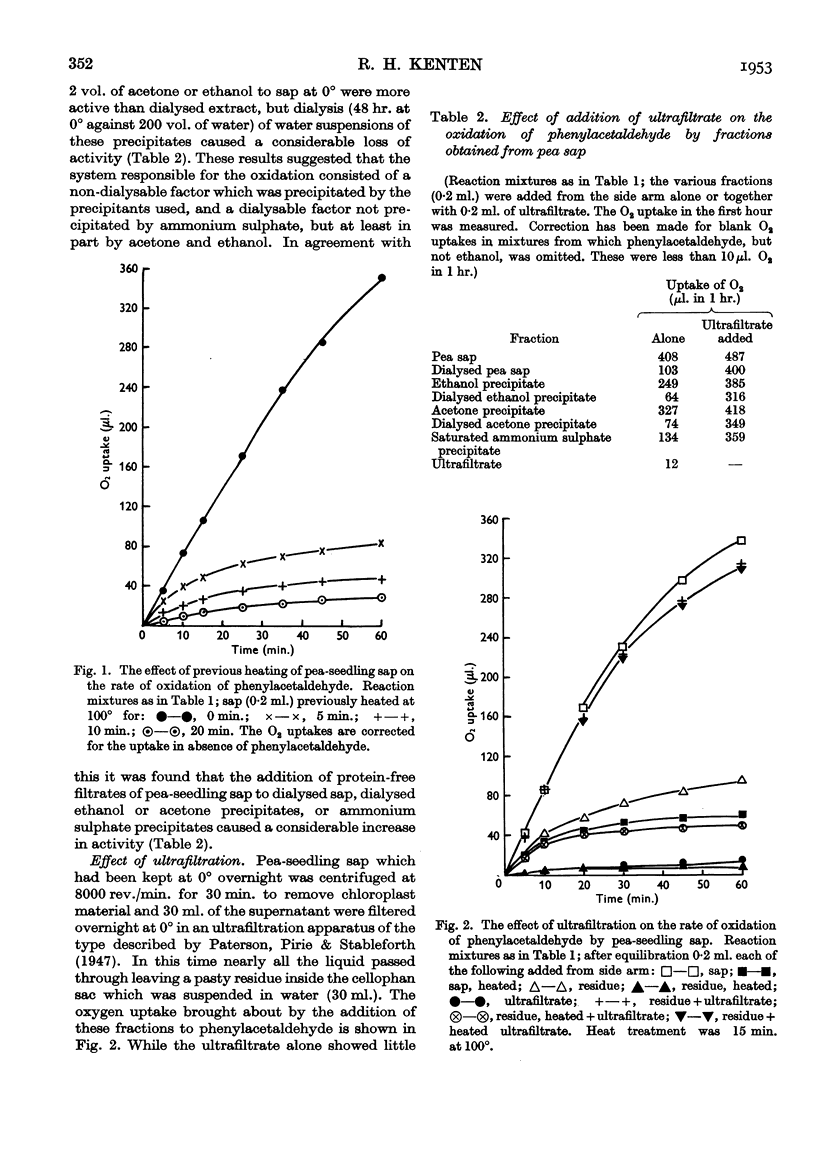
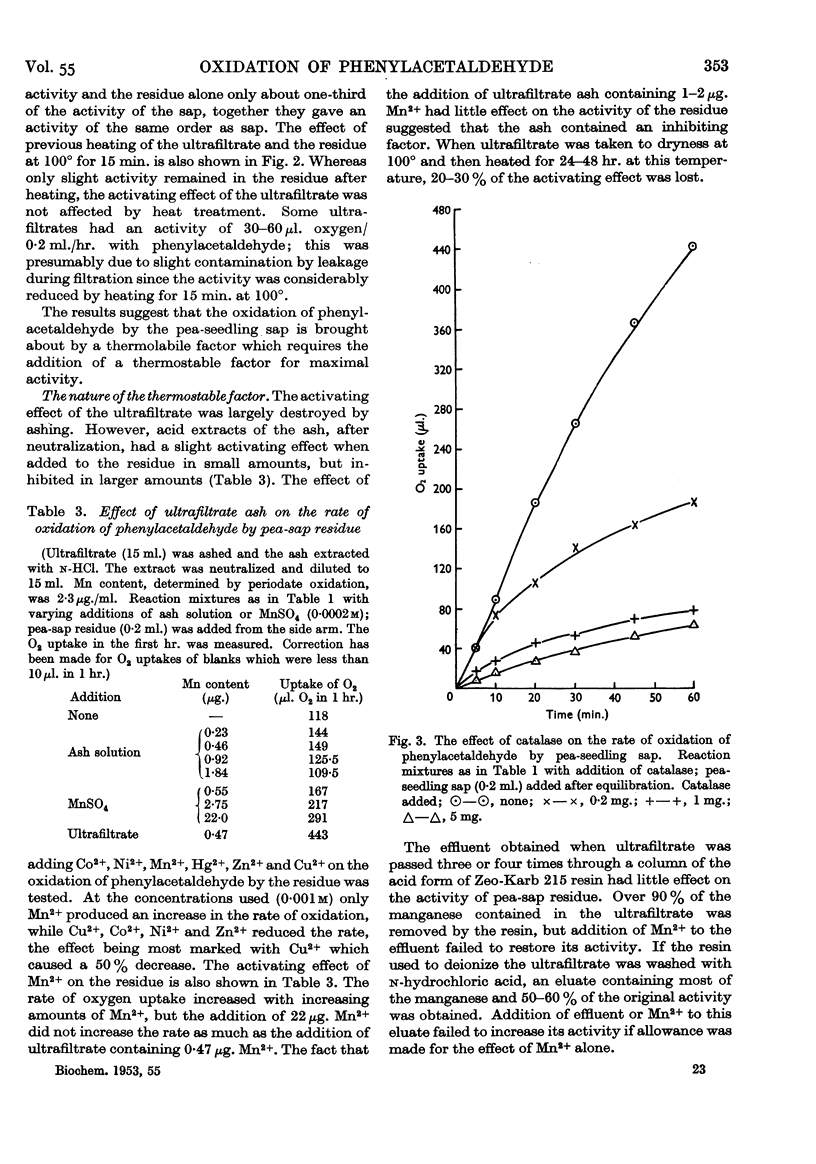
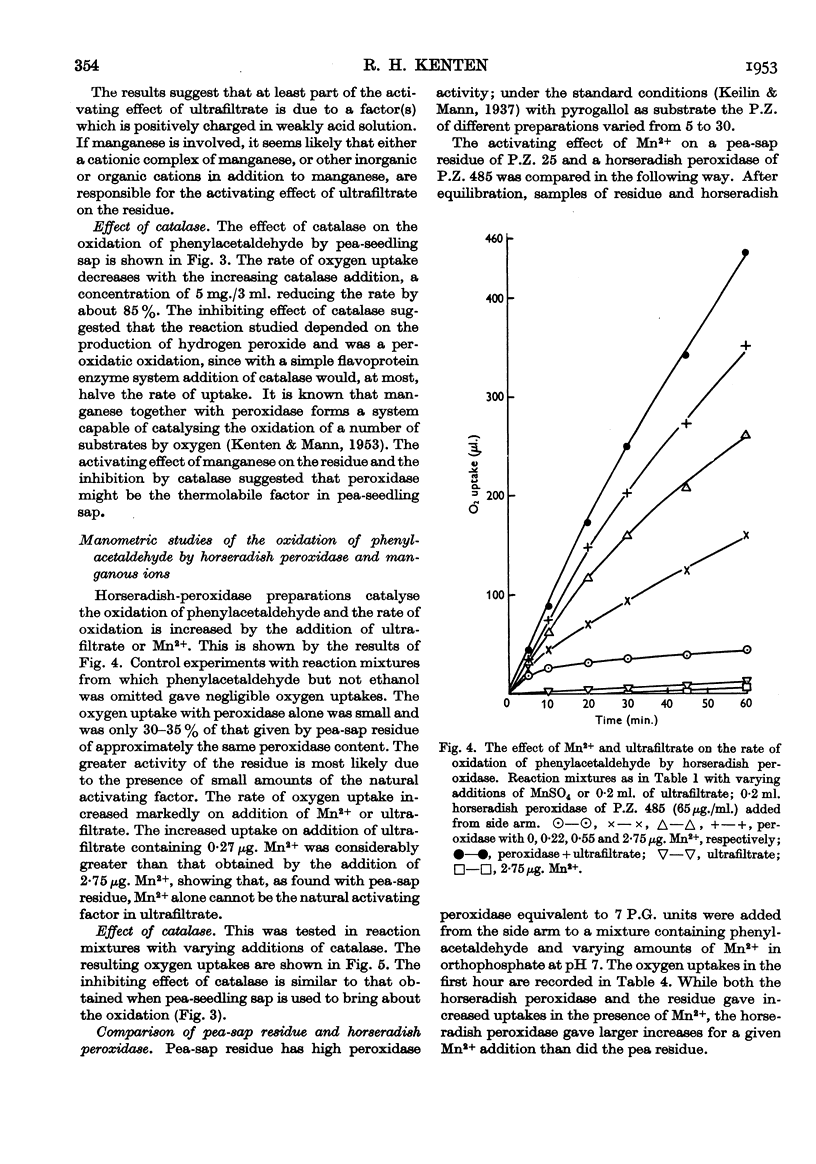
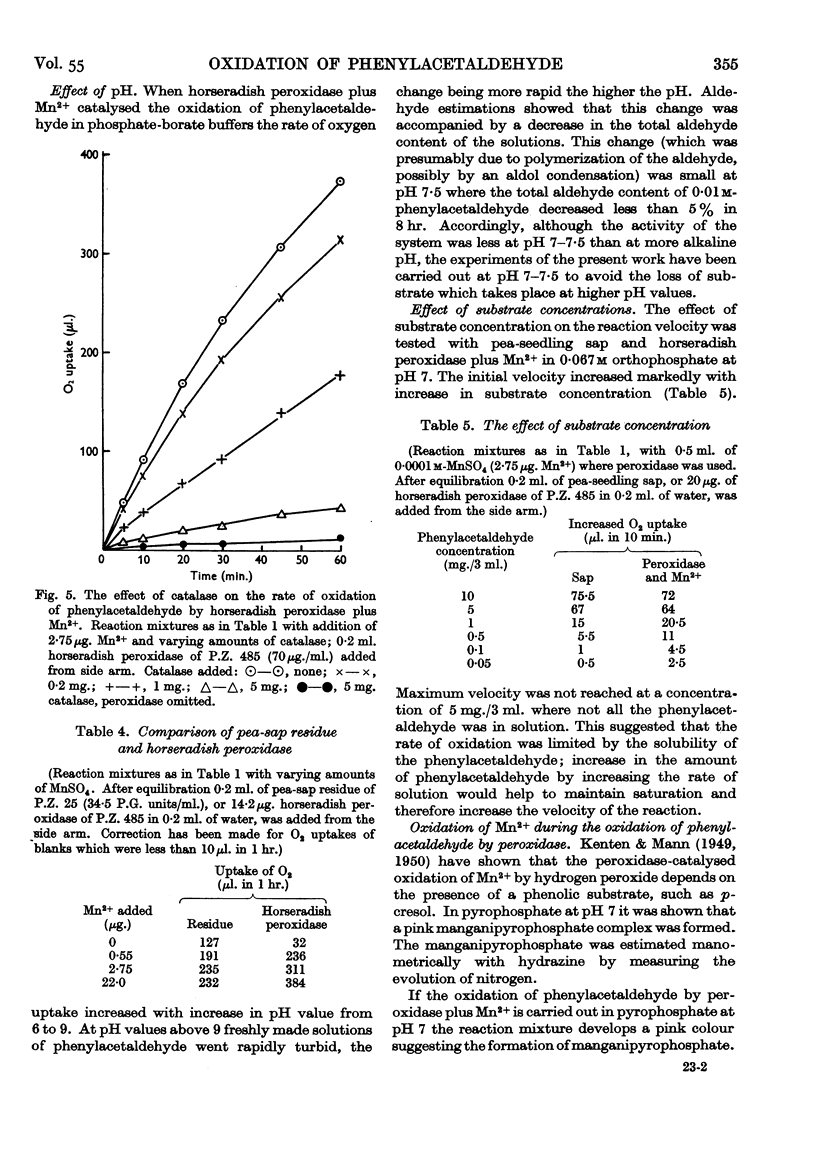
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agner K. The preparation and properties of a highly active catalase from horse liver. Biochem J. 1938 Oct;32(10):1702–1706. doi: 10.1042/bj0321702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN J. B., HENBEST H. B., JONES E. R. H. Synthesis and biological activity of beta-indolylacetaldehyde. Nature. 1952 Feb 23;169(4295):335–335. doi: 10.1038/169335a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HERBERT D. The enzymesubstrate compounds of bacterial catalase and peroxides. Biochem J. 1950 Apr;46(4):402–414. doi: 10.1042/bj0460402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Oxidase and peroxidase reactions in the presence of dihydroxymaleic acid. J Biol Chem. 1952 May;197(2):577–589. [PubMed] [Google Scholar]
- Clift F. P., Cook R. P. A method of determination of some biologically important aldehydes and ketones, with special reference to pyruvic acid and methylglyoxal. Biochem J. 1932;26(6):1788–1799. doi: 10.1042/bj0261788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P. H. Mechanism of Oxidation in the Plant. Part III: Peroxydase. Observations on the Thermostability of the Peroxydase of the Mangold. Biochem J. 1924;18(1):39–46. doi: 10.1042/bj0180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H., MANN P. J. G. The oxidation of amines by extracts of pea seedlings. Biochem J. 1952 Jan;50(3):360–369. doi: 10.1042/bj0500360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H., MANN P. J. G. The oxidation of certain dicarboxylic acids by peroxidase systems in presence of manganese. Biochem J. 1953 Feb;53(3):498–505. doi: 10.1042/bj0530498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H., MANN P. J. G. The oxidation of manganese by enzyme systems. Biochem J. 1952 Sep;52(1):125–130. doi: 10.1042/bj0520125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H., MANN P. J. G. The oxidation of manganese by plant extracts in the presence of hydrogen peroxide. Biochem J. 1949;45(3):255–263. doi: 10.1042/bj0450255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenten R. H., Mann P. J. The oxidation of manganese by peroxidase systems. Biochem J. 1950 Jan;46(1):67–73. doi: 10.1042/bj0460067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. ENZYMATIC CONVERSION OF INDOLE ACETALDEHYDE AND NAPHTHALENE ACETALDEHYDE TO AUXINS. Plant Physiol. 1951 Oct;26(4):697–707. doi: 10.1104/pp.26.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie A. Ascorbic acid content of cornea. Biochem J. 1946;40(1):96–100. doi: 10.1042/bj0400096. [DOI] [PMC free article] [PubMed] [Google Scholar]


