic evaluations in pregnancies with evidence of vertical transmission may help to predict fetal damage (such as hydrocephaly, microcephaly, ventriculitis, or brain calcifications), thus identifying fetuses at significant risk of clinical sequelae (58, 165, 286).
Fetal Prognostic Markers of HCMV Disease
As suggested by previous studies (2, 264), it has recently been documented that fetal HCMV disease is preferentially associated with maternal infection occurring in the first part of pregnancy (164). By combining data on aborted fetuses with severe ultrasonographic abnormalities and infected newborns with poor outcome, it was observed that fetal HCMV disease was more severe if maternal infection occurred prior to 20 weeks of gestation. In fact, in this case the rate of fetal severe HCMV disease was 26% (10 of 38 fetuses), whereas only 1 fetus of 16 infected after 20 weeks of gestation had a minor sequela of retinitis (164).
A potential role of fetal viral load as a prognostic factor has been advocated (58, 149). Lamy et al. (149) observed that viral load was very high in fetuses with brain ultrasonographic anomalies. Donner et al. (58), although not specifically investigating the issue, found that false-negative results in prenatal diagnosis were consistently associated with low viral load and asymptomatic infections both at birth and during follow-up. A correlation of high levels of viral load in the blood and the appearance of HCMV disease has been repeatedly reported in immunocompromised patients, representing the basis for the development of strategies of preemptive therapy in transplant recipients (90, 91, 95, 116). In addition, recent studies have clarified the dynamics of HCMV replication in vivo (63) and have claimed the predictability of HCMV disease based on a single viral DNA quantification in a blood sample drawn early during HCMV infection (64).
However, the clinical significance of HCMV load in fetal blood and amniotic fluid of congenitally infected fetuses has not been fully investigated until very recently. In fetal blood, all virologic parameters tested to determine viral load, i.e., viremia, antigenemia, and leukoDNAemia, were found to be higher in fetuses with abnormalities than in fetuses with normal findings, although only levels of antigenemia were significantly different (217). In addition, as reported above, the level of virus-specific IgM antibody was significantly lower in fetuses with normal findings. These data appeared to justify the conclusion that congenitally infected fetuses with normal biochemical, hematologic, and ultrasound findings and low viral load in blood (together with low or undetectable IgM antibody) might have a more favorable outcome (217).
On the other hand, in the amniotic fluid of mothers of 21 congenitally infected fetuses, quantification of HCMV DNA showed that median levels of HCMV DNA were 1.25 × 108 genome equivalents/ml in the group of fetuses with abnormal ultrasound findings at the time of prenatal diagnosis or with symptomatic infection at birth (n = 7) and 3.75 × 106 genome equivalents/ml in the group of fetuses with normal ultrasound findings at the time of amniocentesis and subclinical infection at birth (n = 14). This difference was not significant (P = 0.09), although this could be due to the small number of fetuses tested (219). In particular, very high levels of viral DNA (108 genome equivalents/ml) could be observed in both asymptomatic and symptomatic fetuses. In addition, some fetuses with asymptomatic infection showed levels of viral DNA in amniotic fluid of <100 genome equivalents/ml. This low level of viral DNA was found not only in fetuses tested in amniotic fluid shortly after maternal infection, but also in fetuses tested several weeks after maternal infection (219). However, all fetuses infected with low viral DNA levels were asymptomatic. In this respect, it is important to stress that all fetuses with viral DNA in amniotic fluid, including those with <100 genome equivalents/ml, were born with congenital infection.
One reason why no correlation was found between HCMV load in amniotic fluid and clinical symptoms may be that viral DNA is accumulating in the amniotic fluid (C. Liesnard, F. Brancart, M. L. Delforge, F. Gosselin, F. Rodesch, and C. Donner, Abstr. 8th International Cytomegalovirus Conference, abstr. p. 15, 2001) instead of being cleared, as also indirectly shown by the lack of degradation of viral DNA in an amniotic fluid sample stored at 37°C for at least 6 months (M. G. Revello, unpublished data). The most recent survey of our series indicates that in the fetal blood of symptomatic fetuses or newborns, all virologic parameters (antigenemia, viremia, and DNAemia) as well as IgM antibody levels were significantly higher than those of asymptomatic fetuses or newborns (Fig. 9), confirming previous results.
The difference in DNA level between fetuses born with symptomatic congenital infection and fetuses born with asymptomatic infection was also found to be significant in the amniotic fluid (Fig. 10) (M. G. Revello and G. Gerna, unpublished data). However, while levels of viral DNA were >105 genome equivalents/ml in all symptomatic fetuses and newborns, only 11 of 18 (61.1%) asymptomatic fetuses and newborns showed DNA levels greater than 105/ml of amniotic fluid, the remaining 7 showing DNA levels of ≤102 genome equivalents/ml. Thus, while the difference in DNA level was not significant between asymptomatic and symptomatic fetuses and newborns when only DNA levels of >105 genome equivalents/ml were considered, such a difference became highly significant (P = 0.0073) when all cases in the asymptomatic group were considered.
FIG. 10.
Comparative median levels (horizontal lines with values beside arrows) of DNA in amniotic fluid of mothers with symptomatic (sympt) and asymptomatic (asympt) fetuses. While in both groups all (symptomatic) or most of the (asymptomatic) fetuses showed high levels of HCMV DNA (>105 genome equivalents [GE]/ml), in the asymptomatic group there were seven fetuses, four of whom had DNA levels of <102 genome equivalents/ml and three with undetectable DNA levels at the time of amniocentesis. (M. G. Revello and G. Gerna, unpublished data.)
These results were in agreement with those reported by Liesnard et al. (164), showing 100% specificity of PCR and culture in detecting HCMV in amniotic fluid, but were in disagreement with those reported by Lazzarotto et al. (153), who suggested that small amounts of viral DNA were eliminated by the fetus without transmission of the infection. While these data were reported on a qualitative basis, the same group subsequently, using quantitative PCR, reported that levels of viral DNA of 103 to 105/ml of amniotic fluid were necessary to transmit the infection, whereas levels >105 were required to cause HCMV disease in the fetus. Thus, levels of <103 genome equivalents/ml were unable to transmit the infection and were cleared by the fetus during fetal life (118, 159, 169).
Following qualitative PCR on amniotic fluid at 21 to 22 weeks of gestation, specificity and positive predictive value with respect to the presence or absence of HCMV infection in the fetus or newborn were only 67.3% and 48.5%, respectively. This means that as many as 17 of 33 (51.5%) fetuses or newborns had viral DNA in the amniotic fluid and were not infected. However, the authors claim that when viral DNA was quantified by PCR, levels of >103 genome equivalents/ml indicated a high probability of infection (12 of 12 were infected, with positive predictive value of 100%), while levels of <103/ml indicated a low probability of infection (only 4 of 21 were infected, with a negative predictive value of 81%). In addition, levels of viral DNA of >105 genome equivalents/ml were selected as a cutoff to indicate a high probability of disease (9 of 9 were ill, with a positive predictive value of 100%), whereas levels of <105 genome equivalents/ml indicated a low probability of disease (2 of 24 were ill, with a negative predictive value of 91.6%).
It has recently been shown that newborns with symptomatic congenital HCMV infection have significantly higher levels of HCMV in the blood at birth and that clearance of virus takes longer than in subclinically infected infants (218). Moreover, it has been reported that infants with symptomatic congenital HCMV infection excrete larger amounts of virus in the first few months of life than those with asymptomatic infection (258). These data seem to indicate that quantification of viral load may correlate with clinical conditions, at least after birth. During fetal life, apart from the peculiar physiopathological condition of the fetus in its relationship with the mother, it is possible that viral load might correlate with clinical symptoms or pathological findings. In this respect, viral load in fetal blood as determined by antigenemia is significantly higher in fetuses with HCMV disease (217). Also, median values of viral DNA in amniotic fluid are markedly higher in fetuses with pathological findings (219). What is surprising in the above-mentioned studies (118, 159) and, more importantly, not confirmed by data from other laboratories is that viral DNA is often detected in amniotic fluid without being transmitted to the fetus.
We conclude that, at this time, multiple difficult to determine variables, such as gestational age at maternal infection, timing of intrauterine transmission of the infection, timing of prenatal diagnosis, and, most important, the unfeasibility of a follow-up of the infection during fetal life represent the major obstacles to identification of a reliable prenatal marker of symptomatic congenital HCMV infection. However, high viral loads in amniotic fluid may be associated with either symptomatic or asymptomatic congenital infections, while low viral loads are consistently associated with asymptomatic congenital infections.
DIAGNOSIS OF CONGENITAL INFECTION IN THE NEWBORN
At birth, or during the first 2 weeks of life, postnatal diagnosis of congenital HCMV infection is required either to confirm the results of prenatal diagnosis or to investigate transmission of the virus to neonates born to women who experienced a suspected or ascertained primary HCMV infection during pregnancy (258). The gold standard method for diagnosis of congenital HCMV infection is represented by virus isolation in human fibroblasts in the first 2 weeks of life, because subsequent virus excretion may represent neonatal infection acquired in the birth canal or following exposure to breast milk or blood products (258).
Urine and saliva are the clinical samples of choice for culture. Urine samples may be stored at 4°C for 7 days, with the isolation rate dropping to only 93%, whereas storage at room temperature or freezing decreases infectivity dramatically (263). In the 1980s, methods for rapid virus isolation were developed, based on the use of monoclonal antibodies to the HCMV major immediate-early protein p72 associated with low-speed centrifugation of clinical samples onto monolayers of human fibroblasts grown on coverslips inserted on the bottom of shell vials (6, 93, 98, 242, 270). The shell vial method was subsequently adapted to 96-well microtiter plates, where it showed a sensitivity of 94.5% and a specificity of 100% compared to standard virus isolation in a series of 1,676 newborn urine specimens (31). The assay retained the same level of sensitivity and specificity when saliva was tested instead of urine (284).
PCR was first used for HCMV DNA detection in the urine of congenitally infected babies at the end of the 1980s (51). Urine samples were repeatedly frozen and thawed. When compared with the standard tissue culture isolation procedure, the PCR assay followed by dot blot hybridization showed a sensitivity and specificity of 100%. Obvious advantages of PCR over culture were the small amount of sample required; the short time required for test results (24 to 48 h versus 2 to 28 days); the ability to use frozen specimens with noninfectious virus; and no need for extensive DNA purification measures.
These results prompted clinical virologists to test for the presence of viral DNA in the blood of congenitally infected newborns. First, Brytting et al. (32) reported detection of HCMV DNA in the serum of five of five congenitally infected infants tested within 2 weeks after birth, while two of these five newborns were negative for HCMV-specific IgM. In 1995, Nelson et al. (182) reported detection of HCMV DNA in the serum of 18 of 18 (100%) infants with symptomatic congenital HCMV infection, 1 of 2 infants with asymptomatic congenital HCMV infection, and 0 of 32 controls. In 1999, Revello et al. (218) investigated the diagnostic and prognostic value of HCMV load as determined by different assays in the blood of 41 newborns with congenital infection and 34 uninfected newborns with respect to conventional virus isolation from urine. Sensitivities of HCMV DNAemia (by PCR), antigenemia, viremia, and IgM determination were 100%, 42.5%, 28.2%, and 70.7%, respectively, while specificity was 100% for all assays. That study concluded the following: (i) determination of viral DNA in blood by PCR at birth appears to be as sensitive and specific as virus recovery from urine for diagnosis of congenital HCMV infection; (ii) significantly higher levels of HCMV load are detected in infants with congenital symptomatic HCMV infection; and (iii) virus clearance from blood occurs spontaneously in both symptomatic and subclinically infected newborns, even though the process takes longer in symptomatic newborns (218).
A further simplification of the procedure for detection of viral DNA in the blood of congenitally infected infants was proposed in 1994 by Shibata et al. (241) with dried blood spots stored on filter paper, as originally suggested for human immunodeficiency virus type 1 by Cassol et al. (35). Although Shibata et al. (241) reported an extraordinarily high rate of viral DNA positivity in the blood of healthy Japanese newborns (25.1%), suggesting a possible carryover contamination in the laboratory, the Japanese approach was verified by others (10, 136). Thus, with dried blood spots collected from babies in the first days of life during routine screening procedures for genetic and metabolic disorders, Barbi et al. (10) reported that eight of eight symptomatic and 11 of 11 asymptomatic congenitally infected babies were positive for HCMV DNA when extraction was done with medium instead of water. Therefore, the method showed 100% sensitivity and specificity with respect to virus recovery by culture.
More recently, determination of HCMV immediate-early mRNA in the blood of congenitally infected newborns by NASBA has been used to diagnose congenital HCMV infection (208). The immediate-early mRNA NASBA assay had 100% sensitivity in detecting 12 congenitally infected newborns examined during the first week of life and previously found to be positive for both HCMV DNAemia and virus recovery from urine. However, immediate-early mRNA was detected for a significantly shorter period of time (median 37 days) than DNAemia (median, 87.5 days; P = 0.04). This trend was attributed to the stricter association of immediate-early mRNA with the early stages of HCMV infection in vivo. Indeed, recently, by using an in vitro model, it has been shown that viral DNA detected in polymorphonuclear leukocytes is transferred from HCMV-infected cells, whereas an aliquot of immediate-early mRNA is synthesized in these cells, which indicates an active, albeit abortive, replication of HCMV (81). Thus, it seems reasonable to speculate that immediate-early mRNA detected in blood might represent a more reliable marker of active HCMV infection. Finally, it is important to stress that the new assay showed 100% specificity, since no immediate-early mRNA was ever found in healthy newborns.
As already mentioned, IgM antibody determination has somewhat limited sensitivity in diagnosis of congenital HCMV infection. The solid-phase radioimmunoassay described by Griffiths and Kangro had a sensitivity of 89% and specificity of 100% (112). With IgM ELISA, the specificity was nearly 95% and the sensitivity approximately 70% when congenitally infected infants were tested (262). Similarly, with a capture ELISA method with enzyme-labeled monoclonal antibody, the level of sensitivity for congenital HCMV infection was found to be 70.7% (218).
TREATMENT OF CONGENITAL INFECTION
Although specific antiviral drugs, such as ganciclovir and foscarnet, have been available for several years for treatment of life-threatening or sight-threatening HCMV disease in immunocompromised patients, their use for treatment of congenital HCMV infection remains undefined due to a paucity of data. In principle, two levels of treatment could be considered, prenatal (during fetal life) and postnatal (based on severity of clinical symptoms). Foscarnet is a competitor of pyrophosphate, while ganciclovir acts as a competitor of guanosine during viral DNA synthesis (41, 70). However, the degree of toxicity of the two drugs must be carefully considered, with special regard to the renal toxicity of foscarnet and the hematologic toxicity of ganciclovir.
Anecdotal studies do not support the efficacy of antiviral drug therapy in fetal HCMV infection. The first was reported in 1993 (212). Ganciclovir was administered in utero for 12 days to a 29-week-old fetus with congenital HCMV infection, thrombocytopenia, and elevated γ-glutamyl transferase levels. Following therapy, the virus titer in amniotic fluid and fetal urine dropped, viral DNA disappeared from the blood, and the platelet count and γ-glutamyl transferase level became normal. However, stillbirth occurred at 32 weeks of gestation, and HCMV inclusion bodies were detected in several organs at autopsy.
Two subsequent reports concerned the administration of HCMV hyperimmune globulin to HCMV-infected fetuses with the intent of mitigating the damaging effects of HCMV infection (181, 186). An additional attempt at fetal therapy in a congenitally infected fetus presenting with high γ-glutamyl transferase values was reported (207). Three subsequent doses of ganciclovir (100 mg, 50 mg, and 200 mg) were administered intra-amniotically 1 week apart starting at 25 weeks of gestation. Viremia dropped after the first drug administration, becoming undetectable at 29 weeks of gestation. However, levels of antigenemia, DNAemia, and infectious virus in amniotic fluid did not change during follow-up. IgM antibody, which was quite high at 23 weeks, decreased progressively and became negative at 29 weeks. At birth, the baby showed petechiae, microcephaly, hepatomegaly, thrombocytopenia, increased alanine transaminase levels, and hearing impairment.
A few anecdotal reports on the use of ganciclovir in congenitally infected infants have also been discouraging (69, 129, 275). In these reports, indications for treatment were acute symptoms of HCMV organ localization (pneumonia, hepatitis) or generalized congenital disease. However, in at least one case, ganciclovir was administered with the specific aim of preventing further involvement of the central nervous system (205). Drug dose, duration of treatment, and age at initiation of treatment varied in single reports. However, in all studies a reduction in or temporary cessation of virus excretion was observed during therapy. In a case of ganciclovir therapy of congenital HCMV hepatitis, viremia was the first parameter to become negative, followed by antigenemia during the first 2 weeks of treatment (271). Clearance of virus from urine required an additional week of treatment. Clinical efficacy was excellent, with improvement of all biochemical parameters by the end of therapy in the absence of side effects. However, 9 days after cessation of therapy, a resumption of virus replication occurred in both blood and urine.
On the other hand, more controlled multicenter clinical trials have begun evaluating the use of antiviral drugs for treatment of infants with symptomatic congenital HCMV infection. A phase II study carried out on a group of 47 infants with congenital infection to investigate the efficacy of ganciclovir treatment following intravenous administration at 12-h intervals for 6 weeks showed that ganciclovir administration had to be stopped in eight infants because of toxicity (mostly neutropenia); the most common side effects were neutropenia and elevation of liver enzymes; excretion of virus with urine decreased during treatment, returning to pretreatment levels after drug discontinuation; and the most significant clinical result was hearing improvement, observed in 5 of 30 infants (16%) after 6 months of follow-up or later (289).
The main goal of antiviral chemotherapy would be to treat pregnant women with primary HCMV infection in order to (hopefully) prevent transmission of the virus to the fetus. In this respect, the combination of hyperimmune globulin with antiviral drugs of low or negligible toxicity could represent the best approach to preventing vertical HCMV transmission in the future (169). Prior to achieving this major goal, it will be highly problematic to identify a truly efficacious treatment because cases of congenital infection are currently diagnosed and, thus, identified weeks or months after virus transmission to the fetus; there are a wide spectrum of congenital HCMV diseases; the natural course of the disease is erratic; and irreversible damage has already occurred before any therapeutic intervention in the fetus or newborn infant can be attempted (258).
PREVENTION OF CONGENITAL INFECTION
Congenital HCMV disease is still a major public health problem, which does not appear to be resolved by means other than active immune prophylaxis, i.e., vaccination (197). Due to the fact that congenital infection is the leading infectious cause of mental retardation in children, HCMV is considered a prime candidate for eradication from the human population through vaccination (109). As reported above, while some data point to the role of recurrent infections in causing defects in infants born to mothers with prepregnancy immunity (28, 75, 266), the overwhelming majority of studies indicate that congenital HCMV disease is the result of primary maternal infection during pregnancy. Thus, the ultimate goal of the HCMV prevention program is to develop a vaccine which can be administered to seronegative women of childbearing age to prevent the occurrence of primary HCMV infection during pregnancy.
Over the last 30 years, attempts to develop an HCMV vaccine have been directed at five major strategic approaches: (i) live attenuated vaccines; (ii) recombinant virus vaccines; (iii) subunit vaccines; (iv) peptide vaccines; and (v) DNA vaccines.
The first attempts were aimed at preparing a vaccine containing live attenuated virus. However, several problems had to be faced from the beginning: the vaccine virus strain may persist in the body as a latent virus and periodically reactivate; reactivations may not be preventable; safe markers of attenuation of vaccine strains had to be identified (80); animal models for HCMV are not available; and HCMV may be oncogenic in vivo, as suggested by its ability to transform both human and embryonic hamster cells in vitro (255).
Live Attenuated Vaccines
The first vaccine was developed by Elek and Stern (61), using a strain of AD169 that was presumed to have been attenuated by propagating the virus 56 times in human fibroblasts. This vaccine, administered subcutaneously, elicited a good, although transitory, neutralizing antibody response in the absence of virus shedding.
Almost simultaneously, a live attenuated virus vaccine was developed at Wistar Institute in Philadelphia, Pa., with the Towne strain, which was recovered from the urine of a newborn with congenital infection and propagated 125 times in human embryonic fibroblasts (203). Shortly thereafter, the vaccine was shown to induce a significant antibody response (199). In three subsequent vaccine trials carried out in renal transplant recipients, it was shown that vaccinated seronegative recipients of kidneys from seropositive donors had a significant reduction in disease severity but not infection with respect to the control group receiving placebo; the Towne vaccine induced not only an antibody response but also a cell-mediated immune response, as determined by a lymphoprolipheration assay; and the vaccine strain was not excreted and was not found to undergo latency (9, 201, 202).
Recombinant Virus Vaccines
The recent finding that laboratory-adapted HCMV strains lack a large DNA fragment found in primary HCMV isolates and a low-passage reference HCMV strain (Toledo) has shed some light on the pathogenesis of HCMV infection (37). In particular, in a DNA fragment referred to as ULb′ (37), as many as 19 open reading frames have been identified, some of which are particularly interesting, such as UL146, coding for an α (CXC) chemokine functionally involved in active recruitment of polymorphonuclear leukocytes (194). It is generally believed that extensive propagation of Towne in human fibroblast cell cultures has been the major factor causing such a significant modification of the viral genome and, potentially, of virus pathogenicity and immune response to vaccine administration. Therefore, new alternative strategies were developed, aimed at inserting the entire genome of Toledo, subdivided into four fragments, in the genetic background of Towne, generating four chimeras, each representing a potential vaccine strain. The efficacy of these four recombinant virus strains in inducing antibody and cell-mediated immune responses in the absence of clinical symptoms is still under evaluation (139).
Subunit Vaccines
Given the teratogenic role of HCMV, over the last decade several investigators have addressed their efforts to the development of a subunit vaccine. In this respect, major antigenic sites of the immune response to HCMV which are potential components of a subunit vaccine are viral glycoproteins gB (UL55) and gH (UL75), the principal targets of the neutralizing antibody response, and viral phosphoproteins pp65 (UL83) and pp150 (UL32), which are the dominant targets of the cytotoxic T-lymphocyte (CTL) immune response (258). The last is also directed to the nonstructural protein p72 (UL122).
One of the most promising subunit vaccines has been based on the use of a recombinant gB molecule which was mutagenized to eliminate a cleavage site (254) and deprived of the transmembrane region prior to being combined with a new adjuvant, MF59, based on an oil-in-water emulsion of squalene (191). Following administration of three doses of this vaccine to seronegative subjects at 0, 1, and 6 months, levels of neutralizing antibody and antibody to gB 2 weeks after the third dose exceeded those in seropositive control subjects, while a fourth dose induced a prompt rise in antibody level. HCMV gB vaccine was also shown to produce significant levels of antibody at mucosal surfaces (282). However, the induction of good levels of neutralizing antibody to gB may not be sufficient to prevent fetal infection and disease. Thus, induction of an immune response to other viral proteins which are targets of neutralizing antibody or CTL may well be required (163, 276, 291).
It has been shown that pp65 is largely dominant in generating in vitro HCMV-specific CTL, followed to a much lesser extent by gB, gH, and p72 (103). In a prevalence study carried out in seropositive healthy individuals, CTL to IE1-exon 4 were nearly as prevalent as to pp65 (76% versus 92%), while gB- and pp150-specific CTL were detected in about one-third of subjects (119). Based on multiple reports showing that gB is the major target of the humoral and pp65 is the major target of the cell-mediated immune response, noninfectious defective enveloped particles of HCMV referred to as dense bodies have recently been proposed as an ideal natural vaccine immunogen, consisting mostly of pp65 and gB and lacking viral DNA (195). Dense bodies have been shown to induce neutralizing antibody and T-helper 1- and CTL-mediated immune responses in mice, thus representing a potential basis for the future development of a recombinant nonreplicating vaccine against HCMV (195).
The most immunogenic viral genes have been inserted in different vector systems to generate recombinant subunit vaccines. This line of research, already active in the past (43, 170), has more recently taken advantage of a nonreplicating canarypox expression vector in which the HCMV gene coding for gB or pp65 has been inserted. Multiple reasons justify the use of canarypox vaccine vectors: avipoxviruses accept large amounts of foreign DNA, thus directing synthesis of foreign proteins, and canarypoxviruses do not produce progeny in mammalian cells and are immunogenic in nonavian species without producing disease (11, 200, 272).
In a group of 20 seronegative adults randomly receiving either a canarypoxvirus (ALVAC) expressing HCMV gB or an ALVAC expressing the rabies virus glycoprotein (controls), with all subjects receiving a dose of Towne vaccine after 90 days, the ALVAC-CMV (gB) was found to prime the humoral immune response to HCMV gB, which was much earlier and higher and persisted longer than in controls (1). In a subsequent study by Berencsy et al. (13) with a canarypox-HCMV pp65 recombinant in a phase I clinical trial on seronegative volunteers, it was found that pp65-specific CTL were elicited after only two vaccinations and were CD8+, while pp65-specific lymphoproliferative response was detected in vitro, revealing stimulation of CD4+ T cells. In addition, a consistent antibody response to pp65 was elicited. Although these results do not provide evidence of the protective effect of the canarypox-HCMV pp65 recombinant vaccine against HCMV disease, immunization with this vaccine seems to confer immunity similar to that provided by natural infection. The protective effect of CTL could be reinforced by the involvement of CD4+ T cells and by the as yet unexplained role of pp65-specific antibodies (13).
Peptide Vaccines
An important advance in viral immunology has been the finding that peptide fragments of immunogenic viral proteins, referred to as minimal cytotoxic epitopes, when properly selected, bind to MHC molecules with high affinity (68, 204). Synthetic versions of these peptides, which are commonly 8 to 11 amino acids long, bind to MHC molecules, sensitizing targets to lysis by CD8+ CTL without requiring any further proteolytic processing to act as CTL epitopes. Peptide vaccines have a disadvantage in that they are of limited efficacy due to their limited HLA specificity. However, by using a computer algorithm, the sequence of HCMV pp65 was scanned for HLA-A∗0201-binding motif peptides, selecting a nonamer peptide (amino acids 495 to 503) capable of sensitizing target cells for lysis in the absence of activity on HLA-mismatched cells (47). However, it was observed that minimal cytotoxic epitopes had to be suspended in a strong adjuvant to be able to elicit an efficient CTL response (233). Lipidated peptides have been shown to confer a good immune response to different pathogens in a safe and effective way (52).
It is known that control of viral infections requires T-helper (TH) besides CD8 cell activity. Thus, efforts have been directed at increasing TH cells by using strong TH epitopes derived from tetanus toxin or synthetic peptides (54). With transgenic mice expressing both HLA class I (A∗0201) and class II DR1 molecules and inoculated with a peptide mixture containing an HCMV-derived class I HLA (A∗0201)-restricted CTL peptide epitope (pp65495-503) and tetanus toxin-derived MHC-binding TH epitope, a significant enhancement in CTL response was observed compared to that in transgenic mice expressing only class I molecules (12).subspecies included in this table.
DNA Vaccines
DNA vaccines are based on the in vivo expression of heterologous genes carried by plasmid vectors. Results of DNA vaccination are determined by both the efficiency of delivery and the level of expression of the heterologous gene. The efficiency of delivery has been improved by using liposomes as an adjuvant (133). The efficiency of expression has been related to the promoter used. In a recent comparative study, the promoter of the HCMV immediate-early gene was shown to be more active than other viral promoters in determining gene expression (161). In the mouse model, inoculation of a plasmid vector expressing pp89 of mouse CMV (homologous to the immediate-early gene of HCMV) conferred protection against subsequent experimental infection with sublethal doses of the virus, as shown by the decrease in viral load in different organs and by the induction of pp89-specific CTL (99). Subsequently, a similar degree of protection and reduction in viral titer were observed in mice inoculated with a plasmid expressing the M84 gene (the homolog of HCMV pp65) (175). Naked-DNA immunization of mice with plasmids expressing gB or pp65 of HCMV has been shown to induce high antibody titers and dose-dependent CTL immune responses, respectively, indicating that both humoral and cellular immune responses to HCMV can be elicited in mice following DNA vaccination (66).
PERCEPTION OF THE PROBLEM
In the last 30 years, more than 800 papers have been published dealing with the epidemiology, diagnosis, and outcome of vertically transmitted HCMV infections. In the introduction of most if not all of these papers, the dreadful scenario of estimated figures of dead and handicapped children (and relevant social and health care costs) due to congenital HCMV infections (Table 3) is duly recounted. Apparently, researchers from different countries on different continents have fully recognized the existence of the problem and its consequences, judging from the huge number of studies funded and performed. Nevertheless, very little has been done in practical terms to face this health problem (see below). A worrisome consideration emerges spontaneously: HCMV appears to be a rewarding topic from a scientific standpoint (let alone a lucrative business for companies), but remains a lingering danger to every pregnant woman.
TABLE 3.
Public health impact of congenital CMV infection in the United Statesa
| Parameter | Estimated value |
|---|---|
| No. of live births per year | 4,000,000 |
| Rate of congenital CMV infection (average) | 1% |
| No. of infected infants | 40,000 |
| No. of infants symptomatic at birth (5-7%) | 2,800 |
| No. with fatal disease (±12%) | 336 |
| No. with sequelae (90% of survivors) | 2,160 |
| No. of infants asymptomatic at birth (93-95%) | 37,200 |
| No. with late sequelae (15%) | 5,580 |
| Total no. with sequelae or fatal outcome | 8,076 |
From Stagno (258), used with permission.
Almost 30 years have elapsed since Elek and Stern reported their study with the compelling title “Development of a Vaccine against Mental Retardation Caused by Cytomegalovirus Infection In Utero” (61), but no licensed vaccine is available yet. Indeed, the absence of an effective means of prevention has been seen by many investigators as an insurmountable obstacle to sensible screening practices. Due to the suggestion reported in recent papers that recurrent maternal HCMV infection can be as dangerous as primary infections (5, 28), preconceptional vaccination is no longer considered a solution by some investigators (5). Thus, it seems that the best and only practical approach suggested by many scientists and health authorities is to ignore the existence of HCMV. However, ignoring the problem does not make it disappear, and it is surprising that in an age in which litigiousness is rising, parents of congenitally HCMV-infected children with severe handicaps have not yet taken legal action against health professionals for not offering the possibility of screening and thus potentially preventing the birth of an affected child.
Finally, to the best of our knowledge, no study has ever investigated the awareness of women of childbearing age about possible risks carried by HCMV infection acquired during pregnancy. It is possible that in this era in which access to information is greatly facilitated by the Internet, knowledge among lay persons might have increased over that just a few years ago. Public awareness can be a potentially strong lever for raising awareness of the problem of congenital HCMV infection. In 1989, during a BBC television program in Britain, parental pressure groups called for a program to identify women susceptible to HCMV in order to prevent congenital HCMV infection and to be counseled about how to avoid the infection. However, an editorial published in The Lancet less than a week after the call concluded that screening for HCMV was inappropriate and avoidance of infection impractical (Editorial, Lancet ii:599-600, 1989). A further plea for screening (D. O. Ho-Yen, letter, Lancet ii:803, 1989) was dismissed shortly afterwards (P. M. Preece, letter, Lancet ii:1101, 1989), and to date, no screening program is available in Britain (P. D. Griffiths, personal communication). Although the success of public initiatives may have been limited in the past, it is doubtful that today a similar call would remain unmet.
UNIVERSAL SEROLOGY SCREENING
Universal screening for HCMV by serology has been and still is a debated issue. In a way, it reflects the same dichotomy in the scientific community's attitude presented above: why study how to diagnose a condition that cannot be treated? An impressive number of papers have been published regarding the diagnosis of HCMV infections with special emphasis on immunocompetent individuals and particularly pregnant women. Different algorithms for HCMV monitoring in pregnant women have been developed and proposed, but none has ever been officially established in any health care program.
To our knowledge, routine serologic screening of pregnant women has never been recommended by any public health authority in any country. The only exception was represented by Italy, where, in 1995 to 1998, determination of HCMV antibody was included in the panel of examinations (ToRCH screening) which could be performed free of charge for pregnant and nonpregnant women. An informal survey performed by us for this review confirmed that, presently, HCMV screening is not officially recommended in any of the following European countries: Austria (T. Popow, personal communication), France (L. Grangeot-Keros, personal communication), Switzerland (W. Wunderli, personal communication), Germany (G. Enders, personal communication), Belgium (C. Liesnard, personal communication), United Kingdom (P. D. Griffiths, personal communication), the Netherlands (A. M. van Loon, personal communication), Spain (F. de Ory, personal communication), or Italy (authors' observation), as well as Israel (S. Lipitz and E. Mendelson, personal communication), Canada (M. Chernesky, personal communication), and Japan (K. Numazaki, personal communication). However, most obstetricians do test pregnant women in Italy (authors' observation), Israel (S. Lipitz and E. Mendelson, personal communication), Belgium (C. Liesnard, personal communication) and France (L. Grangeot-Keros, personal communication), whereas determination of HCMV-specific antibodies is performed only on specific request in Austria (T. Popow, personal communication), Switzerland (W. Wunderli, personal communication), Germany (G. Enders, personal communication), and Japan (K. Numazaki, (personal communication) or in case of symptoms in the mother in the United Kingdom (P. D. Griffiths, personal communication) and the Netherlands (A. M. van Loon, personal communication) or in the presence of possible occupational hazards (pregnant nurses working in obstetrics and pediatrics) in Austria (T. Popow, personal communication) and Japan (K. Numazaki, personal communication).
Similarly, the United States is not committed. In fact, the National Center for Infectious Diseases Internet site on HCMV (http://www.cdc.gov/ncidod/diseases/cmv.htm), among the recommendations for pregnant women with regard to CMV infection, merely indicates that “Laboratory testing for antibody to CMV can be performed to determine if a woman has already had CMV infection” and that “Pregnant women working with infants and children should be informed of the risk of acquiring CMV infection and the possible effects on the unborn child.” It recommends that specific testing should be restricted to “women who develop a mononucleosis-like illness during pregnancy.” The Internet site developed by the National Congenital CMV Disease Registry (http://www.bcm.tmc.edu/pedi/infect/cmv/cmvbroch.htm) is only apparently more committed: “Every woman of childbearing age should consider knowing her CMV status. Prior to pregnancy, consult your doctor to have a blood sample drawn and a CMV antibody test performed.”
It is clear that the scientific community will always be divided into those who support (65, 207, 292) and those who do not support (5, 120, 193, 238) universal screening. However, we believe the time has come for considering this crucial issue under a different perspective. Should women of childbearing age be informed about HCMV so that they can make an informed choice about (voluntary) screening? Or, as advocated by many researchers, should women continue to be denied testing (and, consequently, information), until more data are available about the efficacy of hygienic practice and the outcome of recurrent maternal infection or an appropriate vaccine or specific therapy is licensed? In this era in which much emphasis is being put on the issue of appropriate patient information, there is growing evidence that withholding information on possible medical interventions is unethical (and legally risky).
Indeed, if we consider that reliable and (relatively) inexpensive assays are available for determination of HCMV immune status; precautionary measures can be suggested to seronegative women; prenatal diagnosis procedures can be performed; and voluntary termination is an option in many countries, we believe that it is no longer acceptable or ethically justified to discourage HCMV screening.
However, it must be recognized that we are confronted with two major challenges: educating doctors and offering women the opportunity to know their HCMV immune status prior to pregnancy. The first challenge is the most urgent and basic, as health professionals represent a key factor in screening. In fact, the choice of whether to have a screening test performed is greatly influenced by the personal attitude of the health professionals offering the screening (245). In addition, health professionals who have limited knowledge and thus are unable to inform patients may ultimately affect the choice (248). Therefore, family doctors, obstetricians, internists, pediatricians, and allied professional clinicians as well as medical social workers, nurse practitioners, and physician assistants need to be informed and educated so that they can provide relevant, good-quality, and unbiased information. Unless this step is accomplished, HCMV in pregnancy will remain a problematic issue.
Second, women of childbearing age should be considered the best target population for HCMV prevention. Determination of HCMV immune status before pregnancy carries many advantages: (i) it may be less expensive, as only specific IgG can be safely determined; (ii) IgG-seropositive women could be reassured and informed that they do not need any further testing (this may account for 50 to 70% of the population in Western countries); (iii) IgG-seronegative women could be properly informed, so that whenever they will become pregnant they will already be aware of the possible risks and preventive measures; (iv) monitoring of IgG-seronegative pregnant women would be easier and cheaper without much need to resort to the armamentarium of assays now necessary for the confirmation or interpretation of laboratory test results (IgM antibody and IgG avidity assays) in women of unknown preconceptional serology.
CRUCIAL IMPACT OF CORRECT COUNSELING
Many women receive their first generic (and often misleading) information about HCMV directly from the staff of the laboratory where HCMV-specific IgM is detected, sometimes well before the result is confirmed and correctly interpreted. Consultation with the obstetrician ensues, usually very shortly. Subsequently, depending on how knowledgeable the obstetrician is about HCMV and pregnancy, the woman can be either referred for further testing and counseling or offered the immediate option of terminating the pregnancy. No study has ever investigated how many pregnancies are terminated at an early stage (i.e., <12 weeks of gestation) on the basis of a positive IgM result and inadequate or misleading information (“your baby will be mentally retarded”).
Thus, a great responsibility lies on the health professional providing the first information. Indeed, the first communication to parents is crucial since it may affect how information presented later is accepted or even whether it is sought. In addition, since some time may elapse between the first detection of IgM positivity and the final diagnosis or exclusion of primary HCMV infection, a tremendous level of anxiety, often increased by the opinions of additional “experts,” can be experienced by the woman in the meantime. In our experience, such levels of anxiety cannot be relieved by any subsequent counseling no matter how careful and complete it may be.
On the other hand, falsely reassuring counsel or failure to recognize the potential risk of a positive IgM result (“you are completely protected against the infection because you have both IgG and IgM”) can be equally deleterious. Again, no formal study has specifically addressed the psychological consequences for parents of children with unexpected severe HCMV infection in terms of anxiety, depression, stress, and, most important, attitude towards the disabled child. However, our experience indicates that the impact may be disastrous, and the parents of a severely affected child are more likely to blame doctors for the birth of that child and to pursue legal action against them (authors' personal observation), as already shown for parents of children with Down's syndrome (122; L. Parsons, J. Richards, and R. Garlick, letter, Br. Med. J. 305:1228, 1992).
PRENATAL DIAGNOSIS: IS THERE A ROLE?
In 1992, Pass questioned the usefulness of prenatal testing because predictive values of positive and negative results were unclear and the absence of prognostic markers and fetal therapy prevented both obstetricians and pregnant women from making informed decisions whenever an intrauterine infection was diagnosed (189). After a decade, some of the points raised are still unanswered (fetal therapy is still lacking as well as reliable prognostic markers of fetal disease) and some issues have become even more debated, such as the predictive value of positive results obtained with molecular techniques. On the other hand, the predictive value of negative results is now quite well defined, thanks to the contributions of many European investigators.
Similarly, different diagnostic approaches and techniques have been evaluated and compared, a great deal of information has been obtained, and the overall reliability of prenatal diagnosis has definitely improved over time. Moreover, since both the limitations and benefits of prenatal testing have been better defined, counseling of pregnant women has similarly improved. Thus, prenatal diagnosis has assumed a crucial role in the management of pregnancies complicated by primary HCMV infection. However, the exact role of prenatal diagnosis in the management of pregnancies complicated by primary HCMV infection has not been defined.
We reviewed the records of 179 women with a definite diagnosis of primary HCMV infection and known outcome who were examined at our institute during the period from 1990 to 2000 (M. G. Revello and G. Gerna, unpublished data). In Italy, pregnancy can be terminated in the first 12 weeks of gestation upon the woman's request. Voluntary termination in the period from 13 to 24 weeks is allowed only if continuation of pregnancy will severely affect the mental or physical health of the woman, while voluntary termination is not allowed beyond 24 weeks of gestation. Women included in the survey were thus divided into three groups according to the time of pregnancy at which primary HCMV infection was diagnosed (Table 4). In the group of 73 women in whom primary HCMV infection was diagnosed at ≤12 weeks of gestation, 17 (23.3%) asked for elective termination, 35 (47.9%) decided to undergo prenatal testing at 20 to 22 weeks of gestation, and the remaining 21 (28.8%) women chose to continue the pregnancy without any invasive procedure. In the second group of 68 women with primary HCMV infection diagnosed at 13 to 23 weeks of gestation, as many as 45 (66.1%) chose the option of prenatal testing, 22 (32.4%) continued the pregnancy, and only 1 (1.5%) woman aborted without resorting to prenatal testing. Finally, 3 of 38 (8.5%) women in whom HCMV infection occurred beyond 24 weeks of gestation underwent prenatal diagnosis.
TABLE 4.
Management and outcome of 179 pregnancies complicated by primary HCMV infection according to gestational age at time of diagnosisa
| Wk of pregnancy at diagnosis | No. of women investigated | No. (%) of women choosing:
|
No. of pregnancies terminated/no. of prenatal diagnoses of congenital HCMV infection (% terminated) | ||
|---|---|---|---|---|---|
| No intervention | Voluntary abortion | Prenatal diagnosis | |||
| ≤12 | 73 | 21 (28.8) | 17 (23.3) | 35 (47.9) | 7/14 (50) |
| 13-23 | 68 | 22 (32.3) | 1 (1.5) | 45 (66.1) | 7/21 (30) |
| ≥24 | 38 | 35 (92.1) | 0 | 3 (8.5) | 0/2 (0) |
| Total | 179 | 78 | 18 | 83 | 14/37 (37.8) |
Source: M. G. Revello and G. Gerna, unpublished data.
Thus, altogether, the option of prenatal diagnosis was chosen by 80 of 141 (56.7%) women with primary HCMV infection diagnosed at ≤23 weeks of gestation. However, the most interesting finding comes from examination of the results of prenatal diagnosis and the subsequent behavior of the women who chose antenatal testing. In fact, while all 46 pregnancies with a prenatal diagnosis negative for fetal infection went to term (data not shown), only 14 of 37 (37.8%) pregnancies with documented intrauterine transmission of HCMV infection were terminated.
These data suggest the following. (i) Prenatal testing represents a very important option in the case of primary infection during pregnancy, as documented by the acceptance by the majority of women to whom it was presented. (ii) Prenatal testing appears to be very beneficial in terms of overall reduction in the number of terminated pregnancies, since none of the pregnancies with negative antenatal results and less than half of those with fetal infection were terminated. In fact, although we have no data for comparison, we believe that the toll in terms of voluntary abortions would have been (much) higher without the option of prenatal diagnosis. (iii) Finally, the observation that the majority of women continued the pregnancy despite the knowledge of fetal infection clearly indicates that the main reason for undergoing prenatal testing was not (or not only) selective termination in case of a positive result, but rather eagerness to know whether the infection had been transmitted. Indeed, some women in this group carried long-sought pregnancies or did not contemplate the option of termination for ethical reasons, while we believe that some women changed their mind over time, eventually choosing to continue their pregnancy.
PRECONCEPTIONAL AND PERICONCEPTIONAL HCMV INFECTIONS
While the risks associated with primary HCMV infection during pregnancy have been well assessed and pregnant women can be properly counseled and make informed decisions, no information is available about the outcome of pregnancies complicated by primary HCMV infection acquired shortly before or around the date of conception. Similarly, there is no information about how long conception should be delayed following primary infection.
We performed a retrospective study aimed at defining the risks associated with primary HCMV infection acquired ≤3 months before the last menstrual period (preconceptional infections) or in the first 4 weeks after the last menstrual period (periconceptional infection) (M. G. Revello, M. Zavattoni, and G. Gerna, Abstr. 8th International Cytomegalovirus Conference, abstr. p. 66, 2001).
Back records for 162 consecutive pregnant women with ascertained diagnosis of acute or recent primary HCMV infection and known outcome of pregnancy were thoroughly reviewed. Diagnosis of primary infection was based on either decreasing levels of virus-specific IgM and rising levels of IgG avidity, as determined from sequential serum samples, HCMV detection in blood, or both. Since dating of the infection was crucial for the purpose of the study, only women with a well-defined clinical history were considered. In each case, the kinetics of virologic parameters were considered in relation to symptoms and/or laboratory findings compatible with a primary HCMV infection in order to define the onset of the infection.
By using these strict inclusion criteria, 26 women were identified in whom primary HCMV infection occurred either 2 to 11 weeks (median, 4 weeks) before the last menstrual period (10 women) or 1 to 4 weeks (median, 2 weeks) after the last menstrual period (16 women). In the group of 16 women with periconceptional HCMV infection, 5 (31.2%) elected to terminate their pregnancy before 12 weeks of gestation, while spontaneous abortion occurred in 2 (12.5%) women at 7 weeks of gestation (Table 5). Products of conception were not examined for HCMV. On the other hand, five (31.2%) women underwent prenatal diagnosis at 17 to 23 weeks (median, 20 weeks) of gestation, and all five fetuses were found to be negative for HCMV infection. On the whole, nine pregnancies went to term, and seven of nine (77.8%) newborns examined at birth were found to be uninfected (including the five newborns examined during fetal life), whereas the remaining two (22.2%) were congenitally infected. Of these, one newborn presented with neurologic and metabolic alterations and developed mild sequelae (neuromuscular deficits of the left arm) at 6 months of age (the mother had had HCMV-specific IgM at 6 weeks of gestation and 1 day of moderate fever at 3 weeks of gestation). The other newborn was asymptomatic at birth, and the mother was found to be positive for HCMV-specific IgM at 12 weeks of gestation, while reporting a debilitating and sustained asthenia concomitant with the last menstrual period.
TABLE 5.
Management and outcome of 26 pregnancies complicated by preconceptional or periconceptional primary HCMV infectiona
| Type of HCMV infection | No. of women investigated | No. (%) of pregnancies with the indicated outcome
|
No. of newborns with congenital infection/no. examined (% infected) | |||
|---|---|---|---|---|---|---|
| Spontaneous abortion | Voluntary abortion | Prenatal diagnosis | Delivery | |||
| Preconceptional | 10 | 0 | 0 | 2 (20) | 10 (100) | 1/10 (10) |
| Wk gestation | NAb | NA | 18-22 | Term | ||
| Periconceptional | 16 | 2 (12.5) | 5 (31.2) | 5 (31.2) | 9 (56.2) | 2c/9 (22.2) |
| Wk gestation | 7 | ≤12 | 15-23 | Term | ||
Source: M. G. Revello, M. Zavattoni, and G. Gerna, unpublished data.
NA, not applicable.
One newborn was symptomatic at birth and developed mild neurologic sequelae at 6 months of age.
In the group of 10 women with preconceptional infection, no spontaneous or induced abortion occurred, and two women underwent prenatal diagnosis at 18 and 23 weeks of gestation (Table 5). Neither of the two fetuses was infected. All 10 pregnancies went to term, and 9 of 10 newborns (90%) were found to be HCMV free, while one newborn was subclinically infected. The mother of this newborn infant was sent to our center because of a positive IgM result at 6 weeks of gestation. Retrospective data revealed fever, asthenia, headache, and upper respiratory tract symptoms 8 weeks before the last menstrual period. A prenatal diagnosis procedure performed at 18 weeks of gestation, while the woman was still positive for HCMV DNAemia, gave negative results, as HCMV was neither isolated from nor detected in amniotic fluid or fetal blood. However, at birth the virus was isolated from the urine, and DNAemia was present in neonatal blood together with low levels of HCMV-specific IgM. Both DNAemia and IgM were still positive at 6 months of age, while the infant remained free of symptoms. Possible explanations for the discrepant results obtained antenatally include delayed transmission, iatrogenic transmission, and performance of prenatal procedures too early during pregnancy. All these factors have been repeatedly shown to affect the reliability of prenatal diagnosis, as discussed above.
Although it must be pointed out that the true incidence of intrauterine transmission in our series could not be precisely defined because materials from spontaneous or induced abortions could not be examined for HCMV, the risk of fetal infection following maternal infection acquired before or immediately after conception appears to be substantially lower (10% and 22%, respectively) than that generally reported for infections acquired during gestation (40 to 50%). As for the prognosis of congenitally infected infants born to mothers with periconceptional infection, the numbers are far too small to allow us to draw a sound conclusion. These findings were confirmed in a more extended series (219a).
These results, albeit partial and preliminary, still can be useful in facilitating informed decisions by pregnant women. In particular, from a practical point of view, pregnant women with documented or suspected primary infection acquired before conception can be reassured and counseled to continue their pregnancy without resorting to antenatal testing unless required by parental anxiety. On the other hand, the option of prenatal diagnosis should be offered to women with periconceptional infection in view of the slightly higher incidence of transmission and the uncertainty of clinical outcome. In addition, as more information about the risks to the fetus becomes available from prospective monitoring, the proportion of women electing to undergo voluntary abortion at early stages of gestation will hopefully be reduced.
Finally, as for how long to wait between primary HCMV infection and conception, no definite answer is available yet. However, on the basis of the data reported above, the observation that about 20% of immunocompetent subjects with documented primary HCMV infection are still DNAemia positive at 6 months after onset (208, 216), and the consideration of DNAemia as a marker of potential infectivity, we suggest that at least 6 months should elapse prior to initiation of pregnancy. It must be stressed, however, that this is a general recommendation, and preconceptional primary HCMV infection should not be considered an indication for termination of pregnancy.
CONGENITAL INFECTION FROM IMMUNE MOTHERS AND CLINICAL OUTCOME
The overall incidence of congenital HCMV infection varies from 0.24% to 2.2% (Table 6). This percentage includes newborns born to mothers with primary infection during pregnancy as well as newborns born to mothers with existing immunity. The contribution of either component depends on the seroprevalence in a given population. It is generally recognized that primary HCMV infection carries the highest risk for symptomatic (including sequelae) congenital infection. However, much attention has been paid recently to the possibility that in utero transmission following recurrent maternal infection may result in adverse fetal outcome more frequently than previously thought. The epidemiologic correlation of seroprevalence and incidence of congenital infection supports this observation.
TABLE 6.
Incidence of congenital HCMV infection in relation to rate of maternal immunitya
| Location and dateb | No. of infants studied | % of infants with congenital HCMV infection | % of mothers with maternal immunity |
|---|---|---|---|
| Manchester, England, 1978 | 6,051 | 0.24 | 25 |
| Aarhus-Viborg, Denmark, 1979 | 3,060 | 0.4 | 52 |
| Hamilton, Canada, 1980 | 15,212 | 0.42 | 44 |
| Halifax, Canada, 1975 | 542 | 0.55 | 37 |
| Birmingham, Alabama (upper SES), 1981 | 2,698 | 0.6 | 80 |
| Houston, Texas (upper SES), 1980 | 461 | 0.6 | 50 |
| London, England, 1973 | 720 | 0.69 | 58 |
| Houston, Texas (low SES), 1980 | 493 | 1.2 | 83 |
| Abidjan, Ivory Coast, 1978 | 2,032 | 1.38 | 100 |
| Sendai, Japan, 1970 | 132 | 1.4 | 83 |
| Santiago, Chile, 1976 | 118 | 1.7 | 98 |
| Helsinki, Finland, 1977 | 200 | 2.0 | 85 |
| Birmingham, Alabama (low SES), 1980 | 1,412 | 2.2 | 85 |
From Stagno et al. (265), modified and used with permission.
SES, socieconomic status.
Over time, individual cases of symptomatic infants born to apparently normal (K. Ahlfors, S. Harris, S. Ivarsson, and L. Svanberg, Letter, N. Engl. J. Med. 305:284, 1981; D. Rutter, P. Griffiths, and R. S. Trompeter, letter, Lancet ii:1182, 1985) or variably immunocompromised (18, 147, 176, 239) HCMV-immune mothers have been published, indirectly testifying to the rarity of the event. On the other hand, two large independent studies (75, 290) reported that asymptomatic infants born to immune mothers may develop long-term neurologic sequelae, particularly deafness, in 8% and 22% of cases. Moreover, in the study by Williamson et al. (290), the frequency of sequelae in infants born to immune mothers was comparable to that observed in subclinically infected children born to mothers with primary infection.
In 1999, groups from Sweden, Belgium, and the United States reported long-term prospective studies of congenital HCMV infection. In the Swedish study (5), transient symptoms were noted at birth in an identical number (n = 6) of newborns born to mothers with confirmed primary or recurrent infection, whereas neurological symptoms at follow-up were recorded in four and two children born to mothers with confirmed primary or recurrent HCMV infection, respectively. The Belgian study (36) reported that one of three severe congenital infections was due to a recurrent maternal infection and that one of two infants with late-onset hearing impairment was born to a mother with existing immunity. Finally, the U.S. study (28) reported that of 20 newborns with symptomatic congenital HCMV infection at birth and defined maternal infection, 8 were born to mothers with primary infection, 8 to mothers with confirmed recurrent infection, and 4 to mothers with presumed recurrent infection. No difference was found in the severity of symptoms at birth or in long-term sequelae between children born to mothers with primary or nonprimary HCMV infection. In earlier studies (75, 266) performed by the same group in the same geographic area and on a comparable number of children, no symptomatic infection was observed at birth in newborns of immune mothers and a lower incidence of sequelae was found in newborns from mothers with nonprimary infection (8% versus 25%). No clear explanation for these discrepancies was given by the authors.
Because of the potential implications of these studies as far as screening and vaccination policies are concerned, it is important to stress that all studies mentioned above suffered from a major intrinsic methodological weakness, i.e., none of them, least of all the U.S. study, was specifically designed to investigate the risk of severe HCMV-related abnormalities after recurrent maternal infection. In addition, in none of the quoted studies were mothers of congenitally infected children investigated for other possible causes that might have been responsible for the adverse outcome of pregnancy, such as smoking or drinking habits or illicit-drug consumption. Moreover, since the definition of primary versus recurrent infection relied only on the presence or absence of IgM (except whem seroconversion could be demonstrated or immunity was documented before pregnancy), the type of maternal infection remained uncertain in a fair number of cases in both the Swedish and U.S. studies. Finally, additional assays were not performed to rule out the possibility of a primary infection in the periconceptional period.
Indeed, very large prospective studies of women known to be immune before pregnancy should be performed in order to define this issue. Although we doubt that such studies will ever be funded and carried out (198), still, defining the exact impact of recurrent maternal infections on neonatal disease should be the top priority for those interested in developing and implementing vaccine strategies. While it is possible that the contribution of recurrent maternal infection to symptomatic congenital infection might have been underestimated because of the difficulty in diagnosing it, the available data are still insufficient to allow modification of counseling of immune pregnant women. On the other hand, one wonders whether it would be beneficial to screen newborns for HCMV in view of identifying asymptomatic babies to be enrolled in long-term follow-up. This could be justified because it has been shown that asymptomatic congenitally infected children born to mothers with either primary or recurrent infection face a significant risk of developing sequelae, particularly hearing defects (75, 290); in these silently infected children, hearing loss has been shown to progress silently over time (290); unrecognized hearing loss has a significant negative impact as far as language development, school performance, and communication skills are concerned; and early diagnosis of hearing impairment is mandatory for early intervention. Thus, much effort should be directed to developing rapid, inexpensive, and simple assays for the detection of HCMV in urine so that HCMV screening may become an additional routine test for all newborns.
Finally, since it has been recently proposed (30) that recurrences and unfavorable outcome might be related to reinfection with a new HCMV strain rather than reactivation of the endogenous strain, controlled studies of molecular epidemiology are much needed.
CONGENITAL INFECTION IN TWINS
A fascinating, albeit little investigated aspect of the complex relationship between HCMV and pregnancy concerns congenital HCMV infection in twins. So far, only 12 documented cases of congenital HCMV infection in twins (3) and one case in a quadruplet pregnancy (236) have been described. Two main observations derive from these reports. The first concerns transmission of the infection. In monozygotic twins with a monochorionic placenta, congenital HCMV infection has been observed to occur in both children, while in dizygotic twins with a dichorionic placenta, only one of the twins was generally infected. On the other hand, in three pairs of dizygotic twins with fused placentas, both twins were found to be infected in two pairs, whereas only one twin was infected in the remaining pair (3). Histopathological examination of the placentas showed inflammatory signs in cases of fetal infection, whereas they were apparently normal in noninfected siblings (3). In the case of congenital infection in a quadruplet pregnancy, HCMV immediate-early antigens were detected in three available placentas in the absence of viral inclusions (236).
Our own experience is in keeping with these findings. We observed two twin pregnancies complicated by primary HCMV infection in the mother and subsequent transmission of the infection. In one case, the mother suffered from primary HCMV infection at 16 weeks of gestation. Two baby girls were vaginally delivered at 38 weeks of gestation. HCMV was isolated from urine collected 3 days after birth from one newborn, whereas the second newborn was found to be uninfected. Both newborns were asymptomatic. The placentas were dichorionic-diamniotic and separate. In the second case, the mother had primary HCMV infection at 10 weeks of gestation. The placenta was monochorionic-diamniotic. Both twins were found to excrete HCMV in urine at birth in the absence of signs or clinical symptoms (M. G. Revello and M. Zavattoni, unpublished data).
The second observation is relevant to the clinical outcome of congenitally infected twins. While in the reported cases of congenitally infected monozygotic twins, both members were either severely affected or subclinically infected, the clinical outcome of dizygotic twins appeared more variable. In particular, in one set of congenitally infected twins who appeared asymptomatic at birth, one member was normal at follow-up, while the other had bilateral deafness, was restless, and had poor attention (231). More recently, the variable outcome of three infected survivors of a quadruplet pregnancy has been reported (236). One infant had cholestatic jaundice at birth and died of liver failure at 3 months of age, one infant showed no signs or symptoms at 18 months of age, and the remaining infant had hearing loss and delayed development.
The observed variability in HCMV transmission and clinical outcome in infected twins clearly indicates that twin fetuses may react differently to maternal HCMV infection and that, as anticipated by Ahlfors in 1988 (3), the placenta seems to play a key role in the transmission of or protection from the infection. Moreover, the markedly different clinical outcome of congenital HCMV infection among infants of a multiple pregnancy suggests that genetic determinants might be involved.
In conclusion, these data cast some doubts on the value of some maternal factors, such as humoral and cellular immune response, as possible prognostic markers of intrauterine transmission, as postulated by some researchers (29, 71, 157, 269). They should also be taken into consideration when testing the hypothesis that reinfection with a different HCMV strain may be responsible for the unfavorable outcome observed in some infants born to mothers with existing immunity (30).
Acknowledgments
We thank Fausto Baldanti, Milena Furione, Antonella Sarasini, and Daniele Lilleri for performing molecular assays; Elena Percivalle and Maurizio Zavattoni for performing cellular virology assays; and Maria Torsellini, Maurizio Parea, and Giovanna Gorini for providing serological data. In addition, the technical staff of our Servizio di Virologia (Lucia Chezzi, Cinzia Zanello, Luca Dossena, Gabriella Garbagnoli, Viviana Landini, Simona Pezzaia, and Giuliana Casali) is gratefully acknowledged. We also thank Nazarena Labò for database preparation, Daniela Sartori for manuscript preparation, and Linda D'Arrigo for revision of the English.
Maria Grazia Revello and Giuseppe Gerna were financially supported by the Ministero della Sanità, IRCCS Policlinico San Matteo, Ricerca Finalizzata grants 820RFM95/01, 038RFM98/01, and 820RFM99/01, and Ricerca Corrente grants 80208, 80206, and 80513.
REFERENCES
- 1.Adler, S. P., S. A. Plotkin, E. Gonczol, M. Cadoz, C. Meric, J. B. Wang, P. Dellamonica, A. M. Best, J. Zahradnik, S. Pincus, K. Berencsi, W. I. Cox, and Z. Gyulai. 1999. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne). J. Infect. Dis. 180:843-846. [DOI] [PubMed] [Google Scholar]
- 2.Ahlfors, K., M. Forsgren, S. A. Ivarsson, S. Harris, and L. Svanberg. 1983. Congenital cytomegalovirus infection: on the relation between type and time of maternal infection and infant's symptoms. Scand. J. Infect. Dis. 15:129-138. [DOI] [PubMed] [Google Scholar]
- 3.Ahlfors, K., S. A. Ivarsson, and H. Nilsson. 1988. On the unpredictable development of congenital cytomegalovirus infection. A study in twins. Early Hum. Dev. 18:125-135. [DOI] [PubMed] [Google Scholar]
- 4.Ahlfors, K., S. A. Ivarsson, S. Harris, L. Svanberg, R. Holmqvist, B. Lernmark, and G. Theander. 1984. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand. J. Infect. Dis. 16:129-137. [DOI] [PubMed] [Google Scholar]
- 5.Ahlfors, K., S. A. Ivarsson, and S. Harris. 1999. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand. J. Infect. Dis. 31:443-457. [DOI] [PubMed] [Google Scholar]
- 6.Alpert, G., M. C. Mazeron, R. Colimon, and S. Plotkin. 1985. Rapid detection of human cytomegalovirus in the urine of humans. J. Infect. Dis. 152:631-633. [DOI] [PubMed] [Google Scholar]
- 7.Andersson, A., V. Vetter, L. Kreutzer, and G. Bauer. 1994. Avidities of IgG directed against viral capsid antigen or early antigen: useful markers for significant Epstein-Barr virus serology. J. Med. Virol. 43:238-244. [DOI] [PubMed] [Google Scholar]
- 8.Aono, T., K. Kondo, H. Miyoshi, K. Tanaka-Taya, M. Kondo, Y. Osugi, J. Hara, S. Okada, and K. Yamanishi. 1998. Monitoring of human cytomegalovirus infections in pediatric bone marrow transplant recipients by nucleic acid sequence-based amplification. J. Infect. Dis. 178:1244-1249. [DOI] [PubMed] [Google Scholar]
- 9.Balfour, H. H., Jr., P. K. Velo, and G. W. Saachs. 1985. Cytomegalovirus vaccine trial in 400 renal transplant candidates. Transplant. Proc. 17:81-83.3895674 [Google Scholar]
- 10.Barbi, M., S. Binda, V. Primache, C. Luraschi, and C. Corbetta. 1996. Diagnosis of congenital cytomegalovirus infection by detection of viral DNA in dried blood spots. Clin. Diagn. Virol. 6:27-32. [DOI] [PubMed] [Google Scholar]
- 11.Baxby, D., and E. Paoletti. 1992. Potential use of nonreplicating vectors as recombinant vaccines. Vaccine 10:8-9. [DOI] [PubMed] [Google Scholar]
- 12.BenMohamed, L., R. Krishnan, J. Longmate, C. Auge, L. Low, J. Primus, and D. J. Diamond. 2000. Induction of CTL response by a minimal epitope vaccine in HLA A∗0201/DR1 transgenic mice: dependence on HLA class II restricted TH response. Hum. Immunol. 61:764-779. [DOI] [PubMed] [Google Scholar]
- 13.Berencsi, K., Z. Gyulai, E. Gönczöl, S. Pincus, W. I. Cox, S. Michelson, L. Kari, C. Meric, M. Cadoz, J. Zahradnik, S. Starr, and S. A. Plotkin. 2001. A canarypox vector-rxpressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J. Infect. Dis. 183:1171-1179. [DOI] [PubMed] [Google Scholar]
- 14.Bevan, I. S., R. A. Daw, P. J. Day, F. A. Ala, and M. R. Walker. 1991. Polymerase chain reaction for detection of human cytomegalovirus infection in a blood donor population. Br. J. Hematol. 78:94-99. [DOI] [PubMed] [Google Scholar]
- 15.Bevis, D. C. A. 1952. The antenatal prediction of hemolytic disease of the newborn. Lancet i:395-398. [DOI] [PubMed] [Google Scholar]
- 16.Bitsch, A., H. Kirkner, R. Dupke, and G. Bein. 1992. Failure to detect human cytomegalovirus DNA in peripheral blood leukocytes of healthy blood donors by the polymerase chain reaction. Transfusion 32:612-617. [DOI] [PubMed] [Google Scholar]
- 17.Blackburn, N. K., T. G. Besselaar, B. D. Schoub, and K. F. O'Connell. 1991. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 33:6-9. [DOI] [PubMed] [Google Scholar]
- 18.Blau, E. B., and J. R. Gross. 1997. Congenital cytomegalovirus infection after recurrent infection in a mother with a renal transplant. Pediatr. Nephrol. 11:361-362. [PubMed] [Google Scholar]
- 19.Blok, M. J., V. J. Goossens, S. J. V. Vanherle, B. Top, N. Tacken, J. M. Middeldorp, M. H. L. Christiaans, J. P. van Hooff, and C. A. Bruggeman. 1998. Diagnostic value of monitoring human cytomegalovirus late pp67 mRNA expression in renal-allograft recipients by nucleic acid sequence-based amplification. J. Clin. Microbiol. 36:1341-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodéus, M., and P. Goubau. 1999. Predictive value of maternal IgG avidity for congenital human cytomegalovirus infection. J. Clin. Virol. 12:3-8. [DOI] [PubMed] [Google Scholar]
- 21.Bodéus, M., C. Hubinont, P. Bernard, A. Bouckaert, K. Thomas, and P. Goubau. 1999. Prenatal diagnosis of human cytomegalovirus by culture and polymerase chain reaction: 98 pregnancies leading to congenital infection. Prenatal Diagn. 19:314-317. [DOI] [PubMed] [Google Scholar]
- 22.Bodéus, M., D. Beulné, and P. Goubau. 2001. Ability of three IgG-avidity assays to exclude recent cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 20:248-252. [DOI] [PubMed] [Google Scholar]
- 23.Bodéus, M., S. Feyder, and P. Goubau. 1998. Avidity of IgG antibodies distinguishes primary from nonprimary cytomegalovirus infection in pregnant women. Clin. Diagn. Virol. 9:9-16. [DOI] [PubMed] [Google Scholar]
- 24.Boeckh, M., and G. Boivin. 1998. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin. Microbiol. Rev. 11:533-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeckh, M., R. A. Bowden, J. M. Goodrich, M. Pettinger, and J. D. Meyers. 1992. Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood 80:1358-1364. [PubMed] [Google Scholar]
- 26.Boeckh, M., T. A. Gooley, D. Myerson, T. Cunningham, G. Schoch, and R. A. Bowden. 1996. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 88:4063-4071. [PubMed] [Google Scholar]
- 27.Boivin, G., C. A. Olson, M. R. Quirk, S. M. St-Cyr, and M. C. Jordan. 1995. Quantitation of human cytomegalovirus glycoprotein H gene in cells using competitive PCR and a rapid fluorescence-based detection system. J. Virol. Methods 51:329-342. [DOI] [PubMed] [Google Scholar]
- 28.Boppana, B., S. B., K. B. Fowler, W. J. Britt, S. Stagno, and R. F. Pass. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55-60. [DOI] [PubMed] [Google Scholar]
- 29.Boppana, S. B., and W. J. Britt. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J. Infect. Dis. 171:1115-1121. [DOI] [PubMed] [Google Scholar]
- 30.Boppana, S. B., L. B. Rivera, K. B. Fowler, M. Mach, and W. J. Britt. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344:1366-1371. [DOI] [PubMed] [Google Scholar]
- 31.Boppana, S. B., R. J. Smith, S. Stagno, and W. J. Britt. 1992. Evaluation of a microtiter plate fluorescent-antibody assay for rapid detection of human cytomegalovirus infection. J. Clin. Microbiol. 30:721-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brytting, M., W. Xu, B. Wahren, and V. A. Sundqvist. 1992. Cytomegalovirus DNA detection in sera samples from patients with active cytomegalovirus infections. J. Clin. Microbiol. 30:1937-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carney, W. P., and M. S. Hirsch. 1981. Mechanism of immunosuppression in cytomegalovirus mononucleosis. II. Virus-monocyte interactions. J. Infect. Dis. 144:47-54. [DOI] [PubMed] [Google Scholar]
- 34.Cassol, S. A., M. C. Ponn, R. Pal, and M. J. Naylor. 1989. Primer-mediated enzymatic amplification of cytomegalovirus (CMV) DNA. Application to the early diagnosis of CMV infections in marrow transplant recipients. J. Clin. Investig. 83:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassol, S. A., T. Salas, M. Arella, P. Meumann, M. T. Schechter, and M. O'Shaughnessy. 1991. Use of dried blood spot specimens in the detection of human immunodeficiency virus type 1 by the polymerase chain reaction. J. Clin. Microbiol. 29:667-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casteels, A., A. Naessens, F. Gordts, L. De Catte, A. Bougatef, and W. Foulon. 1999. Neonatal screening for congenital cytomegalovirus infections. J. Perinat. Med. 27:116-121. [DOI] [PubMed] [Google Scholar]
- 37.Cha, T.-H., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. S. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Champsaur, H., M. Fattal-German, and R. Arranhado. 1988. Sensitivity and specificity of viral immunoglobulin M determination by indirect enzyme-linked immunosorbent assay. J. Clin. Microbiol. 26:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, T. Cerny, T. Horsnell, C. A. Hutchinson, T. Kouzarides, and J. A. Martinetti. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 40.Chernoff, D. N., R. C. Miner, B. S. Hoo, L. P. Shen, R. J. Kelso, D. Jekicmcmullen, J. P. Lalezari, S. W. Chou, W. L. Drew, and J. A. Kolberg. 1997. Quantification of cytomegalovirus DNA in peripheral blood leukocytes by a branched-DNA signal amplification assay. J. Clin. Microbiol. 35:2740-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chrisp, P., and S. P. Clissold. 1991. Foscarnet: a review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs 41:104-129. [DOI] [PubMed] [Google Scholar]
- 42.Compton, J. 1991. Nucleic acid sequence-based amplification. Nature 350:91-92. [DOI] [PubMed] [Google Scholar]
- 43.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, and G. L. Smith. 1986. Identification of human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 5:3057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross, J. C., Z. Werb, and S. J. Fisher. 1994. Implantation and the placenta: key pieces of the development puzzle. Science 266:1508-1518. [DOI] [PubMed] [Google Scholar]
- 45.Daffos, F., M. Capella-Paulovsky, and F. Forestier. 1985. Fetal blood sampling during pregnancy with use of a needle guided by ultrasound: a study of 606 consecutive cases. Am. J. Obstet. Gynecol. 153:655-660. [DOI] [PubMed] [Google Scholar]
- 46.Daiminger, A., U. Bader, M. Eggers, T. Lazzarotto, and G. Enders. 1999. Evaluation of two novel immunoassays using recombinant antigens to detect cytomegalovirus-specific immunoglobulin M in sera from pregnant women. J. Clin. Virol. 13:161-171. [DOI] [PubMed] [Google Scholar]
- 47.D'Amaro, J., J. G. Houbiers, J. W. Drijfhout, R. M. Brandt, R. Schipper, J. N. Bavinck, C. J. Melief, and W. M. Kast. 1995. A computer program for predicting possible cytotoxic T lymphocytes epitopes based on HLA class I peptide-binding motifs. Hum. Immunol. 43:13-18. [DOI] [PubMed] [Google Scholar]
- 48.Damsky, C. H., and S. J. Fisher. 1998. Trophoblasts pseudovasculogenesis: faking it with endothelial adhesion receptors. Curr. Opin. Cell Biol. 10:660-666. [DOI] [PubMed] [Google Scholar]
- 49.Davis, L. E., G. V. Tweed, T. D. Y. Chin, and G. L. Miller. 1971. Intrauterine diagnosis of cytomegalovirus infection in mothers and their infants. Am. J. Obstet. Gynecol. 109:1217-1219. [DOI] [PubMed] [Google Scholar]
- 50.Demmler, G. J. 1991. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev. Infect. Dis. 13:315-319. [DOI] [PubMed] [Google Scholar]
- 51.Demmler, G. J., G. J. Buffone, C. M. Schimbor, and R. A. May. 1988. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J. Infect. Dis. 158:1177-1184. [DOI] [PubMed] [Google Scholar]
- 52.Deres, K., H. Schild, K. H. Wiesmuller, G. Jung, and H. G. Rammensee. 1989. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature 342:561-564. [DOI] [PubMed] [Google Scholar]
- 53.Deyi, Y. M., P. Goubau, and M. Bodéus. 2000. False-positive IgM antibody tests for cytomegalovirus in patients with acute Epstein-Barr virus infection. Eur. J. Clin. Microbiol. Infect. Dis. 19:557-560. [DOI] [PubMed] [Google Scholar]
- 54.Diamond, D. J., J. York, J. Sun, C. L. Wright, and S. J. Forman. 1997. Development of a candidate HLA A∗0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood 90:1751-1767. [PubMed] [Google Scholar]
- 55.Di Domenico, N., H. Link, R. Knobel, T. Caratsch, W. Wechsler, Z. G. Loewy, and M. Rosenstraus. 1996. COBAS AMPLICOR: fully automated RNA and DNA amplification and detection system for routine diagnostic PCR. Clin. Chem. 42:1915-1923. [PubMed] [Google Scholar]
- 56.Diosi, P., E. Moldovan, and N. Tomescu. 1965. Latent cytomegalovirus infection in blood donors. Br. Med. J. 4:660-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donner, C., C. Liesnard, F. Brancart, and F. Rodesch. 1994. Accuracy of amniotic fluid testing before 21 weeks’ gestation in prenatal diagnosis of congenital cytomegalovirus infection. Prenatal Diagn. 14:1055-1059. [DOI] [PubMed] [Google Scholar]
- 58.Donner, C., C. Liesnard, J. Content, A. Busine, J. Aderca, and F. Rodesch. 1993. Prenatal diagnosis of 52 pregnancies at risk for congenital cytomegalovirus infection. Obstet. Gynecol. 82:481-486. [PubMed] [Google Scholar]
- 59.Drouet, E., C. Chapuis-Cellier, S. Bosshard, C. Verniol, A. Niveleau, J. L. Touraine, and J. L. Garnier. 1999. Oligo-monoclonal immunoglobulins frequently develop during concurrent cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections in patients after renal transplantation. Clin. Exp. Immunol. 118:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eggers, M., C. Metzger, and G. Enders. 1998. Differentiation between acute primary and recurrent human cytomegalovirus infection in pregnancy, using a microneutralization assay. J. Med. Virol. 56:351-358. [PubMed] [Google Scholar]
- 61.Elek, S. A., and H. Stern. 1974. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet i:1-5. [DOI] [PubMed] [Google Scholar]
- 62.Embil, J. A., R. J. Ozere, and E. V. Haldane. 1970. Congenital cytomegalovirus infection in two siblings from consecutive pregnancies. J. Pediatr. 77:417-421. [DOI] [PubMed] [Google Scholar]
- 63.Emery, V. C., A. V. Cope, E. F. Bowen, D. Gor, and P. D. Griffiths. 1999. The dynamics of human cytomegalovirus replication in vivo. J. Exp. Med. 190:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emery, V. C., C. A. Sabin, A. V. Cope, D. Gor, A. F. Hassan-Walker, and P. D. Griffiths. 2000. Application of viral load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 355:2032-2036. [DOI] [PubMed] [Google Scholar]
- 65.Enders, G., U. Bäder, L. Lindemann, G. Schalasta, and A. Daiminger. 2001. Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenat. Diagn. 21:362-377. [DOI] [PubMed] [Google Scholar]
- 66.Endresz, V., K. Burian, K. Berencsi, Z. Gyulai, L. Kari, H. Horton, D. Virok, C. Meric, S. A. Plotkin, and E. Gönczöl. 2001. Optimization of DNA immunization against human cytomegalovirus. Vaccine 19:3972-3980. [DOI] [PubMed] [Google Scholar]
- 67.Evans, T. J., J. P. K. Mc Collum, and H. Valdimarssan. 1975. Congenital cytomegalovirus infection after maternal renal transplantation. Lancet i:1359-1360. [DOI] [PubMed] [Google Scholar]
- 68.Falk, K., O. Rotzschke, S. Stevanovich, G. Jung, and H. G. Rammensee. 1991. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351:290-296. [DOI] [PubMed] [Google Scholar]
- 69.Fan-Harvard, P., N. C. Nahata, and M. T. Brady. 1989. Ganciclovir - a review of pharmacology, therapeutic efficacy and potential use for treatment of congenital cytomegalovirus pneumonia. J. Clin. Pharmacol. Ther. 14:329-340. [DOI] [PubMed] [Google Scholar]
- 70.Faulds, D., and R. C. Heel. 1990. Ganciclovir: a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 39:597-638. [DOI] [PubMed] [Google Scholar]
- 71.Fernando, S., J. M. Pearce, and J. C. Booth. 1993. Lymphocyte responses and virus excretion as risks factors for intrauterine infection with cytomegalovirus. J. Med. Virol. 41:108-113. [DOI] [PubMed] [Google Scholar]
- 72.Fisher, S. J., M. S. Leitch, M. S. Kantor, C. B. Basbaum, and R. H. Kramer. 1985. Degradation of extracellular matrix by the trophoblastic cells of first trimester human placentas. J. Cell Biochem. 27:31-41. [DOI] [PubMed] [Google Scholar]
- 73.Fisher, S. J., T. Y. Cui, L. Zhang, L. Hartman, K. Grahl, G. Y. Zhang, J. Tarpey, and C. H. Damsky. 1989. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J. Cell Biol. 109:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher, S., O. Genbacev, E. Maidji, and L. Pereira. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74:6808-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fowler, K. B., S. Stagno, R. F. Pass, W. J. Britt, T. J. Boll, and C. A. Alford. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326:663-667. [DOI] [PubMed] [Google Scholar]
- 76.Fox, J. C., P. D. Griffiths, and V. C. Emery. 1992. Quantification of human cytomegalovirus DNA using the polymerase chain reaction. J. Gen. Virol. 73:2405-2408. [DOI] [PubMed] [Google Scholar]
- 77.Geballe, A. P., F. S. Leach, and E. S. Mocarski. 1986. Regulation of cytomegalovirus late gene expression: γ genes are controlled by post transcriptional events. J. Virol. 57:864-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerna, G., D. Zipeto, M. Parea, M. G. Revello, E. Silini, E. Percivalle, M. Zavattoni, P. Grossi, and G. Milanesi. 1991. Monitoring of human cytomegalovirus infections and ganciclovir treatment in heart transplant recipients by determination of viremia, antigenemia, and DNAemia. J. Infect. Dis. 164:488-498. [DOI] [PubMed] [Google Scholar]
- 79.Gerna, G., E. Percivalle, A. Sarasini, and M. G. Revello. 2002. Human cytomegalovirus and human umbilical vein endothelial cells: restriction of primary isolation to blood samples and susceptibility of clinical isolates from other sources to adaptation. J. Clin. Microbiol. 40:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gerna, G., E. Percivalle, F. Baldanti, and M. G. Revello. 2002. Lack of transmission to polymorphonuclear leukocytes and human umbilical vein endothelial cells as a marker of attenuation of human cytomegalovirus. J. Med. Virol. 66:335-339. [DOI] [PubMed] [Google Scholar]
- 81.Gerna, G., E. Percivalle, F. Baldanti, S. Sozzani, P. Lanzarini, E. Genini, D. Lilleri, and M. G. Revello. 2000. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J. Virol. 74:5629-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gerna, G., E. Percivalle, M. G. Revello, and F. Morini. 1993. Correlation of quantitative human cytomegalovirus pp65-, p72- and p150- antigenemia, viremia, and circulating endothelial giant cells with clinical symptoms and antiviral treatment in immunocompromised patients. Clin. Diagn. Virol. 1:47-59. [DOI] [PubMed] [Google Scholar]
- 83.Gerna, G., E. Percivalle, M. Torsellini, and M. G. Revello. 1998. Standardization of the human cytomegalovirus antigenemia assay by means of in vitro-generated pp65-positive peripheral blood polymorphonuclear leukocytes. J. Clin. Microbiol. 36:3585-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gerna, G., F. Baldanti, A. Sarasini, M. Furione, E. Percivalle, M. G. Revello, D. Zipeto, and D. Zella. 1994. Effect of foscarnet induction treatment on quantitation of human cytomegalovirus (HCMV) DNA in peripheral blood polymorphonuclear leukocytes and aqueous humor of AIDS patients with HCMV retinitis. The Italian Foscarnet Study Group. Antimicrob. Agents Chemother. 38:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerna, G., F. Baldanti, D. Lilleri, L. De-Giuli, and M. G. Revello. 1998. Viral infections in bone marrow transplant recipients: from the bench to the bedside. Rev. Clin. Exp. Hematol. 7:84-106. [Google Scholar]
- 86.Gerna, G., F. Baldanti, D. Lilleri, M. Parea, E. Alessandrino, A. Pagani, F. Locatelli, J. Middeldorp, and M. G. Revello. 2000. Human cytomegalovirus immediate-early mRNA detection by nucleic acid sequence-based amplification as a new parameter for preemptive therapy in bone marrow transplant recipients. J. Clin. Microbiol. 38:1845-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerna, G., F. Baldanti, J. M. Middeldorp, M. Furione, M. Zavattoni, D. Lilleri, and M. G. Revello. 1999. Clinical significance of expression of human cytomegalovirus pp67 late transcript in heart, lung, and bone marrow transplant recipients as determined by nucleic acid sequence-based amplification. J. Clin. Microbiol. 37:902-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerna, G., F. Baldanti, M. Zavattoni, A. Sarasini, E. Percivalle, and M. G. Revello. 1992. Monitoring of ganciclovir sensitivity of multiple human cytomegalovirus strains coinfecting blood of an AIDS patient by an immediate-early antigen plaque assay. Antiviral Res. 19:333-345. [DOI] [PubMed] [Google Scholar]
- 89.Gerna, G., F. Baldanti, P. Grossi, F. Locatelli, P. Colombo, M. Viganò, and M. G. Revello. 2001. Diagnosis and monitoring of human cytomegalovirus infection in transplant recipients. Rev. Med. Microbiol. 12:155-175. [Google Scholar]
- 90.Gerna, G., M. Furione, F. Baldanti, and A. Sarasini. 1994. Comparative quantitation of human cytomegalovirus DNA in blood leukocytes and plasma of transplant and AIDS patients. J. Clin. Microbiol. 32:2709-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerna, G., M. Furione, F. Baldanti, E. Percivalle, P. Comoli, and F. Locatelli. 1995. Quantitation of human cytomegalovirus DNA in bone marrow transplant recipients. Br. J. Haematol. 91:674-683. [DOI] [PubMed] [Google Scholar]
- 92.Gerna, G., M. G. Revello, E. Percivalle, and F. Morini. 1992. Comparison of different immunostaining techniques and monoclonal antibodies to the lower matrix phosphoprotein (pp65) for optimal quantitation of human cytomegalovirus antigenemia. J. Clin. Microbiol. 30:1232-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerna, G., M. G. Revello, E. Percivalle, M. Zavattoni, M. Parea, and M. Battaglia. 1990. Quantification of human cytomegalovirus viremia by using monoclonal antibodies to different viral proteins. J. Clin. Microbiol. 28:2681-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerna, G., M. Zavattoni, E. Percivalle, P. Grossi, M. Torsellini, and M. G. Revello. 1998. Rising levels of human cytomegalovirus (HCMV) antigenemia during initial antiviral treatment of solid organ transplant recipients with primary HCMV infection. J. Clin. Microbiol. 36:1113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gerna, G., M. Zavattoni, F. Baldanti, A. Sarasini, L. Chezzi, P. Grossi, and M. G. Revello. 1998. Human cytomegalovirus leukoDNAemia correlates more closely with clinical symptoms than antigenemia and viremia in heart and heart-lung transplant recipients with primary HCMV infection. Transplantation 65:1378-1385. [DOI] [PubMed] [Google Scholar]
- 96.Gerna, G., M. Zavattoni, F. Baldanti, M. Furione, L. Chezzi, M. G. Revello, and E. Percivalle. 1998. Circulating cytomegalic endothelial cells are associated with high human cytomegalovirus (HCMV) load in AIDS patients with late-stage disseminated HCMV disease. J. Med. Virol. 55:64-74. [PubMed] [Google Scholar]
- 97.Gibson, W. 1983. Protein counterparts of human and simian cytomegaloviruses. Virology 128:391-406. [DOI] [PubMed] [Google Scholar]
- 98.Gleaves, C. A., T. F. Smith, E. A. Shuster, and G. R. Pearson. 1984. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J. Clin. Microbiol. 19:917-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzales Armas, J. C., C. S. Morello, L. D. Cranmer, and D. H. Soector. 1996. DNA immunization confers protection against murine cytomegalovirus infection. J. Virol. 70:7921-7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gozlan, J., J. P. Laporte, S. Lesage, M. Labopin, A. Najman, N. C. Gorin, and J. C. Petit. 1996. Monitoring of cytomegalovirus infection and disease in bone marrow recipients by reverse transcription-PCR and blood and urine culture. J. Clin. Microbiol. 34:2085-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grangeot-Keros, L., M. J. Mayaux, P. Lebon, F. Freymuth, G. Eugene, R. Stricker, and E. Dussaix. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944-946. [DOI] [PubMed] [Google Scholar]
- 102.Gray, J. J., B. J. Cohen, and U. Desselberger. 1993. Detection of human parvovirus B19-specific IgM and IgG antibodies using a recombinant viral VP1 antigen expressed in insect cells and estimation of time of infection by testing for antibody avidity. J. Virol. Methods 44:11-24. [DOI] [PubMed] [Google Scholar]
- 103.Greenberg, P. D., P. Reusser, J. M. Goodrich, and S. R. Riddell. 1991. Development of a treatment regimen for human cytomegalovirus (CMV) infection in bone marrow transplantation recipients by adoptive transfer of donor-derived CMV-specific T cell clones expanded in vitro. Ann. N. Y. Acad. Sci. 636:184-195. [DOI] [PubMed] [Google Scholar]
- 104.Grefte, A., M. van der Giessen, W. van Son, and T. H. The. 1993. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J. Infect. Dis. 167:270-277. [DOI] [PubMed] [Google Scholar]
- 105.Grefte, J. M. N., B. T. F. van der Gun, S. Schmolke, M. van der Giessen, W. J. van Son, B. Plachter, G. Jahn, and T. H. The. 1992. The lower matrix protein pp65. is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J. Gen. Virol. 73:2923-2932. [DOI] [PubMed] [Google Scholar]
- 106.Greijer, A. E., E. A. M. Verschuuren, C. A. J. Dekkers, H. M. A. Adriaanse, W. van der Bij, T. H. The, and J. M. Middeldorp. 2001. Expression dynamics of human cytomegalovirus immune evasion genes US3, US6, and US11 in the blood of lung transplant recipients. J. Infect. Dis. 184:247-255. [DOI] [PubMed] [Google Scholar]
- 107.Greijer, A. E., J. M. G. van de Crommert, S. J. C. Stevens, and J. M. Middeldorp. 1999. Molecular fine-specificity analysis of antibody responses to human cytomegalovirus and design of novel synthetic-peptide-based serodiagnostic assays. J. Clin. Microbiol. 37:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Griffith, B. P., S. R. McCormick, C. K. Fong, J. T. Lavallee, H. L. Lucia, and E. Goff. 1985. The placenta as a site of cytomegalovirus infection in guinea pigs. J. Virol. 55:402-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Griffiths, P. D., A. McLean, and V. C. Emery. 2001. Encouraging prospects for immunization against primary cytomegalovirus infection. Vaccine 19:1356-1362. [DOI] [PubMed] [Google Scholar]
- 110.Griffiths, P. D., and C. Baboonian. 1984. A prospective study of primary cytomegalovirus infection during pregnancy: final report. Br. J. Obstet. Gynecol. 91:307-315. [DOI] [PubMed] [Google Scholar]
- 111.Griffiths, P. D., S. Stagno, R. F. Pass, R. J. Smith, and C. A. Alford. 1982. Infection with cytomegalovirus during pregnancy: specific IgM antibodies as a marker of recent primary infection. J. Infect. Dis. 45:647-653. [DOI] [PubMed] [Google Scholar]
- 112.Griffiths. P. D., and H. O. Kangro. 1984. A user's guide to the indirect solid-phase radioimmunoassay for the detection of cytomegalovirus specific IgM antibodies. J. Virol. Methods 8:271-282. [DOI] [PubMed] [Google Scholar]
- 113.Grose, C., and C. P. Weiner. 1990. Prenatal diagnosis of congenital cytomegalovirus infection: two decades later. Am. J. Obstet. Gynecol. 163:447-450. [DOI] [PubMed] [Google Scholar]
- 114.Grose, C., O. Itani, and C. P. Weiner. 1989. Prenatal diagnosis of fetal infection: advances from amniocentesis to cordocentesis—congenital toxoplasmosis, rubella, cytomegalovirus, varicella virus, parvovirus and human immunodeficiency virus. Pediatr. Infect. Dis. J. 8:459-468. [PubMed] [Google Scholar]
- 115.Grose, C., T. Meehan, and C. P. Weiner. 1992. Prenatal diagnosis of congenital cytomegalovirus infection by virus isolation after amniocentesis. Pediatr. Infect. Dis. J. 11:605-607. [PubMed] [Google Scholar]
- 116.Grossi, P., L. Minoli, E. Percivalle, W. Irish, M. Viganò, and G. Gerna. 1995. Clinical and virological monitoring of human cytomegalovirus infection in 294 heart transplant recipients. Transplantation 59:847-851. [PubMed] [Google Scholar]
- 117.Grossi, P., S. Kusne, C. Rinaldo, K. St. George, M. Magnone, J. Rakela, J. Fung, and T. E. Starzl. 1996. Guidance of ganciclovir therapy with pp65 antigenemia in cytomegalovirus-free recipients of livers from seropositive donors. Transplantation 61:1659-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guerra, B., T. Lazzarotto, S. Quarta, M. Lanari, L. Bovicelli, A. Nicolosi, and M. P. Landini. 2000. Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. Am. J. Obstet. Gynecol. 183:476-482. [DOI] [PubMed] [Google Scholar]
- 119.Gyulai, Z., V. Endresz, K. Burian, S. Pincus, J. Toldy, W. I. Cox, C. Meric, S. Plotkin, E. Gönczöl, and K. Berencsi. 2000. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-exon 4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181:1537-1546. [DOI] [PubMed] [Google Scholar]
- 120.Hagay, Z. J., G. Biran, A. Ornay, and A. Reece. 1996. Congenital cytomegalovirus infection: a long-standing problem still seeking a solution. Am. J. Obstet. Gynecol. 174:241-245. [DOI] [PubMed] [Google Scholar]
- 121.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hall, S., M. Bobrow, and T. M. Marteau. 2000. Psycological consequences for parents of false negative results on prenatal screening for Down's syndrome: retrospective interview study. Br. Med. J. 320:407-412. [PMC free article] [PubMed] [Google Scholar]
- 123.Hashido, M., S. Inouye, and T. Kawana. 1997. Differentiation of primary from nonprimary genital herpes infections by a herpes simplex virus specific immunoglobulin G avidity assay. J. Clin. Microbiol. 35:1766-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hayes, K., and H. Gibas. 1971. Placental cytomegalovirus infection without fetal involvement following primary infection in pregnancy. J. Pediatr. 79:401-405. [DOI] [PubMed] [Google Scholar]
- 125.Hedman, K., and S. A. Rousseau. 1989. Measurement of avidity of specific IgG for verification of recent primary rubella. J. Med. Virol. 27:288-292. [DOI] [PubMed] [Google Scholar]
- 126.Hemmings, D. G., R. Kilani, C. Nykiforuk, J. Preiksaitis, and L. J. Guilbert. 1998. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 72:4970-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Higuchi, R., C. Fockler, G. Dollinger, and R. Watson. 1993. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Bio/Technology 11:1026-1030. [DOI] [PubMed] [Google Scholar]
- 128.Hiyoshi, M., S. Tagawa, T. Takubo, K. Tanaka, T. Nakao, Y. Higeno, K. Tamura, M. Shimaoka, A. Fujii, M. Higashihata, Y. Yasui, T. Kim, A. Hiraoka, and N. Tatsumi. 1997. Evaluation of the AMPLICOR CMV test for direct detection of cytomegalovirus in plasma specimens. J. Clin. Microbiol. 35:2692-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hocker, J. R., L. N. Cook, G. Adams, and G. P. F. Rabalais. 1990. Ganciclovir therapy of congenital cytomegalovirus pneumonia. Pediatr. Infect. Dis. J. 9:743-745. [DOI] [PubMed] [Google Scholar]
- 130.Hogge, W. A., G. J. Buffone, and J. S. Hogge. 1993. Prenatal diagnosis of cytomegalovirus (CMV) infection: a preliminary report. Prenatal Diagn. 13:131-136. [DOI] [PubMed] [Google Scholar]
- 131.Hohlfeld, P., Y. Vial, C. Maillard-Brignon, B. Vaudaux, and C. L. Fawer. 1991. Cytomegalovirus fetal infection: prenatal diagnosis. Obstet. Gynecol. 78:615-618. [PubMed] [Google Scholar]
- 132.Humar, A., D. Gregson, A. M. Caliendo, A. McGeer, G. Malkan, M. Krajden, P. Corey, P. Greig, S. Walmsley, G. Levy, and T. Mazzulli. 1999. Clinical utility of quantitative cytomegalovirus viral load determination for predicting cytomegalovirus disease in liver transplant recipients. Transplantation 68:1305-1311. [DOI] [PubMed] [Google Scholar]
- 133.Ishii, N., J. Fukushima, T. Kaneko, E. Okada, K. Tani, S. I. Tanaka, K. Hamajima, K. Q. Xin, S. Kawamoto, W. Koff, K. Nishioka, T. Yasuda, and K. Okuda. 1997. Cationic liposomes are a strong adjuvant for a DNA vaccine of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 13:1421-1428. [DOI] [PubMed] [Google Scholar]
- 134.Jacques, S. M., and F. Qureshi. 1993. Chronic intervillositis of the placenta. Arch. Pathol. Lab. Med. 117:1032-1035. [PubMed] [Google Scholar]
- 135.Jiwa, N. M., G. W. van Gemert, A. K. Raap, F. M. van de Rijke, A. Mulder, P. F. Lens, M. M. Salimans, F. E. Zwaan, W. van Dorp, and M. van der Ploeg. 1989. Rapid detection of human cytomegalovirus DNA in peripheral blood leukocytes of viremic transplant recipients by the polymerase chain reaction. Transplantation 48:72-76. [DOI] [PubMed] [Google Scholar]
- 136.Johansson, P. J. H., M. Jonsson, K. Ahlfors, S. A. Ivarsson, L. Svanberg, and C. Guthenberg. 1997. Retrospective diagnosis of congenital cytomegalovirus infection performed by polymerase chain reaction in blood stored on filter paper. Scand. Infect. Dis. 29:465-468. [DOI] [PubMed] [Google Scholar]
- 137.Jones, M. M., M. D. Lidsky, E. J. Brewer, M. D. Yow, and W. D. Williamson. 1986. Congenital cytomegalovirus infection and maternal systemic lupus erythematosus: a case report. Arthritis Rheum. 29:1402-1404. [DOI] [PubMed] [Google Scholar]
- 138.Jordan, M. C., W. E. Rousseau, J. A. Stewart, G. R. Noble, and T. D. Y. Chin. 1973. Spontaneous cytomegalovirus mononucleosis. Ann. Intern. Med. 79:153-160. [DOI] [PubMed] [Google Scholar]
- 139.Kemble, G., G. Duke, R. Winter, and R. Spaete. 1996. Defined large-scale alterations of the human cytomegalovirus genome constructed by cotransfection of overlapping cosmids. J. Virol. 70:2044-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Klemola, E., and L. Kääriäinen. 1965. Cytomegalovirus as a possible cause of disease resembling infectious mononucleosis. Br. Med. J. 2:1099-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kolberg, J., R. Miner, B. Hoo, R. Kelso, J. Wiegand, D. Jekicmcmullen, and W. L. Drew. 1996. Development of a branched DNA signal amplification assay to monitor patients on anti-HCMV therapies. Biologicals 24:216. [Google Scholar]
- 142.Korhonen, M. H., J. Brunstein, H. Haario, A. Katnikov, R. Rescaldani, and K. Hedman. 1999. A new method with general diagnostic utility for the calculation of immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 6:725-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kovats, S., E. K. Main, C. Librach, M. Stubblebine, S. J. Fisher, and R. DeMars. 1990. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248:220-223. [DOI] [PubMed] [Google Scholar]
- 144.Kraat, Y. J., F. S. Stals, M. H. L. Christiaans, T. Lazzarotto, M. P. Landini, and C. A. Bruggeman. 1996. IgM antibody detection for 38 (ppUL80a) and 150 (ppUL32) kDa proteins by immunoblotting: the earliest parameter for acute cytomegalovirus infection in renal transplant recipients. J. Med. Virol. 48:289-294. [DOI] [PubMed] [Google Scholar]
- 145.Krech, U., Z. Konjajev, and M. Jung. 1971. Congenital cytomegalovirus infection in siblings from consecutive pregnancies. Helv. Pediatr. Acta 26:355-362. [PubMed] [Google Scholar]
- 146.Krishna, R. V., O. H. Meurman, T. Ziegler, and U. H. Krech. 1980. Solid-phase enzyme immunoassay for determination of antibodies to cytomegalovirus. J. Clin. Microbiol. 12:46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Laifer, S. A., G. D. Ehrlich, D. S. Haff, M. J. Balsan, and V. P. Scantlebury. 1995. Congenital cytomegalovirus infection in offspring of liver transplant recipient. Clin. Infect. Dis. 20:52-55. [DOI] [PubMed] [Google Scholar]
- 148.Lam, K. M. C., N. Oldenburg, M. A. Khan, V. Gaylore, G. W. Mikhail, P. D. Strouhal, J. M. Middeldorp, N. Banner, and M. Yacoub. 1998. Significance of reverse transcription polymerase chain reaction in the detection of human cytomegalovirus gene transcripts in thoracic organ transplant recipients. J. Heart Lung Transplant. 17:555-565. [PubMed] [Google Scholar]
- 149.Lamy, M. E., K. N. Mulongo, J. F. Gadisseaux, G. Lyon, V. Gaudy, and M. van Lierde. 1992. Prenatal diagnosis of fetal cytomegalovirus infection. Am. J. Obstet. Gynecol. 166:91-94. [DOI] [PubMed] [Google Scholar]
- 150.Landini, M. P., T. Lazzarotto, A. Ripalti, M. X. Guan, and M. La Placa. 1989. Antibody response to recombinant gt11 fusion proteins in cytomegalovirus infections. J. Clin. Microbiol. 27:2324-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Landini, M. P., T. Lazzarotto, G. T. Maine, A. Ripalti, and R. Flanders. 1995. Recombinant mono- and polyantigens to detect cytomegalovirus-specific immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 33:2535-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lazzarotto, T., A. Ripalti, G. Bergamini, M. C. Battista, P. Spezzacatena, F. Campanini, P. Pradelli, S. Varani, L. Gabrielli, G. T. Maine, and M. P. Landini. 1998. Development of a new cytomegalovirus (CMV) immunoglobulin M (IgM) immunoblot for detection of CMV-specific IgM. J. Clin. Microbiol. 36:3337-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lazzarotto, T., B. Guerra, P. Spezzacatena, S. Varani, L. Gabrielli, P. Pradelli, F. Rumpianesi, C. Banzi, L. Bovicelli, and M. P. Landini. 1998. Prenatal diagnosis of congenital cytomegalovirus infection. J. Clin. Microbiol. 36:3540-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lazzarotto, T., C. Galli, R. Pulvirenti, R. Rescaldani, R. Vezzo, A. La Gioia, C. Martinelli, S. La Rocca, G. Agresti, L. Grillner, M. Nordin, M. van Ranst, B. Combs, G. T. Maine, and M. P. Landini. 2001. Evaluation of the Abbott AxSYM cytomegalovirus (CMV) immunoglobulin M (IgM) assay in conjunction with other CMV IgM tests and a CMV avidity assay. Clin. Diagn. Lab. Immunol. 8:196-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lazzarotto, T., G. T. Maine, P. dal Monte, A. Ripalti, and M. P. Landini. 1997. A novel Western blot test containing both viral and recombinant proteins for anticytomegalovirus immunoglobulin M detection. J. Clin. Microbiol. 35:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lazzarotto, T., P. Spezzacatena, P. Pradelli, D. A. Abate, S. Varani, and M. P. Landini. 1997. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin. Diagn. Lab. Immunol. 4:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lazzarotto, T., P. Spezzacatena, S. Varani, L. Gabrielli, P. Pradelli, B. Guerra, and M. P. Landini. 1999. Anticytomegalovirus (anti-CMV) immunoglobulin G avidity in identification of pregnant women at risk of transmitting congenital CMV infection. Clin. Diagn. Lab. Immunol. 6:127-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lazzarotto, T., S. Brojanac, G. T. Maine, and M. P. Landini. 1997. Search for cytomegalovirus specific IgM: comparison between a new Western blot, conventional Western blot, and nine commercially available assays. Clin. Diagn. Lab. Immunol. 4:483-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lazzarotto, T., S. Varani, B. Guerra, A. Nicolosi, M. Lanari, and M. P. Landini. 2000. Prenatal indicators of congenital cytomegalovirus infection. J. Pediatr. 137:90-95. [DOI] [PubMed] [Google Scholar]
- 160.Leach, J. L., D. D. Seomak, J. Osborne, B. Rahill, M. D. Lairmore, and C. L. Anderson. 1996. Isolation from the human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J. Immunol. 157:3317-3322. [PubMed] [Google Scholar]
- 161.Lee, A. H., Y. S. Suh, J. H. Sung, S. H. Yang, and Y. C. Sung. 1997. Comparison of various expression plasmids for the induction of immune response by DNA immunization. Mol. Cells 7:495-501. [PubMed] [Google Scholar]
- 162.Leiser, R., and P. Kaufmann. 1994. Placental structure: in a comparative aspect. Exp. Clin. Endocrinol. 102:122-134. [DOI] [PubMed] [Google Scholar]
- 163.Li, C., X. Yang, W. Tu, and S. R. Riddell. 1997. Human cytomegalovirus matrix protein pp150 is efficiently presented as one of target antigens for cytotoxic T lymphocyte recognition. Chin. Med. J. 110:397-400. [PubMed] [Google Scholar]
- 164.Liesnard, C., C. Donner, F. Brancart, F. Gosselin, M. L. Delforge, and F. Rodesch. 2000. Prenatal diagnosis of congenital cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstet. Gynecol. 95:881-888. [DOI] [PubMed] [Google Scholar]
- 165.Lipitz, S., S. Yagel, E. Shalev, R. Achiron, S. Mashiach, and E. Schiff. 1997. Prenatal diagnosis of fetal primary cytomegalovirus infection. Obstet. Gynecol. 89:763-767. [DOI] [PubMed] [Google Scholar]
- 166.Locatelli, F., E. Percivalle, P. Comoli, R. Maccario, M. Zecca, G. Giorgiani, P. de Stefano, and G. Gerna. 1994. Human cytomegalovirus (HCMV) infection in pediatric patients given allogeneic bone marrow transplantation: role of early antiviral treatment for HCMV antigenaemia on patients' outcome. Br. J. Haematol. 88:64-71. [DOI] [PubMed] [Google Scholar]
- 167.Lynch, L., F. Daffos, D. Emmanuel, Y. Giovangrandi, R. Meisel, F. Forestier, G. Cathomas, and R. L. Berkowitz. 1991. Prenatal diagnosis of fetal cytomegalovirus infection. Am. J. Obstet. Gynecol. 165:714-718. [DOI] [PubMed] [Google Scholar]
- 168.Maine, G. T., R. Stricker, M. Schuler, J. Spesard, S. Brojanac, B. Iriarte, K. Herwig, T. Gramins, B. Combs, J. Wise, H. Simmons, T. Gram, J. Lonze, D. Ruzicki, B. Byrne, J. D. Clifton, L. E. Chovan, D. Wachta, C. Holas, D. Wang, T. Wilson, S. Tomazic-Allen, M. A. Clements, G. L. Wright, Jr., T. Lazzarotto, A. Ripalti, and M. P. Landini. 2000. Development and clinical evaluation of a recombinant-antigen-based cytomegalovirus immunoglobulin M automated immunoassay using the Abbott AxSYM analyzer. J. Clin. Microbiol. 38:1476-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maine, G. T., T. Lazzarotto, and M. P. Landini. 2001. New developments in the diagnosis of maternal and congenital CMV infection. Expert Rev. Mol. Diagn. 1:19-29. [DOI] [PubMed] [Google Scholar]
- 170.Marshall, G. S., R. P. Ricciardi, R. F. Rando, J. Puck, R. W. Ge, S. A. Plotkin, and E. Gonczol. 1990. An adenovirus recombinant that expresses the human cytomegalovirus major envelope glycoprotein and induces neutralizing antibodies. J. Infect. Dis. 162:1177-1181. [DOI] [PubMed] [Google Scholar]
- 171.Marteau, T. M., E. Dormandy, and S. Michie. 2001. A measure of informed choice. Health Expect. 4:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mazzulli, T., R. H. Rubin, M. J. Ferraro, R. T. D'Aquila, S. A. Doveikis, B. R. Smith, T. H. The, and M. S. Hirsch. 1993. Cytomegalovirus antigenemia: correlation in transplant recipients and in persons with AIDS. J. Clin. Microbiol. 31:2824-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mazzulli, T., S. Wood, R. Chua, and S. Walmsley. 1996. Evaluation of the Digene hybrid capture system for detection and quantitation of human cytomegalovirus viremia in human immunodeficiency virus-infected patients. J. Clin. Microbiol. 34:2959-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McMaster, M. T., C. L. Librach, Y. Zhou, K. H. Lim, J. Janatpour, R. De Mars, S. Kovats, C. Damsky, and S. J. Fisher. 1995. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 154:3771-3778. [PubMed] [Google Scholar]
- 175.Morello, C. S., L. D. Cranmer, and D. H. Spector. 2000. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84, a homolog of human cytomegalovirus pp65. J. Virol. 74:3696-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morris, D. J., D. Sims, M. Chiswick, V. K. Das, and V. E. Newton. 1994. Symptomatic congenital cytomegalovirus infection after maternal recurrent infection. Pediatr. Infect. Dis. J. 13:61-64. [DOI] [PubMed] [Google Scholar]
- 177.Mühlemann, K., R. K. Miller, R. Metlay, and M. A. Menegus. 1992. Cytomegalovirus infection of the human placenta: an immunocytochemical study. Hum. Pathol. 23:1234-1237. [DOI] [PubMed] [Google Scholar]
- 178.Mulongo, K. N., M. E. Lamy, and M. van Lierde. 1995. Requirements for diagnosis of prenatal cytomegalovirus infection by amniotic fluid culture. Clin. Diagn. Virol. 4:231-238. [DOI] [PubMed] [Google Scholar]
- 179.Muss-Pinhata, M. M., A. Y. Yamamoto, L. T. M. Figueiredo, M. C. Cervi, and G. Duarte. 1998. Congenital and perinatal cytomegalovirus infection in infants born to mothers infected with human immunodeficiency virus. J. Pediatr. 132:285-290. [DOI] [PubMed] [Google Scholar]
- 180.Naot, Y., E. V. Barnett, and J. S. Remington. 1981. Method for avoiding false-positive results occurring in immunoglobulin M enzyme-linked immunosorbent assay due to presence of both rheumatoid factor and antinuclear antibodies. J. Clin. Microbiol. 14:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Negishi, H., H. Yamada, E. Hirayama, K. Okuyama, T. Sagawa, Y. Matsumoto, and S. Fujimoto. 1998. Intraperitoneal administration of cytomegalovirus hyperimmunoglobulin to the cytomegalovirus-infected fetus. J. Perinat. 18:466-469. [PubMed] [Google Scholar]
- 182.Nelson, C. T., A. S. Istas, M. K. Wilkerson, and G. J. Demmler. 1995. PCR detection of cytomegalovirus DNA in serum as a diagnostic test for congenital cytomegalovirus infection. J. Clin. Microbiol. 33:3317-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nichols, W. G., L. Corey, T. Gooley, W. L. Drew, R. Miner, M.-L. Huang, C. Davis, and M. Boeckh. 2001. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood 97:867-874. [DOI] [PubMed] [Google Scholar]
- 184.Nicolini, U., A. Kustermann, B. Tassis, R. Fogliani, A. Galimberti, E. Percivalle, M. G. Revello, and G. Gerna. 1994. Prenatal diagnosis of congenital human cytomegalovirus infection. Prenatal Diagn. 14:903-906. [DOI] [PubMed] [Google Scholar]
- 185.Nielsen, C. M., K. Hansen, H. M. K. Andersen, J. Gerstoft, and B. F. Vestergaard. 1987. An enzyme labeled nuclear antigen immunoassay for detection of IgM antibodies in human serum: specific and nonspecific reactions. J. Med. Virol. 22:67-76. [DOI] [PubMed] [Google Scholar]
- 186.Nigro, G., R. La Torre, M. M. Anceschi, M. Mazzocco, and E. V. Cosmi. 1999. Hyperimmunoglobulin therapy for a twin fetus with cytomegalovirus infection and growth restriction. Am. J. Obstet. Gynecol. 180:1222-1226. [DOI] [PubMed] [Google Scholar]
- 187.O'Connor, A., and L. L. O'Brien-Pallas. 1989. Decisional conflict, p. 486-496. In G. K. Mcfarlane and E. A. Mcfarlane (ed.), Nursing diagnosis and intervention. Mosby, Toronto, Canada.
- 188.O'Neill, H. J., P. V. Shirodaria, J. H. Connolly, D. I. Simpson, and M. G. McGeown. 1988. Cytomegalovirus-specific antibody responses in renal transplant patients with primary and recurrent CMV infections. J. Med. Virol. 24:461-470. [DOI] [PubMed] [Google Scholar]
- 189.Pass, R. F. 1992. Commentary: is there a role for prenatal diagnosis of congenital cytomegalovirus infection? Pediatr. Infect. Dis. J. 11:608-609. [PubMed] [Google Scholar]
- 190.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 191.Pass, R. F., A.-M. Duliege, S. Boppana, R. Sekulovich, S. Percell, W. Britt, and R. L. Burke. 1999. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J. Infect. Dis. 180:970-975. [DOI] [PubMed] [Google Scholar]
- 192.Pass, R. F., and S. Boppana. 1999. Cytomegalovirus, p. 35-56. In D. J. Jeffries and C. N. Hudson (ed.), Viral infections in obstetrics and gynaecology. Arnold, New York, N.Y.
- 193.Peckam, C. S., J. C. Cileman, R. Hurley, K. S. Chin, K. Henderson, and P. M. Preece. 1983. Cytomegalovirus infection in pregnancy: preliminary findings from a prospective study. Lancet i:1352-1353. [DOI] [PubMed] [Google Scholar]
- 194.Penfold, M. E. T., D. J. Dairaghi, G. M. Duke, N. Saederup, E. S. Mocarski, G. W. Kemble, and T. Schall. 1999. Cytomegalovirus encodes a potent chemokine. Proc. Natl. Acad. Sci. USA 96:9839-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pepperl, S., J. Munster, M. Mach, J. R. Harris, and B. Plachter. 2000. Dense bodies of human cytomegalovirus induce both humoral and cellular immune responses in the absence of viral gene expression. J. Virol. 74:6132-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Percivalle, E., M. G. Revello, L. Vago, F. Morini, and G. Gerna. 1993. Circulating endothelial giant cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infection with organ involvement. J. Clin. Investig. 92:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Plotkin, S. A. 1999. Cytomegalovirus vaccine. Am. Heart J. 138:S484-S487. [DOI] [PubMed] [Google Scholar]
- 198.Plotkin, S. A. 1999. Vaccination against cytomegalovirus, the changeling demon. Pediatr. Infect. Dis. J. 18:313-326. [DOI] [PubMed] [Google Scholar]
- 199.Plotkin, S. A., J. Farquhar, and E. Horberger. 1976. Clinical trials of immunization with the Towne 125 strain of human cytomegalovirus. J. Infect. Dis. 134:470-475. [DOI] [PubMed] [Google Scholar]
- 200.Plotkin, S. A., M. Cadoz, B. Meignier, C. Meric, O. Leroy, J. L. Excler, J. Tartaglia, E. Paoletti, E. Gonczol, and G. Chappuis. 1995. The safety and use of canarypox vectored vaccines. Dev. Biol. Stand. 84:165-170. [PubMed] [Google Scholar]
- 201.Plotkin, S. A., R. Higgins, J. B. Kurtz, P. J. Morris, D. A. Campbell Jr., T. C. Shope, S. A. Spector, and W. M. Dankner. 1994. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation 58:1176-1178. [PubMed] [Google Scholar]
- 202.Plotkin, S. A., S. E. Starr, H. M. Friedman, K. Brayman, S. Harris, S. Jackson, N. B. Tustin, R. Grossman, D. Dafoe, and C. Barker. 1991. Effect of Towne live virus vaccine on cytomegalovirus disease after renal transplant. A controlled trial. Ann. Intern. Med. 114:525-531. [DOI] [PubMed] [Google Scholar]
- 203.Plotkin, S. A., T. Furukawa, N. Zygraich, and C. Huygelen. 1975. Candidate cytomegalovirus strain for human vaccination. Infect. Immun. 12:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rammensee, H. G., K. Falk, and O. Rotzschke. 1993. MHC molecules as peptide receptors. Curr. Opin. Immunol. 5:35-44. [DOI] [PubMed] [Google Scholar]
- 205.Reigstad, H., R. Bjerknes, T. Markestad, and H. Myrmel. 1992. Ganciclovir therapy of congenital cytomegalovirus disease. Acta Pediatr. 81:707-708. [DOI] [PubMed] [Google Scholar]
- 206.Revello, M. G., A. Sarasini, M. Zavattoni, F. Baldanti, and G. Gerna. 1998. Improved prenatal diagnosis of congenital human cytomegalovirus infection by a modified nested polymerase chain reaction. J. Med. Virol. 56:99-103. [DOI] [PubMed] [Google Scholar]
- 207.Revello, M. G., and G. Gerna. 1999. Diagnosis and implications of human cytomegalovirus infection in pregnancy. Fet. Matern. Med. Rev. 11:117-134. [Google Scholar]
- 208.Revello, M. G., D. Lilleri, M. Zavattoni, M. Stronati, L. Bollani, J. M. Middeldorp, and G. Gerna. 2001. Human cytomegalovirus immediate-early messenger RNA in blood of pregnant women with primary infection and of congenitally infected newborns. J. Infect. Dis. 184:1078-1081. [DOI] [PubMed] [Google Scholar]
- 209.Revello, M. G., E. Percivalle, A. Di Matteo, F. Morini, and G. Gerna. 1992. Nuclear expression of the lower matrix protein of human cytomegalovirus in peripheral blood leukocytes of immunocompromised viraemic patients. J. Gen. Virol. 73:437-442. [DOI] [PubMed] [Google Scholar]
- 210.Revello, M. G., E. Percivalle, A. Sarasini, F. Baldanti, M. Furione, and G. Gerna. 1994. Diagnosis of human cytomegalovirus infection of the nervous system by pp65 detection in polymorphonuclear leukocytes of cerebrospinal fluid from AIDS patients. J. Infect. Dis. 170:1275-1279. [DOI] [PubMed] [Google Scholar]
- 211.Revello, M. G., E. Percivalle, E. Arbustini, R. Pardi, S. Sozzani, and G. Gerna. 1998. In vitro generation of human cytomegalovirus pp65 antigenemia, viremia, and leukoDNAemia. J. Clin. Investig. 101:2686-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Revello, M. G., E. Percivalle, F. Baldanti, G. Gerna, A. Kustermann, S. Nava, and U. Nicolini. 1993. Prenatal treatment of congenital human cytomegalovirus infection by fetal intravascular administration of ganciclovir. Clin. Diagn. Virol. 1:61-67. [DOI] [PubMed] [Google Scholar]
- 213.Revello, M. G., E. Percivalle, M. Zannino, V. Rossi, and G. Gerna. 1991. Development and evaluation of a capture ELISA for IgM antibody to the human cytomegalovirus major DNA binding protein. J. Virol. Methods 35:315-329. [DOI] [PubMed] [Google Scholar]
- 214.Revello, M. G., E. Percivalle, M. Zavattoni, M. Parea, P. Grossi, and G. Gerna. 1989. Detection of human cytomegalovirus immediate early antigen in leukocytes as a marker of viremia in immunocompromised patients. J. Med. Virol. 29:88-93. [DOI] [PubMed] [Google Scholar]
- 215.Revello, M. G., F. Baldanti, M. Furione, A. Sarasini, E. Percivalle, M. Zavattoni, and G. Gerna. 1995. Polymerase chain reaction for prenatal diagnosis of congenital human cytomegalovirus infection. J. Med. Virol. 47:462-466. [DOI] [PubMed] [Google Scholar]
- 216.Revello, M. G., M. Zavattoni, A. Sarasini, E. Percivalle, L. Simoncini, and G. Gerna. 1998. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J. Infect. Dis. 177:1170-1175. [DOI] [PubMed] [Google Scholar]
- 217.Revello, M. G., M. Zavattoni, A. Sarasini, F. Baldanti, C. De Julio, L. De-Giuli, U. Nicolini, and G. Gerna. 1999. Prenatal diagnostic and prognostic value of human cytomegalovirus load and IgM antibody response in blood of congenitally infected fetuses. J. Infect. Dis. 180:1320-1323. [DOI] [PubMed] [Google Scholar]
- 218.Revello, M. G., M. Zavattoni, F. Baldanti, A. Sarasini, S. Paolucci, and G. Gerna. 1999. Diagnostic and prognostic value of human cytomegalovirus load and IgM antibody in blood of congenitally infected newborns. J. Clin. Virol. 14:57-66. [DOI] [PubMed] [Google Scholar]
- 219.Revello, M. G., M. Zavattoni, M. Furione, F. Baldanti, and G. Gerna. 1999. Quantification of human cytomegaloviral DNA in amniotic fluid of mothers of congenitally infected fetuses. J. Clin. Microbiol. 37:3350-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219a.Revello M. G., M. Zavattoni, M. Furione, D. Lilleri, G. Gorini, and G. Gerna. 2002. Diagnosis and outcome of preconceptional and periconceptional primary human cytomegalovirus infections. J. Infect. Dis. 186:553-557. [DOI] [PubMed] [Google Scholar]
- 220.Reynolds, D. W., and S. Stagno. 1979. Laboratory diagnosis of cytomegalovirus infections, p. 399-439. In E. H. Lennette and N. J. Schmidt (ed.). Diagnostic procedures for viral, rickettsial, and chlamydial infections, 5th ed. American Public Health Association, Washington, D.C.
- 221.Rinaldo, C. R., P. H. Black, and M. S. Hirsch. 1977. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J. Infect. Dis. 136:667-668. [DOI] [PubMed] [Google Scholar]
- 222.Rinaldo, C. R., W. Carney, B. S. Richter, P. H. Black, and M. S. Hirsch. 1980. Mechanism of immunosuppression in cytomegaloviral mononucleosis. J. Infect. Dis. 141:488-495. [DOI] [PubMed] [Google Scholar]
- 223.Ripalti, A., Q. Ruan, M. C. Boccuni, F. Campanini, G. Bergamini, and M. P. Landini. 1994. Construction of polyepitope fusion antigens of human cytomegalovirus ppUL32: reactivity with human antibodies. J. Clin. Microbiol. 32:358-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ripalti, A., P. dal Monte, M. C. Boccuni, F. Campanini, G. Bergamini, T. Lazzarotto, B. Campisi, Q. Ruan, and M. P. Landini. 1994. Procaryotic expression of a large fragment of the most antigenic cytomegalovirus DNA-binding protein (pp UL44) and its reactivity with human antibodies. J. Virol. Methods 46:39-50. [DOI] [PubMed] [Google Scholar]
- 225.Roberts, T. C., D. C. Brennan, R. S. Buller, M. Gaudreault-Keener, M. A. Schnitzler, K. E. Sternhell, K. A. Garlock, G. G. Singer, and G. A. Storch. 1998. Quantitative polymerase chain reaction to predict occurrence of symptomatic cytomegalovirus infection and assess response to ganciclovir therapy in renal transplant recipients. J. Infect. Dis. 178:626-635. [DOI] [PubMed] [Google Scholar]
- 226.Roth, I., and S. J. Fisher. 1999. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev. Biol. 205:194-204. [DOI] [PubMed] [Google Scholar]
- 227.Roth, I., D. B. Corry, R. M. Locksley, J. S. Abrams, M. J. Litton, and S. J. Fisher. 1996. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 184:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rowe, W. P., J. W. Hartley, S. Waterman, H. C. Turner, and R. J. Huebner. 1956. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc. Soc. Exp. Biol. Med. 92:418-422. [PubMed] [Google Scholar]
- 229.Rowley, A. H., S. M. Wolinski, S. P. Sambol, L. Barkholt, A. Ehrnst, and J. P. Andersson. 1991. Rapid detection of cytomegalovirus DNA and RNA in blood of renal transplant patients by in vitro enzymatic amplification. Transplantation 51:1028-1033. [DOI] [PubMed] [Google Scholar]
- 230.Ruellan-Eugene, G., P. Barjot, M. Campet, A. Vabret, M. Herlicoviez, G. Muller, G. Levy, B. Guillois, and F. Freymuth. 1996. Evaluation of virological procedures to detect fetal human cytomegalovirus infection: avidity of IgG antibodies, virus detection in amniotic fluid and maternal serum. J. Med. Virol. 50:9-15. [DOI] [PubMed] [Google Scholar]
- 231.Saigal, S., W. A. Eisele, and M. A. Chernesky. 1982. Congenital cytomegalovirus infection in a pair of dizygotic twins. Am. J. Dis. Child. 136:1094-1095. [DOI] [PubMed] [Google Scholar]
- 232.Salzberger, B., D. Myerson, and M. Boeckh. 1997. Circulating cytomegalovirus (CMV)-infected endothelial cells in marrow transplant recipients with CMV disease and CMV infection. J. Infect. Dis. 176:778-781. [DOI] [PubMed] [Google Scholar]
- 233.Schild, H., K. Deres, K. H. Wiesmuller, G. Jung, and H. G. Rammensee. 1991. Efficiency of peptides and lipopeptides for in vivo priming of virus-specific cytotoxic T cells. Eur. J. Immunol. 21:2649-2654. [DOI] [PubMed] [Google Scholar]
- 234.Schmitz, H., H. W. Doerr, D. Kampa, and A. Vogt. 1977. Solid-phase enzyme immunoassay for immunoglobulin M antibodies to human cytomegalovirus. J. Clin. Microbiol. 5:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schmitz, H., U. von Deimling, and B. Fleming. 1980. Detection of IgM antibodies to cytomegalovirus (CMV) using an enzyme-labeled antigen [ELA]. J. Gen. Virol. 50:59-68. [DOI] [PubMed] [Google Scholar]
- 236.Schneeberger, P. M., F. Groenendaal, L. S. de Vries, A. M. van Loon, and T. M. Vroom. 1994. Variable outcome of a congenital cytomegalovirus infection in a quadruplet after primary infection of the mother during pregnancy. Acta Paediatr. 83:986-989. [DOI] [PubMed] [Google Scholar]
- 237.Schopfer, K., E. Lauber, and U. Krech. 1978. Congenital cytomegalovirus infection in newborn infants of mothers infected before pregnancy. Arch. Dis. Child. 53:536-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schoub, B. D., S. Johnson, J. M. McAnerney, N. K. Blackburn, F. Guidozzi, D. Ballot, and A. Rothberg. 1993. Is antenatal screening for rubella and cytomegalovirus justified? South Afr. Med. J. 83:108-110. [PubMed] [Google Scholar]
- 239.Schwebke, K., K. Henry, H. H. Balfour, D. Olson, R. T. Crane, and M. C. Jordan. 1995. Congenital cytomegalovirus infection as a result of nonprimary cytomegalovirus disease in a mother with acquired immunodeficiency syndrome. J. Pediatr. 126:293-295. [DOI] [PubMed] [Google Scholar]
- 240.Shibata, D., W. J. Martin, M. D. Appleman, D. M. Causey, J. M. Leedom, and N. Arnheim. 1988. Detection of cytomegalovirus in peripheral blood of patients infected with human immunodeficiency virus. J. Infect. Dis. 158:1185-1192. [DOI] [PubMed] [Google Scholar]
- 241.Shibata, M., H. Takano, T. Hironaka, and K. Hirai. 1994. Detection of human cytomegalovirus DNA in dried newborn blood filter paper. J. Virol. Methods 46:279-285. [DOI] [PubMed] [Google Scholar]
- 242.Shuster, E. A., J. S. Beneke, G. E. Tegtmeier, G. P. Pearson, C. A. Gleaves, A. D. Wold, and T. F. Smith. 1985. Monoclonal antibody for rapid laboratory detection of cytomegalovirus infections: characterization and diagnostic application. Mayo Clin. Proc. 60:577-585. [DOI] [PubMed] [Google Scholar]
- 243.Sia, I. G., J. A. Wilson, C. Groettum, M. J. Epsy, T. F. Smith, and C. V. Paya. 2000. Cytomegalovirus DNA load predicts relapsing CMV infection after solid organ transplantation J. Infect. Dis. 181:717-720. [DOI] [PubMed] [Google Scholar]
- 244.Simister, N. E., C. M. Story, H. L. Chen, and J. S. Hunt. 1996. An IgG transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur. J. Immunol. 26:1527-1531. [DOI] [PubMed] [Google Scholar]
- 245.Simpson, W. M., F. D. Johnstone, F. M. Boyd, D. J. Goldberg, G. J. Hart, and R. J. Prescott. 1998. Uptake and acceptability of antenatal human immunodeficiency virus testing: randomised controlled trial of different methods of offering the test. Br. Med. J. 316:262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sinzger, C., A. Grefte, B. Plachter, A. S. Gouw, T. H. The, and G. Jahn. 1995. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76:741-750. [DOI] [PubMed] [Google Scholar]
- 247.Sinzger, C., H. Müntefering, T. Löning, H. Stöss, B. Plachter, and G. Jahn. 1993. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch. A Pathol. Anat. Histopathol. 423:249-256. [DOI] [PubMed] [Google Scholar]
- 248.Smith, D., R. W. Shaw, and T. Marteau. 1994. Lack of knowledge in health professionals: a barrier to providing information to patients. Quality Health Care 3:75-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Smith, K. L., J. K. Kulski, T. Cobain, and R. A. Dunstan. 1993. Detection of cytomegalovirus in blood donors by the polymerase chain reaction. Transfusion 33:497-503. [DOI] [PubMed] [Google Scholar]
- 250.Smith, M. G. 1956. Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc. Soc. Exp. Biol. Med. 92:424-430. [DOI] [PubMed] [Google Scholar]
- 251.Smith, S. C., P. N. Baker, and E. M. Symonds. 1997. Placental apoptosis in normal human placenta. Am. J. Obstet. Gynecol. 177:57-65. [DOI] [PubMed] [Google Scholar]
- 252.Söderberg-Nauclér, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 253.Söderberg-Nauclér, C., D. N. Streblow, K. N. Fish, J. Allan-Yorke, P. P. Smith, and J. A. Nelson. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Spaete, R. R. 1991. A recombinant subunit vaccine approach to HCMV vaccine development. Transplant Proc. 23:90-96. [PubMed] [Google Scholar]
- 255.Spector, D. H., and S. A. Spector. 1984. The oncogenic potential of human cytomegalovirus. Prog. Med. Virol. 29:45-89. [PubMed] [Google Scholar]
- 256.Spencer, E. S., and H. K. Andersen. 1972. The development of immunofluorescent antibodies compared with complement-fixing and virus-neutralizing antibodies in human cytomegalovirus infection. Scand. J. Infect. Dis. 4:109-112. [DOI] [PubMed] [Google Scholar]
- 257.Sperling, R. S., D. E. Shapiro, R. W. Coombs, J. A. Todd, S. A. Herman, G. D. McSherry, M. J. O'Sullivan, R. B. van Dyke, E. Jimenez, C. Rouzioux, P. M. Flynn, and U. L. Sullivan. 1996. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N. Engl. J. Med. 335:1621-1629. [DOI] [PubMed] [Google Scholar]
- 258.Stagno, S. 2001. Cytomegalovirus, p. 389-424. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant. W. B. Saunders Co., Philadelphia, Pa.
- 259.Stagno, S., and R. J. Whitley. 1985. Herpesvirus infection of pregnancy. N. Engl. J. Med. 313:1270-1274. [DOI] [PubMed] [Google Scholar]
- 260.Stagno, S., D. W. Reynolds, A. Lakeman, L. J. Charamella, and C. A. Alford. 1973. Congenital cytomegalovirus infection: consecutive occurrence due to viruses with similar antigenic compositions. Pediatrics 52:788-794. [PubMed] [Google Scholar]
- 261.Stagno, S., D. W. Reynolds, E. S. Huang, S. D. Thames, R. J. Smith, and C. A. Alford. 1977. Congenital cytomegalovirus infection: occurrence in an immune population. N. Engl. J. Med. 296:1254-1258. [DOI] [PubMed] [Google Scholar]
- 262.Stagno, S., M. K. Tinker, C. Elrod, D. A. Fuccillo, G. Cloud, and A. J. O'Beirne. 1985. Immunoglobulin M antibodies detected by enzyme-linked immunosorbent assay and radioimmunoassay in the diagnosis of cytomegalovirus infections in pregnant women and newborn infants. J. Clin. Microbiol. 21:930-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Stagno, S., R. F. Pass, D. W. Reynolds, M. A. Moore, A. J. Nahmias, and C. A. Alford. 1980. Comparative study of diagnostic procedures for congenital cytomegalovirus infection. Pediatrics 65:251-257. [PubMed] [Google Scholar]
- 264.Stagno, S., R. F. Pass, G. Cloud, W. J. Britt, R. E. Henderson, P. D. Walton, D. A. Veren, F. Page, and C. A. Alford. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to the fetus and clinical outcome. JAMA 256:1904-1908. [PubMed] [Google Scholar]
- 265.Stagno, S., R. F. Pass, M. E. Dworsky, and C. A. Alford. 1982. Maternal cytomegalovirus infection and perinatal transmission. Clin. Obstet. Gynecol. 25:563-576. [DOI] [PubMed] [Google Scholar]
- 266.Stagno, S., R. F. Pass, M. E. Dworsky, R. E. Henderson, E. G. Moore, P. D. Walton, and C. A. Alford. 1982. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 306:945-949. [DOI] [PubMed] [Google Scholar]
- 267.Stalder, H., and A. Ehrensberger. 1980. Microneutralization of cytomegalovirus. J. Infect. Dis. 142:102-105. [DOI] [PubMed] [Google Scholar]
- 268.Stanier, P., D. L. Taylor, A. D. Kitchen, N. Wales, Y. Tryhorn, and A. S. Tyms. 1989. Persistence of cytomegalovirus in mononuclear cells in peripheral blood from blood donors. Br. Med. J. 299:897-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stern, H., G. Hannington, J. Booth, and D. Moncrieff. 1986. An early marker of fetal infection after primary cytomegalovirus infection in pregnancy. Br. Med. J. 15:718-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Stirk, P. R., and P. D. Griffiths. 1987. Use of monoclonal antibodies for the diagnosis of cytomegalovirus infection by the detection of early antigen fluorescent foci (DEAFF) in cell culture. J. Med. Virol. 21:329-337. [DOI] [PubMed] [Google Scholar]
- 271.Stronati, M., M. G. Revello, R. M. Cerbo, M. Furione, G. Rondini, and G. Gerna. 1995. Ganciclovir therapy of congenital human cytomegalovirus hepatitis. Acta Pediatr. 84:340-341. [DOI] [PubMed] [Google Scholar]
- 272.Taylor, J., R. Weinberg, J. Tartaglia, C. Richardson, G. Alkhatib, D. Briedis, M. Appel, E. Norton, and E. Paoletti. 1992. Nonreplicating viral vectors as potential vaccines: recombinant canarypox virus expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology 187:321-328. [DOI] [PubMed] [Google Scholar]
- 273.Taylor-Wiedeman, J., J. G. Sissons, L. K. Borysiewicz, and J. H. Sinclair. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059-2064. [DOI] [PubMed] [Google Scholar]
- 274.The, T. H., M. van der Ploeg, A. P. van den Berg, A. M. Vlieger, M. van der Giessen, and W. J. van Son. 1992. Direct detection of cytomegalovirus in peripheral blood leukocytes: a review of the antigenemia assay and polymerase chain reaction. Transplantation 54:193-198. [DOI] [PubMed] [Google Scholar]
- 275.Tricoire, J., M. Rolland, and C. Regnier. 1993. Traitment par ganciclovir des infections congenitales à cytomegalovirus. Arch. Fr. Pediatr. 50:17. [PubMed] [Google Scholar]
- 276.Urban, M., M. Klein, W. J. Britt, E. Hassfurther, and M. Mach. 1996. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J. Gen. Virol. 77:1537-1547. [DOI] [PubMed] [Google Scholar]
- 277.Van den Berg, A. P., W. van der Bij, W. J. van Son, J. Anema, M. van der Giessen, J. Schirm, A. M. Tegzess, and T. H. The. 1989. Cytomegalovirus antigenemia as a useful marker of symptomatic cytomegalovirus infection after renal transplantation -a report of 130 consecutive patients. Transplantation 48:991-995. [DOI] [PubMed] [Google Scholar]
- 278.Van der Bij, W., R. Torensma, W. J. van Son, J. Anema, J. Schirm, A. M. Tegzess, and T. H. The. 1988. Rapid immunodiagnosis of active cytomegalovirus infection by monoclonal antibody staining of blood leukocytes. J. Med. Virol. 25:179-188. [DOI] [PubMed] [Google Scholar]
- 279.Van Loon, A. M., F. W. A. Heessen, J. Th. M. van der Logt, and J. van der Veen. 1981. Direct enzyme-linked immunosorbent assay that uses peroxidase-labeled antigen for determination of immunoglobulin M antibody to cytomegalovirus. J. Clin. Microbiol. 13:416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Vornhagen, R., B. Plachter, W. Hinderer, T. H. The, J. Van Zanten, L. Matter, C. A. Schmidt, H. H. Sonneborn, and G. Jahn. 1994. Early serodiagnosis of acute human cytomegalovirus infection by enzyme-linked immunosorbent assay using recombinant antigens. J. Clin. Microbiol. 32:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Vornhagen, R., W. Hinderer, H. H. Sonneborn, G. Bein, L. Matter, T. H. The, G. Jahn, and B. Plachter. 1995. The DNA-binding protein pUL57 of human cytomegalovirus is a major target antigen for the immunoglobulin M antibody response during acute infection. J. Clin. Microbiol. 33:1927-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang, J. B., S. P. Adler, S. Hempfling, R. L. Burke, A. M. Duliege, S. E. Starr, and S. A. Plotkin. 1996. Mucosal antibodies to human cytomegalovirus glycoprotein B occur following both natural infection and immunization with human cytomegalovirus vaccines. J. Infect. Dis. 174:387-392. [DOI] [PubMed] [Google Scholar]
- 283.Ward, K. N., W. Dhaliwal, K. L. Ashworth, E. J. Clutterbuck, and C. G. Teo. 1994. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J. Med. Virol. 43:367-372. [DOI] [PubMed] [Google Scholar]
- 284.Warren, W. P., K. Balcarek, R. J. Smith, and R. F. Pass. 1992. Comparison of rapid methods of detection of cytomegalovirus in saliva with virus isolation in tissue culture. J. Clin. Microbiol. 30:786-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Weiner, C. P. 1988. Cordocentesis. Obstet. Gynecol. Clin. North Am. 15:283-301. [PubMed] [Google Scholar]
- 286.Weiner, C. P., C. F. Grose, and S. J. Naides. 1993. Diagnosis of fetal infection in the patient with an ultrasonographically detected abnormality but a negative clinical history. Am. J. Obstet. Gynecol. 168:6-11. [DOI] [PubMed] [Google Scholar]
- 287.Weller, T. H. 1970. Cytomegaloviruses: the difficult years. J. Infect. Dis. 122:532-539. [DOI] [PubMed] [Google Scholar]
- 288.Weller, T. H., J. C. Macaulay, J. M. Craig, and P. Wirth. 1957. Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Proc. Soc. Exp. Biol. Med. 94:4-12. [DOI] [PubMed] [Google Scholar]
- 289.Whitley, R. J., G. Cloud, W. Gruber, G. A. Storch, G. J. Demmler, R. F. Jacobs, W. Dankner, S. A. Spector, S. Starr, R. F. Pass, S. Stagno, W. J. Britt, C. Alford Jr., S. Soong, X. J. Zhou, L. Sherrill, J. M. Fitzgerald, and J. P. Sommadossi. 1997. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: results of a Phase II study. J. Infect. Dis. 175:1080-1086. [DOI] [PubMed] [Google Scholar]
- 290.Williamson, D. W., G. J. Demmler, A. K. Percy, and F. I. Catlin. 1992. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics 90:862-866. [PubMed] [Google Scholar]
- 291.Wills, M. R., A. J. Carmichael, K. Mynard, X. Jin, M. P. Weekes, B. Plachter, and J. G. Sissons. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70:7569-7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yow, M. D., and G. J. Demmler. 1992. Congenital cytomegalovirus disease - 20 years is long enough. N. Engl. J. Med. 326:702-703. [DOI] [PubMed] [Google Scholar]
- 293.Yow, M. D., D. W. Williamson, L. J. Leeds, P. Thompson, R. M. Woodward, B. F. Walmus, J. W. Lester, H. R. Six, and P. D. Griffiths. 1988. Epidemiologic characteristics of cytomegalovirus infection in mothers and their infants. Am. J. Obstet. Gynecol. 158:1189-1195. [DOI] [PubMed] [Google Scholar]
- 294.Zanghellini, F., S. B. Boppana, V. C. Emery, P. D. Griffiths, and R. F. Pass. 1999. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J. Infect. Dis. 180:702-707. [DOI] [PubMed] [Google Scholar]
- 295.Zhou, Y., S. J. Fisher, M. Janatpour, O. Genbacev, E. Dejana, M. Wheelock, and C. H. Damsky. 1997. Human cytotrophoblasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J. Clin. Investig. 99:2139-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]