Abstract
Acute septic arthritis may develop as a result of hematogenous seeding, direct introduction, or extension from a contiguous focus of infection. The pathogenesis of acute septic arthritis is multifactorial and depends on the interaction of the host immune response and the adherence factors, toxins, and immunoavoidance strategies of the invading pathogen. Neisseria gonorrhoeae and Staphylococcus aureus are used in discussing the host-pathogen interaction in the pathogenesis of acute septic arthritis. While diagnosis rests on isolation of the bacterial species from synovial fluid samples, patient history, clinical presentation, laboratory findings, and imaging studies are also important. Acute nongonococcal septic arthritis is a medical emergency that can lead to significant morbidity and mortality. Therefore, prompt recognition, rapid and aggressive antimicrobial therapy, and surgical treatment are critical to ensuring a good prognosis. Even with prompt diagnosis and treatment, high mortality and morbidity rates still occur. In contrast, gonococcal arthritis is often successfully treated with antimicrobial therapy alone and demonstrates a very low rate of complications and an excellent prognosis for full return of normal joint function. In the case of prosthetic joint infections, the hardware must be eventually removed by a two-stage revision in order to cure the infection.
SOURCE OF INFECTION
Most septic joints develop as a result of hematogenous seeding of the vascular synovial membrane due to a bacteremic episode (86, 113). Although a rare cause, acute septic arthritis may also occur as a result of joint aspiration or local corticosteroid joint injection (74, 86). In addition, bacterial arthritis may arise secondary to penetrating trauma (such as human or animal bite or nail puncture) or after trauma to a joint without an obvious break in the skin. The direct introduction of bacteria during joint surgery has increasingly been a source of bacterial arthritis, particularly in association with knee and hip arthroplasties. When a bone infection breaks through the outer cortex and into the intracapsular region, a joint infection may also result, especially in children (9, 117). In infants, small capillaries cross the epiphyseal growth plate and permit extension of infection into the epiphysis and joint space (22). In children older than 1 year, osteomyelitis infection presumably starts in the metaphyseal sinusoidal veins and is usually contained by the growth plate. The joint is spared unless the metaphysis is intracapsular. The infection spreads laterally, where it breaks through the cortex and lifts the loose periosteum to form a subperiosteal abscess. In adults, the growth plate has resorbed and the infection may again extend to the joint spaces.
MICROBIOLOGY
Virtually every bacterial organism has been reported to cause septic arthritis. The microorganisms responsible for bacterial arthritis are largely dependent on host factors (see “Risk Factors” below). The most common etiological agent of all septic arthritis cases in Europe and all nongonococcal cases in the United States is Staphylococcus aureus (9, 34, 39, 94, 141). The representation of S. aureus is more pronounced in patients with either rheumatoid arthritis or diabetes. After S. aureus, Streptococcus spp. are the next most commonly isolated bacteria from adult patients with septic arthritis (58, 94, 113, 141, 153, 183). While one study had a high representation of Streptococcus pneumoniae (113), Streptococcus pyogenes is usually the most common streptococcal isolate, often associated with autoimmune diseases, chronic skin infections, and trauma (94, 113, 141, 153). Groups B, G, C, and F, in order of decreasing preponderance, are also isolated, especially in patients with immunocompromise, diabetes mellitus, malignancy, and severe genitourinary or gastrointestinal infections (94, 113, 141, 153). Gram-negative bacilli account for approximately 10 to 20% of cases (34, 94, 113, 141, 153, 183). Patients with a history of intravenous drug abuse, extremes of age, or immunocompromise display a higher prevalence of infection by gram-negative organisms. The most common gram-negative organisms are Pseudomonas aeruginosa and Escherichia coli. Anaerobes are also isolated in a small percentage of cases, usually in diabetic patients and patients with prosthetic joints. Approximately 10% of patients with nongonococcal septic arthritis have polymicrobial infections.
Historically, Haemophilus influenzae, S. aureus, and group A streptococci were the most common causes of infectious arthritis in children younger than 2 years. However, the overall incidence of H. influenzae as a cause of septic arthritis is decreasing because of the H. influenzae type b (Hib) vaccine now given to children (35). A recent study of 165 cases of acute hematogenous osteomyelitis or septic arthritis treated in the years before and after the advent of the Hib vaccine demonstrated that musculoskeletal infections due to this bacterial species were reduced to nearly nonexistent levels (18). Therefore, the coverage of H. influenzae as part of the empiric antibiotic coverage may no longer be needed in the management of acute septic arthritis in Hib-vaccinated children. While H. influenzae has lost its predominance as the most commonly identified gram-negative pathogen in pediatric populations, the normal oropharyngeal resident of young children, Kingella kingae, may have taken its place, specifically in patients younger than 2 year (102, 103, 190, 192). In fact, a recent study found that the nearly half of the clinical isolates from patients younger than 2 years with acute septic arthritis were K. kingae (190). However, these results have yet to be repeated in other regions. Clinical data suggest that the organism may gain access to the bloodstream in the course of an upper respiratory infection or stomatitis (191). In children older than 2 years, S. aureus, streptococci, H. influenzae, and N. gonorrhoea have usually been isolated (33, 47, 185), although H. influenzae may have also lost its predominance in patients in this age group (102).
Microbiological associations exist with concomitant disease states. Septic arthritis following cases of infectious diarrhea may be caused by Shigella spp., Salmonella spp., Campylobacter spp., or Yersinia spp. (50, 82). However, these cases may reflect a form of reactive arthritis. A rare form of migrating polyarthritis may be caused by Streptobacillus moniliformis. In human immunodeficiency virus (HIV)-infected patients, S. aureus continues to be the most common isolate (approximately 30%) (178). However, increased numbers of opportunistic pathogens are isolated from this patient subset, including S. pneumoniae, mycobacterial species, and fungal species (149, 178).
While relatively rare in Western Europe, the diplococcus gram-negative bacterial species Neisseria gonorrhoeae is the most common cause of septic arthritis in United States (94, 122, 141). The number of cases of gonorrhea decreased by 72% between 1975 and 1997, and this decrease was correlated with a reduction in disseminated gonococcal infection and arthritis (26). However, the reported rate has increased by 9.2% between 1997 and 1999 and now stands at 133.2 cases per 100,000 per year (26). Specifically, the rate of gonococcal infection in men who have sex with men has demonstrated an alarming increase. These increased incidence rates may also cause larger numbers of observed gonococcal arthritis cases.
PATHOGENESIS
The pathogenesis of acute septic arthritis is multifactorial and depends on the interaction of the host immune response and the invading pathogen. By taking into account the steps of bacterial colonization, infection and induction of the host inflammatory response, one may gain a greater understanding of this joint disease.
Nongonococcal Arthritis
Since S. aureus has been extensively studied with regard to its role in septic arthritis and causes the majority of cases in most nations (and the majority of nongonococcal cases in the United States), we will use this bacterial species as the “typical” pathogen in the discussion of acute nongonococcal septic arthritis.
Joint colonization and bacterial adherence.
The synovial membrane has no limiting basement plate under the well-vascularized synovium; this allows easy hematogenous entry of bacteria. As mentioned above, bacteria may also gain entry into the joint by direct introduction or extension from a contiguous site of infection. Once bacteria are seeded within the closed joint space, the low fluid shear conditions enable bacterial adherence and infection. Colonization may also be aided in cases where the joint has undergone recent injury. In this environment, the production of host-derived extracellular matrix proteins that aid in joint healing (e.g., fibronectin) may promote bacterial attachment and progression to infection. The virulence and tropism of the microorganisms, combined with the resistance or susceptibility of the synovium to microbial invasion, are major determinants of joint infection. S. aureus, Streptococcus spp., and N. gonorrhoeae are examples of bacteria that have a high degree of selectivity for the synovium, probably related to adherence characteristics and toxin production. Aerobic gram-negative bacilli such as Escherichia coli rarely infect the synovium except in the presence of an underlying and compromising condition. The virulence of the organism once inside the joint varies. In rabbits, intra-articular injection of 10 5 S. aureus organisms into the knee joint resulted in major joint destruction but identical injections of N. gonorrhoeae or S. epidermidis caused no joint inflammation (57).
S. aureus has a variety of receptors, termed microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), for host proteins that mediate adherence to the joint extracellular matrix or implanted medical devices (68, 142, 189). Some of the host matrix proteins include fibronectin and laminin (adherence proteins), elastin (imparts elastic properties), collagen (structural support), and hyaluronic acid (a glycosaminoglycan that is rich in the joints and the matrix and provides cushioning through hydration of its polysaccharides). A number of adhesin genes have been determined and include genes encoding fibrinogen binding proteins (fib, cflA, and fbpA) (17, 27, 105), fibronectin binding proteins (fnbA and fnbB) (76), a collagen receptor (cna) (126), an elastin binding protein (ebpS) (124), and a broad-specificity adhesin (map) that mediates low-level binding of several proteins including osteopontin, collagen, bone sialoprotein, vitronectin, fibronectin, and fibrinogen (106). Also, S. aureus possesses a number of other host protein binding receptors whose genes have not yet been determined. These include a laminin binding protein (52 kDa) (99), a lactoferrin binding protein (450 kDa) (114), and a transferrin binding protein (42 kDa) (112). The staphylococcal receptor that binds laminin may be used in extravasation (100). These receptors specific to S. aureus were absent from the noninvasive pathogen S. epidermidis (100). The lactoferrin and transferrin receptors bind to host iron acquisition proteins and may be used as adhesins and/or as iron acquisition mechanisms.
Increasing evidence supports the importance of staphylococcal surface components as virulence determinants by enabling initial colonization. In a number of studies, mutations in these receptors strongly reduced the ability of staphylococci to produce infection. In a murine septic arthritis model, inoculation of mice with mutants containing mutations of the collagen adhesin gene showed that septic arthritis occurred 43% less often than in the corresponding wild type (166). Also, vaccination with a recombinant fragment of the S. aureus collagen adhesin was able to reduce the sepsis-induced mortality rate to 13%, compared with 87% in the control group (120). However, the role of collagen adhesion of S. aureus as a major virulence factor has recently been questioned since approximately 30 to 60% of clinical isolates do not display collagen binding in vitro or the cna-encoded collagen adhesin (170). Staphyloccal fibronectin binding proteins (FbpA and FbpB) may play a major role in the colonization and virulence of septic arthritis. In a recent study, all of the tested clinical isolates (n = 163) contained one or both of the coding regions for these binding proteins and 95% of these strains had a comparable fibronectin binding capacity to that seen in a staphylococcal reference strain known to efficiently bind fibronectin (127). In addition, an in vivo study of endocarditis in a rat model showed that mutants deficient for fibronectin binding protein were 250-fold less adherent to traumatized heart valves (90). Also, S. aureus adherence to miniplates from iliac bones of guinea pigs was three times higher for the wild-type strain than for the adhesin-defective mutant strain (48). It is likely fibronectin binding proteins play an important role in joint infections, especially those associated with joint trauma or implanted medical devices (125).
These receptors may play an additional role in an intracellular immunoavoidance strategy. S. aureus survives intracellularly after internalization by cultured osteoblasts (72). Staphylococci have demonstrated internalization into other cultured mammalian cells as well as osteoblasts; these include bovine mammary gland epithelial cells, human umbilical vein endothelial cells, and pulmonary epithelial cells isolated from a cystic fibrosis patient (80, 91, 108). Initial adherence to glandular epithelial cells is mediated by S. aureus fibronectin receptors (91), possibly using fibronectin as a bridge between the host cell and the bacterial receptors. Following adherence, bacteria may be internalized by host mechanisms involving membrane pseudopod formation (seen in established bovine mammary epithelial cell lines) or through receptor-mediated endocytosis via clathrin-coated pits (seen in mouse osteoblasts and epithelial cells) (42, 91). In either case, the dependence on the action of host cytoskeletal rearrangements through microfilaments is evident.
Following internalization, staphylococci may induce apoptosis (via a host caspase-dependent mechanism) or survive intracellularly (12, 91, 108, 186). Induced apoptosis may exacerbate the host cell damage seen in septic arthritis. Also, staphylococci may escape clearance by the immune system and antimicrobial therapy by persisting within these host cells. This survival was recently demonstrated in vivo when S. aureus cells were found in the cytoplasm of embryonic chicken osteoblasts and osteocytes in mineralized bone matrix (131). In another study, S. aureus was found within polymorphonuclear neutrophils in an in vivo infection model, and these infected host cells were able to establish infection in naïve animals (65). Therefore, this pathogen may utilize invasion as an immunoavoidance technique during the host inflammatory response. After the downregulation of the adaptive immune response through T-cell apoptosis (mediated by superantigens, other toxins, and invasion), fulminant and/or persistent infection may result.
Joint infection and the host immune response.
Once colonized, bacteria are able to rapidly proliferate and activate an acute inflammatory response. Initially, host inflammatory cytokines, including interleukin 1-β (IL-1β) and interleukin 6 (IL-6), are released into the joint fluid by synovial cells (88). These cytokines activate the release of acute-phase proteins (e.g., C-reactive protein) from the liver that bind to the bacterial cells and thereby promote opsonization and activation of the complement system. In addition, there is an accompanying influx of host inflammatory cells into the synovial membrane early in the infection. Phagocytosis of the bacteria by macrophages, synoviocytes, and polymorphonuclear cells occurs and is associated with the release of other inflammatory cytokines that include tumor necrosis factor alpha (TNF-α), IL-8, and granulocyte-macrophage colony-stimulating factor, in addition to increasing the levels of IL-1β and IL-6, which are already present. It was demonstrated in a recent clinical study that IL-6 and TNF-α concentrations were persistently high even 7 days after treatment was initiated while IL-1β levels decreased significantly after 7 days (123). Many of these cytokines and the associated immune response have been shown in animal models to be required for bacterial clearance and the prevention of mortality due to bacteremia and septic shock (180). Nitric oxide, a common mediator of inflammatory cytokines, is also required (143).
The T-cell mediated (Th1) and humoral (Th2) adaptive immune responses may also play a role in the clearance and/or pathogenesis of acute septic arthritis. T cells enter the joint within a few days following infection (1). The role of CD4 + T cells in joint destruction has been demonstrated by showing that their in vivo depletion resulted in a considerably milder course of staphylococcal arthritis (1). These lymphocytes are specifically activated by bacterial antigens in association with host antigen-presenting cells or nonspecifically in the case of bacterial superantigens (e.g., toxic shock syndrome toxin 1 [TSST-1]). The cytokine, gamma interferon (IFN-γ), produced by these activated T cells reduced the level of mortality and joint destruction in a mouse model of group B Streptococcus when delivered 18 h after bacterial inoculation (129). However, when S. aureus was used as the infecting organism in this model, IFN-γ increased the frequency and severity of septic arthritis while simultaneously protecting mice from septicemia (194). Also, it has been found in a recent study in mice that a high level of IFN-γ (a Th1 cytokine) plays a detrimental role in staphylococcal infection and that IL-4 and IL-10, both being Th2 cytokines, are involved in host resistance to infection through regulation of IFN-γ (150). However, the necessity of the Th2 response to clear S. aureus infection has lately been questioned in a study utilizing IL-4-deficient mice (73). It seems that a Th2 response is required for S. aureus infection clearance only in certain mice, depending on their genetic background. Therefore, the exact role of T cells in host tissue damage and infection clearance is still being elucidated.
Joint damage.
Under most circumstances, the host is able to mount a protective inflammatory response that contains the invading pathogen and resolves the infection. However, when the infection is not quickly cleared by the host, the potent activation of the immune response with the associated high levels of cytokines and reactive oxygen species leads to joint destruction. High cytokine concentrations increase the release of host matrix metalloproteinases (including stromelysin and gelatinase A/B) and other collagen-degrading enzymes. When monoclonal antibodies or steroids attenuate these cytokines, cartilage degradation is minimized. The joint is further damaged by the release of lysosomal enzymes and bacterial toxins (139). Host proteoglycans are initially degraded, and this is followed by collagen degradation. In fact, the polymorphonuclear response with subsequent release of these proteolytic enzymes can lead to permanent destruction of intra-articular cartilage and subchondral bone loss in as little as 3 days. Metalloproteinases and the antigen-induced inflammatory response may persist and continue to damage the joint architecture even after the infection has been cleared (134, 162). The infectious process induces a joint effusion that increases intra-articular pressure, mechanically impeding blood and nutrient supply to the joint. Thus, increased pressure destroys the synovium and cartilage. Because of the proximity of the epiphyseal growth plate to the joint, direct extension of a joint infection to any of the articulating bones may lead to decreased bone growth in infants and children (87, 117). While bone mineralization is preserved, cartilage destruction causes joint space narrowing and erosive damage to the cartilage and bone if left untreated (110). In addition, the infection can spread to surrounding soft tissue, form sinus tracts, and disrupt ligaments and tendons in the untreated state (137).
Bacterial products and their pathogenic role.
While bacterial attachment proteins promote colonization and initiate the infectious process, a number of bacterial products activate the host immune response and increase tissue damage in cases of septic arthritis. S. aureus has a large variety of factors that have been implicated in host virulence. Many of these factors have been tested for their ability to increase the morbidity and mortality associated with acute septic arthritis. Most studies evaluating the potential role of these bacterial products have been performed using the murine model of septic arthritis (167).
During acute septic arthritis, the innate immune system responds to the presence of the peptidoglycan wall (via N-formylmethionine proteins and teichoic acids) of S. aureus to produce proinflammatory cytokines (such as IL-1β, IL-6, and TNF-α) and C-reactive protein. Bacterial DNA (specifically unmethylated CpG motifs) also elicits an intense inflammatory response (37, 38). When bacterial DNA from S. aureus or E. coli or synthetic, unmethylated oligonucleotides containing CpG motifs were injected into the knee joint of mice, arthritis developed quickly and lasted up to 14 days, while methylated DNA had no significant effect. Also, the affected tissue was characterized by monocyte and macrophage influx with the release of their associated cytokines and chemokines and the absence of T cells.
It has been noted that bacterial superantigens such as staphylococcal TSST-1 and enterotoxins may play a major role in the potent activation of the host inflammatory response, thereby increasing the mortality rates and exacerbating host inflammatory-cell invasion, cytokine release, and joint degradation (20). Most animals infected with strains of S. aureus isogenic for TSST-1 or enterotoxins (A through D) developed frequent and severe arthritis (20). However, most animals (80%) infected with strains devoid of these toxins had no symptoms and the animals with symptoms had only mild or transient arthritis infections (20). Also, vaccination with a recombinant form of staphylococcal enterotoxin A devoid of superantigenicity was able to generate significant protection from S. aureus sepsis in mice (121).
Superantigens act by binding to the conserved lateral regions of the host major histocompatibility complex class II molecule and T-cell receptor. While only approximately 1 in 10 4 T cells are activated during normal presentation of a nonself antigen, a superantigen may activate 2 to 20% of all T cells (154). These activated T cells are then able to increase the release of a number of cytokines, such as IL-2 (154), IFN-γ, and TNF-α (98). This upregulated production of cytokines causes a significant systemic toxicity and suppression of the adaptive immune responses and inhibits plasma cell differentiation. Also, the stimulated T cells proliferate and then rapidly disappear, apparently due to apoptosis (132). Therefore, immune suppression may be due to generalized immunosuppression and T-cell deletion. Human B cells are also stimulated by these staphylococcal superantigens.
Besides the role that superantigens play in the mortality and morbidity associated with septic arthritis, other staphylococcal toxins may also contribute to the disease process. One study was able to demonstrate that alpha-hemolysin was a significant mediator of virulence in arthritis (53). Alpha-hemolysin is secreted as a monomer that attaches to host membranes and polymerizes into a hexameric ring channel (174). While this hemolysin binds to human erythrocytes in a nonspecific manner, it can still mediate significant host cell lysis when produced in high concentrations in the infection environment (69). Also, alpha-hemolysin promotes significant blood coagulation by neutrophil adhesion (85), platelet aggregation (via a fibrinogen-dependent mechanism) (11), and its nonlytic attack on human platelets (6). In addition, this hemolysin can form channels in nucleated cells (e.g., endothelial cells) through which calcium ions freely pass (62, 175). The calcium influx is responsible for the vasoregulatory process and inflammatory- response disturbances seen in severe infection (66). Lastly, alpha-hemolysin interferes with lymphocyte DNA replication (85). These multiple effects of alpha-hemolysin on the host contribute to the vascular disturbances and immunodeficiency seen in staphylococcal infections.
The pathogenic properties of alpha-hemolysin were recently found to only occur when another staphylococcal toxin, the leukocyte-specific gamma-toxin, was also present in the infecting strain (118). Gamma-hemolysin (HlgAB and HlgCB) and a related S. aureus leukocidin (LukSF-PV) specifically lyse leukocytes. Each of these toxins is composed of an interchangeable two-component system. The active toxin is formed by taking one protein from the S-component family (LukS-PV, HlgA, and HlgC) and one from the F-component family (LukF-PV and HlgB) (46, 89). The S component is most probably responsible for the specific cytopathic effect of each of the toxins, while the F component is responsible for the common leukocyte binding activity. While LukF and HlgA proteins show very strong similarity, they are encoded on different gene loci (128). Since these cytotoxins specifically interact and lyse leukocytes, they contribute to the inhibition of infection clearance by the host immune system, thereby enabling staphylococcal species to persist. Therefore, it is the combined effects of the hemolysin and leukotoxins that increase the ability of S. aureus to cause acute septic arthritis.
These factors enable the host to mount a protective inflammatory response that contains this pathogen and often resolves the infection. However, when the infection is not cleared by the host innate immune system, S. aureus is well equipped to persist by possessing a number of virulence factors and strategies, including but not limited to invading and surviving in mammalian cells, hiding within a biofilm, or producing a thick, antiphagocytic capsule.
The difficulty in treating septic arthritis and the ability of the bacteria to evade clearance by the host immune response reside in a number of staphylococcal defense mechanisms. Such characteristics are expressed at both the cellular and matrical levels. As mentioned above, protein A is bound covalently to the outer peptidoglycan layer of their cell walls. This receptor binds to the Fc portion of immunoglobulin G and presents the Fab fragment of the antibody to the external environment. Therefore, the Fc portion is unable to either bind complement or signal polymorphonuclear leukocytes, thereby interfering with staphylococcal opsonization and phagocytosis. This interference has been demonstrated in vitro and in animal models with subcutaneous abscesses and peritonitis. Also, protein A coats the staphylococcal cell in a coat of host Fab fragments, and the ability of the immune system to recognize the pathogen as nonself is hindered. The importance of protein A in S. aureus septic arthritis was demonstrated in a recent study in which strains that obtained this virulence factor caused greater inflammation and cartilage destruction (53).
Capsular polysaccharide may interfere with opsonization and phagocytosis. Among the 11 reported serotypes, capsule types 5 and 8 (microcapsule producers) comprise the vast majority (75 to 94%) of clinical isolates (2, 44, 115). The capsule of these two serotypes is much smaller than the capsule of other serotypes of S. aureus (such as capsule type 1) or pathogenic species such as Streptococcus pneumoniae. Unencapsulated and microencapsulated strains demonstrated a high rate of serum clearance compared to fully encapsulated strains. Therefore, the role of capsular polysaccharide in opsonization and phagocytosis was questioned (2). However, the thin capsule may be necessary in early bone infection stages in order to allow the interaction of staphylococcal adhesion factors with host proteins (such as fibrin and fibronectin). In one study, it was shown that a small capsule was necessary for fibroblast attachment by protein A of S. aureus and that a fully encapsulated strain reduced the binding efficiency (104). In another study, the thin capsule was shown to be necessary for binding to bone collagen type 1, since high capsular expression actually inhibited binding (23). Once these microorganisms adhere to solid surfaces (such as bone), both in vitro and in vivo, staphylococci produce larger quantities of cell-associated capsule than do those grown in liquid cultures (95). Specifically, type 5 and type 8 capsule production is strongly upregulated during postexponential growth (i.e., after adhesion and colonization) (177). This upregulated capsule production makes them resistant to antimicrobial treatment and host immune clearance. Therefore, once staphylococcal adherence proteins establish the infection, the pathogen enters the postexponential growth phase and begins producing a thicker capsule that covers and hides the highly immunogenic adherence proteins. This thicker type 5 and type 8 capsule is serum resistant through inhibition of phagocytosis and opsonization (2, 119). The effect of the staphylococcal polysaccharide microcapsule in murine arthritis was recently explored. In this study, strains expressing type 5 capsule were shown to cause a higher rate of mortality, a higher frequency of arthritis, and a more severe form of the disease compared to capsule mutants (119). In a clinical trial, a vaccine (Staph Vax) that consists of isolated type 5 and 8 capsular polysaccharides was able to significantly reduce infection rates (by 57%) in a high-risk population for as long as 10 months (179).
As mentioned above, S. aureus also survives intracellularly after internalization by cultured osteoblasts (72). Type 5 capsule production by in vivo-grown S. aureus (i.e., internalized in cultured osteoblasts) was recently shown to be upregulated compared to that by S. aureus grown in vitro (101). Therefore, the capsule may not only resist phagocytosis and opsonization but may also contribute to intracellular survival.
In summary, S. aureus infects and elicits a strong native immune response, cytokine release, and high T-cell activation. This pathogen is able to use a number of immunoavoidance strategies during this time while the host immune system simultaneously causes damage to “self” tissues and blood vessels in the area of infection. This damage may cause local circulatory and immune system compromise. The high T-cell activation eventually results in apoptosis and a weakened immune system, enabling the pathogen to effectively produce a sustained and destructive infection. While the bacterial products discussed above have been shown to increase joint damage in acute septic arthritis, many more S. aureus virulence factors have not yet been tested. Therefore, we would expect that number of factors implicated as playing a role in septic arthritis would undoubtedly increase, and their relative roles will be more clearly elucidated in future studies.
Bacterial clearance versus joint damage.
The interaction of the bacteria and host is of utmost importance in the initiation and prolongation of infection and cartilage damage. There is a subtle balance between an effective immune response to eliminate the infecting organism from the host and the overactivation of this response that causes the majority of infection-related joint destruction. Therefore, care must be exercised and further studies must be performed in regard to using agents that suppress the inflammatory response in the treatment of septic arthritis.
Gonococcal Arthritis
Gonococcal arthritis occurs in approximately 42 to 85% of patients with disseminated gonococcal infection (DGI) and begins with a localized mucosal infection (4, 122). DGI-producing strains are unusually sensitive to in vitro killing by penicillin G and possess unique nutritional requirements for arginine, hypoxanthine, and uracil. N. gonorrhoeae possesses a number of virulence factors. It is the combined effects of these factors, their phase and antigenic variation, and properties of the host immune response that enable this pathogen to persist and allow the localized infection to become DGI.
Gonococcal virulence factors.
N. gonorrhoeae possesses a number of cell surface structures that have been implicated in virulence. Initial attachment to host epithelium is mediated by long, hair-like protein projections called pili Phase variation, i.e., the question of whether this membrane structure is assembled (Pil +) or not (Pil −), is determined by posttranslational proteolytic cleavage, variations in homologous recombination, and slipped-strand DNA replication resulting in frameshift mutations (193). In addition, the antigenic character of the pili is altered by homologous recombination between coding regions for the various pilin subunits.
Protein I is the main protein on the outer membrane. It is a porin that is expressed in two different forms, a protein IA variant that is almost always associated with disseminated infection and a protein IB variant that is associated with strains causing localized infections. Strains that are able to cause a disseminated infection in hosts with a normal immune system display serum resistance (21). Protein IA enables stable serum resistance by binding the host factor H. This bacterially- bound host factor efficiently inactivates C3b (a central factor in both the classical and alternative complement cascades) into iC3b (130), thereby reducing the efficacy of the host complement system. This porin may also be responsible for the prevention of phagolysosomal fusion in polymorphonuclear leukocytes and a reduced oxidative burst, thereby enabling survival within these cells. Another extracellular gonococcal protein is protein II, which is also called Opa since colonies expressing protein II on their surface have a more opaque appearance. This protein is thought to cooperate in the more intimate attachment following initial pilus interaction. In addition, protein II is able to attach to the lipooligosaccharide (LOS) of other N. gonorrhoeae organisms, thereby enabling the cells to bind to one other and form microcolonies. These microcolonies may also aid in the initiation of mucosal surface attachment. Protein II is capable of avoiding clearance by the host immune system by phase and antigenic variation (93). Phase variation occurs through slipped-strand synthesis that produces a frameshift mutation and produces a prematurely terminated form of the protein. In addition, multiple variants of the protein II gene exist, and therefore the antigenic character of protein II can be changed by homologous recombination between these variants. While this protein is important for mucosal infections, most isolates from patients with DGI are missing protein II from their outer membrane and grow to form transparent colonies. Protein III is another porin that is prevalent on the bacterial surface. The antibodies directed against protein III are not bactericidal, and they sterically inhibit antibody binding to protein I and unsialylated LOS that would probably result in bactericidal action (133). Therefore, the generation of these blocking antibodies may prevent serum bactericidal action.
LOS is like the lipopolysaccharide of other gram-negative bacteria except that its carbohydrate portion does not have the complex structure of the repeating O side chain. LOS has endotoxin activity and is largely responsible for the synovial damage produced in gonococcal arthritis (60, 64). While stable serum resistance is due to protein IA, unstable resistance is mediated by the ability of some gonococcal strains to covalently attach activated forms of host sialic acid to the galactose residues on LOS (187). This covalent attachment coats the bacterial cell in host proteins and avoids complement activation. In addition, opsonization by complement components and the formation of the membrane attack complex of the complement system are inhibited. N. gonorrhoeae also produces an immunoglobulin A protease that may aid in colonization. However, the relevance of this potential virulence factor in gonococcal pathogenesis needs further study.
Host factors.
The host may contain a gonococcal infection through the action of the innate immune response, with particular dependence on the complement system. This system is largely responsible for attracting polymorphonuclear leukocytes and the resulting cascade of inflammatory cytokines and chemokines. However, during periods surrounding early pregnancy, puerperium, and menstruation, the accompanying alterations in vaginal pH, cervical mucus, and genital flora and the endometrial exposure of submucosal vessels may predispose the female patient to N. gonorrhoeae invasion and DGI (21, 122). As mentioned above, defects in the complement and/or reticuloendothelial systems may also inhibit the host's ability to contain gonococcal infection.
RISK FACTORS
Besides the obvious risk of septic arthritis associated with age older than 60 years and recent bacteremia, certain medical conditions predispose joints to nongonococcal infection. Degenerative joint disease, rheumatoid arthritis, and corticosteroid therapy are the most common predisposing conditions. Specifically, patients with rheumatoid arthritis have an approximately 10-fold-higher incidence of septic arthritis than does the general population (79, 113). Patients with diabetes mellitus, leukemia, cirrhosis, granulomatous diseases, cancer, hypogammaglobulinemia, intravenous substance abuse, or renal disease and patients undergoing cytotoxic chemotherapy also have an increased incidence of septic arthritis (3, 39, 140). Total joint arthroplasties are susceptible to intraoperative or hematogenous seeding and subsequent prosthetic joint infections. While patients infected with HIV demonstrate a higher prevalence of musculoskeletal infections than does the general population (approximately 60 and 2 to 10 cases per 100,000 persons per year, respectively), it is unclear if this higher occurrence is due to the common septic arthritis risk factors due to intravenous drug abuse and multiple transfusions in this patient population (79, 113, 178).
In 0.5 to 3% of gonorrhea infections, the pathogen is able to gain access to the bloodstream from the primary mucosal site of infection and produce DGI (71, 84, 122). A number of risk factors have been epidemiologically associated with the development of DGI; these include infection with transparent, piliated N. gonorrhoeae strains capable of phase variation; diagnosis delay (especially in females due to asymptomatic nature of the infection); complement system deficiency; systemic lupus erythematosus; menstration, pregnancy, and puerperium; male homosexuality; urban residence; promiscuity; and low socioeconomic and educational status. Females are four times as likely to develop DGI as males (4). This prevalence in women may be due to the asymptomatic nature of gonorrhea infections in women and the associated delay in diagnosis, thereby providing time for the bacteria to gain access to the bloodstream. In addition, many affected females are either pregnant or menstruating at the time of the infection (122). Also, since the clearance of gonococcal infection depends on an effective complement-mediated immunity and a functional reticuloendothelial system, complement deficiencies and systemic lupus erythematosus are risk factors in this patient subset.
DIAGNOSIS OF NONGONOCOCCAL ARTHRITIS
Nongonococcal septic arthritis is a medical emergency that can lead to serious sequelae and mortality. Therefore, prompt recognition and treatment are critical to ensuring a good prognosis.
Clinical Presentation
The classical presentation of acute nongonococcal septic arthritis includes recent onset of fever, malaise, and local findings of pain, warmth, swelling, and decreased range of motion in the involved joint (87, 117). A significant number of patients have mild fever and may not demonstrate localized heat and erythema around the affected joint (10). The clinician should obtain a detailed history with special emphasis on determining the presence of any risk factors discussed above. However, the diagnosis of infectious arthritis rests on the isolation of the pathogen(s) from aspirated joint fluid.
While any joint can become infected, the most commonly involved joints in nongonococcal septic arthritis are the knee and hip, followed by the shoulder and ankle (9). The hip may be more frequently involved in children. Also, infectious nongonococcal arthritis is monoarticular in 80 to 90% of cases (70, 75, 156). Atypical joint infection, including the sternoclavicular, costochondral, and sacroiliac joints, may be common in intravenous drug users. Also, penetrating trauma, including human or animal bites, and local corticosteroid therapy may cause septic arthritis in atypical joints. Polyarticular septic arthritis is usually accompanied by a number of risk factors (see above).
Laboratory Findings
Peripheral blood leukocyte counts are usually elevated in children but are often within normal limits in adults. Most patients display elevated C-reactive protein levels and erythrocyte sedimentation rates. Synovial fluid analysis is also very important and usually reveals turbid, low-viscosity fluid with leukocyte counts usually in excess of 50,000/mm3. However, nonbacterial inflammatory processes, such as acute crystalline joint disease or reactive arthritis, may have counts above this level while gonococcal and granulomatous arthritis may have counts below 50,000/mm3. In nongonococcal arthritis, the fraction of polymorphonuclear leukocytes approaches 90% (59, 158). Even though low joint fluid glucose levels (<40 mg/dl or less than half the serum glucose concentration) and high lactate levels are nonspecific, they are suspicious for bacterial arthritis. Normal joint glucose and lactate levels are usually found in patients with viral arthritis (156, 158). Synovial fluid from any adult with monarticular arthritis should be examined by compensated polarizing light microscopy for negatively birefringent (uric acid) and positively birefringent (calcium pyrophosphate dihydrate) crystals in order to rule out crystalline joint disease. However, simultaneous bacterial infection and crystalline disease has been reported (8, 158). Gram stains of synovial fluids may support the diagnosis of septic arthritis. In addition, it may differentiate between infections by gram-positive and gram-negative bacteria, thereby directing initial antimicrobial therapy before antibiotic sensitivity results are obtained. The synovial fluid should be sent for aerobic, anaerobic, mycobacterial, and fungal culture prior to the initiation of antimicrobial therapy. In addition, antibiotic sensitivities should be determined. Cultures are positive in nongonococcal arthritis approximately 90% of the time, while Gram stain is effective only 50% of the time (141). These cultures may be negative in patients in whom treatment has already been initiated.
Once aspirated joint samples are obtained, it is imperative that they be quickly transported to clinical microbiology and not be allowed to stand for a long time without processing or culturing. In one study, it was found that by directly inoculating the aspirated sample into blood culture tubes, even very small numbers of viable bacteria in infected fluid could be detected (182). However, an increase in false-positive results due to general skin or other contaminants may also occur with this technique. If fluid cultures are sterile but the suspicion of septic arthritis persists, tissue samples of the synovial membrane may also be cultured for microbial isolation and identification. Sputum, urine, and blood cultures are also often required. Around half of all patients with nongonococcal arthritis show positive blood cultures (59). A summary of the diagnosis and management of acute nongonococcal arthritis can be seen in Table 1.
TABLE 1.
Principles of diagnosis and management of acute nongonococcal septic arthritis
| Indicators at presentation |
| Recent onset of fever and malaise |
| Local pain, warmth, swelling, and decreased range of motion in the involved joint |
| The presence of any risk factors as determined by a detailed history |
| Diagnosis by synovial fluid testing |
| Synovial culture and Gram stain |
| Leukocyte counts in excess of 50,000/mm3 |
| Glucose level of <40 mg/dl or less than half that seen in the serum |
| High concentration of lactate |
| >90% polymorphonuclear leukocytes |
| Lack of bifringent crystals (Note: simultaneous crystalline and bacterial arthritis has been reported) |
| Other laboratory indicators |
| May have an elevated erythrocyte sedimentation rate, C-reactive protein levels, and/or peripheral leukocyte levels |
| Sputum, urine, and blood cultures may be warranted; blood cultures are positive in 50% of cases |
| Management |
| Antibiotics adjusted based on culture and sensitivity results |
| Adequate drainage of joint |
| Needle aspiration |
| Arthroscopic drainage |
| Open drainage in difficult and deep joints |
| Monitoring of synovial fluid leukocyte counts and cultures |
| Acute phase of disease — patient rest and optimal joint position |
| Following the acute phase — early physical therapy and aggressive mobilization |
Imaging Studies
Imaging studies of septic arthritis can be used only to support or dissuade a clinical suspicion of the disease; they should not be used as an absolute diagnostic indicator. Because the approaches and techniques are both numerous and diverse, there is confusion about which technique is most effective. Radiographic images are usually not revealing in the first few days of infection since they are usually normal or show only preexisting joint disease. However, swelling of capsule and soft tissue around the affected joint, fat pad displacement, and in some cases joint space widening due to localized edema and effusion may be seen. Also, the initial radiographic image may be used to determine associated conditions, such as osteoarthritis or simultaneous osteomyelitis, or may be used as a baseline image in monitoring the response to treatment. As the infection progresses, radiographic detection of diffuse joint space narrowing due to cartilage destruction is possible. Radiographs can also evaluate late, inadequately treated stages of septic arthritis in which generalized joint destruction, osteomyelitis, osteoarthritis, joint fusion, calcifications in the periarticular tissues, or subchondral bone loss followed by reactive sclerosis are seen.
Ultrasonography is capable of showing both intra- and extra-articular abnormalities not apparent by plain radiography and is a very powerful tool to detect early fluid effusions and to guide initial joint aspiration and drainage procedures (157, 195). Even small collections of fluid (1 to 2 ml) can be accurately detected (195). Non-echo-free effusions (due to clotted hemorrhagic collections) are very characteristic of a septic joint. It has been suggested that the presence of only an echo-free effusion (caused by transient synovitis and fresh hemorrhagic effusions) may rule out the diagnosis of septic arthritis (195). This imaging technique is also useful for detecting collections of fluids in deep joints, including the hip. In addition, the status of the intra-articular compartment, joint capsule, bony surface, and adjacent soft tissues and the patient's response to therapy can be monitored. Since ultrasonography is also noninvasive, inexpensive, easy to use, and devoid of irradiation or any other known complications, more clinicians should use it in the diagnosis of septic arthritis in the future.
To diagnose ambiguous cases of septic arthritis or to determine the extent of bone and soft tissue infections, computed tomography (CT), magnetic resonance imaging (MRI), and radionuclide scans may be obtained. In most cases, these diagnostic tests are not required for septic arthritis.
Like radiographs, CT scans have limited use during the early stages of septic arthritis. However, they may enable the visualization of joint effusion, soft tissue swelling, and para-articular abscesses. In addition, CT is more sensitive than plain radiography in the imaging of joint space widening due to localized edema, bone erosions, foci of osteitis, and scleroses. This scanning technique may be useful in the diagnoses of arthritis cases that are difficult to assess, including infections of the hip, sacroiliac, and sternoclavicular joints. In addition, it may assist in guiding joint aspiration, selecting the surgical approach, and monitoring therapy in these difficult infections (155).
MRI has become a useful diagnostic tool for the early determination of musculoskeletal infection and its extent (111, 169). As with CT, MRI may be particularly useful in aiding the diagnosis of joint infections that are difficult to access, such as sacroiliitis (145). MRI displays greater resolution for soft tissue abnormalities than CT or radiography and greater anatomical detail than radionuclide scans. The spatial resolution of MRI makes it useful in visualizing joint effusion and differentiating between bone and soft tissue infections. Furthermore, patients do not have to be exposed to ionizing radiation. The main disadvantages to MRI are high cost, lack of universal availability, imaging interference due to metal implants, and lower resolution of calcified bone structures and the cortex (43). Initial MRI screening usually consists of a T1-weighted and T2-weighted spin-echo pulse sequence. In a T1-weighted study, edema and fluid are dark while fat is bright. In a T2-weighted study, the reverse is true. Therefore, joint effusions, abscesses, and soft tissue edema generate a high signal on T2-weighted images. As with the other imaging techniques, MRI is nonspecific and is unable to differentiate between infectious and noninfectious inflammatory arthropathies (63).
Radionuclide scans are often able to detect localized areas of inflammation. The 99mTc methyldiphosphonate scan demonstrates increases isotope accumulation in areas of osteoblast activity and increased vascularity (138). However, this radionuclide scan may be normal in the early stages of septic arthritis. A second class of radiopharmaceuticals used for the evaluation of septic arthritis includes 67Ga citrate and 111In chloride scans. 67Ga citrate and 111In chloride attach to serum proteins, including transferrin, lactoferrin, haptoglobin, and albumin, that leak from the bloodstream into areas of inflammation. Gallium and indium scans also show increased isotope uptake in areas of concentrated polymorphonuclear leukocytes, macrophages, and malignant tumors. While these scans are more specific and sensitive in the detection of active infection than 99mTc methyldiphosphonate (15), they do not show bone or joint detail well, and it is often difficult to distinguish between bone, joint, and soft tissue inflammation. Three-phase 99mTc methyldiphosphonate scans may help resolve this problem. In 111In-labeled leukocyte scanning, a sample of the patient's leukocytes is isolated, labeled with 111In, and injected back into the patient. These radiolabeled leukocytes localize into areas of acute infection according to host inflammatory cytokine and chemokine gradients. While this scan is positive in approximately 60% of patients with septic arthritis, false-positive results may occur in patients with synovitis secondary to active osteoarthritis (172).
Differential Diagnosis
Preexisting joint infection.
A number of associated arthropathies should be considered in the differential diagnosis of acute septic arthritis. Patients with underlying chronic joint disease (including rheumatoid arthritis, osteoarthritis, and other connective tissue diseases) have a poor prognosis when suffering from acute septic arthritis (41). The poor prognosis associated with this patient population is mainly due to diagnostic delays since clinicians incorrectly ascribe symptoms to the preexisting arthropathy (55). Also, these patients are often afebrile and demonstrate an indolent presentation (56). Therefore, a diagnosis of septic arthritis must be entertained whenever a sudden onset of inflammatory arthritis in one or two joints occurs in these patients.
Endocarditis.
Patients with infective endocarditis also demonstrate relatively high incidence rates (23 to 44%) of musculoskeletal abnormalities (61, 96, 109, 135, 148, 171). Specifically, many of these patients have sterile myalgias and althragias, and the joint symptoms are usually polyarticular and symmetric, affecting both the large and small joints. In addition, septic arthritis is seen in approximately 5 to 15% of these patients, especially in intravenous drug abusers. Cases of endocarditis-associated septic arthropathies are usually mediated by Streptococcus spp. or S. aureus.
Chronic infectious arthritis.
The diagnosis of mycobacterial or fungal arthritis should be entertained when a patient presents with chronic monarticular arthritis. Both of these arthritides have increased in prevalence, largely due to increased incidence rates seen in HIV-infected patients (56, 141). Synovial fluid cultures for acid-fast bacteria and fungi should be considered for any patient who is immunocompromised or receiving immunosuppressive therapy or who has a persistent effusion. A culture of a synovial biopsy specimen should be done for fungi and acid-fast organisms in any person with a chronic monarticular involvement whose synovial fluid cultures are negative (32, 52). In mycobacterial arthritis, macrophages may predominate in the synovial fluids. The delayed onset and insidious progression of this disease are markedly different from those of acute septic arthritis. Therefore, a thorough history of the illness in the patient, appropriate culture, and monitoring of clinical progression should provide reasonable clues to distinguish acute infectious joint arthropathies from cases of fungal or mycobacterial arthritis. Lyme disease may also present with chronic monoarticular arthritis. About 60% of untreated patients with Lyme disease in the United States have intermittent attacks of joint swelling and pain, especially in the knee, even months after the onset of illness (164). Demonstration of the typical and slowly expanding erythema migrans skin lesion at the site of the tick bite and the development of influenza-like symptoms (including malaise, fatigue, headache, fever, regional lymphadenopathy, and migratory polyarthragia) lasting weeks to months are highly indicative of Lyme disease (165). This is especially true in patients living in areas where this disease is endemic. While culture of Borellia burgdorferi (the spirochete responsible for Lyme disease) from specimens in Barbour-Stoenner-Kelly medium permits a definitive diagnosis, determination of an antibody response to B. burgdorferi by enzyme-linked immunosorbent assay is also possible (24, 165). Mycoplasma spp. have also been repeatedly isolated in cases of chronic erosive septic arthritis, particularly in patients suffering from hypogammaglobulinemia (51).
Viral arthritis.
Patients with viral arthritis usually present with polyarthritis, fever, lymphadenopathy and characteristic rash (56). Also, synovial fluid samples reveal an abundant presence of mononuclear leukocytes, and normal joint glucose and lactate levels are usually found (156, 158). Clinical and epidemiological clues often lead the clinician to perform appropriate serological studies via antibody titers.
Crystal-induced arthritis.
Gout and pseudogout may mimic many of the symptoms associated with septic arthritis. Therefore, synovial fluid samples should be examined by compensated polarizing light microscopy for the presence of negatively birefringent (uric acid) and positively birefringent (calcium pyrophosphate dihydrate) crystals to rule out crystalline joint disease. However, it must be noted that simultaneous bacterial infection and crystalline disease has been reported (8, 158).
Reactive arthritis.
An inflammatory joint response to extra-articular rather than intra-articlar presence of microorganisms may be defined as reactive arthritis (168). Therefore, while infection can be demonstrated at a distant site, joint inflammation occurs without traditional evidence of sepsis at the affected joint(s). Most cases are associated with patients with the major histocompatibility complex antigen HLA-B27. Also, patients usually have recent microbial infections in distal sites that include the gastrointestinal (e.g., Shigella spp., Salmonella spp., Campylobacter spp., or Yersinia spp.), genitourinary (e.g., chlamydiae and mycoplasmas), and respiratory (e.g., Streptococcus pyogenes) tracts (83). Patients present with a sterile, inflamed joint and may also demonstrate enthesopathy, uveitis, conjunctivitis, or skin and mucous membrane lesions (56). Specifically, poststreptococcal reactive arthritis can follow group A streptococcal infection and presents with nonmigratory arthritis, lack of response to aspirin or nonsteroidal anti-inflammatory agents, and the presence of extra-articular manifestations, including vasculitis and glomerulonephritis (7).
Recent studies utilizing immunofluorescence, immunohistochemical, and PCR techniques have detected persistent microbial antigens within joints affected with reactive arthritis (168). These results may be explained by one hypothesis that describes the presence of bacteria and/or their antigens as a reflection of the persistence of small numbers of latent nonculturable microbes in the joint space. This hypothesis may be valid in specific cases (e.g., chlamydia-triggered reactive arthritis), since the early administration of tetracycline therapy may reduce the length of disease and the associated articular damage (173). However, antibiotics are usually ineffective, especially when given at later stages of reactive arthritis. Therefore, another hypothesis is that the detection of microbial products may just reflect the natural filtering action of the synovium and the subsequent concentration of these products, thereby stimulating inflammation.
DIAGNOSIS OF GONOCOCCAL ARTHRITIS
Clinical Presentation
Gonococcal arthritis may present as part of a disseminated infection or as arthritis (36) and usually affects young, healthy, and sexually active individuals. It is important to obtain a complete patient history in order to identify the presence of individual risk factors (a full list of potential risk factors for DGI is given in “Risk Factors” above). The presenting symptoms in DGI may include migratory arthralgias, moderate fever, chills, dermatitis, and tenosynovitis. The large majority of these patients have asymptomatic genital, anal, or pharyngeal gonococcal infections (122). The classic skin lesion manifests as small erythematous papules which progress to vesicular or pustular lesions and are often limited to the extremities and the trunk. If the papules are present on the affected joint, there are typically 5 to 10 lesions. The tenosynovitis is characterized by pain, swelling, and periarticular erythema.
Some patients develop septic gonococcal arthritis without prior polyarthralgia, tenosynovitis, or dermatitis. In fact, while most patients with DGI present with tenosynovitis, only 21% of patients with confirmed suppurative arthritis display this clinical sign (122). Therefore, properties of the host and the serological properties of the infecting N. gonorrhoeae strain may be responsible for determining whether the DGI will result in tenosynovitis and dermatitis or will produce arthritis. In the absence of the characteristic dermatitis or overt genital infection, septic gonococcal arthritis is often clinically indistinguishable from other forms of septic arthritis (10, 159).
In contrast to nongonococcal arthritis, distal joints including the fingers, wrists, elbows, knees, and ankles are most often affected in gonococcal arthritis. Also, migratory asymmetric joint pain followed by polyarticular infection is common in this patient population.
Laboratory Findings
Peripheral leukocytosis and elevated erythrocyte sedimentation rates are present in more than half of these patients. Also, N. gonorrhoeae is isolated from synovial cultures in only approximately 50% of patients with gonococcal arthritis, and Gram stains are even less reliable (122, 188). Therefore, there is a high dependence on clinical presentation, accurate history, and positive cultures from affected sites for the diagnosis of this disease. Cultures derived from the uterine endocervix are positive in approximately 90% of women, while urethral, pharyngeal, and rectal mucosal cultures are positive in approximately 50 to 75, 20, and 15% of men, respectively. Blood and skin lesion cultures are rarely positive. It is extremely important for the bedside clinician to be aware of the specific requirements for correctly culturing N. gonorrhoeae from patient samples. Briefly, blood and synovial fluid samples should be plated immediately on prewarmed chocolate agar while genitourinary, rectal, and pharyngeal samples should be plated on prewarmed Thayer-Martin or modified New York medium with appropriate antibiotic supplementation (31). The plates should then be incubated at 37°C in a moist 5% CO 2 environment within 15 min of sample harvest. It is also important to note that N. gonorrhoeae growth is inhibited in blood culture tubes containing polyanethol sulfate.
Since N. gonorrhoeae DNA has been detected in culture-negative synovial samples by PCR amplification (97), this molecular biology tool may have a future widespread role in accurate gonococcal arthritis diagnosis. The specificity and sensitivity of this technique were 96.4 and 78.6%, respectively, and the false-positive rate was 3.6% (97). However, it is unclear whether a positive PCR result represents viable but nonculturable bacteria or nonviable bacteria with an associated reactive arthropathy. As with all PCR-based techniques, careful sample preparation and the inclusion of proper positive and negative controls are essential to maintaining the efficacy of this diagnostic tool. However, the generalized use of this molecular technique for routine screening and detection of N. gonorrhoeae will not replace the “gold standard” of culture since PCR-based methods do not yet provide information about antibiotic sensitivity.
Imaging Studies
The utility of the imaging studies discussed above also applies to gonococcal arthritis, especially with regard to advanced cases and monitoring treatment success. However, these diagnostic tools are generally not used in the diagnosis of this infection. The extremely rapid clinical response to treatment, the lack of complicating manifestations, and distal joint involvement often make imaging unnecessary.
Differential Diagnosis
The symptoms associated with N. gonorrhoeae joint infection can be mimicked by arthritis due to other bacteria. Arthritis due to N. meningitides is nearly indistinguishable from DGI, especially with regard to the musculoskeletal manifestations and arthritis-dermatitis syndrome. Skin lesions, similar to those produced in cases of gonococcal arthritis, are also occasionally induced by infection with other pathogenic species, including H. influenzae, Streptobacillus monoliformis, and Streptococcus pyogenes. However, patients with nonneisserial joint infections usually present with distinct clinical characteristics and laboratory findings (see above).
As described above for nongonococcal septic arthritis, preexisting joint infection (including systemic lupus erythematosus, rheumatoid arthritis, and other connective tissue disorders), endocarditis, chronic infectious arthritis, viral arthritis, and crystal-induced arthritis should be considered in the differential diagnosis. Also, cases of gonococcal arthritis and reactive arthritis (specifically sexually acquired reactive arthritis) may be hard to distinguish since both involve sexually active people and common symptoms are seen, including urethritis, conjunctivitis, oral ulcers, and genitourinary manifestations. However, the patients are usually HLA-B27 positive, the onset is often slower and less acute, and the skin lesions (if present) are usually keratoderma blenorrhagicum and circinate balanitis in this form of reactive arthritis. Also, antibiotic therapy is usually ineffective. Preicteric hepatitis may also be confused with DGI since it commonly presents with tenosynovitis, polyarthritis, and skin rash. However, in hepatitis-associated arthritis, the rash usually resembles hives, the concentrations of synovial leukocyte are lower, and the hepatitis surface antigen can usually be detected in the blood.
TREATMENT OF NONGONOCOCCAL ARTHRTIS
Acute nongonococcal septic arthritis is a medical emergency that can lead to significant morbidity and mortality. Therefore, prompt recognition and rapid and aggressive treatments are critical to ensuring a good prognosis. The treatment of this form of septic arthritis includes both appropriate antimicrobial therapy and joint drainage (Table 1).
Antibiotic Therapy
Most people with suppurative arthritis respond clinically to appropriate antimicrobial agents after the initial diagnostic joint aspiration. Initial antimicrobial therapy is based on the clinical presentation, a thorough history, initial Gram stain, and joint fluid analysis. The patient's history and clinical course often provide clues to distinguish between gonococcal, nongonococcal, and granulomatous arthritis. Joint culture collection followed by initiation of treatment with an effective broad-spectrum antibiotic should be done as soon as possible. The initial antibiotic therapy is adjusted, if necessary, based on appropriate culture and antibiotic sensitivity results (Table 2).
TABLE 2.
Initial choice of antibiotics for therapy of infectious arthritis (adult doses)
| Organism | Antibiotics of first choice | Alternative antibiotics | |
|---|---|---|---|
| Methicillin resistant | |||
| Staphylococcus aureus | Vancomycin 1 g every 12 h or | SXTa or minocycline ± rifampin | |
| linezolid 600 mg every 12 h | |||
| Coagulase-negative Staphylococcus spp. | Vancomycin 1 g every 12 h or | SXT or minocycline ± rifampin, clindamycinb | |
| linezolid 600 mg every 12 h | |||
| Methicillin sensitive | |||
| Staphylococcus aureus | Nafcillin 2 g every 6 h or | Cefazolin, vancomycin | |
| clindamycin 900 mg every 8 h | |||
| Coagulase-negative Staphylococcus spp. | Nafcillin 2 g every 6 h or | Cefazolin, vancomycin | |
| clindamycin 900 mg every 8 h | |||
| Group A streptococcus, S. pyogenes | Penicillin G 2 million every 4 h or | Clindamycin, cefazolin | |
| ampicillin 2 g every 6 h | |||
| Group B streptococcus, S. agalactiae | Penicillin G 2 million every 4 h or | Clindamycin, cefazolin | |
| ampicillin 2 g every 6 h | |||
| Enterococcus spp. | Ampicillin 2 g every 6 hc or | Ampicillin-sulbactam, linezolid | |
| vancomycin 1 g every 12 h | |||
| Escherichia coli | Ampicillin-sulbactam 3 g every 6 h | Cefazolin, levofloxacin, gentamicin, SXT | |
| Proteus mirabilis | Ampicillin 2 g every 6 h or | Cefazolin, SXT, gentamicin | |
| Levofloxacin 500 mg daily | |||
| Proteus vulgaris, Proteus rettgeri, Morganella morganii | Cefotaxime 2 g every 6 h, imipenem 500 mg every 6 h, or levofloxacin 500 mg daily | Mezlocillin, gentamicin, or ticarcillin-clavulanate | |
| Serratia marcescens | Cefotaxime 2 g every 6 h | Levofloxacin, gentamicin, imipenem | |
| Pseudomonas aeruginosa | Cefepimed 2 gm every 12 h or | Ticarcillin-clavulanate, tobramycin, amikacin, ciprofloxacine | |
| Piperacillind 3 gm every 6 h or | |||
| Imipenem 500 every 6 h | |||
| Neisseria gonorrhea | Ceftriaxone 2 g daily or | Levofloxacin, ampicillin | |
| Cefotaxime 1 g every 8 h | |||
| Bacteroides fragilis group | Clindamycin 900 mg every 8 h or | Ampicillin-sulbactam, ticarcillin-clavulanic acid | |
| metronidazole 500 mg every 8 h |
SXT, sulfamethoxazole-trimethoprim.
If sensitive to clindamycin.
In a serious Enterococcus infection, ampicillin plus an aminoglycoside is used.
In a serious infection, those drugs should be used with an aminoglycoside.
Increasing resistance of the quinolones including ciprofloxacin.
The usual course of therapy for nongonococcal arthritis is 2 weeks for arthritis due to H. influenzae or Streptococcus spp. and 3 weeks for arthritis due to S. aureus or gram-negative bacilli. Initial antibiotic therapy in children younger than 5 years includes cefuroxime, cefotaxime, or ceftriaxone depending on the blood and joint culture results. Initial therapy for patients older than 5 years is aided by the Gram stain. If clusters of gram-positive organisms suggestive of S. aureus are seen, treatment with intravenous (i.v.) penicillinase-resistant penicillin is begun. If gram-positive organisms in chains consistent with Streptococcus spp. are seen, penicillin G is used for therapy. If the Gram stain is negative, an extended-spectrum or broad-spectrum cephalosporin or semisynthetic penicillin is appropriate. Ceftriaxone is a reasonable initial antibiotic in sexually active adults. The initial antibiotic therapy is adjusted, if necessary, on receipt of appropriate culture and sensitivity results.
Few controlled studies exist assessing the optimal duration, dose, or route of administration of antibiotics in nongonococcal arthritis (16). Most antibiotics achieve excellent bactericidal concentrations in synovial fluid following parenteral or oral administration (116, 151). Intra-articular antimicrobial administration is usually not necessary and may cause a chemical synovitis.
Antibiotic Administration in the Elderly
Most cases of nongonococcal arthritis occur in the elderly, even though persons older than 65 years account for only 12% of the population; this relative percentage will only increase with our aging population (28, 144, 160). The choice of antibiotic therapy for these patients must be carefully made due to the decreased organ reserve capacity, altered pharmacokinetics and pharmacodynamics, and polypharmacy with associated drug-drug and drug-disease interactions characteristic of older patients, all of which cause a high rate of adverse drug effects in this patient population. In addition, low compliance with prolonged or complicated oral regimens must be considered. The physician can help to minimize adverse drug reactions and improve outcomes by being aware of the principles of clinical pharmacology, the characteristics of specific drugs, and the special physical, psychological, and social needs of older patients.
The most important and best-studied pharmocokinetic alteration that occurs in the elderly is the age-associated decline in normal renal function. The creatinine clearance is a very useful measure of renal function in elderly patients and can be estimated by the Cockroft-Gault equation (29) in which the creatinine clearance (in milliliters per minute) is assumed to equal the percentage of normal renal function:
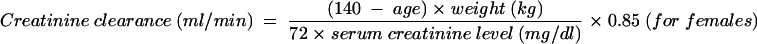 |
Antibiotic loading and maintenance doses should be estimated and confirmed by measuring peak and trough concentrations in serum after the fourth dose. The loading dose may be calculated by using the ideal body weight to estimate lean mass (28):
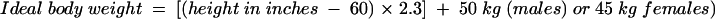 |
The dose may be adjusted upward or downward to compensate for increased or decreased extracellular fluid volume. The maintenance dose should be estimated using ideal body weight and percentage of normal renal function.
Prolonged use of aminoglycosides should be avoided if possible because of the increased risk for ototoxicity and nephrotoxicity in elderly patients. It is important to note that for drugs with appreciable renal clearance, such as vancomycin and aminoglycosides, monitoring of plasma drug levels is wise (28). In Australia, cases of cholestatic hepatitis were found in elderly patients (predominantly women) following 3 weeks of flucloxacillin treatment (45). Augmentin (amoxacillin-clavulanate) was also associated with instances of cholestatic hepatitis. However, this side effect was noted primarily in elderly men following Augmentin treatment for more than 2 weeks (92). Due to reports of seizures, the intravenous dose of 0.5 g every 6 h should be reduced in elderly patients with decreased renal function, cerebrovascular disease, or seizure disorders (152). Cefamandole may increase creatinine levels in the elderly. Seizures due to hypo- or hyperglycemia were noted in four elderly patients being treated with ofloxacin (176). Quinolones, fluoroquinolones, and tetracycline may have decreased oral absorption when coadministered with aluminum- or magnesium-containing antacids or sucralfate (Carafate). Quimapril (Accupril), a newly released angiotensin-converting enzyme inhibitor, contains a high concentration of magnesium, which may also decrease the oral absorption of fluoroquinolones and tetracycline. The interaction of rifampin with a large number of therapeutic agents requires close patient monitoring and follow-up (54). It is important to note that potent loop diuretics decrease the extracellular fluid volume, thereby elevating the levels of antibiotics in serum and requiring further reductions in dose levels.
Surgical Therapy
There are a variety of methods to drain the purulent fluid from the infected joint. Presented in ascending order of invasiveness, cost, and effectiveness in the thoroughness of drainage, they include needle aspiration, tidal irrigation, arthroscopy, and arthrotomy. There is no set of universally accepted criteria for choosing the drainage method. It is agreed that the specific method of drainage used should be tailored to the clinical situation of the patient. However, some general guidelines can be listed.
Patients should be initially treated with needle aspiration if a joint infection is easily accessible, if the vast majority of the purulent fluid can be removed, and if the patient does not suffer from negative prognostic indicators (see below). Although no prospective studies of these methods exist, most retrospective studies suggest that peripheral joints such as the knee, elbow, ankle, and wrist receive needle aspiration initially whereas axial joints, such as the hip, shoulder and sternoclavicular joint undergo open drainage (59, 137). Repeated needle aspiration for recurrent joint effusions has been used with success during the first 7 days of treatment (59, 70). If the volume of synovial fluid, the cell count, and the percentage of polymorphonuclear leukocytes decrease with each aspiration, then the combination of antimicrobial therapy and aspiration as needed is probably adequate (70). Persistence of effusion beyond 7 days is evidence that arthroscopy or open drainage should be performed. Tidal irrigation is as effective as arthoscopy and can be performed at the bedside. This closed-system irrigation method may be useful when needle aspiration results in incomplete evacuation or when multiple synovial fluid samples demonstrate different characteristics, indicating the presence of loculating pockets of infection. Arthroscopic lavage has been increasingly used in the treatment of septic arthritis of the knee. A recent study demonstrated that this method may also be effective for deep joints, such as the hip. Arthroscopy is advantageous in that extensive debridement can be performed with a small incision, thereby allowing for a more rapid and effective rehabilitation period. Further study of the efficacy of tidal irrigation and arthroscopy needs to be performed. Arthroscopy is a less invasive technique than open surgery and provides much better irrigation and visualization than needle aspiration (58). Aspiration under radiologic imaging or open surgical drainage with vigorous exploration and debridement is recommended for hip infections as well as for joint infections possessing adhesions or loculated areas of abscess (59, 87).
Arthrotomy should be used when an infected joint must be decompressed urgently because of neuropathy or compromised blood supply, when the infected joint is inaccessible by less invasive methods (such as the hip and sometimes the shoulder), when the joint has been damaged by preexisting disease, when bacterial arthritis is complicated by osteomyelitis, and when the less invasive methods of treatment fail. Also, when the isolated pathogen (e.g., P. aeruginosa) can be treated only with aminoglycosides, arthrotomy is often required to overcome the low oxygen tensions and pH of the infected joint. A number of patient factors have also been implicated as negative prognostic indicators in septic arthritis and may increase the need for invasive surgical intervention. Some of these factors include a long duration between symptom onset and treatment, complicated joint site, extremes of age, underlying illness, immunosuppressive drugs, underlying joint diseases, presence of juxta-articular osteomyelitis, and chronic failure of less invasive methods to clear the infection as demonstrated by positive blood or syovial fluid cultures, continued back pain, and restriction of motion.
During the acute phase of bacterial arthritis, patient rest and optimal joint position are absolutely required to prevent the occurrence of joint deformation and deleterious contractures. Splints may be used to maintain proper joint position (hip in neutral rotation in some abduction, knee in full extension, elbow in flexion at 90°, and forearm in neutral rotation). Isotonic exercise is often helpful in preventing muscular atrophy. Following the acute phase, early physical therapy and aggressive mobilization are vital for optimal recovery (156, 161).
TREATMENT OF GONOCOCCAL ARTHRITIS
The treatment of gonococcal arthritis strongly relies on appropriate antimicrobial therapy, and surgical procedures besides aspiration are rarely indicated. Patients should initially be hospitalized and should remain in this setting until 1 or 2 days following symptom resolution or for the entire length of therapy for patients who cannot be relied on to comply with treatment. The patient should also return 1 week after completion of the prescribed antibiotic regimen for follow-up, and clinicians should obtain and analyze synovial fluid samples of all previously affected joints at this time.
In the United States, nearly 30% of all N. gonorrhoeae isolates are resistant to penicillin, tetracycline, or both (26). Therefore, the Centers for Disease Control and Prevention suggest that patients with gonococcal arthritis should be treated initially with parenteral ceftriaxone (1 g intramuscularly [i.m.] or i.v. every 24 h) (25). Therapeutically equivalent doses of other broad-spectrum cephalosporins (e.g., cefotaxime 1 g i.v. every 8 h or ceftizoxime 1 g i.v. every 8 h) are effective (10). The tetracyclines (except in pregnant women) or penicillins may be used if the infecting organism is proven to be susceptible. Skin lesions may continue to develop for up to 2 days following the initiation of antibiotic therapy. These lesions are often due to the localization of host complement complexes in the skin. The treatment may be switched to oral antibiotic therapy with a quinolone (ciprofloxacin 500 mg orally twice a day or ofloxacin 400 mg orally twice a day), except in pregnant women or young children, or cefixime (400 mg orally twice a day) to complete 7 to 10 days of total therapy 48 h after clinical improvement begins (39). It should be noted that resistance to ceftriaxone and cefixime is rare in the United States. Also, only approximately 1.4% of all N. gonorrhoeae isolates demonstrate intermediate or full resistance to ciprofloxacin (26). Therefore, these antibiotics are still highly effective in the treatment of DGI.
Patients indicating penicillin allergies should be given spectinomycin (2 g i.m. every 12 h). However, the clinician must be aware that this antibiotic shows poor activity against pharyngeal N. gonorrhoeae infection. Therefore, cultures should be performed on these patients 3 to 5 days following treatment. In the Western world, spectinomycin-resistant gonococcal isolates are a rare occurrence (26). However, resistance rates of up to 10% of isolates for this antibiotic have been demonstrated in a few countries (67). Alternative antibiotics in the β-lactam-allergic patient may be ciprofloxacin (500 mg i.v. every 12 h) or ofloxacin (400 mg i.v. every 12 h). Children weighing more than 45 kg should be treated with a single daily dose of ceftriaxone (50 mg/kg and a maximum dose of 2 g, i.m. or i.v.) for 10 to 14 days. For children weighing less than 45 kg, a 7-day parenteral ceftriaxone regimen (50 mg/kg and a maximum dose of 1 g, i.m. or i.v. in a single daily dose) is recommended. In geographic areas with high rates of N. gonorrhoeae and Chlamydia trachomatis coinfection, doxycycline or azithromycin may be added to the antibiotic treatment regimen since the cost of therapy for chlamydia is often lower than the cost of testing (25).
Surgical management of the affected joint is usually not necessary, with the exception of the initial joint aspiration for synovial fluid sample collection at presentation. The diminution of symptoms is often rapid in these patients, and so subsequent joint drainage is often unnecessary. However, in cases of persistent effusion, the affected joint should be repeatedly drained as needed. In rare, very advanced cases, tidal irrigation, arthroscopy, and arthrotomy may play a role in disease resolution. While most patients display a dramatic response to therapy, some patients, especially those with large joint effusions or high erythrocyte sedimentation rates, may require longer hospital stays.
A SPECIAL CASE: PROSTHETIC JOINT INFECTIONS
The increased use of implanted prosthetic joints has provided a physiological niche for pathogenic organisms to cause septic arthritis. In fact, prosthetic joint implantation and replacement is the single most common cause of joint infections. The prevalence of infection after total knee or hip arthroplasty is estimated to be approximately 1 to 2%, while in patients with rheumatoid arthritis, the incidence rate can climb to 4.4% (13).
If the infection is of recent onset (<3 months after surgery), it was probably the result of surgical contamination. In this setting, Staphylococcus epidermidis predominates as the major isolate. However, late-onset infection is usually caused by hematogenous seeding, and S. aureus is the most common isolate, followed by Streptococcus spp., gram-negative bacilli, and anaerobes.
An inherent problem associated with implants is their propensity to be coated by host proteins such as fibrinogen and fibronectin shortly after implantation (49). In the short term, fibrinogen and fibrin seem to be the dominant coating host proteins, while fibronectin becomes dominant in the long term since fibrinogen and fibrin are degraded. Implants can then act as a colonization surface to which bacteria readily adhere, like the fibrinogen and fibrin binding receptors of S. aureus.
Also, implants are often responsible for reduced blood flow and local immunocompromise by impairing natural killer, lymphocytic, and phagocytic cell activities. These implanted devices have also been linked to decreases in the amount of superoxide, a mediator of bacterial killing within professional phagocytic blood cells (136). Another mechanism by which implanted medical devices produce local immune compromise is through frustrated phagocytosis (136). In this case, professional phagocytes may undergo apoptosis when encountering a substrate of a size that is beyond their phagocytic capability. The resulting release of reactive products of oxygen and lysosomal enzymes may cause accidental host tissue damage and local vascular insufficiency, thereby increasing the predisposition of osteomyelitis development. Also, some of the normal phagocytic processes are devoted to removal of the implant foreign material (particularly with metals, methylmethacrylate, and polyglycolic acid), thereby utilizing the energy and resources of the immune system that would normally be used to fight infection (146, 147, 184). Therefore, prosthetic implants not only provide a substrate for bacterial adherence but also limit the ability of the host to adequately deal with the infection. Once colonized, bacteria (such as staphylococcal species) are able to synthesize a “slime” layer, termed the glycocalyx or biofilm. This layer prevents the inward diffusion of a number of antimicrobials and host phagocytic cells, allowing bacteria to escape from the effects of antimicrobial therapy and host clearance (19). Once an implant is colonized and osteomyelitis ensues, the only treatment option is implant removal. Finally, it has been shown that nasal carriage of the organism by the patient was the most important risk factor associated with surgical site infection (81). Therefore, it may be a worthwhile goal to eliminate S. aureus nasal carriage prior to invasive procedures.
The risk of implant infection may be increased by a number of factors. First, certain joint replacements are more susceptible to infection because they remain close to the surface and have poor soft tissue coverage (e.g., total elbow arthroplasties) (163) or require prolonged surgery. Second, certain patient populations are at increased risk because of underlying conditions or systemic diseases, including patients with diabetes mellitus and rheumatoid arthritis (40); also, patients who are elderly, obese, or malnourished or who have undergone prior surgery at the implantation site are at risk. Third, polymethylmethacrylate bone cement may be inhibitory to the activity of leukocytes and complement function; also, the heat released during polymethylmethacrylate polymerization may kill the juxtaposed cortical bone, thereby creating a nonvascularized area and providing the bacteria with a lush growth environment while being sealed off from the circulating host defenses.
The clinical presentation of prosthetic joint infections of early onset (<3 months postimplantation) is much like that of acute septic arthritis and includes joint swelling, pain, leukocytosis, and a febrile response. In contrast, patients with late-onset infections, while demonstrating an elevated erythrocyte sedimentation rate, are often afebrile (50%), lack leukocytosis, and have less pronounced clinical features and gradually progressive joint pain. Imaging studies can be used but are often unable to distinguish between hardware loosening, a noninfectious inflammatory response, and active infection. Therefore, accurate diagnosis depends on collecting periprosthetic and fluid samples by needle aspiration in suspected cases of infected prosthetic knees or by arthrotomy in cases involving infected hips.
If caught rapidly, early-onset prosthetic joint infections may be successfully treated with antibiotics alone or in combination with debridement without prosthesis removal, especially when rifampin in combination with another culture-directed antibiotic is used. However, in most cases, the disease has progressed to a state in which the hardware must be eventually removed to cure or arrest the bone infection. A two-stage procedure of implant removal and debridement (stage 1) and reimplantation (stage 2) is recommended. This procedure should focus on the state of the patient rather than the specific organism when determining the interval between stages since recurrence is usually associated with the quality of the initial debridement, not with the infecting organism. Earlier attempts at arthrodesis rather than repeated attempts at reimplantation are recommended. On occasion, patients can be given suppressive oral antibiotic therapy if they refuse implant replacement or if surgery is prohibitive. Nevertheless, prosthesis removal will eventually have to be performed. Another 4- to 6-week course of culture directed antibiotic therapy should be administered following the last major surgery. Since recurrence rates have been found to be up to 60% in patients with rheumatoid arthritis, these patients should be monitored.
PROGNOSIS
A permanent reduction in joint function is seen in approximately 40% of patients with nongonococcal septic arthritis but ranges between 10 and 73% (5, 77-79). This wide range of observed morbidity reflects the dependence of therapy success on host, bacterial, and diagnostic and treatment factors. The mortality associated with this disease is usually between 5 and 20% and is often a result of the transient or chronic bacteremia that causes most cases of septic arthritis (5, 77-79, 86). This high rate has not changed significantly over the last 40 years, even with present-day diagnostic and treatment options (41).
The results of treatment vary greatly with the number of indicators of poor prognosis. Patients who start treatment after experiencing symptoms for 7 days or more demonstrate a poor outcome. Therefore, prompt diagnosis and rapid initiation of therapy are of the utmost importance in limiting the morbidity associated with septic arthritis. In addition, early physical therapy and aggressive mobilization are important for optimal recovery (156, 161). A delay in diagnosis can also lead to a longer time being taken to clear the joint infection with appropriate therapy. An extended time (>6 days) required to sterilize the joint is another indicator of poor prognosis (70, 137).
The outcome in patients with septic arthritis due to some of the more virulent organisms such as superantigen-producing S. aureus and certain gram-negative bacilli is poor in spite of the use of optimal therapy (58, 87). Elderly patients demonstrate a high mortality (19 to 33%) associated with septic arthritis since they often have preexisting medical conditions (e.g., diabetes mellitus) and joint diseases (e.g., osteoarthritis and rheumatoid arthritis) (30, 107, 181). In addition, these patients are more susceptible to a number of infections than are younger adults (30, 107, 181). The decline in natural and induced immunity in elderly patients causes a generalized reduction in the immune response to foreign antigens. The greater susceptibility to infections is due to the effects of age on the immune system and to immuno suppression caused by age-related illnesses. Specifically, the deficient immune response to foreign antigens results from the loss of thymic and T-lymphocyte function (mainly related to the production and response to IL-2) and associated decrease in antibody production by B cells (14). Underlying joint disease (e.g., osteoarthritis or rheumatoid arthritis) is another indicator that despite optimal treatment, the patient has a poor prognosis despite optimal treatment (41, 55). This poor prognosis is often due to a delayed diagnosis since the clinical symptoms of septic arthritis are often mistaken for symptoms related to the preexisting joint disease.
Patients who present with polyarticular septic nongonococcal arthritis have a very poor prognosis (aa. 30% mortality) due to the associated bacteremia and a reduced ability to resist the infection. Polyarticular septic nongonococcal arthritis may result in even higher rates of mortality when seen in patients infected with staphylococcal species (up to 56% mortality) or those with concomitant diagnosis of rheumatoid arthritis (up to 49% mortality) (41).
The prognosis for patients with gonococcal arthritis is very favorable, with a rapid diminution of symptoms and a full return of joint function. In rare cases of DGI (i.e., 1 to 3% of cases), complications such as endocarditis, pericarditis, osteomyelitis, pyomyositis, perihepatitis, and meningitis may occur.
REFERENCES
- 1.Abdelnour, A., T. Bremell, R. Holmdahl, and A. Tarkowski. 1994. Role of T lymphocytes in experimental Staphylococcus aureus arthritis. Scand. J. Immunol. 39:403-408. [DOI] [PubMed] [Google Scholar]
- 2.Albus, A., R. D. Arbeit, and J. C. Lee. 1991. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect. Immun. 59:1008-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Eissa, Y. A., A. M. Kambal, M. N. al-Nasser, S. A. al-Habib, I. M. al-Fawaz, and F. A. al-Zamil. 1990. Childhood brucellosis: a study of 102 cases. Pediatr. Infect. Dis. J. 9:74-79. [PubMed] [Google Scholar]
- 4.Al-Suleiman, S. A., E. M. Grimes, and H. S. Jonas. 1983. Disseminated gonococcal infections. Obstet. Gynecol. 61:48-51. [PubMed] [Google Scholar]
- 5.Andersen, K., F. N. Bennedbaek, and B. L. Hansen. 1994. Septic arthritis. Ugeskr. Laeg. 156:3871-3875. (In Danish.) [PubMed]
- 6.Arvand, M., S. Bhakdi, B. Dahlback, and K. T. Preissner. 1990. Staphylococcus aureus alpha-toxin attack on human platelets promotes assembly of the prothrombinase complex. J. Biol. Chem. 265:14377-14381. [PubMed] [Google Scholar]
- 7.Ayoub, E. M., and H. A. Majeed. 2000. Poststreptococcal reactive arthritis. Curr. Opin. Rheumatol. 12:306-310. [DOI] [PubMed] [Google Scholar]
- 8.Baer, P. A., J. Tenenbaum, A. G. Fam, and H. Little. 1986. Coexistent septic and crystal arthritis. Report of four cases and literature review. J Rheumatol. 13:604-607. [PubMed] [Google Scholar]
- 9.Barton, L. L., L. M. Dunkle, and F. H. Habib. 1987. Septic arthritis in childhood. A 13-year review. Am. J. Dis. Child. 141:898-900. [DOI] [PubMed] [Google Scholar]
- 10.Bayer, A. S. 1980. Gonococcal arthritis syndromes: an update on diagnosis and management. Postgrad. Med. 67:200-208. [DOI] [PubMed] [Google Scholar]
- 11.Bayer, A. S., P. M. Sullam, M. Ramos, C. Li, A. L. Cheung, and M. R. Yeaman. 1995. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect. Immun. 63:3634-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bengtson, S., and K. Knutson. 1991. The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop. Scand. 62:301-311. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Yehuda, A., and M. E. Weksler. 1992. Host resistance and the immune system. Clin. Geriatr. Med. 8:701-711. [PubMed] [Google Scholar]
- 15.Bittini, A., P. L. Dominguez, P. M. Martinez, L. F. Lopez, I. Monteagudo, and L. Carreno. 1985. Comparison of bone and gallium-67 imaging in heroin users' arthritis. J. Nucl. Med. 26:1377-1381. [PubMed] [Google Scholar]
- 16.Black, J., T. L. Hunt, P. J. Godley, and E. Matthew. 1987. Oral antimicrobial therapy for adults with osteomyelitis or septic arthritis. J. Infect. Dis. 155:968-972. [DOI] [PubMed] [Google Scholar]
- 17.Boden, M. K., and J. I. Flock. 1994. Cloning and characterization of a gene for a 19 kDa fibrinogen-binding protein from Staphylococcus aureus. Mol. Microbiol. 12:599-606. [DOI] [PubMed] [Google Scholar]
- 18.Bowerman, S. G., N. E. Green, and G. A. Mencio. 1997. Decline of bone and joint infections attributable to Haemophilus influenzae type b. Clin. Orthop. Relat. Res. 341:128-133. [PubMed] [Google Scholar]
- 19.Brause, B. D. 1986. Infections associated with prosthetic joints. Clin. Rheum. Dis. 12:523-536. [PubMed] [Google Scholar]
- 20.Bremell, T., and A. Tarkowski. 1995. Preferential induction of septic arthritis and mortality by superantigen-producing staphylococci. Infect. Immun. 63:4185-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Britigan, B. E., M. S. Cohen, and P. F. Sparling. 1985. Gonococcal infection: a model of molecular pathogenesis. N. Engl. J. Med. 312:1683-1694. [DOI] [PubMed] [Google Scholar]
- 22.Buckholz, J. M. 1987. The surgical management of osteomyelitis: with special reference to a surgical classification. J. Foot Surg. 26:S17-S24. [PubMed] [Google Scholar]
- 23.Buxton, T. B., J. P. Rissing, J. A. Horner, K. M. Plowman, D. F. Scott, T. J. Sprinkle, and G. K. Best. 1990. Binding of a Staphylococcus aureus bone pathogen to type I collagen. Microb. Pathog. 8:441-448. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. 1998. 1998 guidelines for treatment of sexually transmitted diseases. Morb. Mortal. Wkly. Rep. 47:1-111. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. 2000. Sexually transmitted disease surveillance 1999, p. 15-24. U.S. Department of Health and Human Services, Public Health Service, Washington, D.C.
- 27.Cheung, A. I., S. J. Projan, R. E. Edelstein, and V. A. Fischetti. 1995. Cloning, expression, and nucleotide sequence of a Staphylococcus aureus gene (fbpA) encoding a fibrinogen-binding protein. Infect. Immun. 63:1914-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chutka, D. S., J. M. Evans, K. C. Fleming, and K. G. Mikkelson. 1995. Symposium on geriatrics. Part I. Drug prescribing for elderly patients. Mayo Clin. Proc. 70:685-693. [DOI] [PubMed] [Google Scholar]
- 29.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 30.Cooper, C., and M. I. Cawley. 1986. Bacterial arthritis in the elderly. Gerontology 32:222-227. [DOI] [PubMed] [Google Scholar]
- 31.Cucurull, E., and L. R. Espinoza. 1998. Gonococcal arthritis. Rheum. Dis. Clin. North Am. 24:305-322. [DOI] [PubMed] [Google Scholar]
- 32.Cuellar, M. L., L. H. Silveira, and L. R. Espinoza. 1992. Fungal arthritis. Ann. Rheum. Dis. 51:690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dagan, R. 1993. Management of acute hematogenous osteomyelitis and septic arthritis in the pediatric patient. Pediatr. Infect. Dis. J. 12:88-92. [DOI] [PubMed] [Google Scholar]
- 34.Deesomchok, U., and T. Tumrasvin. 1990. Clinical study of culture-proven cases of non-gonococcal arthritis. J. Med. Assoc. Thail. 73:615-623. [PubMed] [Google Scholar]
- 35.De Jonghe, M., and G. Glaesener. 1995. Type B Haemophilus influenzae infections. Experience at the Pediatric Hospital of Luxembourg]. Bull. Soc. Sci. Med. Grand-Duche Luxemb. 132:17-20. (In French.) [PubMed] [Google Scholar]
- 36.Delauche, M. C., M. F. Kahn, and A. Ryckewaert. 1981. Gonococcal arthritis. Rev. Rhum. Mal. Osteoartic. 48:127-132. (In French.) [PubMed] [Google Scholar]
- 37.Deng, G. M., I. M. Nilsson, M. Verdrengh, L. V. Collins, and A. Tarkowski. 1999. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 5:702-705. [DOI] [PubMed] [Google Scholar]
- 38.Deng, G. M., and A. Tarkowski. 2000. The features of arthritis induced by CpG motifs in bacterial DNA. Arthritis Rheum. 43:356-364. [DOI] [PubMed] [Google Scholar]
- 39.Dickie, A. S. 1986. Current concepts in the management of infections in bones and joints. Drugs 32:458-475. [DOI] [PubMed] [Google Scholar]
- 40.Dougherty, S. H., and R. L. Simmons. 1989. Endogenous factors contributing to prosthetic device infections. Infect. Dis. Clin. North Am. 3:199-209. [PubMed] [Google Scholar]
- 41.Dubost, J. J., I. Fis, P. Denis, R. Lopitaux, M. Soubrier, J. M. Ristori, J. L. Bussiere, J. Sirot, and B. Sauvezie. 1993. Polyarticular septic arthritis. Medicine (Baltimore) 72:296-310. [DOI] [PubMed] [Google Scholar]
- 42.Ellington, J. K., S. S. Reilly, W. K. Ramp, M. S. Smeltzer, J. F. Kellam, and M. C. Hudson. 1999. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb. Pathog. 26:317-323. [DOI] [PubMed] [Google Scholar]
- 43.Erdman, W. A., F. Tamburro, H. T. Jayson, P. T. Weatherall, K. B. Ferry, and R. M. Peshock. 1991. Osteomyelitis: characteristics and pitfalls of diagnosis with MR imaging. Radiology 180:533-539. [DOI] [PubMed] [Google Scholar]
- 44.Essawi, T., T. Na'was, A. Hawwari, S. Wadi, A. Doudin, and A. I. Fattom. 1998. Molecular, antibiogram and serological typing of Staphylococcus aureus isolates recovered from Al-Makased Hospital in East Jerusalem. Trop. Med. Int. Health 3:576-583. [DOI] [PubMed] [Google Scholar]
- 45.Fairley, C. K., I. Boyd, P. Purcell, and J. McNeil. 1992. Flucloxacillin jaundice. Lancet 339:679. [DOI] [PubMed] [Google Scholar]
- 46.Ferreras, M., F. Hoper, S. M. Dalla, D. A. Colin, G. Prevost, and G. Menestrina. 1998. The interaction of Staphylococcus aureus bi-component gamma-hemolysins and leucocidins with cells and lipid membranes. Biochim. Biophys. Acta 1414:108-126. [DOI] [PubMed] [Google Scholar]
- 47.Fink, C. W., and J. D. Nelson. 1986. Septic arthritis and osteomyelitis in children. Clin. Rheum. Dis. 12:423-435. [PubMed] [Google Scholar]
- 48.Fischer, B., P. Vaudaux, M. Magnin, Y. el Mestikawy, R. A. Proctor, D. P. Lew, and H. Vasey. 1996. Novel animal model for studying the molecular mechanisms of bacterial adhesion to bone-implanted metallic devices: role of fibronectin in Staphylococcus aureus adhesion. J. Orthop. Res. 14:914-920. [DOI] [PubMed] [Google Scholar]
- 49.Francois, P., P. Vaudaux, and P. D. Lew. 1998. Role of plasma and extracellular matrix proteins in the physiopathology of foreign body infections. Ann. Vasc. Surg. 12:34-40. [DOI] [PubMed] [Google Scholar]
- 50.Fryden, A., A. Bengtsson, U. Foberg, B. Svenungsson, B. Castor, A. Karnell, R. Schvarcz, B. Lindblom, and E. Kihlstrom. 1990. Early antibiotic treatment of reactive arthritis associated with enteric infections: clinical and serological study. Br. Med. J. 301:1299-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furr, P. M., D. Taylor-Robinson, and A. D. Webster. 1994. Mycoplasmas and ureaplasmas in patients with hypogammaglobulinaemia and their role in arthritis: microbiological observations over twenty years. Ann. Rheum. Dis. 53:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garrido, G., J. J. Gomez-Reino, P. Fernandez-Dapica, E. Palenque, and S. Prieto. 1988. A review of peripheral tuberculous arthritis. Semin. Arthritis Rheum. 18:142-149. [DOI] [PubMed] [Google Scholar]
- 53.Gemmell, C. G., S. C. Goutcher, R. Reid, and R. D. Sturrock. 1997. Role of certain virulence factors in a murine model of Staphylococcus aureus arthritis. J. Med. Microbiol. 46:208-213. [DOI] [PubMed] [Google Scholar]
- 54.Gleckman, R. A. 1995. Antibiotic concerns in the elderly. A clinician's perspective. Infect. Dis. Clin. North Am. 9:575-590. [PubMed] [Google Scholar]
- 55.Goldenberg, D. L. 1989. Infectious arthritis complicating rheumatoid arthritis and other chronic rheumatic disorders. Arthritis Rheum. 32:496-502. [DOI] [PubMed] [Google Scholar]
- 56.Goldenberg, D. L. 1998. Septic arthritis. Lancet 351:197-202. [DOI] [PubMed] [Google Scholar]
- 57.Goldenberg, D. L., P. L. Chisholm, and P. A. Rice. 1983. Experimental models of bacterial arthritis: a microbiologic and histopathologic characterization of the arthritis after the intraarticular injections of Neisseria gonorrhoeae, Staphylococcus aureus, group A streptococci, and Escherichia coli. J. Rheumatol. 10:5-11. [PubMed] [Google Scholar]
- 58.Goldenberg, D. L. and A. S. Cohen. 1976. Acute infectious arthritis. A review of patients with nongonococcal joint infections (with emphasis on therapy and prognosis). Am. J. Med. 60:369-377. [DOI] [PubMed] [Google Scholar]
- 59.Goldenberg, D. L., and J. I. Reed. 1985. Bacterial arthritis. N. Engl. J. Med. 312:764-771. [DOI] [PubMed] [Google Scholar]
- 60.Goldenberg, D. L., J. I. Reed, and P. A. Rice. 1984. Arthritis in rabbits induced by killed Neisseria gonorrhoeae and gonococcal lipopolysaccharide. J. Rheumatol. 11:3-8. [PubMed] [Google Scholar]
- 61.Gonzalez-Juanatey, C., M. A. Gonzalez-Gay, J. Llorca, F. Crespo, C. Garcia-Porrua, J. Corredoira, J. Vidan, and J. R. Gonzalez-Juanatey. 2001. Rheumatic manifestations of infective endocarditis in non-addicts. A 12-year study. Medicine (Baltimore) 80:9-19. [DOI] [PubMed] [Google Scholar]
- 62.Gouaux, E., M. Hobaugh, and L. Song. 1997. alpha-Hemolysin, gamma-hemolysin, and leukocidin from Staphylococcus aureus: distant in sequence but similar in structure. Protein Sci. 6:2631-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graif, M., M. E. Schweitzer, D. Deely, and T. Matteucci. 1999. The septic versus nonseptic inflamed joint: MRI characteristics. Skeletal Radiol. 28:616-620. [DOI] [PubMed] [Google Scholar]
- 64.Gregg, C. R., M. A. Melly, C. G. Hellerqvist, J. G. Coniglio, and Z. A. McGee. 1981. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J. Infect. Dis. 143:432-439. [DOI] [PubMed] [Google Scholar]
- 65.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 66.Grimminger, F., F. Rose, U. Sibelius, M. Meinhardt, B. Potzsch, R. Spriestersbach, S. Bhakdi, N. Suttorp, and W. Seeger. 1997. Human endothelial cell activation and mediator release in response to the bacterial exotoxins Escherichia coli hemolysin and staphylococcal alpha-toxin. J. Immunol. 159:1909-1916. [PubMed] [Google Scholar]
- 67.Guoming, L., C. Qun, and W. Shengchun. 2000. Resistance of Neisseria gonorrhoeae epidemic strains to antibiotics: report of resistant isolates and surveillance in Zhanjiang, China: 1998 to 1999. Sex. Transm. Dis. 27:115-118. [DOI] [PubMed] [Google Scholar]
- 68.Herrmann, M., P. E. Vaudaux, D. Pittet, R. Auckenthaler, P. D. Lew, F. Schumacher-Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158:693-701. [DOI] [PubMed] [Google Scholar]
- 69.Hildebrand, A., M. Pohl, and S. Bhakdi. 1991. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. J. Biol. Chem. 266:17195-17200. [PubMed] [Google Scholar]
- 70.Ho, G. J., and E. Y. Su. 1982. Therapy for septic arthritis. JAMA 247:797-800. [PubMed] [Google Scholar]
- 71.Holmes, K. K., G. W. Counts, and H. N. Beaty. 1971. Disseminated gonococcal infection. Ann. Intern. Med. 74:979-993. [DOI] [PubMed] [Google Scholar]
- 72.Hudson, M. C., W. K. Ramp, N. C. Nicholson, A. S. Williams, and M. T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19:409-419. [DOI] [PubMed] [Google Scholar]
- 73.Hultgren, O., M. Kopf, and A. Tarkowski. 1999. Outcome of Staphylococcus aureus-triggered sepsis and arthritis in IL-4-deficient mice depends on the genetic background of the host. Eur. J. Immunol. 29:2400-2405. [DOI] [PubMed] [Google Scholar]
- 74.Hunter, J. A., and T. H. Blyth. 1999. A risk-benefit assessment of intra-articular corticosteroids in rheumatic disorders. Drug Saf. 21:353-365. [DOI] [PubMed] [Google Scholar]
- 75.Jackson, M. A., and J. D. Nelson. 1982. Etiology and medical management of acute suppurative bone and joint infections in pediatric patients. J. Pediatr. Orthop. 2:313-323. [PubMed] [Google Scholar]
- 76.Jonsson, K., C. Signas, H. P. Muller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 77.Kaandorp, C. J., H. J. Dinant, M. A. van de Laar, H. J. Moens, A. P. Prins, and B. A. Dijkmans. 1997. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann. Rheum. Dis. 56:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaandorp, C. J., P. Krijnen, H. J. Moens, J. D. Habbema, and D. Van Schaardenburg. 1997. The outcome of bacterial arthritis: a prospective community-based study. Arthritis Rheum. 40:884-892. [DOI] [PubMed] [Google Scholar]
- 79.Kaandorp, C. J., D. Van Schaardenburg, P. Krijnen, J. D. Habbema, and M. A. van de Laar. 1995. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. 38:1819-1825. [DOI] [PubMed] [Google Scholar]
- 80.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalmeijer, M. D., E. van Nieuwland-Bollen, D. Bogaers-Hofman, and G. A. de Baere. 2000. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect. Control Hosp. Epidemiol. 21:319-323. [DOI] [PubMed] [Google Scholar]
- 82.Keat, A. 1990. Sexually transmitted arthritis syndromes. Med. Clin. North Am. 74:1617-1631. [DOI] [PubMed] [Google Scholar]
- 83.Keat, A. 1999. Reactive arthritis. Adv. Exp. Med. Biol. 455:201-206. [DOI] [PubMed] [Google Scholar]
- 84.Kerle, K. K., J. R. Mascola, and T. A. Miller. 1992. Disseminated gonococcal infection. Am. Fam. Physician 45:209-214. [PubMed] [Google Scholar]
- 85.Khachapuridze, G. G., S. G. Nergadze, N. N. Lapiashvili, I. N. Panieva, L. S. Barenfel'd, N. M. Pleskach, and M. S. Imedadze. 1991. The effect of staphylococcal alpha-toxin on DNA replication in human cells. Tsitologiia 33:82-89. (In Russian.) [PubMed] [Google Scholar]
- 86.Klein, R. S. 1988. Joint infection, with consideration of underlying disease and sources of bacteremia in hematogenous infection. Clin. Geriatr. Med 4:375-394. [PubMed] [Google Scholar]
- 87.Knights, E. M. 1982. Infectious arthritis. J. Foot Surg. 21:229-233. [PubMed] [Google Scholar]
- 88.Koch, B., P. Lemmermeier, A. Gause, H. Wilmowsky, J. Heisel, and M. Pfreundschuh. 1996. Demonstration of interleukin-1beta and interleukin-6 in cells of synovial fluids by flow cytometry. Eur. J. Med. Res. 1:244-248. [PubMed] [Google Scholar]
- 89.Konig, B., G. Prevost, and W. Konig. 1997. Composition of staphylococcal bi-component toxins determines pathophysiological reactions. J. Med. Microbiol. 46:479-485. [DOI] [PubMed] [Google Scholar]
- 90.Kuypers, J. M., and R. A. Proctor. 1989. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect. Immun. 57:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lammers, A., P. J. Nuijten, and H. E. Smith. 1999. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol. Lett. 180:103-109. [DOI] [PubMed] [Google Scholar]
- 92.Larrey, D., T. Vial, A. Micaleff, G. Babany, M. Morichau-Beauchant, H. Michel, and J. P. Benhamou. 1992. Hepatitis associated with amoxycillin-clavulanic acid combination report of 15 cases. Gut 33:368-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larribe, M., M. K. Taha, A. Topilko, and C. Marchal. 1997. Control of Neisseria gonorrhoeae pilin gene expression by environmental factors: involvement of the pilA/pilB regulatory genes. Microbiology 143:1757-1764. [DOI] [PubMed] [Google Scholar]
- 94.Le Dantec, L., F. Maury, R. M. Flipo, S. Laskri, B. Cortet, B. Duquesnoy, and B. Delcambre. 1996. Peripheral pyogenic arthritis. A study of one hundred seventy-nine cases. Rev. Rhum. Engl. Ed. 63:103-110. [PubMed] [Google Scholar]
- 95.Lee, J. C., S. Takeda, P. J. Livolsi, and L. C. Paoletti. 1993. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 61:1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levo, Y., and M. Nashif. 1983. Musculoskeletal manifestations of bacterial endocarditis. Clin. Exp. Rheumatol. 1:49-52. [PubMed] [Google Scholar]
- 97.Liebling, M. R., D. G. Arkfeld, G. A. Michelini, M. J. Nishio, B. J. Eng, T. Jin, and J. S. Louie. 1994. Identification of Neisseria gonorrhoeae in synovial fluid using the polymerase chain reaction. Arthritis Rheum. 37:702-709. [DOI] [PubMed] [Google Scholar]
- 98.Littlewood-Evans, A. J., M. R. Hattenberger, C. Luscher, A. Pataki, O. Zak, and T. O'Reilly. 1997. Local expression of tumor necrosis factor alpha in an experimental model of acute osteomyelitis in rats. Infect. Immun. 65:3438-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lopes, J. D., G. F. Da-Mota, C. R. Carneiro, L. Gomes, F. Costa-e-Silvo-Filho, and R. R. Brentani. 1988. Evolutionary conservation of laminin-binding proteins. Brazilian J. Med. Biol. Res. 21:1269-1273. [PubMed] [Google Scholar]
- 100.Lopes, J. D., M. dos Reis, and R. R. Brentani. 1985. Presence of laminin receptors in Staphylococcus aureus. Science 229:275-277. [DOI] [PubMed] [Google Scholar]
- 101.Lowe, A. M., D. T. Beattie, and R. L. Deresiewicz. 1998. Identification of novel staphylococcal virulence genes by in vivo expression technology. Mol. Microbiol. 27:967-976. [DOI] [PubMed] [Google Scholar]
- 102.Luhmann, J. D., and S. J. Luhmann. 1999. Etiology of septic arthritis in children: an update for the 1990s. Pediatr. Emerg. Care 15:40-42. [DOI] [PubMed] [Google Scholar]
- 103.Lundy, D. W., and D. K. Kehl. 1998. Increasing prevalence of Kingella kingae in osteoarticular infections in young children. J. Pediatr. Orthop. 18:262-267. [PubMed] [Google Scholar]
- 104.Martin, D., L. G. Mathieu, J. Lecomte, and J. deRepentigny. 1986. Adherence of gram-positive and gram-negative bacterial strains to human lung fibroblasts in vitro. Exp. Biol. 45:323-334. [PubMed] [Google Scholar]
- 105.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 106.McGavin, M. H., D. Krajewska-Pietrasik, C. Ryden, and M. Hook. 1993. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect. Immun. 61:2479-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGuire, N. M., and C. A. Kauffman. 1985. Septic arthritis in the elderly. J. Am. Geriatr. Soc. 33:170-174. [DOI] [PubMed] [Google Scholar]
- 108.Menzies, B. E., and I. Kourteva. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 66:5994-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meyers, O. L., and P. J. Commerford. 1977. Musculoskeletal manifestations of bacterial endocarditis. Ann. Rheum. Dis. 36:517-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mitchell, M., B. Howard, J. Haller, D. J. Sartoris, and D. Resnick. 1988. Septic arthritis. Radiol. Clin. North Am. 26:1295-1313. [PubMed] [Google Scholar]
- 111.Modic, M. T., W. Pflanze, D. H. Feiglin, and G. Belhobek. 1986. Magnetic resonance imaging of musculoskeletal infections. Radiol. Clin. North Am. 24:247-258. [PubMed] [Google Scholar]
- 112.Modun, B., D. Kendall, and P. Williams. 1994. Staphylococci express a receptor for human transferrin: identification of a 42-kilodalton cell wall transferrin-binding protein. Infect. Immun. 62:3850-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morgan, D. S., D. Fisher, A. Merianos, and B. J. Currie. 1996. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol. Infect. 117:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naidu, A. S., M. Andersson, and A. Forsgren. 1992. Identification of a human lactoferrin-binding protein in Staphylococcus aureus. J. Med. Microbiol. 36:177-183. [DOI] [PubMed] [Google Scholar]
- 115.Na'was, T., A. Hawwari, E. Hendrix, J. Hebden, R. Edelman, M. Martin, W. Campbell, R. Naso, R. Schwalbe, and A. I. Fattom. 1998. Phenotypic and genotypic characterization of nosocomial Staphylococcus aureus isolates from trauma patients. J. Clin. Microbiol. 36:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nelson, J. D. 1971. Antibiotic concentrations in septic joint effusions. N. Engl. J. Med. 284:349-353. [DOI] [PubMed] [Google Scholar]
- 117.Nelson, J. D., and W. C. Koontz. 1966. Septic arthritis in infants and children: a review of 117 cases. Pediatrics 38:966-971. [PubMed] [Google Scholar]
- 118.Nilsson, I. M., O. Hartford, T. Foster, and A. Tarkowski. 1999. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect. Immun. 67:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nilsson, I. M., J. C. Lee, T. Bremell, C. Ryden, and A. Tarkowski. 1997. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect. Immun. 65:4216-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nilsson, I. M., J. M. Patti, T. Bremell, M. Hook, and A. Tarkowski. 1998. Vaccination with a recombinant fragment of collagen adhesin provides protection against Staphylococcus aureus-mediated septic death. J. Clin. Investig. 101:2640-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nilsson, I. M., M. Verdrengh, R. G. Ulrich, S. Bavari, and A. Tarkowski. 1999. Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J. Infect. Dis. 180:1370-1373. [DOI] [PubMed] [Google Scholar]
- 122.O'Brien, J. P., D. L. Goldenberg, and P. A. Rice. 1983. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 62:395-406. [PubMed] [Google Scholar]
- 123.Osiri, M., K. Ruxrungtham, S. Nookhai, Y. Ohmoto, and U. Deesomchok. 1998. IL-1beta, IL-6 and TNF-alpha in synovial fluid of patients with non-gonococcal septic arthritis. Asian Pac. J. Allergy Immunol. 16:155-160. [PubMed] [Google Scholar]
- 124.Park, P. W., J. Rosenbloom, W. R. Abrams, and R. P. Mecham. 1996. Molecular cloning and expression of the gene for elastin-binding protein (ebpS) in Staphylococcus aureus. J. Biol. Chem. 271:15803-15809. [DOI] [PubMed] [Google Scholar]
- 125.Patel, A. H., P. Nowlan, E. D. Weavers, and T. Foster. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55:3103-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Hook. 1992. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 127.Peacock, S. J., N. P. Day, M. G. Thomas, A. R. Berendt, and T. J. Foster. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23-31. [DOI] [PubMed] [Google Scholar]
- 128.Prevost, G., B. Cribier, P. Couppie, P. Petiau, G. Supersac, V. Finck-Barbancon, H. Monteil, and Y. Piemont. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63:4121-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Puliti, M., C. von Hunolstein, F. Bistoni, P. Mosci, G. Orefici, and L. Tissi. 2000. Influence of interferon-gamma administration on the severity of experimental group B streptococcal arthritis. Arthritis Rheum. 43:2678-2686. [DOI] [PubMed] [Google Scholar]
- 130.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, U. Vogel, M. Frosch, C. Elkins, H. K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 36:915-928. [DOI] [PubMed] [Google Scholar]
- 131.Reilly, S. S., M. C. Hudson, J. F. Kellam, and W. K. Ramp. 2000. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone 26:63-70. [DOI] [PubMed] [Google Scholar]
- 132.Renno, T., M. Hahne, and H. R. MacDonald. 1995. Proliferation is a prerequisite for bacterial superantigen-induced T cell apoptosis in vivo. J. Exp. Med. 181:2283-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rice, P. A., and D. L. Kasper. 1982. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J. Clin. Investig. 70:157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riegels-Nielsen, P., N. Frimodt-Moller, M. Sorensen, and J. S. Jensen. 1989. Antibiotic treatment insufficient for established septic arthritis. Staphylococcus aureus experiments in rabbits. Acta Orthop. Scand. 60:113-115. [DOI] [PubMed] [Google Scholar]
- 135.Roberts-Thomson, P. J., M. Rischmueller, R. A. Kwiatek, M. Soden, M. J. Ahern, W. R. Hill, and R. A. Geddes. 1992. Rheumatic manifestations of infective endocarditis. Rheumatol. Int. 12:61-63. [DOI] [PubMed] [Google Scholar]
- 136.Roisman, F. R., D. T. Walz, and A. E. Finkelstein. 1983. Superoxide radical production by human leukocytes exposed to immune complexes: inhibitory action of gold compounds. Inflammation 7:355-362. [DOI] [PubMed] [Google Scholar]
- 137.Rosenthal, J., G. G. Bole, and W. D. Robinson. 1980. Acute nongonococcal infectious arthritis. Evaluation of risk factors, therapy, and outcome. Arthritis Rheum. 23:889-897. [DOI] [PubMed] [Google Scholar]
- 138.Rosenthall, L., R. Lisbona, M. Hernandez, and A. Hadjipavlou. 1979. 99mTc-PP and 67Ga imaging following insertion of orthopedic devices. Radiology 133:717-721. [DOI] [PubMed] [Google Scholar]
- 139.Roy, S., and J. Bhawan. 1975. Ultrastructure of articular cartilage in pyogenic arthritis. Arch. Pathol. 99:44-47. [PubMed] [Google Scholar]
- 140.Rozadilla, A., J. M. Nolla, L. Mateo, J. del Blanco, J. Valverde, and D. Roig. 1992. Septic arthritis induced by pyogenic germs in patients without parenteral drug addiction. Analysis of 44 cases. Med. Clin. 98:527-530. (In Spanish.) [PubMed] [Google Scholar]
- 141.Ryan, M. J., R. Kavanagh, P. G. Wall, and B. L. Hazleman. 1997. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br. J. Rheumatol. 36:370-373. [DOI] [PubMed] [Google Scholar]
- 142.Ryden, C., H. S. Tung, V. Nikolaev, A. Engstrom, and A. Oldberg. 1997. Staphylococcus aureus causing osteomyelitis binds to a nonapeptide sequence in bone sialoprotein. Biochem. J. 327:825-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sakiniene, E., T. Bremell, and A. Tarkowski. 1997. Inhibition of nitric oxide synthase (NOS) aggravates Staphylococcus aureus septicaemia and septic arthritis. Clin. Exp. Immunol. 110:370-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salom, I. L., and K. Davis. 1995. Prescribing for older patients: how to avoid toxic drug reactions. Geriatrics 50:37-40, 43. [PubMed] [Google Scholar]
- 145.Sandrasegaran, K., A. Saifuddin, A. Coral, and W. P. Butt. 1994. Magnetic resonance imaging of septic sacroiliitis. Skeletal Radiol. 23:289-292. [DOI] [PubMed] [Google Scholar]
- 146.Santavirta, S., Y. T. Konttinen, V. Bergroth, and M. Gronblad. 1991. Lack of immune response to methyl methacrylate in lymphocyte cultures. Acta Orthop. Scand. 62:29-32. [DOI] [PubMed] [Google Scholar]
- 147.Santavirta, S., Y. T. Konttinen, T. Saito, M. Gronblad, E. Partio, P. Kemppinen, and P. Rokkanen. 1990. Immune response to polyglycolic acid implants. J. Bone Joint Surg. [Br.] 72:597-600. [DOI] [PubMed] [Google Scholar]
- 148.Sapico, F. L., J. A. Liquete, and R. J. Sarma. 1996. Bone and joint infections in patients with infective endocarditis: review of a 4-year experience. Clin. Infect. Dis. 22:783-787. [DOI] [PubMed] [Google Scholar]
- 149.Saraux, A., H. Taelman, P. Blanche, J. Batungwanayo, J. Clerinx, A. Kagame, L. Kabagabo, J. Ladner, P. Van de Perre, P. Le Goff, and J. Bogaerts. 1997. HIV infection as a risk factor for septic arthritis. Br. J. Rheumatol. 36:333-337. [DOI] [PubMed] [Google Scholar]
- 150.Sasaki, S., S. Nishikawa, T. Miura, M. Mizuki, K. Yamada, H. Madarame, Y. I. Tagawa, Y. Iwakura, and A. Nakane. 2000. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect. Immun. 68:2424-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sattar, M. A., S. P. Barrett, and M. I. Cawley. 1983. Concentrations of some antibiotics in synovial fluid after oral administration, with special reference to antistaphylococcal activity. Ann. Rheum. Dis. 42:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Saxon, A., D. C. Adelman, A. Patel, R. Hajdu, and G. B. Calandra. 1988. Imipenem cross-reactivity with penicillin in humans. J. Allergy Clin. Immunol. 82:213-217. [DOI] [PubMed] [Google Scholar]
- 153.Schattner, A., and K. L. Vosti. 1998. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore) 77:122-139. [DOI] [PubMed] [Google Scholar]
- 154.Schlievert, P. M. 1993. Role of superantigens in human disease. J. Infect. Dis. 167:997-1002. [DOI] [PubMed] [Google Scholar]
- 155.Seltzer, S. E. 1984. Value of computed tomography in planning medical and surgical treatment of chronic osteomyelitis. J. Comput. Assist. Tomogr. 8:482-487. [DOI] [PubMed] [Google Scholar]
- 156.Sharp, J. T., M. D. Lidsky, J. Duffy, and M. W. Duncan. 1979. Infectious arthritis. Arch. Intern. Med. 139:1125-1130. [PubMed] [Google Scholar]
- 157.Shiv, V. K., A. K. Jain, K. Taneja, and S. K. Bhargava. 1990. Sonography of hip joint in infective arthritis. Can. Assoc. Radiol. J. 41:76-78. [PubMed] [Google Scholar]
- 158.Shmerling, R. H., T. L. Delbanco, A. N. Tosteson, and D. E. Trentham. 1990. Synovial fluid tests. What should be ordered? JAMA 264:1009-1014. [PubMed] [Google Scholar]
- 159.Silva, J. J., and K. Wilson. 1979. Disseminated gonococcal infections (DGI). Cutis 24:601-606. [PubMed] [Google Scholar]
- 160.Sloan, R. W. 1992. Principles of drug therapy in geriatric patients. Am. Fam. Physician. 45:2709-2718. [PubMed] [Google Scholar]
- 161.Smith, J. W. 1990. Infectious arthritis. Infect. Dis. Clin. North Am. 4:523-538. [PubMed] [Google Scholar]
- 162.Smith, R. L., D. J. Schurman, G. Kajiyama, M. Mell, and E. Gilkerson. 1987. The effect of antibiotics on the destruction of cartilage in experimental infectious arthritis. J. Bone Joint Surg. [Am.] 69:1063-1068. [PubMed] [Google Scholar]
- 163.Sourmelis, S. G., F. D. Burke, and J. P. Varian. 1986. A review of total elbow arthroplasty and an early assessment of the Liverpool elbow prosthesis. J. Hand Surg. [Br.] 11:407-413. [DOI] [PubMed] [Google Scholar]
- 164.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 165.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 166.Switalski, L. M., J. M. Patti, W. Butcher, A. G. Gristina, P. Speziale, and M. Hook. 1993. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol. Microbiol. 7:99-107. [DOI] [PubMed] [Google Scholar]
- 167.Tarkowski, A., L. V. Collins, I. Gjertsson, O. H. Hultgren, I. Jonsson, E. Sakiniene, and M. Verdrengh. 2001. Model systems: modeling human staphylococcal arthritis and sepsis in the mouse. Trends Microbiol. 9:321-326. [DOI] [PubMed] [Google Scholar]
- 168.Taylor-Robinson, D., and A. Keat. 1999. Septic and aseptic arthritis: a continuum? Baillières Best Pract. Res. Clin. Rheumatol. 13:179-192. [DOI] [PubMed] [Google Scholar]
- 169.Tehranzadeh, J., F. Wang, and M. Mesgarzadeh. 1992. Magnetic resonance imaging of osteomyelitis. Crit. Rev. Diagn. Imaging 33:495-534. [PubMed] [Google Scholar]
- 170.Thomas, M. G., S. Peacock, S. Daenke, and A. R. Berendt. 1999. Adhesion of Staphylococcus aureus to collagen is not a major virulence determinant for septic arthritis, osteomyelitis, or endocarditis. J. Infect. Dis. 179:291-293. [DOI] [PubMed] [Google Scholar]
- 171.Thomas, P., J. Allal, D. Bontoux, F. Rossi, J. Y. Poupet, J. P. Petitalot, and B. Becq-Giraudon. 1984. Rheumatological manifestations of infective endocarditis. Ann. Rheum. Dis. 43:716-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thomas, P. A., and B. Mullan. 1995. Avid In-111 labeled WBC accumulation in a patient with active osteoarthritis of both knees. Clin. Nucl. Med. 20:973-975. [DOI] [PubMed] [Google Scholar]
- 173.Toivanen, A., and P. Toivanen. 2000. Reactive arthritis. Curr. Opin. Rheumatol. 12:300-305. [DOI] [PubMed] [Google Scholar]
- 174.Tokunaga, H., and T. Nakae. 1992. Calcium ion-mediated regulation of the alpha-toxin pore of Staphylococcus aureus. Biochim. Biophys. Acta 1105:125-130. [DOI] [PubMed] [Google Scholar]
- 175.Tomita, T., and Y. Kamio. 1997. Molecular biology of the pore-forming cytolysins from Staphylococcus aureus, alpha- and gamma-hemolysins and leukocidin. Biosci. Biotechnol. Biochem. 61:565-572. [DOI] [PubMed] [Google Scholar]
- 176.Traeger, S. M., M. F. Bonfiglio, J. A. Wilson, B. R. Martin, and N. A. Nackes. 1995. Seizures associated with ofloxacin therapy. Clin. Infect. Dis. 21:1504-1506. [DOI] [PubMed] [Google Scholar]
- 177.Vandenesch, F., S. J. Projan, B. Kreiswirth, J. Etienne, and R. P. Novick. 1993. Agr-related sequences in Staphylococcus lugdunensis. FEMS Microbiol. Lett. 111:115-122. [DOI] [PubMed] [Google Scholar]
- 178.Vassilopoulos, D., P. Chalasani, R. L. Jurado, K. Workowski, and C. A. Agudelo. 1997. Musculoskeletal infections in patients with human immunodeficiency virus infection. Medicine (Baltimore) 76:284-294. [DOI] [PubMed] [Google Scholar]
- 179.Vastag, B. 2001. New vaccine decreases rate of nosocomial infections. JAMA 285:1565-1566. [PubMed] [Google Scholar]
- 180.Verdrengh, M., and A. Tarkowski. 1998. Granulocyte-macrophage colony-stimulating factor in Staphylococcus aureus-induced arthritis. Infect. Immun. 66:853-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vincent, G. M., and J. D. Amirault. 1990. Septic arthritis in the elderly. Clin. Orthop. 251:241-245. [PubMed] [Google Scholar]
- 182.von Essen, R., and A. Holtta. 1986. Improved method of isolating bacteria from joint fluids by the use of blood culture bottles. Ann. Rheum. Dis. 45:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vyskocil, J. J., M. A. McIlroy, T. A. Brennan, and F. M. Wilson. 1991. Pyogenic infection of the sacroiliac joint. Case reports and review of the literature. Medicine (Baltimore) 70:188-197. [DOI] [PubMed] [Google Scholar]
- 184.Wang, J. Y., B. H. Wicklund, R. B. Gustilo, and D. T. Tsukayama. 1997. Prosthetic metals impair murine immune response and cytokine release in vivo and in vitro. J. Orthop. Res. 15:688-699. [DOI] [PubMed] [Google Scholar]
- 185.Welkon, C. J., S. S. Long, M. C. Fisher, and P. D. Alburger. 1986. Pyogenic arthritis in infants and children: a review of 95 cases. Pediatr. Infect. Dis. J. 5:669-676. [DOI] [PubMed] [Google Scholar]
- 186.Wesson, C. A., J. Deringer, L. E. Liou, K. W. Bayles, G. A. Bohach, and W. R. Trumble. 2000. Apoptosis induced by Staphylococcus aureus in epithelial cells utilizes a mechanism involving caspases 8 and 3. Infect. Immun. 68:2998-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wetzler, L. M., K. Barry, M. S. Blake, and E. C. Gotschlich. 1992. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect. Immun. 60:39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wise, C. M., C. R. Morris, B. L. Wasilauskas, and W. L. Salzer. 1994. Gonococcal arthritis in an era of increasing penicillin resistance. Presentations and outcomes in 41 recent cases (1985-1991). Arch. Intern. Med. 154:2690-2695. [DOI] [PubMed] [Google Scholar]
- 189.Yacoub, A., P. Lindahl, K. Rubin, M. Wendel, D. Heinegard, and C. Ryden. 1994. Purification of a bone sialoprotein-binding protein from Staphylococcus aureus. Eur. J. Biochem. 222:919-925. [DOI] [PubMed] [Google Scholar]
- 190.Yagupsky, P., Y. Bar-Ziv, C. B. Howard, and R. Dagan. 1995. Epidemiology, etiology, and clinical features of septic arthritis in children younger than 24 months. Arch. Pediatr. Adolesc. Med. 149:537-540. [DOI] [PubMed] [Google Scholar]
- 191.Yagupsky, P., R. Dagan, C. B. Howard, M. Einhorn, I. Kassis, and A. Simu. 1993. Clinical features and epidemiology of invasive Kingella kingae infections in southern Israel. Pediatrics 92:800-804. [PubMed] [Google Scholar]
- 192.Yagupsky, P., R. Dagan, C. W. Howard, M. Einhorn, I. Kassis, and A. Simu. 1992. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J. Clin. Microbiol. 30:1278-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang, Q. Y., D. DeRyckere, P. Lauer, and M. Koomey. 1992. Gene conversion in Neisseria gonorrhoeae: evidence for its role in pilus antigenic variation. Proc. Natl. Acad. Sci. USA 89:5366-5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhao, Y. X., I. M. Nilsson, and A. Tarkowski. 1998. The dual role of interferon-gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology 93:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zieger, M. M., U. Dorr, and R. D. Schulz. 1987. Ultrasonography of hip joint effusions. Skeletal Radiol. 16:607-611. [DOI] [PubMed] [Google Scholar]


